Abstract
Background
Despite the strong association of smoking with cardiovascular disease (CVD) and cerebral stroke, the consequences of smoking have not been elucidated among Iranian populations. This study aimed to assess sex-specific incidence of CVDs among an urban Iranian population with different smoking habits.
Methods
Participants were recruited from the Tehran Lipid and Glucose Study (TLGS). Data on socio-demographic features and smoking habits from a sample of 10,400 individuals (4378 men and 6022 women), aged ≥20 years without prior CVD history were analyzed. Participants were followed up for 12 years for incidence of CVD/CHD events. Men were categorized in six groups, including never-, passive, ex-, passive and ex-, occasional and daily smokers. Women were categorized in three groups, i.e. never smokers, passive smokers and ever smokers. Using cox regression model, adjusted hazard ratios (HRs) of incident CVD/CHD were calculated for each group, given never smokers as the reference.
Results
In men, HR of CVD was 1.13 (95%CI: 0.80–1.59) in passive smokers, 1.23 (95%CI: 0.91–1.66) in ex-smokers, 1.46 (95%CI: 0.90–2.36) in passive and ex-smokers, 2.33 (95%CI: 1.25–4.33) in occasional smokers and 2.05 (95%CI: 1.57–2.67) in daily smokers. In smokers of ≥21 cigarettes/day, HR of CVD was 3.79 (95%CI: 2.25–6.37), with less risk observed in those who smoked lesser numbers of cigarettes/day. Quitters of ≥15 years were almost risk free. In women, none of the HRs of CVD/CHD were significant.
Conclusion
An increased risk of incidence of CVD/CHD was found in current male smokers. To confirm and further elaborate these findings, more data of sex-specific studies are required from culturally diverse urban and rural areas of Iran.
Keywords: Smoking habits, Cardio-vascular outcomes, Iran, TLGS
Background
Cardiovascular diseases (CVDs) are among the most prevalent non-communicable diseases (NCDs) responsible for 31% of all global mortality [1]. Due to fast increasing changes in human lifestyles, prevalence of CVDs is markedly increasing in both the developed and developing countries [2]. According to the recent assessment of GBD (Global Burden of Disease), an estimated 422.7 million individuals suffer from CVDs and 17.9 million annual deaths are attributed to these diseases [3]. The burden of CVDs and its related risk factors as well as its rising trend in Eastern Mediterranean communities, including Iran is very alarming [2]. Ischemic heart disease was found to be the main cause of death in Iran [4], with a 5.9% prevalence for coronary heart disease (CHD) and 3% for stroke, reported recently by a prospective study [5]. Regarding the high prevalence and incidence of CVDs, there has been an increasing trend to ascertain the main determinants and underlying causes of these diseases. Meanwhile, the most prominent risk factors of CVDs are obesity, low physical activity, glucose intolerance, hypertension, emotional stress and smoking [6].
Smoking is the second major modifiable risk factor of CVDs [7] which directly harms and affects cardiac vasculature, and also contributes to development of other cardiovascular risk factors, such as glucose intolerance, dyslipidemia and thrombus formation [8]. Iran has been the most successful country in implementing world health organization (WHO) measures for tobacco control in the Eastern Mediterranean region [9]; according to the latest reports, however, smoking prevalence is high and about a fifth of Iranian men are still smokers [4]. Aside from active smoking, passive smoking or exposure to environmental tobacco smoke (ETS) directly plays a substantial role in CVDs as well [10], and can aggravate the contributing risk factors, viz. metabolic syndrome, vascular inflammation, thrombus formation and atherosclerosis [11].
Despite the relationship between smoking and CVDs being well documented [12], because of geographical variations in patterns, the burden of cardiovascular risk factors [13–15] and the lack of longitudinal studies among Iranians, especially in women and passive smokers [16–18], data available cannot be generalized to all Iranians. The Tehran Lipid and Glucose Study (TLGS), is a large prospective cohort aimed to investigate the risk factors of NCDs among Iranian populations. However, previous studies on the association of smoking with CVDs among the TLGS populations did not include passive and female smokers [19–21]. Thus, the current study, for the first time in Iran, aims to assess the association between smoking and the incidence of CVD/CHD from two aspects, i.e. the smoking habits and smoking intensity among Tehranian men and women.
Methods
Study population
The TLGS is an ongoing community-based study initiated in 1999, and designed to be continued for at least 20 years. The main goal of study is to assess risk factors and different aspects of life style in relation with NCDs among a representative urban Tehranian population [22]. Details of its goals and design have been published previously [22, 23].
The current analysis has been conducted on all TLGS participants who participated in the study between 1999 and 2002; those aged under 20 years, those with positive CVD history, those with missing smoking data and those lost to follow up were excluded. A final sample of 10,400 adult individuals (4378 men and 6022 women) was followed for a median of 12 years. The sampling frame of the current analysis is shown in Fig. 1. The study was approved by the research ethics committee of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences. Written informed consents were obtained from all participants.
Fig. 1.
