Abstract
Background
High-risk patients in the pediatric intensive care unit (PICU) contribute substantially to PICU-mortality. Complex chronic conditions (CCCs) are associated with death. However, it is unknown whether CCCs also increase mortality in the high-risk PICU-patient. The objective of this study is to determine if CCCs or other factors are associated with mortality in this group.
Methods
Retrospective cohort study from a national PICU-database (2006–2012, n = 30,778). High-risk PICU-patients, defined as patients < 18 years with a predicted mortality risk > 30% according to either the recalibrated Pediatric Risk of Mortality-II (PRISM) or the Paediatric Index of Mortality 2 (PIM2), were included. Patients with a cardiac arrest before PICU-admission were excluded.
Results
In total, 492 high-risk PICU patients with mean predicted risk of 24.8% (SD 22.8%) according to recalibrated PIM2 and 40.0% (SD 23.8%) according to recalibrated PRISM were included of which 39.6% died. No association was found between CCCs and non-survival (odds ratio 0.99; 95% CI 0.62–1.59). Higher Glasgow coma scale at PICU admission was associated with lower mortality (odds ratio 0.91; 95% CI 0.87–0.96).
Conclusions
Complex chronic conditions are not associated with mortality in high-risk PICU patients.
Electronic supplementary material
The online version of this article (10.1186/s12887-019-1646-9) contains supplementary material, which is available to authorized users.
Keywords: Child, Critical care, Mortality, Outcome assessment (healthcare)
Background
Patients with a high predicted mortality risk in the pediatric intensive care unit (PICU) are a challenge to the clinical team. The relatively small subset of these patients contributes substantially to the number of non-survivors and to PICU-resources. Around 1% of the PICU-admissions in the Australian and New Zealand Paediatric Intensive Care Registries (ANZPIC) has a predicted mortality risk between 30 and 100%, but this small cohort contributes to one third of all deaths [1–3].
Complex chronic conditions (CCCs) are associated with prolonged length of stay in PICU patients, unplanned readmissions and death [4, 5]. A CCC is defined as ‘any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 organ system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center’ [6]. There are many CCCs in several organ systems. Examples are spinal cord malformations, cystic fibrosis, hypoplastic left heart syndrome, extreme immaturity, metabolic disorders, etc. [7] Besides CCCs there are so called ‘noncomplex chronic conditions’ (NCCCs), diagnoses that could be expected to last > 12 months but not meeting the additional CCC criteria. Examples of NCCCs are asthma, atrial septal defect, obesity, etc. [4]. The prevalence of CCCs among hospitalized patients and among PICU patients is increasing [4]. Only few CCCs are incorporated in severity-of illness models like Paediatric Index of Mortality (PIM (2,3)) and Pediatric Risk of Mortality (PRISM (II, III, IV) [4, 8–12]. In low-risk PICU-patients (patients with predicted mortality risk < 1%) CCCs and unplanned admissions are associated with death (OR 3.29, 95% CI 1.97–5.50) [13, 14]. It is unknown whether CCCs increase mortality in the high-risk PICU patient as well.
Therefore, the aim of the present study is to determine if CCCs or other identifiable factors are associated with death in high-risk PICU-patients.
Methods
Study population
Patients were derived from a national PICU database containing data from all pediatric intensive care departments in the Netherlands (2006–2012, n = 30,778); the ‘PICE-registry’ [13, 15]. The same cohort was used in a previous study on low-risk PICU-patients [13]. Patients < 18 years old with a high predicted mortality risk were included in the study. High-risk was defined as a predicted mortality risk > 30% according to either the PRISM II (referred to as PRISM) or the PIM2 risk score [9, 10]. In this study, as described before, both models were recalibrated to predict the overall mortality in the total population in this particular 6-year period without altering the relative weights of risk factors in the models and thus retaining the discriminative power of the original models [13, 15].
Patients who were already dead before PICU admission (e.g., patients admitted for organ transplantation already being brain-dead) or patients admitted for palliative care, patients dying within 2 h of PICU admission, and patients transferred to another ICU during their PICU treatment were excluded from the study. Data of patients that did not pass quality control during local site audit visits and were excluded from the annual reports were also excluded from the study [13]. Patients with a cardiac arrest prior to PICU admission were excluded due to possible bias of the results [16, 17].
Design
Retrospective cohort study based on data prospectively collected in a national registry.
