Abstract
Background:
The aim of this study was to perform a literature-based meta-analysis to assess the efficacy of the novel immune checkpoint inhibitors (ICIs) in first-line metastatic renal cell carcinoma (RCC), focusing on the predictive role of PD-L1 expression.
Methods:
The primary outcome was overall survival, and secondary outcomes were progression-free survival (PFS) and objective response. We planned a subgroup analysis for overall survival according to PD-L1 status.
Results:
Five studies were included in the analysis for a total of 4063 cases. Overall survival was greater in PD-L1 positive tumours (HR = 0.49, 95% CI: 0.36–0.67; p < 0.001). The pooled analysis of the unselected cases showed a statistically significative improvement in PFS with the use of ICIs (HR = 0.85, 95% CI: 0.72–0.99; p = 0.04) and we found a greater PFS benefit (HR = 0.65, 95% CI: 0.57–0.74; p < 0.001) in patients with PD-L1 positive tumours.
Conclusions:
This study supports the efficacy of ICIs and, although a significant clinical benefit has been reported in PD-L1 negative patients, a greater efficacy of ICIs was observed in PD-L1 positive patients. More prospective randomized studies are needed to clarify the role of PDL-1 status in metastatic RCC treated with ICIs.
Keywords: immunotherapy, PD-1, PD-L1, renal cancer
Introduction
Renal cell carcinoma (RCC) is the 6th most common malignancy among men and the 10th among women.1 About one in four patients with RCC present with metastatic disease at diagnosis.2 Although vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors were considered the standard first-line treatment for metastatic RCC,3 immune checkpoint inhibitors (ICIs) have recently been added to the therapeutic armamentarium of metastatic RCC on the basis of the CheckMate 025 trial results,4 which demonstrated a survival advantage of the anti-programmed death-1 (PD-1) antibody nivolumab compared with everolimus.
The use of immunotherapy in RCC is not a novelty, as the disease was historically managed with cytokine therapies (interleukin-2 and interferon-alfa). After the good results demonstrated in second-line treatment, ICIs were investigated also in the first-line setting. In 2018, CheckMate 214 showed an overall survival (OS) benefit for first-line nivolumab plus ipilimumab in comparison with sunitinib.5 In addition, atezolizumab in combination with bevacizumab also demonstrated higher efficacy when compared with sunitinib in first-line metastatic RCC.6,7 Finally, two very recent phase III studies investigated the combination of an ICI with axitinib, a highly selective VEGFR inhibitor, in comparison with sunitinib.8,9 The JAVELIN Renal101 study compared avelumab (an anti-PD-L1 antibody) plus axitinib to sunitinib, while KEYNOTE-426 investigated the combination of pembrolizumab (an anti-PD-1 monoclonal antibody) plus axitinib. The aim of this work was to analyse all available clinical data from randomized clinical trials (RCTs), evaluating the impact of ICIs on the outcomes of patients with metastatic RCC, with a main focus on PD-L1 status.
Materials and methods
Data retrieval strategies
We conducted a meta-analysis of RCTs in accordance with the preferred reporting items for systematic reviews guidelines (PRISMA). The databases of PubMed, Embase, ASCO meeting library and Web of Sciences (WOS) were searched for relevant publications using the following search strategy (Supplementary File 1). Publications available in these databases up to 1 February 2019 were analysed. The search criteria were limited to phase II or III studies. The computer search was supplemented with a manual search of the primary studies referenced in all the retrieved review articles.
Inclusion criteria
The studies were screened independently by two authors. Decisions regarding contentious studies were made in consultation with a third author. The studies were identified according to the following inclusion criteria: participants with first line metastatic renal cell carcinoma; a novel immune checkpoint inhibitor alone or in combination treatment as the experimental drug; the presence of a control arm for comparison (sunitinib); a primary outcome of OS and secondary outcomes of progression-free survival (PFS) expressed as hazard ratio (HR), objective response (partial + complete response) expressed as the number of patients over the total who experienced a tumour response. The following exclusion criteria were used: insufficient data were available to estimate the outcomes; animal studies; the size of each arm was fewer than 10 participants; nonrandomized studies; studies in second or further lines of treatment for metastatic renal cell carcinoma.
