Abstract
Background
Positron emission tomography (PET) and PET/computed tomography (PET/CT) imaging with 3,4-dihydroxy-6-[18F] fluoro-L-phenylalanine (18F-FDOPA) has been used in the evaluation of gliomas. We performed a meta-analysis to obtain the diagnostic and grading accuracy of 18F-FDOPA PET and PET/CT in patients with gliomas.
Methods
PubMed, Embase, Cochrane Library and Web of Science were searched through 13 May 2019. We included studies reporting the diagnostic performance of 18F-FDOPA PET or PET/CT in glioma patients. Pooled sensitivity, specificity, and area under the summary receiver operating characteristic (SROC) curve were calculated from eligible studies on a per-lesion basis.
Results
Eventually, 19 studies were included. Across 13 studies (370 patients) for glioma diagnosis, the pooled sensitivity and specificity of 18F-FDOPA PET and PET/CT were 0.90 (95%CI: 0.86–0.93) and 0.75 (95%CI: 0.65–0.83). Across 7 studies (219 patients) for glioma grading, 18F-FDOPA PET and PET/CT showed a pooled sensitivity of 0.88 (95%CI: 0.81–0.93) and a pooled specificity of 0.73 (95%CI: 0.64–0.81).
Conclusions
18F-FDOPA PET and PET/CT demonstrated good performance for diagnosing gliomas and differentiating high-grade gliomas (HGGs) from low-grade gliomas (LGGs). Further studies implementing standardized PET protocols and investigating the grading parameters are needed.
Keywords: PET, 18F-FDOPA, Glioma, Meta-analysis
Background
Glioma is the most common primary brain tumor, accounting for 81% of all malignant brain tumors with an annual incidence of 5.26 per 100,000 individuals [1]. According to the World Health Organization (WHO) 2007 classification, grade I and II tumors are together referred to as low-grade gliomas (LGGs), while grade III and IV tumors are categorized into high-grade gliomas (HGGs) [2]. The treatment of gliomas requires multidisciplinary care. Appropriate surgical or radiotherapy regimen is highly dependent on the delineation and grade of tumors, and therefore imaging assessment is critical to the clinical management of affected patients. For the past few decades, conventional magnetic resonance imaging (MRI) has been the method of choice for glioma diagnosis. However, it lacks sensitivity in non-enhancing gliomas and cannot reliably provide the differentiation between tumor recurrence and radiation-induced changes (e.g., pseudoprogression and radionecrosis) [3, 4]. Thus, more accurate imaging modalities need to be found.
The Response Assessment in Neuro-Oncology (RANO) working group has recently recommended the use of positron emission tomography (PET) imaging for gliomas complementary to MRI [5]. Compared with MRI, PET provides additional insight into tumor metabolism and has been shown to improve tumor delineation and grading [6]. The glucose metabolic agent 18-fluoro-deoxyglucose (18F-FDG) has been the classic PET tracer used in tumor imaging, but it is not ideal in detecting gliomas due to the high physiologic uptake in normal brain tissue. Given the diagnostic limitations of 18F-FDG, amino acid tracers have been extensively investigated, such as 3,4-dihydroxy-6-[18F] fluoro-L-phenylalanine (18F-FDOPA). Unlike gadolinium, 18F-FDOPA is transported across the intact blood brain barrier (BBB) [7]. Hence the use of 18F-FDOPA PET enables the depiction of tumor components beyond contrast enhancement in MRI [8]. Furthermore, the accuracy of 18F-FDOPA PET and PET/CT in detecting gliomas has been reported to be superior as compared with 18F-FDG PET and PET/CT in previous studies [9, 10]. However, existing studies are inconclusive because of relatively small sample sizes and heterogeneous designs. Also, previous studies have provided contradictory conclusions on whether there are significant differences in 18F-FDOPA PET and PET/CT between low-grade gliomas (LGGs) and high-grade gliomas (HGGs) [11–13].
We performed this meta-analysis to systemically review all relevant publications in attempt to (1) evaluate the overall diagnostic performance of 18F-FDOPA PET and PET/CT in patients with gliomas and to (2) access the ability of 18F-FDOPA PET and PET/CT in discriminating HGGs from LGGs.
Methods
Search strategy
A systematic search of the PubMed, Embase, Cochrane Library and Web of Science databases was performed for English and non-English publications through 13 May 2019 using the following search: “(DOPA [all fields] OR FDOPA [all fields] OR fluorodihydroxyphenylalanine [all fields]) AND (positron emission tomography [all fields] OR PET [all fields]) AND (glioma [all fields] OR gliomas [all fields] OR brain tumor [all fields] OR brain tumors [all fields])”. References to retrieved articles and unpublished clinical trials were also checked for potential findings.
Study selection
All records were screened independently for eligibility by 2 reviewers (JX and YJ), and discrepancies were resolved by consensus. The inclusion criteria were: (1) Original studies investigating the diagnostic or grading capacity of 18F-FDOPA PET or PET/CT in patients with gliomas; (2) Studies with histopathology and/or clinical and imaging follow-up as reference standards; (3) Certain numbers of true-positive (TP), false-positive (FP), false-negative (FN) and true-negative (TN) results in diagnostic or grading tests can be derived from sufficient data. Case reports, reviews, letters and in vitro studies were excluded. Meanwhile, studies involving diagnosing brain metastases were also excluded (unless single cases can be differentiated). When study populations overlapped, we only included the most recent one to avoid data duplication [14, 15]. Eligible literatures were then classified into two different groups according to different aims of study: (1) Studies focused on the diagnosis of gliomas; (2) Studies focused on the grading of gliomas.