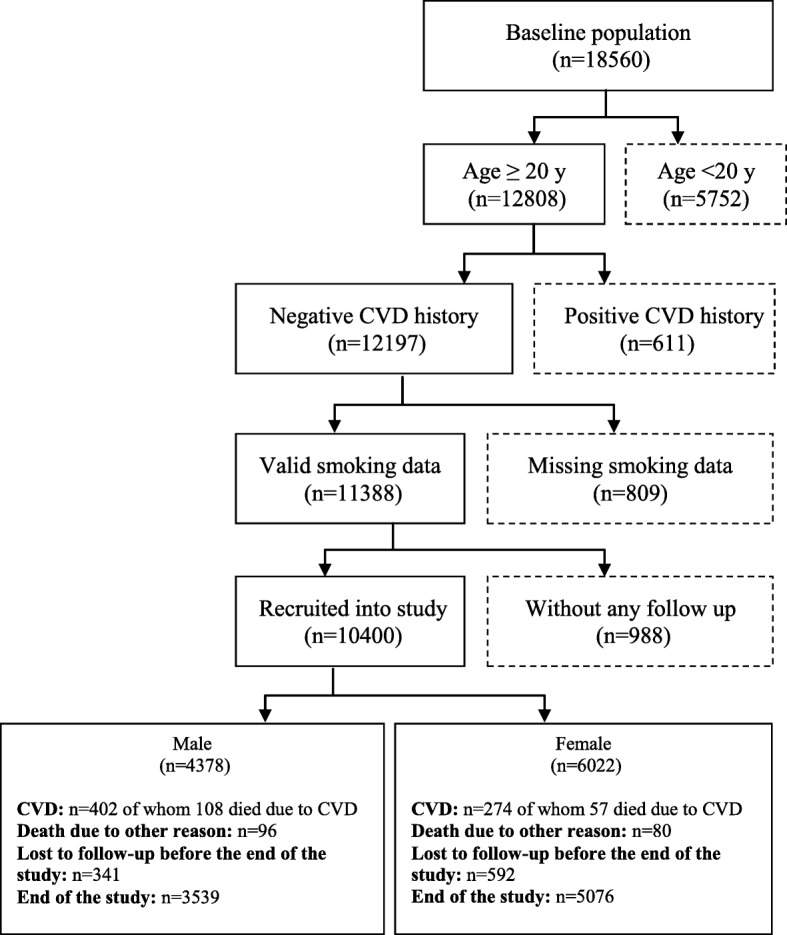
Flow diagram of participants recruitment. Dashed boxes represent exclusion. CVD. Cardiovascular diseases
Baseline measurements
Data on socio-demographic information (age, education level, marital status and occupation), smoking habits and exposure to ETS and any familial history of NCDs were collected by trained interviewers. For physical activity, data was collected using a validated, reliable questionnaire entitled the Modifiable Activity Questionnaire (MAQ) [24]. Psychometric properties of the Iranian version of MAQ have been assessed in the TLGS population and showed acceptable validity and high reliability [25]. Weight was measured by digital scales and was recorded to the nearest 100 g, without shoes and with light clothing. Height was measured standing, without shoes. Body mass index (BMI) was calculated by dividing weight (kg) by square of height (m2). After participants rested for 15 min, a physician measured blood pressure using a mercury sphygmomanometer with appropriate cuff size for each individual. Systolic and diastolic blood pressure were measured twice, at least 30-s apart and the mean of the two measurements was recorded as the participant’s blood pressure. After 12 to 14 h of overnight fasting, blood samples were collected between 7:00 and 9:00 AM to be sent to TLGS research laboratory and biochemically analyzed on the same day. Levels of fasting blood glucose (FBS), total cholesterol, high density lipoprotein (HDL-c) and triglycerides (TG) were enzymatically measured. Low density lipoprotein (LDL-c) was calculated using Friedewald formula [26]. The detailed process of socio-demographic, anthropometric and biochemical assessments has previously been published elsewhere [22, 23].
Definitions of smoking groups
Male participants were categorized into the six following groups: 1) never smokers, 2) passive smokers (exposure to ETS), 3) ex-smokers (quitter since at least a month before interview), 4) passive and ex-smokers (quitters exposed to ETS), 5) active occasional smokers (non-routine smokers) and 6) active daily smokers. In another grouping pattern, based on number of cigarettes smoked per day, active male smokers (whether occasional or daily) have been further subdivided in to three groups: ≤10, 11–20 and ≥ 21 cigarettes per day. Ex-smokers were also subcategorized into those who quit smoking in less than 15 years, and those that had quit 15 years ago or earlier, regardless of passive smoking. Female participants were categorized as never smokers, passive smokers (same as males) and ever smokers (active and ex-smokers pooled together). Different categorization in men and women in the current study was mainly due to the limited number of women who reported themselves as active (n = 146) or past smokers (n = 94) and consequently the limited number of CVD events that occurred in these groups (n = 15) through the study period.
Outcome measures
Participants were followed up annually for any cardiovascular event through phone call interviews by trained nurses. A physician confirmed the diagnosis by home visits or data collection from the medical file or death certification, in case of mortality. A committee consisting of an internist, endocrinologist, cardiologist, epidemiologist and other professionals when needed, confirmed the outcome data; CHD event was defined as any definite myocardial infarction (MI) (diagnostic electrocardiogram (ECG) and biomarkers), probable MI (positive ECG findings and cardiac symptoms plus missing biomarkers or positive ECG findings plus equivocal biomarkers), sudden cardiac death, unstable angina pectoris (new cardiac symptoms or changing symptom pattern and positive ECG findings with normal biomarkers) and angiographically proved coronary artery disease [27]. The definition of CVD event was that of CHD, plus fatal or non-fatal stroke (defined as a new neurological deficit lasting ≥24 h). Details of outcome data analysis and definitions have been described elsewhere [22, 23, 28].