Data extraction
Two authors independently extracted the relevant data, including name of the trial, first author, publication year, patient characteristics, median treatment duration, study design, survival outcomes expressed as HRs for OS and PFS, number of patients over the total who experienced a response or disease control and toxicity outcomes expressed as the number of patients over the total who experienced a grade 3–4 adverse event. For each trial, the ICI was the experimental arm and sunitinib the control.
Quality assessment
Study quality was assessed using the Jadad 5-item scale, considering randomization, double blinding and withdrawals. The final score ranged from 0 to 5.10 In the event of disagreements, the consensus was achieved in discussion with the corresponding author (GR). The protocol for this systematic review was registered on the PROSPERO International prospective register of systematic reviews (CRD42019125277) and is available in full on the website at http://www.crd.york.ac.uk/PROSPERO.
Statistical analysis
Statistical analysis was performed with Revman 5.3. The summary estimates were generated using a fixed-effect (Mantel–Haenszel method) or a random-effect (DerSimonian–Laird method) model,11,12 depending on the absence or presence of heterogeneity. Statistical heterogeneity was assessed with the Q-test and the I2 statistic. An I2 value of 25%, 50% and 75% was considered to indicate low, moderate and high heterogeneity, respectively.13 When p > 0.1 and I2 < 50%, the fixed-effects model was used; otherwise, the random-effects model was used. Time-to-event variables, including OS, PFS, HRs with 95% confidence intervals (CIs) were calculated for each study. For the dichotomous variables, risk ratios (RRs) with 95% CIs were calculated for each study. A value of p < 0.05 was regarded as statistically significant, and all tests were two-sided. We planned a subgroup analysis for OS according to PD-L1 status, age (with a cut-off of 65 years), sex, previous nephrectomy, Eastern Cooperative Oncology Group (ECOG) performance status (1 versus 0), intermediate- and poor-risk patients (IMDC) prognostic risk. Unfortunately, we were able to perform only the PD-L1 status analysis because we had insufficient data for the analysis of the other subgroups.
Results
Literature review and characteristics of the studies
The search yielded 1554 potentially relevant articles, of which 1103 were excluded as duplicates. After viewing the titles and abstracts of the 451 remaining studies, the full text of 47 studies was retrieved and 5 studies5–9 were ultimately included in the analysis (Figure 1A). The characteristics of the studies are summarized in Table 1. A total of 4063 cases were included; among these, 2082 cases were in the experimental group and 1981 cases in the control group. All studies, with the exception of one,6 were phase III studies. Sunitinib was the control arm in all studies. The median Jadad score was 5, showing a good quality of the included studies (Table 1). Owing to the small number of trials that were included, no publication bias was estimated.
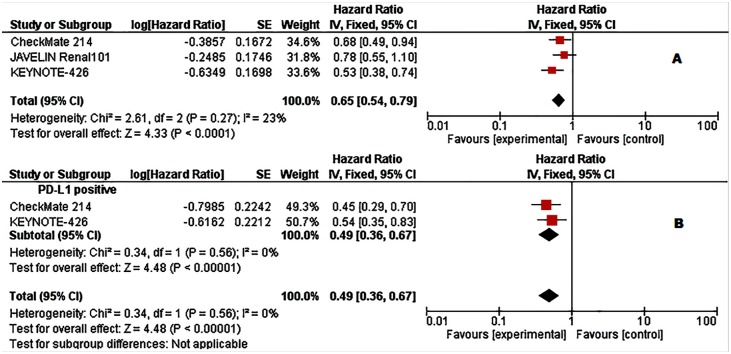
Figure 1.
Forest plots of hazard ratios (HRs) for overall survival (OS) comparing immune checkpoint inhibitors with sunitinib. (A) Unselected patients. (B) PD-L1 positive patients. Data from Checkmate 214 trial has been performed among intermediate- and poor-risk patients.