Data extraction and quality assessment
Two reviewers (JX and YJ) independently went through all eligible studies and extracted essential information, including name of principal author, year of publication, study country, type of study design (retrospective or prospective), number of specimens, number of patients, mean or median age of patients, male to female ratio, index test, prior treatment, tumor occurrence (newly diagnosed or recurrent), reference standard, comparative imaging approaches, parameters and threshold (if existed), and the number of TP, FP, FN, TN.
Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [16] was applied to evaluate the quality of all included studies in Review Manager 5.3 software (Cochrane Collaboration, Oxford, England). QUADAS-2 tool is composed of four aspects, of which each item can be defined as “yes”, “no” or “unclear”. The overall assessments of each aspect interpret the bias risk as low, high or unclear. Two reviewers (JX and YJ) independently assessed each article with disagreements resolved.
Statistical analysis
Meta-DiSc software, version 1.4 (Clinical Biostatistics Unit, Ramón y Cajal Hospital, Madrid, Spain) was used to calculate pooled data including sensitivity, specificity, positive likelihood (LR+), negative likelihood (LR-), diagnostic odds ratio (DOR) (with 95% confidence intervals (CI)), and construct summary receiver operating characteristic (SROC) curves [17]. The Area Under the Curve (AUC) was computed to measure overall performance of tests (AUC is positively correlated with diagnostic value, 0.5 ≤ AUC < 0.7 representing a low accuracy; 0.7 ≤ AUC < 0.9 indicating a moderate accuracy; AUC ≥ 0.9 indicating a high diagnostic value) [18, 19]. StataSE, version 12 (StataCorp, College Station, TX, USA) was employed to assess publication bias through Deeks’ Funnel Plot Asymmetry Test [20]. The Spearman’s rank correlation coefficient was calculated between logarithmic sensitivity and logarithmic (1-specificity), and a strong positive correlation indicates the existence of threshold effect [17]. We used Chi-square, Cochran-Q, and I-squared test to evaluate the heterogeneity between studies [17]. The Random-Effects Model would be applied, unless no significant heterogeneity was detected between studies [21].
Considering the differences between primary and recurrent tumors in clinical management and post-treatment changes, we divided all studies into two subgroups: detecting (1) newly diagnosed gliomas and (2) recurrent (including residual) gliomas. TP, FP, FN, TN were recalculated in studies investigating in a mixed population of primary and recurrent gliomas when feasible. (Significant level: two-tailed p-value< 0.05).
Results
Study selection
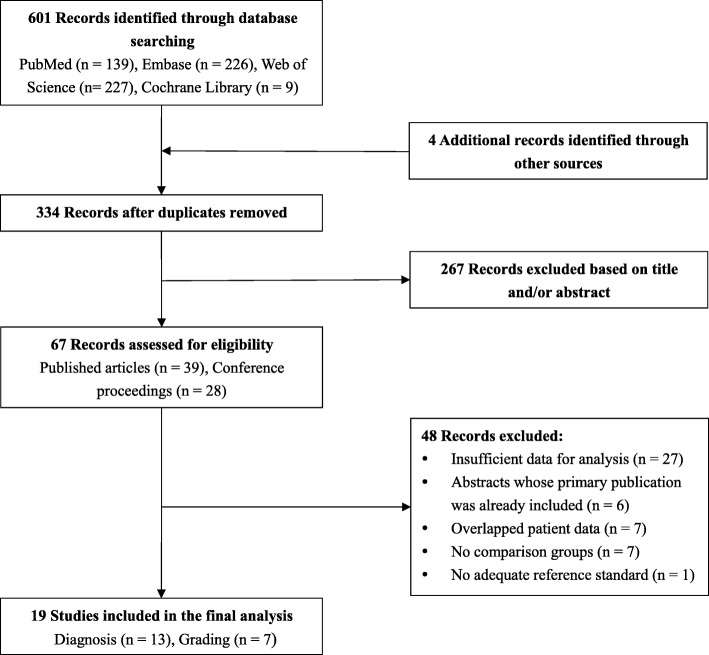
The comprehensive literature search yielded 605 relevant records in total. After excluding duplicates and screening through titles and abstracts, 67 records went into full-text review, with 19 studies fulfilling the inclusion criteria for the final meta-analysis. Eligible studies were further classified for the intended subanalyses: 13 studies for glioma diagnosis and 7 studies for glioma grading. The details of the selection process are illustrated in Fig. 1.
Fig. 1.
Flow chart of study selection. 19 studies are included eventually
Study characteristics
The characteristics of the included studies are demonstrated in Table 1 and Table 2.
Table 1.