Statistical analysis
All analyses and baseline characteristics are classified by sex. Continuous variables are expressed as mean ± (SD) for normally distributed and as median (Q1-Q3) for skewed ones. To compare continuous variables between different CVD groups, Student t-test and Mann-Whitney test were used for normally distributed and skewed variables, respectively. In different smoking groups, continuous variables were compared by ANOVA in normally distributed- and by Kruskal-Wallis test in skewed ones. Categorical variables are expressed as number (%) and have been compared with Chi-square test, both within different smoking groups and CVD groups. Cox proportional hazard model was used to study the association between different smoking patterns and cardiovascular outcomes. The follow-up duration was defined as the period starting from entrance to study and ending in occurrence of any predefined CVD/CHD event or any attributed mortality or censoring, whichever happened first. Censoring was defined as loss to follow-up or mortality, not attributable to CVD or CHD. Univariate regression model was first performed for all potential confounding factors and those with p-values less than 0.2 were selected for multivariate regression. Adjustment of potential confounding factors was conducted in three cumulative models; the first model was adjusted for age, the second for age and cardio-metabolic risk factors (BMI, systolic and diastolic blood pressure, fasting blood sugar, total cholesterol and triglycerides) and the third model was adjusted for all the above mentioned variables, plus socio-economic factors (education level, reference: college degree; marital status, reference: married and occupation, reference: employed). Within each model, adjusted hazard ratios (HRs) of outcome events were estimated in men and women with 95% confidence intervals (95% CIs) for each smoking pattern, given never smokers as reference. Proportionality assumption of Cox models was assessed using the Schoenfeld residual test and confirmed. Statistical analyses were performed by STATA software version 12, and SPSS software version 15. P-values below 0.05 were considered statistically significant.
Results
A total of 10,400 CVD free participants, aged ≥20 years with complete data on smoking status, were followed during 12 years for occurrence of CVD or CHD events. Mean ages of participants at baseline were 42.73 ± 15.30 and 40.33 ± 14.00 years in men and women, respectively. Over a median 12 year follow up, incidence rate of CVD was 10.6 (95% CI: 9.6–11.7) and 5.1 (95% CI: 4.5–5.8) per 1000 person years, in men and women respectively. Incidence rate of CHD was 9.0 (95% CI: 8.1–10.0) in men and 4.4 (95% CI: 3.9–5.0) in women, per 1000 person years.
Table 1 shows baseline characteristics and CVD/CHD incidence of study participants based on their smoking status. In men, occasional smokers had the highest levels of BMI, TG, total cholesterol and LDL-C; lowest levels of physical activity were found among daily smokers. In women, ever smokers had higher levels of illiteracy compared to never- and passive smokers. The highest rate of CVD/CHD incidence was among ex-smokers in males and among ever smokers in female participants.
Table 1.
Baseline characteristics and CVD/CHD incidence of study participants based on smoking status
| Men (n = 4378) | P value | Women (n = 6022) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Never smoker (n = 1895) (43.3%) | Passive smoker (n = 831) (19%) | Ex-smoker (n = 454) (10.4%) | Passive & ex-smoker (n = 207) (4.7%) | Occasional smoker (n = 98) (2.2%) | Daily smoker (n = 893) (20.4) | Never smoker (n = 4531) (75.2%) | Passive smoker (n = 1251) (20.8%) | Ever smoker (n = 240) (4%) | |||
| Age | 43.97 ± 16.4 | 36.64 ± 13.1 | 51.13 ± 15.7 | 41.40 ± 13.1 | 39.56 ± 12.9 | 42.17 ± 12.6 | < 0.001 | 40.74 ± 14.2 | 37.72 ± 12.9 | 46.28 ± 13.3 | < 0.001 |
| Education level n (%) | |||||||||||
| Illiterate | 107(5.6) | 17(2.1) | 37(8.1) | 10(4.8) | 3(3.1) | 28(3.1) | < 0.001 | 490(10.8) | 111(8.9) | 46(19.2) | < 0.001 |
| Lower than diploma | 663(35.0) | 230(27.7) | 210(46.3) | 72(34.8) | 34(34.7) | 364(40.9) | 1828(40.4) | 517(41.4) | 88(36.7) | ||
| Diploma | 731(38.6) | 426(51.4) | 129(28.4) | 95(45.9) | 40(40.8) | 383(43.0) | 1695(37.4) | 502(40.2) | 84(35.0) | ||