Table 1.
Characteristics of the analysed trials.
| Study/Reference | Primary endpoint | Number of patients experimental arm | Number of patients control arm | Experimental drug/control arm | PD-L1 positivity |
|---|---|---|---|---|---|
| CheckMate 214/5 | OS, PFS, ORR | 550 | 546 | Nivolumab+ipilimumab/ Sunitinib | ⩾1% Dako PD-L1 IHC 28-8 pharmDx test |
| IMmotion150/6 | PFS | 101/103 | 101 | Atezolizumab+bevacizumab/ Atezolizumab/Sunitinib | ⩾1% (SP142 IHC assay) |
| IMmotion151/7 | OS/PFS | 454 | 461 | Atezolizumab+bevacizumab/ Sunitinib |
⩾1% (SP142 IHC assay) |
| KEYNOTE-426/9 | OS, PFS |
432 | 429 | Pembrolizumab+axitinib/ Sunitinib |
NR |
| JAVELIN Renal 101/8 | OS, PFS | 442 | 444 | Avelumab+axitinib/ Sunitinib |
⩾1% Ventana PD-L1 (SP263) assay |
ORR, objective response rate; OS, overall survival; PFS, progression free survival.
Efficacy data
Patient characteristics, together with efficacy data, are summarized in Tables 2 and 3. Among the total 4063 cases, 1797 (44.2%) were positive for PD-L1 expression (895 in the experimental arm and 902 in control arm). The pooled analysis in unselected cases showed improved OS in the experimental arm (HR = 0.65, 95% CI: 0.54–0.79; p < 0.001, Figure 1A). The analysis was performed using a fixed-effects model (I2 = 23%). However, this analysis involved three studies only.5,8,9
Table 2.
Data on overall survival, progression free survival, objective response of the included studies in unselected and PD-L1 positive patients.
| Study | OS (months) |
PFS (months) |
Objective response rate (%) |
|||
|---|---|---|---|---|---|---|
|
Exp
arm |
C
arm |
Exp
arm |
C
arm |
Exp
arm |
C
Arm |
|
| CheckMate 214/5 | NR | 32.9 | 12.4 | 12.3 | 39 | 32 |
| IMmotion150/6 | NA | NA | 11.7/6.1° | 8.4 | 32/25° | 29 |
| IMmotion151/7 | NA | NA | 11.2 | 8.4 | 37 | 33 |
| KEYNOTE-4269 | NR | NR | 15,1 | 11.1 | 59.3 | 35.7 |
| JAVELIN Renal 101/8 | NR | NR | 13.8 | 8.4 | 51.4 | 25.7 |
| PD-L1 positive patients | ||||||
| CheckMate 214/5 | NR | 19.6* | 22.8* | 5.9* | 58 | 22 |
| IMmotion150/6 | NA | NA | 14.7/5.5° | 7.8 | 46/28° | 27 |
| IMmotion151/7 | NA | NA | 11.2 | 7.7 | 43 | 35 |
| KEYNOTE-4269 | NA | NA | 15,3 | 8.9 | NA | NA |
| JAVELIN Renal 101/8 | NR | NR | 13.8 | 7.2 | 55.2 | 25.2 |
C, control; Exp, Experimental; NA, Not available; NR, not reached.
IMDC Intermediate- and Poor-Risk Patients.
Atezolizumab arm.
Table 3.