Baseline information of included studies for glioma diagnosis
| Reference | Year | Country | Design | Specimens No. | Patients No. | Age, yr | M/F | Test | Prior treatment | Occurrence | Gold Standard | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | Median | |||||||||||
| Chen et al. [9] | 2006 | US | Prospective | 27 | 30 (27b) | – | – | – | PET | With or without | 7 New+ 20 Recur | Histo+Radio+follow-up |
| Tripathi et al. [10] | 2009 | India | Prospective | 15 | 15 | 28.4 ± 11.1 | – | 9/6 | PET/CT | With (Sx/CT/RT) or without | 3 New+ 12 Recur | Histo+Radio+follow-up |
| Sellam et al. [22] | 2010 | India | Prospective | 30 | 30 | – | – | – | PET/CT | Sx+/−RT | Recur | Histo+Radio+follow-up |
| Jora et al. [23] | 2011 | India | Prospective | 23 | 23 | 43.25 ± 14.9 | – | – | PET/CT | 15 with (Sx + RT) + 8 without | 8 New+ 15 Recur | Histo+Radio+follow-up |
| Karunanithi et al. [15] | 2013 | India | Prospective | 35 | 35 | 36.62 ± 0.86 | – | 28/7 | PET/CT | Sx + RT+/−CT | Recur | Histo+Radio+follow-up |
| Pafundi et al. [24] | 2013 | US | Prospective | 23 | 10 | 40.8 ± 18.9 | – | 9/1 | PET/CT | With or without | 8 New+ 2 Recur | Histo |
| Herrmann et al. [25] | 2014 | US | Retrospective | 110 | 110 | 51.7 ± 12.1 | 52.5 | 72/38 | PET/CT | Sx | Recur | Histo+Radio+follow-up |
| Moran et al. [26] | 2015 | Italy | Retrospective | 27 | 27 | 10 | 10 | 15/12 | PET | 1 with (Sx + CT + RT) + 20 without | 20 New+ 1 Recur | Histo+Radio+follow-up |
| Sharma et al. [27] | 2016 | India | Prospective | 12 | 11 | 34 | – | 6/5 | PET/CT | Sx | Recur | Histo+Radio+follow-up |
| Paquet et al. [28] | 2017 | France | Prospective | 60 | 35 | 60 | – | – | PET | Sx + CT + RT | Recur | Histo+Radio |
| Evangelista et al. [29] | 2018 | Italy | Retrospective | 13 | 13 | – | 60 | – | PET/CT | Unclear | Recur | Radio+follow-up |
| Youland et al. [30] | 2018 | US | Prospective | 37 | 13 | – | 40 | 9/4 | PET | Sx/CT/RT | Recur | Histo |
| Evangelista et al. [31] | 2019 | Italy | Retrospective | 21 | 21 | 58 ± 11 | – | – | PET/CT | Sx + CT/RT/immunotherapy | Recur | Radio+follow-up |
M male, F female, New newly-diagnosed, Recur recurrent, Histo histopathology, Radio radiology, Sx surgery, CT chemotherapy, RT radiation therapy
aMean age is expressed as mean ± standard deviation
bThree patients with brain metastases were excluded
Table 2.
Baseline information of included studies for glioma grading
| Reference | Year | Country | Design | Specimens No. | Patients No. | Age, yr | M/F | Test | Prior treatment | Occurrence | Gold Standard | Parameter | Cut-off | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | Median | |||||||||||||
| Fueger et al. [12] | 2010 | US | 17 Prospective+ 42 Retrospective | 59 | 59 (22b) | – | 44.5 | 13/9 | PET or PET/CT | With (Sx + CT/RT) or without | New | Histo | SUVmax | 2.72 |
| Nioche et al. [32] | 2013 | France | Prospective | 33 | 33 | 51 ± 16 | 51 | 28/5 | PET/CT | With (Sx + CT/RT) or without | 20 New+ 13 Recur | Histo | SUVmean | 2.2 |
| Pafundi et al. [24] | 2013 | US | Prospective | 23 | 10 (9b) | 42.9 ± 19.2 | – | 8/1 | PET/CT | With or without | 7 New+ 2 Recur | Histo | SUVmax T/SUVmean N | 2.0 |
| Janvier et al. [13] | 2015 | France | Retrospective | 31 | 31 | 36.8 ± 12.1 | – | 13/18 | PET | With (Sx/CT/RT) or without | 25 New+ 6 Recur | Histo+Radio+follow-up | SUVmean T/N | 1.33 |
| Bund et al. [33] | 2017 | France | Prospective | 53 | 53 | 38 | – | 23/30 | PET/CT | Without | New | Histo | SUVmax T/N | 2.16 |
| Morana et al. [34] | 2017 | Italy | Retrospective | 26 | 26 | 10.2 ± 4.6 | 9.5 | 15/11 | PET | Without | New | Histo | SUVmax T/S | 0.90 |
| Patel et al. [35] | 2018 | US | Prospective | 45 | 45 | 46.4 ± 16.2 | – | 22/23 | PET | Without | New | Histo | SUVmax T/N | 1.7 |
M, male; F, female; Sx, surgery; CT, chemotherapy; RT, radiation therapy; New, newly-diagnosed; Recur, recurrent; Histo, histopathology; SUV, standardized uptake value; Radio, radiology; T, tumor; N, normal; S, Striatum
aMean age is expressed as mean ± standard deviation
bPatients that are finally included in the quantitative analysis by authors
Aim 1: Investigating the accuracy of 18F-FDOPA PET and PET/CT for diagnosing gliomas.
Altogether, 13 studies (370 patients) [9, 10, 15, 22–31] were included in this meta-analysis. 4 of the 13 studies (31%) were retrospective studies, whereas the others (69%) were designed prospectively. Other than Morana et al. [26], which focused on the diagnostic performance of 18F-FDOPA PET on pediatric gliomas, all researches were carried out among adults. 8 of 13 studies focused only on diagnosing recurrent gliomas with previous treatment, while the rest studies were conducted jointly in newly diagnosed and pretreated patients, and all of them can be further stratified into corresponding subgroups. All TP, FP, FN and TN results extracted were based on visual analysis.