| Higher | 394(20.8) | 156(18.8) | 78(17.2) | 30(14.5) | 21(21.4) | 116(13.0) | 515(11.4) | 120(9.6) | 22(9.2) | ||
| Marital status n (%) | |||||||||||
| Single | 380(20.1) | 259(31.2) | 40(8.8) | 28(13.5) | 18(18.4) | 131(14.7) | < 0.001 | 573(12.6) | 204(16.3) | 12(5.0) | < 0.001 |
| Widowed/divorced | 14(0.7) | 3(0.4) | 8(1.8) | 1(0.5) | 1(1.0) | 9(1.0) | 428(9.4) | 74(5.9) | 48(20.0) | ||
| Married | 1501(79.2) | 569(68.4) | 406(89.4) | 178(86.0) | 79(80.6) | 753(84.3) | 3530(78.0) | 973(77.8) | 180(75.0) | ||
| Occupation n(%) | |||||||||||
| Unemployed | 231(12.2) | 98(11.8) | 37(8.2) | 12(5.8) | 12(12.2) | 55(6.2) | < 0.001 | 2832(84.6) | 1049(83.9) | 197(82.1) | < 0.001 |
| Unemployed with income | 374(19.7) | 35(4.2) | 137(30.2) | 23(11.1) | 10(10.2) | 102(11.4) | 192(4.2) | 28(2.2) | 19(7.9) | ||
| Employed | 1289(68.1) | 698(84.0) | 279(61.6) | 172(83.1) | 76(77.6) | 735(82.4) | 507(11.2) | 174(13.9) | 24(10.0) | ||
| Physical activity n(%) | |||||||||||
| Low | 1036(56.9) | 446(55.7) | 264(59.6) | 101(51.6) | 54(55.7) | 606(68.2) | < 0.001 | 2533(59.4) | 710(59.3) | 143(60.8) | 0.655 |
| Moderate | 307(16.9) | 145(18.1) | 59(13.3) | 34(17.3) | 22(22.7) | 116(13.0) | 619(14.5) | 167(14.0) | 26(11.1) | ||
| High | 477(26.2) | 210(26.2) | 120(27.1) | 61(31.1) | 21(21.6) | 167(18.8) | 1115(26.1) | 320(26.7) | 66(28.1) | ||
| BMI (kg/m2) | 25.95 ± 4.0 | 25.71 ± 4.2 | 26.22 ± 4.0 | 26.28 ± 3.9 | 26.55 ± 4.5 | 25.12 ± 4.2 | < 0.001 | 27.39 ± 5.1 | 27.35 ± 5.2 | 27.94 ± 4.3 | 0.147 |
| FBS (mmol/l) | 5.43 ± 1.60 | 5.19 ± 1.21 | 5.70 ± 1.90 | 5.46 ± 1.74 | 5.45 ± 1.64 | 5.32 ± 1.63 | < 0.001 | 5.36 ± 1.80 | 5.28 ± 1.70 | 5.53 ± 2.12 | 0.094 |
| TG (mmol/l)* | 1.67 (1.11–2.42) | 1.63 (1.10–2.41) | 1.83 (1.31–2.60) | 1.65 (1.15–2.56 | 1.93 (1.34–2.70 | 1.80 (1.20–2.61) | < 0.001 | 1.47 (0.99–2.21) | 1.40 (0.96–2.07) | 1.55 (1.08–2.23) | 0.004 |
| Chol (mmol/l) | 5.17 ± 1.08 | 5.04 ± 1.12 | 5.35 ± 1.14 | 5.11 ± 1.31 | 5.44 ± 1.06 | 5.25 ± 1.14 | < 0.001 | 5.39 ± 1.26 | 5.23 ± 1.19 | 5.55 ± 1.26 | < 0.001 |
| HDL-C (mmol/l) | 1.01 ± 0.24 | 0.98 ± 0.23 | 0.98 ± 0.22 | 0.99 ± 0.23 | 0.97 ± 0.26 | 0.96 ± 0.25 | < 0.001 | 1.17 ± 0.29 | 1.16 ± 0.30 | 1.13 ± 0.27 | 0.179 |
| LDL-C (mmol/l) | 3.29 ± 0.90 | 3.21 ± 0.95 | 3.45 ± 0.94 | 3.21 ± 0.97 | 3.52 ± 0.90 | 3.36 ± 0.96 | < 0.001 | 3.43 ± 1.04 | 3.30 ± 0.96 | 3.62 ± 1.04 | < 0.001 |
| SBP (mmHg) | 122.1 ± 18.9 | 118.04 ± 14.8 | 124.4 ± 19.7 | 119.5 ± 17.5 | 118.2 ± 16.7 | 114.6 ± 15.8 | < 0.001 | 117.4 ± 19.4 | 115.3 ± 18.6 | 117.7 ± 20.4 | 0.002 |
| DBP (mmHg) | 78.6 ± 11.1 | 77.8 ± 10.2 | 78.1 ± 11.2 | 78.4 ± 11.6 | 78.1 ± 11.6 | 74.0 ± 10.46 | < 0.001 | 76.8 ± 10.9 | 76.1 ± 10.4 | 76.2 ± 11.1 | 0.153 |
| With CVD | 161 (8.5) | 42 (5.5) | 61 (13.4) | 19 (9.2) | 11 (11.2) | 104 (11.2) | < 0.001 | 213 (4.7) | 46 (3.7) | 15 (6.3) | 0.133 |
| With CHD | 133 (7.0) | 42 (5.1) | 49 (10.8) | 19 (9.2) | 9 (9.2) | 92 (10.3) | < 0.001 | 183 (4.0) | 40 (3.2) | 13 (5.4) | 0.189 |
Normally distributed continuous variables are presented as mean ± SD and p value was calculated with ANOVA; * Skewed continuous variables are presented as median (Q1-Q3) and p value was calculated with Kruskal-Wallis; Categorical variables are presented as number (%) and p value was calculated with Chi-square test. BMI body mass index, FBS fasting blood sugar, TG triglycerides, Chol cholesterol, HDL-C high density lipoprotein-cholesterol, LDL-C low density lipoprotein-cholesterol, SBP systolic blood pressure, DBP diastolic blood pressure, CVD cardiovascular disease, CHD coronary heart disease. P < 0.05 has been considered significant
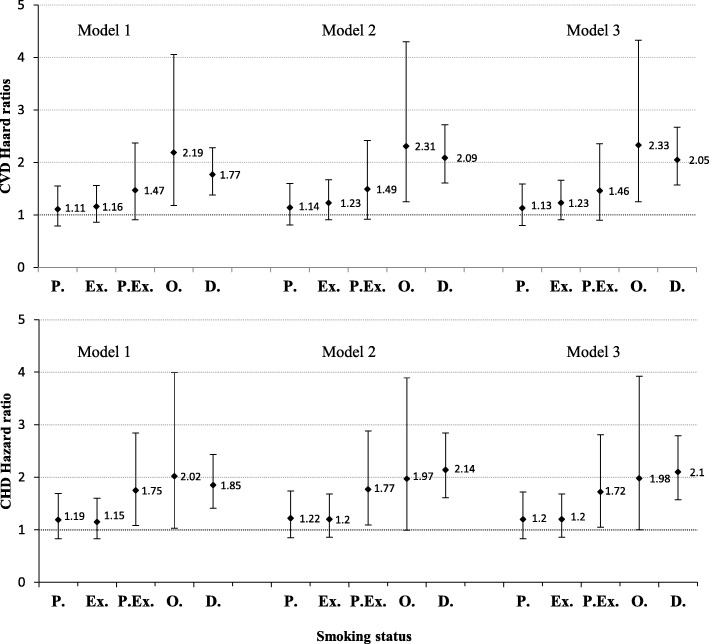
Hazard ratios of CVD/CHD incidence among different smoking groups of men, are shown in Fig. 2. In men, compared with never smokers, even after adjusting for potential confounders (model 3), HRs of CVD incidence were 1.13 (95% CI: 0.80–1.59) in passive smokers, 1.23 (95% CI: 0.91–1.66) in ex-smokers, 1.46 (95% CI: 0.90–2.36) in passive and ex-smokers, 2.33 (95% CI: 1.25–4.33) in occasional smokers and 2.05 (95% CI: 1.57–2.67) in daily smokers. Adjusted HRs of CHD incidence were 1.20 (95% CI: 0.83–1.72) in passive smokers, 1.20 (95% CI: 0.86–1.68) in past smokers, 1.72 (95% CI: 1.05–2.81) in past and passive smokers, 1.98 (95% CI: 1.00–3.92) in occasional smokers and 2.10 (95% CI: 1.57–2.79) in daily smokers, all compared with never smokers.