Risk variables and data-handling
Variables that were analysed represented many aspects of the PICU stay, including admission characteristics, physiological state, diagnoses and outcome. Non-survivors were defined as patients who died in the PICU. The ANZPIC diagnostic code list was used in the PICE-registry [18]. Patients were classified as patients with a CCC if either the primary diagnosis, underlying diagnosis or first additional diagnosis was a CCC [6, 7]. Patients were classified as having a NCCC if the primary diagnosis, underlying diagnosis or first additional diagnosis was a diagnosis defined as a NCCC. A modified Feudtner’s list was used to classify diagnoses into CCC or NCCC [4, 6, 7, 18]. ANZPIC diagnoses not appearing on these lists were classified according to expert opinion (C.V. and J.L.). The list of CCC-diagnoses was recently published [13]. Definitions of ‘Admission outside office hours’, ‘readmission’ and ‘specialized transport’ were published previously [13]. The data were checked for non-valid data. Illogical and impossible values that surpassed physiologic threshold values were excluded if the value likely resulted from a typo or measurement error, as described before. (Examples of typo/measurement errors: diastolic blood pressure > 400 mmHg, low paO2 in combination with cyanotic congenital heart disease which by definition should be excluded from PRISM score.) [13].
Statistical analysis
Depending on distribution, continuous variables were tested using an independent T test or Mann-Whitney U test. For dichotomous variables, chi-square test or, in case of small expected frequencies, Fisher’s exact test was used. To adjust for multiple testing, Bonferroni correction was performed and differences were considered statistically significant if p-value was < 0.001.
For the multivariable logistic regression analysis, only risk factors that were present at the time of admission were included in the regression analysis. Because the selection of the study population was based on PIM2 and PRISM scores, predictors from these scores were not included in the multivariable logistic regression analysis, except for the Glasgow Coma Scale (GCS) at admission.
Statistical analyses were carried out using IBM SPSS Statistics Version 22.1.
Results
Population characteristics
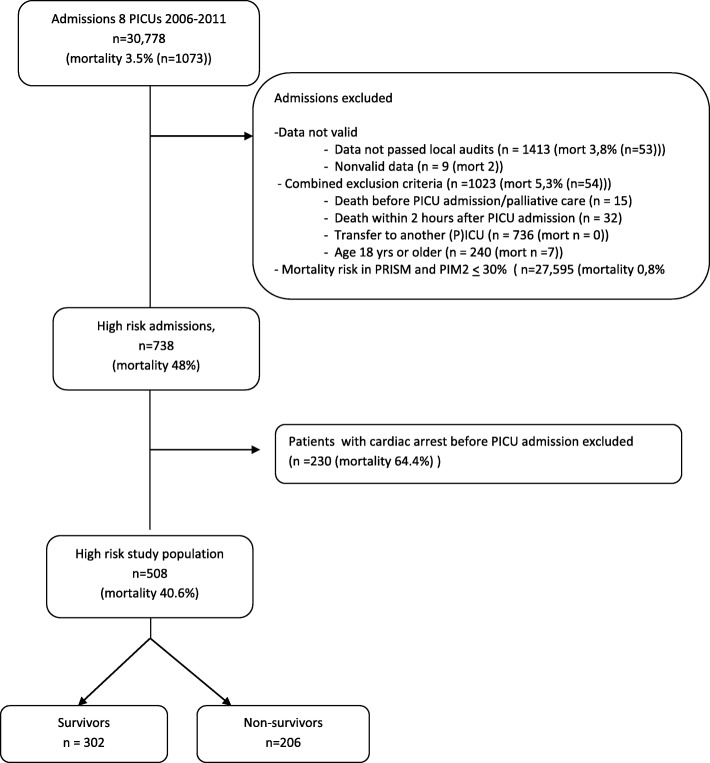
In total, there were 30,778 admissions of which 738 patients were high-risk patients (Fig. 1, Additional file 1: Table S3). After excluding patients with cardiac arrest before PICU admission, a total of 492 high-risk patients was included with a mortality rate of 39.6%. The mean predicted mortality risk of these 492 patients was 24.8% (SD: 22.8%) according to the recalibrated PIM2 and 40.0% (SD: 23.8%) according to the recalibrated PRISM. The majority of the high-risk patients had an unplanned admission for medical reasons.
Fig. 1.
Flowchart of the population
Analysis of differences
Baseline characteristics are shown in Table 1. The median GCS at time of admission was significantly higher in survivors compared to non-survivors (median 15 vs. median 12, respectively; p < 0.001). Both PRISM and PIM2 mortality risks were significantly lower in survivors compared to non-survivors. Ventilator-days and length of stay were longer in survivors compared to non-survivors. No other significant differences were found.