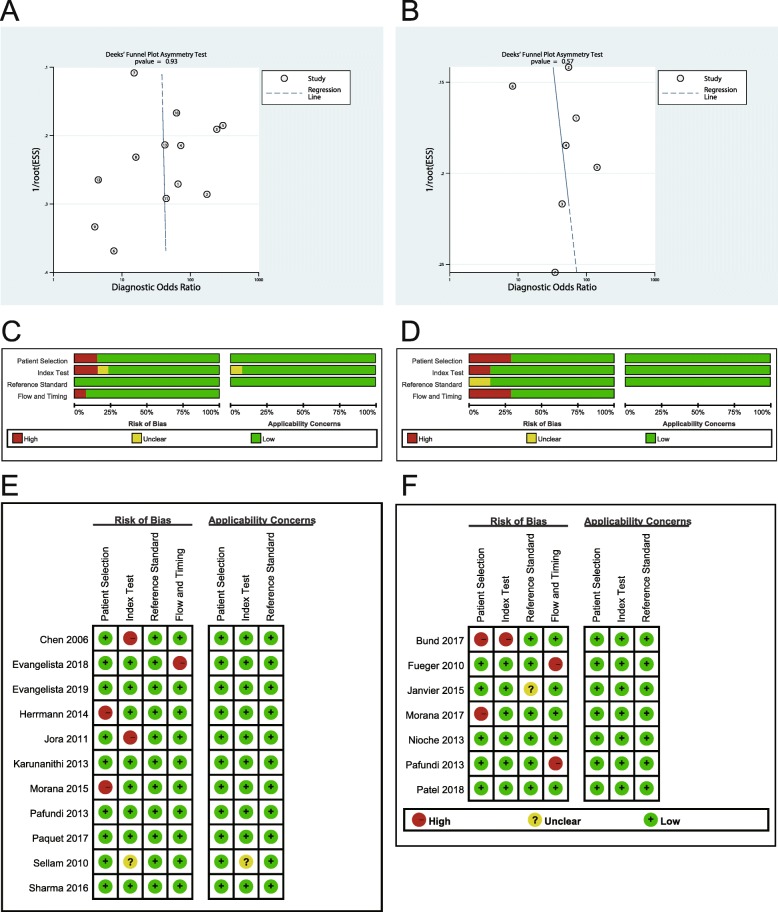
Spearman correlation coefficient was − 0.18 (p-value = 0.56), displaying no threshold effect. Deeks’ Funnel Plot test demonstrated no publication bias (p-value = 0.93, Fig. 2a).
Fig. 2.
Publication bias assessment of included studies (a, b) and quality assessment of included studies (c-f). a, b: Deeks’ Funnel Plot shows no publication bias in both detecting (a) and grading (b) gliomas. c-f: The graphs show risk of bias and applicability concerns regarding each study. Quality assessment result for diagnosis (c, e); for grading (d, f)
Aim 2: Investigating the performance of 18F-FDOPA PET and PET/CT for grading gliomas.
As for the meta-analysis of grading gliomas, 7 studies [12, 13, 24, 32–35] with 219 patients were involved in total, with male/female ratio of 122/97 (1.26). 193 (88%) patients were newly diagnosed, and 26 (12%) patients were diagnosed with recurrent gliomas. All studies were performed at the patient level. The number of TN represents the number of discriminating HGGs from LGGs. All the interpretations were based on quantitative analysis, and the cut-off value of each study was determined through ROC analysis. For those used various parameters, the most predictive index was included in this analysis.
Neither threshold effect nor publication bias was detected through Spearman correlation coefficient (= 0.67, p-value = 0.10) and Deeks’ Funnel Plot test (p-value = 0.57, Fig. 2b) respectively.
Quality assessment
We used QUADAS-2 to estimate the quality of literature in Review Manager 5.3. The assessment results were shown in Fig. 2(c-f).
Meta-analysis of Diagnostic/Grading Performance in Gliomas.
Aim 1: Investigating the accuracy of 18F-FDOPA PET for diagnosing gliomas.
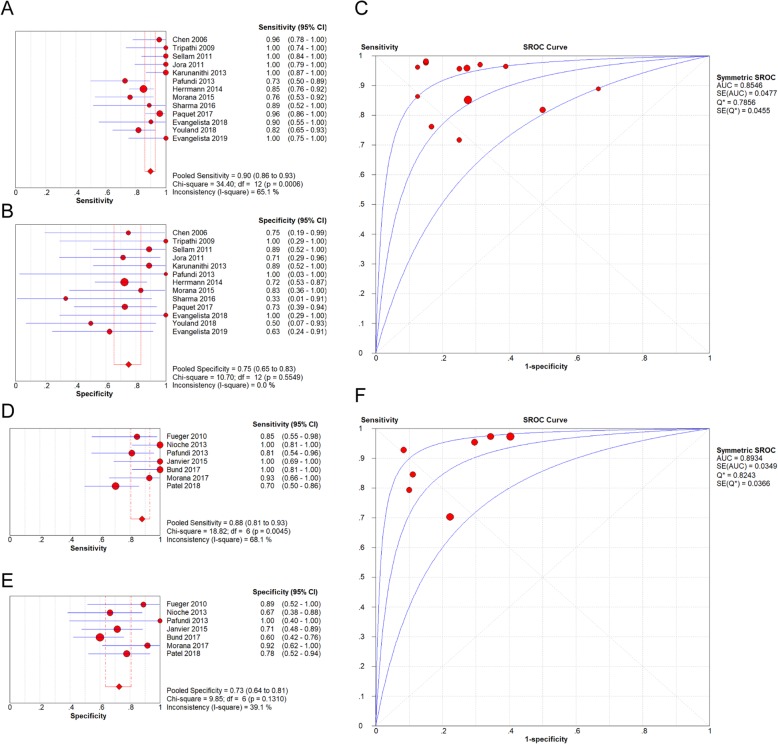
Meta-analysis resulted in a pooled sensitivity of 0.90 (95% CI: 0.86–0.93), and a pooled specificity of 0.75 (95% CI: 0.65–0.83). The pooled LR+ was 2.84 (95% CI, 2.09–3.85), and the pooled LR- was 0.15 (95% CI: 0.09–0.26). The DOR was 24.05 (95% CI: 12.62–45.85) (Fig. 3a/b).