Fig. 2.
Hazard ratios and 95% confidence intervals (CI) for CVD/CHD incidence in men, among different smoking groups (Ref: never smoker). Tehran Lipid and Glucose study 1999–2010. Model 1. Adjusted for age. Model 2. Adjusted for age and cardio-metabolic risk factors including: body mass index, systolic blood pressure, diastolic blood pressure, triglyceride levels, cholesterol level and fasting blood sugar. Model 3. Adjusted for age, cardio-metabolic risk factors (as above) and socio-economic features including: education (Ref: college degree), marital status (Ref: married) and occupation (Ref: employed). P. Passive smoker; Ex. Ex-smoker; P.Ex. Passive and Ex-smoker; O. Occasional smoker; D. Daily smoker
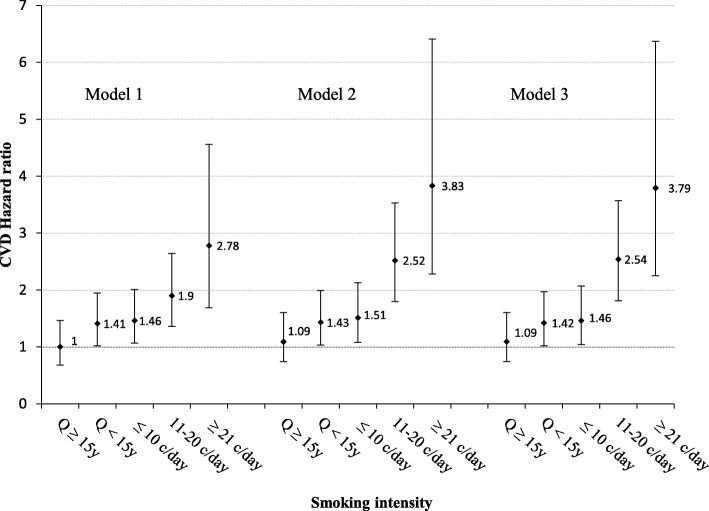
Figure 3 illustrates HRs of CVD based on the number of cigarettes smoked per day or the time passed since last cessation, in active and ex-smoker males respectively. Adjusted for all potential confounders in the third model, HRs of CVD incidence were 1.09 (95% CI: 0.74–1.60) in quitters of ≥15 years ago, 1.42 (95% CI: 1.02–1.97) in quitters of < 15 years ago, 1.46 (95% CI: 1.04–2.07) in smokers of ≤10 cigarettes/day, 2.54 (95% CI: 1.81–3.57) in smokers of 11–20 cigarettes/day and 3.79 (95% CI: 2.25–6.37) in smokers of ≥21 cigarettes/day all compared with never smokers. As shown in Fig. 3, higher CVD risk was observed with higher numbers of cigarettes smoked per day. Male ex-smokers who had quit 15 years ago or earlier had the same CVD risk as never smokers.
Fig. 3.
Hazard ratios and 95% confidence intervals (CI) for CVD incidence in male smokers and ex-smokers, based on the number of cigarettes smoked per day, or the time spent since the last quit, respectively (ref: never smoker). Model 1. Adjusted for age. Model 2. Adjusted for age and cardio-metabolic risk factors including: body mass index, systolic blood pressure, diastolic blood pressure, triglycerides level, cholesterol level and fasting blood sugar. Model 3. Adjusted for age, cardio-metabolic risk factors (as above) and socio-economic features including: education (Ref: college degree), marital status (Ref: married) and occupation (Ref: employed). Q ≥ 15y, Quit 15 years ago or before; Q < 15y, Quit in the last 15 years; ≤ 10 c/day, Smoking ≤10 cigarettes/day; 11–20 c/day, Smoking 11–20 cigarettes/day; ≥ 21 c/day, Smoking ≥21 cigarettes/day
Figure 4 shows hazard ratio of CVD/CHD among different smoking groups of women, in whom, HRs of CVD incidence were 1.03 (95% CI: 0.74–1.43) in passive smokers and 1.03 (95% CI: 0.61–1.81) in ever smokers. Hazard ratios of CHD incidence were 1.01 (95% CI: 0.71–1.43) in passive smokers and 1.05 (95% CI: 0.58–1.9) in ever smokers, compared with never smokers.
Fig. 4.