Characteristics of patients in the evaluated studies.
| Study | Median age/ male patients % |
ECOG >0% |
Previous nephrectomy % |
PD-L1 positive % |
IMDC prognostic risk poor/ intermediate/ favourable % |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | S | E | S | E | S | E | S | E | S | |
| CheckMate 214/5 | 62/75 | 62/72 | NR | NR | 82 | 80 | 2326* | 25 29* |
23/61/17 | 23/61/16 |
| IMmotion150/6 | 62/7361/75° | 61/78 | 99/ 99° |
93°° | 87/ 86 |
87 | 50/ 52° |
59 | 30/61/ 9 25/67/8° |
21/69/10 |
| IMmotion151/7 | NR | NR | NR | NR | NR | NR | 39 | 40 | NR | NR |
| KEYNOTE-4269 | 62/71 | 61/75 | NR | NR | 83 | 83 | 59 | 62 | 32/55/13 | 30/57/12 |
| JAVELIN Renal 101/8 | 62/71 | 61/77 | NR | NR | 80 | 80 | 62 | 65.3 | 21/61/16 | 22/62/16 |
E, experimental arm; NR, Not reported; S: sunitinib.
IMDC Intermediate- and Poor-Risk Patients.
Atezolizumab arm.
KPS ⩾ 80, n (%).
When we explored the OS in PD-L1 positive tumours, we found an even greater OS improvement in the experimental arm (HR = 0.49, 95% CI: 0.36–0.67; p < 0.001; I2 = 0 Figure 1B). Unfortunately, this analysis was performed using only two of the five studies.5,9
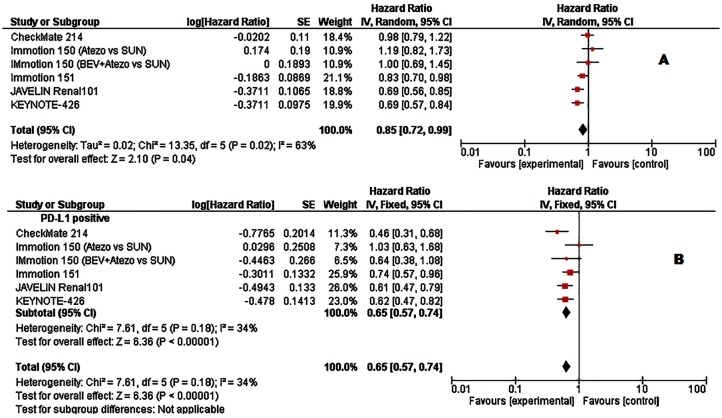
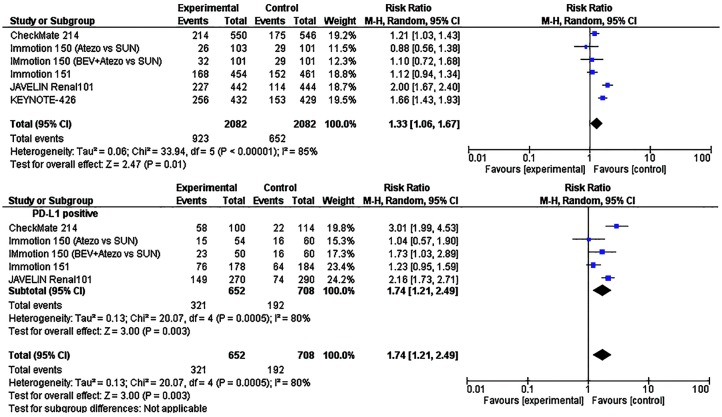
When we looked at PFS, the pooled analysis of the unselected cases showed a statistically significative improvement in PFS with the use of ICIs (HR = 0.85, 95% CI: 0.72–0.99; p = 0.04, Figure 2A). The analysis was performed using a random-effects model (I2 = 64%). As was the case for OS, we found a greater PFS benefit (HR = 0.65, 95% CI: 0.57–0.74; p < 0.001; I2 = 34%, Figure 2B) in patients with PD-L1 positive tumours. Lastly, the objective response was investigated in all studies: a total of 923/2082 (44.3%) patients in the experimental arm and of 623/1981 (31.4%) patients in the control arm showed an objective tumour response. Using the Mantel–Haenszel method, the pooled RR was 1.33 (95% CI 1.06–1.67; p = 0.01; I2 = 85%, random effect model; Figure 3A). Among PD-L1 positive tumours, a total of 321/652 (49.2%) patients in the experimental arm and 176/642 (27.4%) patients in the control arm showed an objective tumour response. Stratification according to PD-L1 expression showed that ICIs significantly improved objective response rate in PDL-1 positive tumours (RR = 1.74 95% CI: 1.21–2.49; p = 0.003, I2 = 80%, random effect model; Figure 3B).