Fig. 3.
Forest plots of sensitivity and specificity, and summary receiver operating characteristic curve of diagnosing gliomas (a-c) and differentiating HGGs from LGGs (d-f)
SROC curve was established based on pooled sensitivity and specificity, and the overall AUC was 0.85 [Standard Error (SE) = 0.05], indicating a moderately high overall diagnostic value of 18F-FDOPA PET and PET/CT on gliomas among individuals (Fig. 3c).
Only a significant heterogeneity for sensitivity was detected (Chi-square = 34.40, p-value = 0.0006, I-square = 65.1%) through Chi-square, Cochran-Q, and I-squared tests (Fig. 3a).
Subgroup analysis
No threshold effect was detected in two subgroups. Pooled data and SROC curve manifested a high diagnostic value of 18F-FDOPA PET and PET/CT in recurrent subgroup, with sensitivity of 0.92 (95% CI: 0.88–0.95), specificity of 0.76 (95% CI: 0.66–0.85), LR+ of 2.87 (95% CI: 2.01–4.11), LR- of 0.13 (95% CI: 0.07–0.23), DOR of 29.65 (95% CI: 13.09–67.15), and AUC of 0.90 (SE = 0.06). In the newly-diagnosed subgroup, only two studies were eligible for meta-analysis (in another three studies only sensitivity can be computed after stratification). In comparison with recurrent group, pooled data of newly-diagnosed group revealed a lower sensitivity of 0.71 (95% CI: 0.54–0.85) and a higher specificity of 0.86 (95% CI: 0.42–1.00). The LR+, LR- and DOR were 3.71 (0.87–15.81), 0.36 (0.19–0.68) and 10.88 (1.57–75.31) respectively.
Aim 2: Investigating the Performance of 18F-FDOPA PET and PET/CT for Grading Gliomas.
As illustrated in the Fig. 3(d-f), 18F-FDOPA PET and PET/CT presented a pooled sensitivity of 0.88 (95% CI: 0.81–0.93), specificity of 0.73 (95% CI: 0.64–0.81), LR+ of 2.90 (95% CI: 2.19–3.85), LR- of 0.16 (95% CI: 0.08–0.36), DOR of 25.87 (95% CI: 10.53–63.54), and AUC of 0.89 (SE = 0.03) for differentiating HGGs from LGGs.
Other than sensitivity (p-value = 0.0045), no significant heterogeneity was found among studies for specificity (p-value = 0.13), LR+ (p-value = 0.42), LR- (p-value = 0.07) and DOR (p-value = 0.53).
Discussion
This meta-analysis demonstrated a pooled sensitivity of 0.90 and specificity of 0.75 for 18F-FDOPA PET and PET/CT in diagnosing gliomas, and a pooled sensitivity of 0.88 and specificity of 0.73 in grading gliomas. Subgroup analysis showed that 18F-FDOPA PET and PET/CT had good diagnostic performance in both newly-diagnosed and recurrent groups. The specificity for detecting recurrent gliomas was slightly lower, which may be explained by the increased false positive rate caused by treatment response such as edema and inflammatory tissue. However, it is worth mentioning that the limited number of studies in newly-diagnosed group may impair the reliability to some degree.
The diagnostic accuracy of 18F-FDOPA PET for distinguishing radiation necrosis from brain tumor recurrence has been previously investigated by Yu J et al. [36]. However, the use of 18F-FDOPA PET or PET/CT in accessing newly-diagnosed gliomas has not been systematically studied before. Moreover, the recommendations for the use of 18F-FDOPA in glioma grading remained controversial [5]. Our findings confirmed there is a meaningful role of 18F-FDOPA PET and PET/CT for the evaluation of glioma patients.
Of increased interest is the value of 18F-FDOPA PET and PET/CT in differentiating tumor recurrence from radiation-induced changes. At present, MRI remains the standard imaging method for assessing treatment effects in glioma patients [37]. However, the utility of contrast enhancement MRI mainly relies on the disruption of blood-brain barrier (BBB), which impairs its specificity in differentiating radiation-induced changes from recurrent or residual brain tumors, such as pseudoprogression and radionecrosis. New amino acid tracers including 18F-FDOPA PET and PET/CT have been investigated to address this problem. Several studies have directly compared the performance of contrast enhanced MRI with 18F-FDOPA PET or PET/CT in the identification of residual and recurrent gliomas. Jora et al. [23] reported 18F-FDOPA PET to have higher overall sensitivity and specificity over MRI, although their sample size was too limited to show any statistical significance. Karunanithi et al. [15] proved significantly higher specificity of 18F-FDOPA PET in diagnosing recurrence than contrast enhanced MRI overall (p-value = 0.002), and for HGGs (p-value = 0.006) and LGGs (p-value = 0.004) respectively. Besides the evidence presented above, our systematical analysis has also shown that 18F-FDOPA PET and PET/CT provide more reliable results in discriminating recurrent lesions from treatment related changes, with a pooled sensitivity and specificity of 0.92 and 0.76.