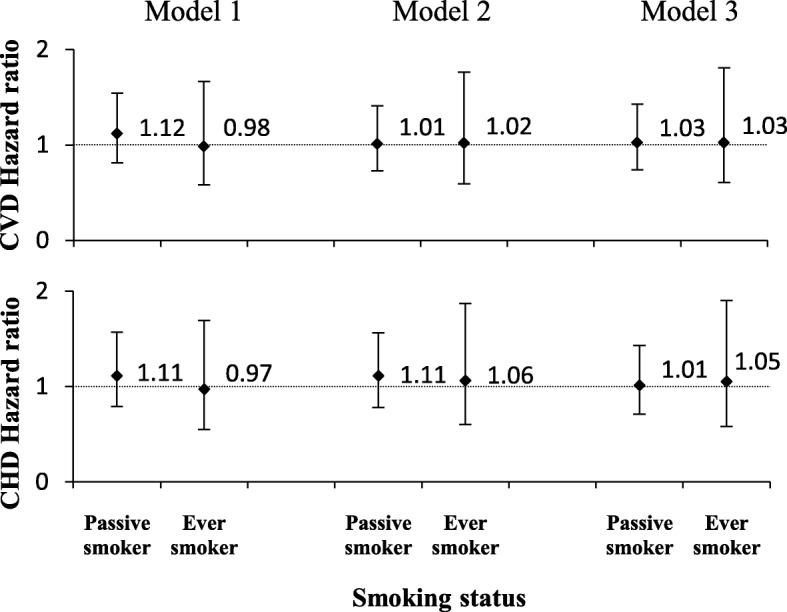
Hazard ratios and 95% confidence intervals (CI) for CVD/CHD incidence in women, among different smoking groups (Ref: never smoker). Tehran Lipid and Glucose study 1999–2010. Model 1. Adjusted for age. Model 2. Adjusted for age and cardio-metabolic risk factors including: body mass index, systolic blood pressure, diastolic blood pressure, triglycerides level, cholesterol level and fasting blood sugar. Model 3. Adjusted for age, cardio-metabolic risk factors (as above) and socio-economic features including: education (Ref: college degree), marital status (Ref: married) and occupation (Ref: employed)
Discussion
Our results showed that different smoking habits and higher rates of cigarette smoking increased the risk of CVD/CHD in men. In women, however, the relation between different smoking patterns and the risk of CVD/CHD was not established with these data. According to our findings, smoking rates were significantly higher among men than women, which is consistent with previous findings from Iran and other Middle Eastern countries. In this regard, a recent meta-analysis indicated that the prevalence of smoking in Iran ranged between 19.8 and 21.7% in men, while it was estimated to be 0.94 to 3.6% in women [18]. According to the latest WHO report (2017), the same pattern was also seen in other Middle Eastern countries including Kuwait (35.4% in men and 2.0% in women), Oman (12.3% in men and 0.1% in women) and Qatar (18.2% in men and 0.1% in women) [29]; gender differences however, were lower in East-Asian communities, including Japan (28.0% in men and 8.8% in women) [30] and Korea (37.6% in men and 4.9% in women) [31]; these gender differences in smoking were even less in Western countries such as Britain (19.1% in men and 15.7% in women) [32] and United States (15.8% in men and 12.4% in women) [33]. The geographical variation of smoking prevalence in men and women may be due to under-reporting of smoking in women of the Middle Eastern countries, where smoking is usually associated with social stigma [34].
Our findings regarding the prognostic value of current smoking both occasionally and daily in the prediction of CVD/CHD incidence are in agreement with the findings of Birgitte et al. who reported an age-adjusted relationship of active- and passive smoking with MI incidence in males and females [35]. Shields and Wilkins also reported that active daily smokers had a 60% higher risk of CHD incidence in Canada [36]. Several other studies also show that passive smoking may result in increment in the risk of MI [37–39]. Further analysis showed that the relation between smoking and risk of CVD followed a dose-dependent association, i.e. the “more cigarettes smoked daily, the higher the risk of CVD”, a finding in line with previous studies reporting that smoking intensity is significantly related with an increased risk of CVD [40–42]. Furthermore, the risk of CVD in men who had quit smoking for over 15 years, tends to be similar to that in never smokers, results in accordance with previous findings of the Surgeon General’s Report on the health benefits of smoking cessation, reporting that reduced risk of heart diseases may not show itself until ≥15 years after complete abstinence from smoking [43]. Shields and Wilkins also reported that at least 20 years of smoking cessation was required for the risk of heart diseases to normalize the risk of heart disease in former smokers [36].
Based on our findings, no association between various smoking habits and risk of CVD/CHD was found in females, findings in contrast with those of previous studies, reporting that smoking is the leading contributing factor in development and progression of CVDs in both men and women [44]. Furthermore, it is well established that smoking is associated with a wide range of risk factors which increase the risk of CVD, e.g. endothelial dysfunction, decreased insulin sensitivity, increased heart rate and blood pressure, hypercoagulable and hyper inflammatory status and altered serum lipoproteins and lipid related enzymes [45]. Some studies even propose that women may be more susceptible than men to some of the smoking-related CVD consequences and have a significant 25% increased risk for CHD, compared to their male counterparts [46–48]. The current findings regarding lack of association between smoking pattern and CVD/CHD outcomes in women may be due to the under reporting of their smoking habits. While smoking in men is considered a common habit, smoking in women is often seen as inappropriate and is associated with social stigma in developing countries [49]; these socio-cultural factors may raise questions about the accuracy and validity of self-reported data of smoking in women [34]. Another reason that may increase the risk of misreporting is the presence of other family members who usually accompany younger or female participant during the interview [50]. In this situation, some researchers found that women tend to conceal their smoking habits from family members, mainly because of the shame and stigma attached [51].
Regarding the strengths of the current study to the best of our knowledge, this is the first longitudinal study to assess the association of smoking habits with the risk of cardiovascular diseases among Iranian populations among women and passive smokers. Among the limitations of the study was the possible information bias of data collection using a questionnaire-based method. In addition the lack of significant findings in women which can be explained in light of the potential under-reporting of smoking as well as the lack of statistical power due to low number of current smokers; limitations which need to be further investigated in future studies in Iran.