Figure 2.
Forest plots of hazard ratios (HRs) for progression-free survival (PFS) comparing immune checkpoint inhibitors with sunitinib. (A) Unselected patients. (B) PD-L1 positive patients. Data from Checkmate 214 trial has been performed among intermediate- and poor-risk patients.
Figure 3.
Forest plots of hazard ratios (HRs) for overall survival (OS) comparing immune checkpoint inhibitors with sunitinib. (A) Unselected patients. (B) PD-L1 positive patients. Data from IMmotion150; IMmotion151 and CheckMate 214 were estimated from a percentage. Data from Checkmate 214 trial has been performed among intermediate- and poor-risk patients.
Discussion
Immune checkpoint inhibitors have revolutionized the treatment of several tumours.14 These inhibitors were also evaluated in first-line patients with metastatic RCC. The present literature-based meta-analysis of five RCTs involving a total of 4063 patients indicates that the use of ICIs in unselected patients with metastatic RCC results in a statistically significant improvement in OS, PFS and objective response over sunitinib, confirming the clinical efficacy of immunotherapy in first-line treatment of metastatic RCC. In addition, after stratification according to PD-L1 expression, we observed that the efficacy of ICIs was greater in patients with PD-L1 positive tumours (Figures 1–3).
Poor prognosis is reported in patients with PD-L1 overexpression.15 In particular, the prognostic significance of PD-L1 expression in RCC is well established: in fact, PD-L1 positivity was associated with a more aggressive disease in terms of TNM stage, tumour size and outcomes in several studies.16–18 Although PD-L1 positivity has been investigated in several tumour types for its prognostic function, the role of PD-L1 expression as a predictive marker of response to therapy in patients treated with ICIs is still not well defined. In 2016, a meta-analysis involving about 6000 patients with different cancers, including RCC, enrolled in 20 trials, did not show any significant difference in response to treatment in patients with RCC stratified according to PD-L1 status.19 In the Checkmate 025 study, nivolumab was compared with everolimus in second line treatment: however, the activity and efficacy of nivolumab was not influenced by PD-L1 expression.4 Conversely, in the Checkmate 214 study, OS benefit was observed in patients treated with nivolumab plus ipilimumab across PD-L1 expression levels among intermediate- and poor-risk patients.5 In line with these data, a phase Ib trial investigating the combination of axitinib and avelumab in metastatic RCC, showed a higher percentage of objective responses in patients with PD-L1 expression of at least 1%.20 Intra-tumour heterogeneity of PD-L1 expression data may explain these discrepancies. PD-L1 is mainly investigated in primary nephrectomy specimens. However, about 20% of patients with metastatic RCC show a discordance in tumour PD-L1 staining between primary tumours and corresponding metastases.21 Conversely, a higher concordance has been found when only metastatic samples from the same patients were investigated for the presence of PD-L1 expression.21
PFS was the only end point investigated in all 5 studies included in the meta-analysis. We found a high heterogeneity (I2 = 63%), which could be explained by differences in the definition of experimental arm (Tables 1 and 3). In fact, although sunitinib is the control arm in all studies, in the checkmate 214 study, the experimental arm was the combination of nivolumab and ipilimumab; in the Immotion 150 study, one of two experimental arms was atezolizumab in monotherapy. In the remaining studies, the experimental arm was a combination of ICI with an antiangiogenetic agent. To try reducing this heterogeneity, we performed a subgroup analysis including all the studies which have an ICI combination with anti-angiogenic agent in the experimental arm. Data on this analysis are reported in Table 4. Interestingly, the HR of PFS was equal in the analysis of all studies and in the subgroup analysis when we evaluated PD-L1 positive patients.
Table 4.