Another advantage of 18F-FDOPA PET and PET/CT is the utility in the detection of HGGs. Due to the differences between LGGs and HGGs in therapeutic regimen and prognosis evaluation, initial grading for gliomas through imaging approaches is of great significance. This meta-analysis proved the high overall value of 18F-FDOPA PET and PET/CT in evaluating tumor grade, with the AUC of 0.89. However, studies with respect to grading of recurrent gliomas demonstrated controversial results. In Fueger et al. [12], 18F-FDOPA uptake manifested no significant correlation with different tumor grades in recurrent gliomas, whereas Nioche et al. [32] reported a threshold of 1.8 to identify HGGs in pre-treated patients, with a sensitivity and specificity of 1.0 and 0.80 respectively. The discrepancy between limited studies concerned with grading recurrent gliomas made it unachievable for subgroup analysis. It is also worth noting that in Fueger et al. [12], only the data of newly-diagnosed group is available for meta-analysis. The exclusion of recurrent group might therefore introduce bias. In addition, since most patients included in our study are with newly-diagnosed gliomas (90%), our synthetic results are not representative enough for grading accuracy within the recurrent population, which still requires further investigation with larger sample size.
Evidence for comparisons of 18F-FDOPA with other PET tracers are available in several existing studies. As the most predominant PET tracer in oncological diagnostics so far, 18F-FDG shares the feature of being transported through integrate BBB. However, the high glucose metabolism within normal brain parenchyma may considerably hamper the differentiation between brain tumor and nonneoplastic tissue [38]. 18F-FDOPA has demonstrated better contrast than 18F-FDG in tumor versus normal tissues surrounding, and a higher sensitivity for detecting brain tumors than 18F-FDG [5, 10, 23]. The superiority in sensitivity of 18F-FDOPA can be further proved in comparison with previous meta-analysis of 18F-FDG (pooled sensitivity of 0.38, 95% CI: 0.27–0.50) [39]. The longest established amino acid tracer for brain tumor imaging is [11C]-methyl-L-methionine (11C-MET). In the only study with direct comparison between 18F-FDOPA PET and 11C-MET, 18F-FDOPA performed equally well as 11C-MET in the imaging of brain tumors [11]. However, 11C-MET is being replaced by tracers labelled with fluorine-18 (half-life of 109.8 min) due to its short half-life (20 min). The use of another amino acid tracer O-(2-[18F]-fluorethyl)-1-tyrosine (18F-FET) has rapidly grown in recent years. A previous meta-analysis of 5 studies including 180 patients reported a sensitivity and specificity of 0.82 and 0.76 of 18F-FET PET for the differential diagnosis of primary brain tumor [40]. In later comparison studies revealed that 18F-DOPA and 18F-FET shared similar uptake pattern in primary and recurrent HGGs and had similar diagnostic accuracy in recurrent HGGs [31, 41]. However, compared with 18F-FET, the elevated physiological 18F-FDOPA uptake in striatum has been a concern which may limits its use to detect brain tumor with striatum involvement [5, 42, 43]. Therefore, Kratochwil et al. recommended 18F-FET to examine patients with possible involvement of basal ganglia irrespective of tumor grade. Similarly, Morana et al. [44] reported that the diagnostic ability of 18F-FDOPA PET/CT in dorsal striatum was concordant with its overall accuracy, while not in the ventral striatum, which required fused MRI to improve its performance.
Several study limitations also need to be addressed. Although 19 studies were included in this meta-analysis, the sample size of each study tended to be small, and the number of true negative cases was particularly limited, which might possibly yield less replicable results, especially the pooled specificity. Limited sample size also led to data loss in stratification of subgroups when true negative case was less than one (specificity could not be calculated in this case). Besides, multiple specimens were obtained from one patient in three studies [24, 27, 30], which impeded our analysis on a per-patient basis. In addition, different imaging protocols and grading parameters from separate studies limited the quantitative analysis we performed for glioma grading. Finally, since most studies included in our analysis were published before the WHO 2016 classification system [45], the correlation of 18F-FDOPA uptake with glioma molecular characteristics cannot be analyzed.
Conclusions
In conclusion, this meta-analysis provides evidence that 18F-FDOPA PET and PET/CT have high diagnostic accuracy for the detection of gliomas, especially the discrimination between tumor recurrence and radiation-induced changes. 18F-FDOPA PET and PET/CT also demonstrate good grading performance for the distinction between HGGs and LGGs. However, the grading parameters needs further investigation with standardized imaging protocols in prospective studies.
Acknowledgements
None.
Abbreviation
- 11C-MET
[11C]-methyl-L-methionine
- 18F-FDG
18-fluoro-deoxyglucose
- 18F-FDOPA
3,4-dihydroxy-6-[18F] fluoro-L-phenylalanine
- 18F-FET
O-(2-[18F]-fluorethyl)-1-tyrosine
- AUC
Area under the curve
- BBB
Blood brain barrier
- CI
Confidence intervals
- CT
Computed tomography
- DOR
Diagnostic odds ratio
- FN
False negative
- FP
False positive
- HGGs
High-grade gliomas
- LGGs
Low-grade gliomas
- LR-
Negative likelihood ratio
- LR +
Positive likelihood ratio
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies 2
- SE
Standard Error
- SROC
Summary receiver operating characteristic
- TN
True negative
- TP
True positive
- WHO
World Health Organization
Authors’ contributions
JX, YJ, and JN worked together on the conception, data analysis and interpretation, and drafting of the manuscript. They contributed equally to our work. FC and XM supervised development of work, helped to evaluate the manuscript and acted as corresponding author. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiarui Xiao, Yizi Jin and Ji Nie, contributed equally to this work.