Conclusion
In conclusion, although an increased risk of CVD/CHD incidence was found in current male smokers, this finding was not supported by our data in women. This result might be attributed to under-reporting of smoking in female participants due to the existing social stigma regarding smoking of women in Iran. Our findings indicated the importance of smoking cessation to prevent cardio-vascular outcomes in an Iranian urban population. To confirm and further elucidate these findings, more studies are required in both urban and rural areas of Iran.
Acknowledgements
The authors wish to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of the manuscript and would like to express their appreciation to all participants of this study for their cooperation.
Abbreviations
- BMI
Body mass index
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- ECG
Diagnostic electrocardiogram
- ETS
Environmental tobacco smoke
- FBS
Fasting blood glucose
- GBD
Global Burden of Disease
- HDL-c
High density lipoprotein
- HRs
Hazard ratios
- LDL-c
Low density lipoprotein
- MAQ
Modifiable Activity Questionnaire
- MI
Myocardial infarction
- NCDs
Non-communicable diseases
- RIES
Institute for Endocrine Sciences
- TG
Triglycerides
- TLGS
Tehran Lipid and Glucose Study
- WHO
World health organization
Authors’ contributions
PA, AA and KMN designed the study. PA, KMN, BA, SJF, contributed to interpretation of data and drafted the manuscript. AAM, participated in acquisition of data. LCH carried out the statistical analysis and contributed to interpretation of data. AA, FH, FA supervised and revised the manuscript. All authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
Data would be available on the request of corresponding author based on TLGS rules.
Ethics approval and consent to participate
The study was approved by the research ethics committee of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences. Written informed consents were obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kendir C, van den Akker M, Vos R, Metsemakers J. Cardiovascular disease patients have increased risk for comorbidity: a cross-sectional study in the Netherlands. Eur J Gen Pract. 2018;24:45–50. doi: 10.1080/13814788.2017.1398318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk-Adawi K, et al. Cardiovascular disease in the eastern Mediterranean region: epidemiology and risk factor burden. Nat Rev Cardiol. 2018;15:106. doi: 10.1038/nrcardio.2017.138. [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forouzanfar MH, et al. Evaluating causes of death and morbidity in Iran, global burden of diseases, injuries, and risk factors study 2010. Arch Iran Med. 2014;17:304. [PubMed] [Google Scholar]
- 5.Teo K, et al. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: the prospective urban rural epidemiology (PURE) study. Jama. 2013;309:1613–1621. doi: 10.1001/jama.2013.3519. [DOI] [PubMed] [Google Scholar]
- 6.Bandara P, Weller S. Cardiovascular disease: time to identify emerging environmental risk factors. Eur J Prev Cardiol. 2017;24:1819–1823. doi: 10.1177/2047487317734898. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Brunner H, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the working group on Endothelins and endothelial factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Organization, W. H . WHO report on the global tobacco epidemic 2017: monitoring tobacco use and prevention policies. 2017. [Google Scholar]
- 10.Lee PN, Thornton AJ, Forey BA, Hamling JS. Environmental tobacco smoke exposure and risk of stroke in never smokers: an updated review with meta-analysis. J Stroke Cerebrovasc Dis. 2017;26:204–216. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Yankelevitz DF, et al. The Association of Secondhand Tobacco Smoke and CT angiography-verified coronary atherosclerosis. J Am Coll Cardiol Img. 2017;10:652–659. doi: 10.1016/j.jcmg.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Health, U. D. o. & services, H . The health consequences of smoking—50 years of progress: a report of the surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. p. 17. [Google Scholar]
- 13.Ezzati M, Lopez AD. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tob Control. 2004;13:388–395. doi: 10.1136/tc.2003.005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization, W. H. Global health risks: mortality and burden of disease attributable to selected major risks: World Health Organization; 2009.
- 15.Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–2864. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 16.Namayandeh SM, Sadr S, Ansari Z, Rafiei M. A cross-sectional study of the prevalence of coronary artery disease traditional risk factors in Yazd urban population, Yazd healthy heart project. Iran Cardiovasc Res J. 2011;5:7–13. [Google Scholar]
- 17.Moosazadeh M, Ziaaddini H, Mirzazadeh A, Ashrafi-Asgarabad A, Haghdoost AA. Meta-analysis of smoking prevalence in Iran. Addiction & health. 2013;5:140–153. [PMC free article] [PubMed] [Google Scholar]
- 18.Jalilian F, et al. Socio-demographic characteristics associated with cigarettes smoking, drug abuse and alcohol drinking among male medical university students in Iran. J Res Health Sci. 2015;15:42–46. [PubMed] [Google Scholar]
- 19.Ehteshami-Afshar S, Momenan A, Hajshekholeslami F, Azizi F, Hadaegh F. The impact of smoking status on 9.3 years incidence of cardiovascular and all-cause mortality among Iranian men. Ann Hum Biol. 2014;41:249–254. doi: 10.3109/03014460.2013.853834. [DOI] [PubMed] [Google Scholar]
- 20.Hadaegh F, et al. Twelve-year cardiovascular and mortality risk in relation to smoking habits in type 2 diabetic and non-diabetic men: Tehran lipid and glucose study. PLoS One. 2016;11:e0149780. doi: 10.1371/journal.pone.0149780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afsharian S, et al. Risk factors for cardiovascular disease and mortality events in adults with type 2 diabetes—a 10-year follow-up: Tehran lipid and glucose study. Diabetes Metab Res Rev. 2016;32:596–606. doi: 10.1002/dmrr.2776. [DOI] [PubMed] [Google Scholar]