Data from the subgroup analysis of ICIs in combination with anti-angiogenetic agent compared to sunitinib in RCC.
| PFS HR |
95% CI | p value |
I2
% |
p value | Model | |
|---|---|---|---|---|---|---|
| Unselected patients PD-L1 positive | 0.76 0.65 |
0.69–0.85 0.56–0.76 |
<0.01 <0.01 |
39 0 |
0.18 0.73 |
Fixed |
CI, confidence interval; HR, Hazard ratio; PFS, progression free survival.
This study has several limitations. First, the study was a literature-based rather than patient-based meta-analysis. Second, only a few studies were included in the meta-analysis. There were also differences in the type of experimental arms among the studies, with ICIs alone or in combination with anti-angiogenic agents or with other ICIs. Heterogeneous disease characteristics and treatments could impact on outcome measures; moreover, we did not perform an adverse events analysis. In addition, it should be mentioned that PD-L1 testing itself still has several limitations, such as intra-tumour heterogeneity, the effect of PD-L1 expression on immune cells versus tumour cells, assay procedures and interpretation variability. Finally, data from the Checkmate 214 trial derived from a subgroup of IMDC patients.
Treatment of metastatic RCC is an evolving scenario. Immunotherapy has changed the treatment paradigm of several cancers, including RCC. Although a significant clinical benefit has been reported in PD-L1-negative patients, a greater efficacy of ICIs has been observed in PD-L1-positive patients, underlying the need for better selection of patients in clinical trials and the identification of other predictive biomarkers that would help the selection of optimal candidates for ICIs. More prospective randomized studies are needed to clarify the role of PDL-1 status in metastatic RCC treated with ICIs.
Supplemental Material
Supplemental material, Supplementary_files for Results from a meta-analysis of immune checkpoint inhibitors in first-line renal cancer patients: does PD-L1 matter? by Giandomenico Roviello, Silvia Paola Corona, Gabriella Nesi and Enrico Mini in Therapeutic Advances in Medical Oncology
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Authors declare that no potential conflicts of interest exist (e.g. personal or financial relationships that could influence their actions or sources of funding) and disclose they have received no writing assistance for the manuscript.
ORCID iD: Giandomenico Roviello  https://orcid.org/0000-0001-5504-8237
https://orcid.org/0000-0001-5504-8237
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Giandomenico Roviello, Department of Health Sciences, University of Florence, viale Pieraccini, 6, 50139, Italy.
Silvia Paola Corona, Department of Medical, Surgical and Health Sciences, University of Trieste, Italy.
Gabriella Nesi, Department of Health Sciences, University of Florence, Section of Pathological Anatomy, University Hospital of Florence, Italy.
Enrico Mini, Department of Health Sciences, University of Florence, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999; 17: 2530–2540. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 804–834. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018; 24: 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Motzer RJ, Powles T, Atkins M, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma. J Clin Oncol 2018: 36: 578. [Google Scholar]
- 8. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. Epub ahead of print 16 February 2019. DOI: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. Epub ahead of print 16 February 2019. DOI: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 10. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 12. Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koppolu V, Rekha Vasigala VK. Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma. J Cancer Res Ther 2018; 14: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 15. Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine (Baltimore) 2017; 96: e6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer 2005; 104: 2084–2091. [DOI] [PubMed] [Google Scholar]
- 17. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66: 3381–3385. [DOI] [PubMed] [Google Scholar]
- 18. Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 2007; 13: 1757–1761. [DOI] [PubMed] [Google Scholar]
- 19. Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2016; 100: 88–98. [DOI] [PubMed] [Google Scholar]
- 20. Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose finding and dose-expansion, phase 1b trial. Lancet Oncol 2018; 19: 451–460. [DOI] [PubMed] [Google Scholar]
- 21. Callea M, Albiges L, Gupta M, et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res 2015; 3: 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_files for Results from a meta-analysis of immune checkpoint inhibitors in first-line renal cancer patients: does PD-L1 matter? by Giandomenico Roviello, Silvia Paola Corona, Gabriella Nesi and Enrico Mini in Therapeutic Advances in Medical Oncology