Contributor Information
Fukun Chen, Phone: +86-13987169627, Email: cfk007@126.com.
Xuelei Ma, Phone: +86-28-85475576, Email: drmaxuelei@gmail.com.
References
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 4.Galldiks N, Law I, Pope WB, Arbizu J, Langen KJ. The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. NeuroImage Clin. 2017;13:386–394. doi: 10.1016/j.nicl.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, La Fougere C, Pope W, Law I, Arbizu J, et al. Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-oncology. 2016;18(9):1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-oncology. 2011;13(8):806–819. doi: 10.1093/neuonc/nor054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youland Ryan S., Kitange Gaspar J., Peterson Timothy E., Pafundi Deanna H., Ramiscal Judi A., Pokorny Jenny L., Giannini Caterina, Laack Nadia N., Parney Ian F., Lowe Val J., Brinkmann Debra H., Sarkaria Jann N. The role of LAT1 in 18F-DOPA uptake in malignant gliomas. Journal of Neuro-Oncology. 2012;111(1):11–18. doi: 10.1007/s11060-012-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. doi: 10.1038/nrneurol.2017.44. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nuclear Med. 2006;47(6):904–911. [PubMed] [Google Scholar]
- 10.Tripathi M, Sharma R, D'Souza M, Jaimini A, Panwar P, Varshney R, Datta A, Kumar N, Garg G, Singh D, et al. Comparative evaluation of F-18 FDOPA, F-18 FDG, and F-18 FLT-PET/CT for metabolic imaging of low grade gliomas. Clin Nucl Med. 2009;34(12):878–883. doi: 10.1097/RLU.0b013e3181becfe0. [DOI] [PubMed] [Google Scholar]
- 11.Becherer A, Karanikas G, Szabo M, Zettinig G, Asenbaum S, Marosi C, Henk C, Wunderbaldinger P, Czech T, Wadsak W, et al. Brain tumour imaging with PET: a comparison between [18F] fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30(11):1561–1567. doi: 10.1007/s00259-003-1259-1. [DOI] [PubMed] [Google Scholar]
- 12.Fueger BJ, Czernin J, Cloughesy T, Silverman DH, Geist CL, Walter MA, Schiepers C, Nghiemphu P, Lai A, Phelps ME, et al. Correlation of 6-18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J Nuclear Med. 2010;51(10):1532–1538. doi: 10.2967/jnumed.110.078592. [DOI] [PubMed] [Google Scholar]
- 13.Janvier L, Olivier P, Blonski M, Morel O, Vignaud JM, Karcher G, Taillandier L, Verger A. Correlation of suv-derived indices with tumoral aggressiveness of gliomas in static <sup>18</sup>F-FDOPA PET: use in clinical practice. Clin Nucl Med. 2015;40(9):e429–e435. doi: 10.1097/RLU.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 14.Karunanithi S, Bandopadhyaya GP, Sharma P, Kumar A, Singla S, Malhotra A, Gupta DK, Bal C. Prospective comparison of (99m)Tc-GH SPECT/CT and (18) F-FDOPA PET/CT for detection of recurrent glioma: a pilot study. Clin Nucl Med. 2014;39(2):e121–e128. doi: 10.1097/RLU.0b013e318279bcd8. [DOI] [PubMed] [Google Scholar]
- 15.Karunanithi S, Sharma P, Kumar A, Khangembam BC, Bandopadhyaya GP, Kumar R, Goenka A, Gupta DK, Malhotra A, Bal C. Comparative diagnostic accuracy of contrast-enhanced MRI and (18) F-FDOPA PET-CT in recurrent glioma. Eur Radiol. 2013;23(9):2628–2635. doi: 10.1007/s00330-013-2838-6. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 19.Swets JA. Measuring the accuracy of diagnostic systems. Science (New York, NY) 1988;240(4857):1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 20.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 22.Sellam K, Bal C, Gupta D, Malhotra A, Kumar A, Bandopadhyaya G. Inter comparison of 18F-FDOPA PET-CT, 99mTc-GHA SPECT-CT and 18F- FDG PET-CT in cases with recurrent glioma. J Nucl Med. 2010;51.