- 22.Azizi F, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials. 2009;10:5. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azizi F, et al. Tehran lipid and glucose study (TLGS): rationale and design. Iran J Endocrinol Metab. 2000;2:77–86. [Google Scholar]
- 24.Kriska AM, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 25.Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012; 15(5):279-82. 10.2155/AIM.007. [PubMed]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Luepker RV, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 28.Hadaegh F, Harati H, Ghanbarian A, Azizi F. Association of total cholesterol versus other serum lipid parameters with the short-term prediction of cardiovascular outcomes: Tehran lipid and glucose study. Eur J Cardiovasc Prev Rehabil. 2006;13:571–577. doi: 10.1097/01.hjr.0000216552.81882.ca. [DOI] [PubMed] [Google Scholar]
- 29.Stamatakis KA, Brownson RC. Sleep duration and obesity-related risk factors in the rural Midwest. Prev Med. 2008;46:439–444. doi: 10.1016/j.ypmed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda Y, Nakamura K, Takano T. Socioeconomic pattern of smoking in Japan: income inequality and gender and age differences. Ann Epidemiol. 2005;15:365–372. doi: 10.1016/j.annepidem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Khang Y-H, Cho H-J. Socioeconomic inequality in cigarette smoking: trends by gender, age, and socioeconomic position in South Korea, 1989–2003. Prev Med. 2006;42:415–422. doi: 10.1016/j.ypmed.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Jarvis MJ, Cohen JE, Delnevo CD, Giovino GA. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tob Control. 2013;22:356–360. doi: 10.1136/tobaccocontrol-2011-050279. [DOI] [PubMed] [Google Scholar]
- 33.Taylor WC, Shugart D, Paxton RJ. Healthy lifestyle behaviors and disparities between the United States mainland compared to Puerto Rico, Guam, and United States Virgin Islands (ie, United States territories) J Health Disparities Res Pract. 2018;12:3. [Google Scholar]
- 34.Khattab A, et al. Smoking habits in the Middle East and North Africa: results of the BREATHE study. Respir Med. 2012;106:S16–S24. doi: 10.1016/S0954-6111(12)70011-2. [DOI] [PubMed] [Google Scholar]
- 35.Iversen B, Jacobsen BK, Løchen M-L. Active and passive smoking and the risk of myocardial infarction in 24,968 men and women during 11 year of follow-up: the Tromsø study. Eur J Epidemiol. 2013;28:659–667. doi: 10.1007/s10654-013-9785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shields M, Wilkins K. Smoking, smoking cessation and heart disease risk: a 16-year follow-up study. Health Rep. 2013;24:12. [PubMed] [Google Scholar]
- 37.Whincup PH, et al. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. Bmj. 2004;329:200–205. doi: 10.1136/bmj.38146.427188.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi M, Negri E, Vecchia CL, Campos H. Smoking habits and the risk of non-fatal acute myocardial infarction in Costa Rica. Eur J Cardiovasc Prev Rehabil. 2011;18:467–474. doi: 10.1177/1741826710389381. [DOI] [PubMed] [Google Scholar]
- 39.Jefferis B, et al. Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart. 2010;96:854–859. doi: 10.1136/hrt.2009.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan Jibin, Zhang Xiumin, Wang Weihua, Yin Peng, Guo Xiaomin, Zhou Maigeng. Smoking, Blood Pressure, and Cardiovascular Disease Mortality in a Large Cohort of Chinese Men with 15 Years Follow-up. International Journal of Environmental Research and Public Health. 2018;15(5):1026. doi: 10.3390/ijerph15051026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huxley R. R., Yatsuya H., Lutsey P. L., Woodward M., Alonso A., Folsom A. R. Impact of Age at Smoking Initiation, Dosage, and Time Since Quitting on Cardiovascular Disease in African Americans and Whites: The Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 2012;175(8):816–826. doi: 10.1093/aje/kwr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehteshami-Afshar Solmaz, Momenan Amirabbas, Hajshekholeslami Farhad, Azizi Fereidoun, Hadaegh Farzad. The impact of smoking status on 9.3 years incidence of cardiovascular and all-cause mortality among Iranian men. Annals of Human Biology. 2013;41(3):249–254. doi: 10.3109/03014460.2013.853834. [DOI] [PubMed] [Google Scholar]
- 43.Novello AC. Surgeon General’s report on the health benefits of smoking cessation. Public Health Rep. 1990;105:545–548. [PMC free article] [PubMed] [Google Scholar]
- 44.Akcay M, Yuksel S. Smoking and cardiovascular diseases. J Exp Clin Med. 2017;34:21–25. [Google Scholar]
- 45.Campbell SC, Moffatt RJ, Stamford BA. Smoking and smoking cessation—the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201:225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 46.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson PM, Nilsson JA, Berglund G. Population-attributable risk of coronary heart disease risk factors during long-term follow-up: the Malmo preventive project. J Intern Med. 2006;260:134–141. doi: 10.1111/j.1365-2796.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 48.Schnohr P, Jensen JS, Scharling H, Nordestgaard BG. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from the Copenhagen City heart study. Eur Heart J. 2002;23:620–626. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]
- 49.Nasir K, Rehan N. Epidemiology of cigarette smoking in Pakistan. Addiction. 2001;96:1847–1854. doi: 10.1080/09652140120089599. [DOI] [PubMed] [Google Scholar]
- 50.Jarallah JS, Al-Rubeaan KA, Al-Nuaim ARA, Al-Ruhaily AA, Kalantan KA. Prevalence and determinants of smoking in three regions of Saudi Arabia. Tob Control. 1999;8:53–56. doi: 10.1136/tc.8.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bush J, White M, Kai J, Rankin J, Bhopal R. Understanding influences on smoking in Bangladeshi and Pakistani adults: community based, qualitative study. BMJ. 2003;326:962. doi: 10.1136/bmj.326.7396.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data would be available on the request of corresponding author based on TLGS rules.