- 23.Jora C, Mattakarottu JJ, Aniruddha PG, Mudalsha R, Singh DK, Pathak HC, Sharma N, Sarin A, Prince A, Singh G. Comparative evaluation of 18F-FDOPA, 13N-AMMONIA, 18F-FDG PET/CT and MRI in primary brain tumors - a pilot study. Indian J Nuclear Med. 2011;26(2):78–81. doi: 10.4103/0972-3919.90256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pafundi DH, Laack NN, Youland RS, Parney IF, Lowe VJ, Giannini C, Kemp BJ, Grams MP, Morris JM, Hoover JM, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro-oncology. 2013;15(8):1058–1067. doi: 10.1093/neuonc/not002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann K, Czernin J, Cloughesy T, Lai A, Pomykala KL, Benz MR, Buck AK, Phelps ME, Chen W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro-oncology. 2014;16(4):603–609. doi: 10.1093/neuonc/not166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morana G, Piccardo A, Puntoni M, Nozza P, Cama A, Raso A, Mascelli S, Massollo M, Milanaccio C, Garre ML, et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: a comparative study. Neuro-oncology. 2015;17(12):1637–1647. doi: 10.1093/neuonc/nov099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Singh M, Garg A, Tripathi M, Gupta S, Kp H, Bal C. Comparison of F18-Fluorodopa, F18-Fluorocholine and F18- Fluorodeoxyglucose PET/CT for detection of recurrence in patients with primary brain tumors. Eur J Nucl Med Mol Imaging. 2016;43(1):S194. [Google Scholar]
- 28.Paquet M, Doyen J, Mondot L, Bouzid ES, Bondiau P, Almairac F, Fontaine D, Chanalet S, Ouvrier M, Zwarthoed C, et al. Value of early and delayed imaging for 18F-FDOPA PET high grade gliomas evaluation. Eur J Nucl Med Mol Imaging. 2017;44:S642–S643. [Google Scholar]
- 29.Evangelista L, Burei M, Rita Cervino A, Reccia P, Cuppari L. Suspicious for recurrent low and high grade glioma and indeterminate MRI: the role of 18F-DOPA PET/CT. J Nucl Med. 2018;59.
- 30.Youland RS, Pafundi DH, Brinkmann DH, Lowe VJ, Morris JM, Kemp BJ, Hunt CH, Giannini C, Parney IF, Laack NN. Prospective trial evaluating the sensitivity and specificity of 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (18F-DOPA) PET and MRI in patients with recurrent gliomas. J Neuro-Oncol. 2018;137(3):583–591. doi: 10.1007/s11060-018-2750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evangelista L, Lea C, Luisa B, Daniele B, Mario C, Pasquale R, Vittorina Z, Giuseppe L. Comparison between 18f-Dopa and 18f-Fet pet/Ct in patients with suspicious recurrent high grade glioma: a literature review and our experience. Curr Radiopharm. 2019. [DOI] [PubMed]
- 32.Nioche C, Soret M, Gontier E, Lahutte M, Dutertre G, Dulou R, Capelle L, Guillevin R, Foehrenbach H, Buvat I. Evaluation of quantitative criteria for glioma grading with static and dynamic 18F-FDopa PET/CT. Clin Nucl Med. 2013;38(2):81–87. doi: 10.1097/RLU.0b013e318279fd5a. [DOI] [PubMed] [Google Scholar]
- 33.Bund C, Heimburger C, Imperiale A, Lhermitte B, Chenard MP, Lefebvre F, Kremer S, Proust F, Namer IJ. FDOPA PET-CT of nonenhancing brain tumors. Clin Nucl Med. 2017;42(4):250–257. doi: 10.1097/RLU.0000000000001540. [DOI] [PubMed] [Google Scholar]
- 34.Morana G, Piccardo A, Tortora D, Puntoni M, Severino M, Nozza P, Ravegnani M, Consales A, Mascelli S, Raso A, et al. Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur J Nucl Med Mol Imaging. 2017;44(12):2084–2093. doi: 10.1007/s00259-017-3777-2. [DOI] [PubMed] [Google Scholar]
- 35.Patel CB, Fazzari E, Chakhoyan A, Yao J, Raymond C, Nguyen H, Manoukian J, Nguyen N, Pope W, Cloughesy TF, et al. (18) F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naive gliomas: a cross-sectional study. J Neuro-Oncol. 2018;139(2):399–409. doi: 10.1007/s11060-018-2877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Zheng J, Xu W, Weng J, Gao L, Tao L, Liang F, Zhang J. Accuracy of (18) F-FDOPA positron emission tomography and (18) F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg. 2018;114:e1211–e1224. doi: 10.1016/j.wneu.2018.03.179. [DOI] [PubMed] [Google Scholar]
- 37.Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. doi: 10.1016/S1470-2045(17)30194-8. [DOI] [PubMed] [Google Scholar]
- 38.Galldiks N, Langen KJ, Pope WB. From the clinician's point of view - what is the status quo of positron emission tomography in patients with brain tumors? Neuro-oncology. 2015;17(11):1434–1444. doi: 10.1093/neuonc/nov118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro-oncology. 2016;18(3):426–434. doi: 10.1093/neuonc/nov148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunet V, Rossier C, Buck A, Stupp R, Prior JO. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nuclear Med. 2012;53(2):207–214. doi: 10.2967/jnumed.111.096859. [DOI] [PubMed] [Google Scholar]
- 41.Lapa C, Linsenmann T, Monoranu CM, Samnick S, Buck AK, Bluemel C, Czernin J, Kessler AF, Homola GA, Ernestus RI, et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55(10):1611–1616. doi: 10.2967/jnumed.114.140608. [DOI] [PubMed] [Google Scholar]
- 42.Puttick S, Bell C, Dowson N, Rose S, Fay M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov Today. 2015;20(3):306–317. doi: 10.1016/j.drudis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Salmon E, Bernard Ir C, Hustinx R. Pitfalls and limitations of PET/CT in brain imaging. Semin Nucl Med. 2015;45(6):541–551. doi: 10.1053/j.semnuclmed.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Morana G, Puntoni M, Garre ML, Massollo M, Lopci E, Naseri M, Severino M, Tortora D, Rossi A, Piccardo A. Ability of (18) F-DOPA PET/CT and fused (18) F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur J Nucl Med Mol Imaging. 2016;43(9):1664–1672. doi: 10.1007/s00259-016-3333-5. [DOI] [PubMed] [Google Scholar]
- 45.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.