Table 1.
Population characteristics and differences between high-risk survivors and non-survivors
| Characteristic | Survivors n = 297 | Non-survivors n = 195 | p value |
|---|---|---|---|
| Male | 179 (60.3) | 105 (53.8) | 0.16 |
| Age < 12 months | 161 (54.2) | 90 (46.2) | 0.08 |
| Unplanned admission | 275 (92.6) | 182 (93.3) | 0.76 |
| Medical admission | 227 (76.4) | 146 (74.9) | 0.69 |
| Readmission < 48 h | 4 (1.3) | 3 (1.5) | 0.86 |
| Admission outside office hours | 151 (50.8) | 96 (49.2) | 0.73 |
| Mode of transport upon admission | |||
| None | 159 (53.5) | 104 (53.3) | 0.97 |
| Non-specialized transport | 52 (17.5) | 20 (10.3) | 0.03 |
| Specialized transport | 107 (36.0) | 84 (43.1) | 0.12 |
| Season of admission | |||
| Winter | 74 (24.9) | 46 (23.6) | 0.74 |
| Spring | 64 (21.5) | 51 (26.1) | 0.24 |
| Summer | 59 (19.8) | 50 (25.6) | 0.13 |
| Autumn | 100 (33.7) | 48 (24.6) | 0.03 |
| Recovery as reason for PICU admission | 22 (7.4) | 15 (7.7) | 0.91 |
| PRISM recalibrated mortality risk, median [IQR] | 0.36 [0.15–0.48] | 0.44 [0.31–0.66] | < 0.001 |
| PIM2 recalibrated mortality risk, median [IQR] | 0.14 [0.05–0.34] | 0.21 [0.09–0.46] | < 0.001 |
| Patients with | |||
| PRISM > 30% (and PIM < 30%) | 190 (64.0) | 115 (59.0) | 0.26 |
| PIM2 > 30% (and PRISM < 30%) | 89 (30.0) | 43 (22.1) | 0.05 |
| PRISM and PIM2 > 30% | 18 (6.1) | 37 (19.1) | < 0.001 |
| Chronic conditions | |||
| No chronic condition | 82 (27.6) | 68 (34.9) | 0.09 |
| NCCC | 19 (6.4) | 7 (3.6) | 0.17 |
| CCC | 196 (66.0) | 120 (61.5) | 0.31 |
| Diagnose groups | |||
| Trauma | 9 (3.0) | 16 (8.2) | 0.01 |
| Cardiovascular | 30 (10.1) | 22 (11.3) | 0.68 |
| Neurological | 30 (10.1) | 31 (15.9) | 0.06 |
| Respiratory | 79 (26.6) | 29 (14.9) | 0.002 |
| Renal | 2 (0.7) | 1 (0.5) | 0.82 |
| Gastrointestinal | 14 (4.7) | 12 (6.2) | 0.49 |
| Post procedure diagnosis | 45 (15.2) | 32 (16.4) | 0.71 |
| Miscellaneous | 88 (29.6) | 52 (26.7) | 0.48 |
| Glasgow Coma Scale at admission | 15 [9–15] | 12 [3–15] | < 0.001 |
| Mechanically ventilated (n = 660) | 260 (91.5) | 178 (97.8) | 0.01 |
| Outcome | |||
| Number of days mechanically ventilated, median [IQR] | 7 [4–13] | 3 [2–7] | < 0.001 |
| Length of stay PICU, median [IQR] | 12 [7–21] | 3 [2–7] | < 0.001 |
Data are presented as n (%), unless mentioned otherwise
[IQR] is defined as interquartile range: [25th percentile – 75th percentile]
NCCC non-complex chronic condition, CCC complex chronic condition
The physiological parameters are the most abnormal values collected in the first 24 h after admission
Factors associated with survival
Higher GCS at admission was associated with lower mortality (OR 0.91; 95% CI 0.87–0.96) (Table 2). No association was found between CCCs and non-survival (OR 0.99; 95% CI 0.62–1.59). No other factors were associated with mortality. Results from the unadjusted ORs are shown in (Additional file 1: Table S3).
Table 2.
Variables associated with non-survival in the high-risk group
| Factor | OR | 95% CI |
|---|---|---|
| Male | 0.75 | 0.51–1.12 |
| Age < 1 yr. | 0.84 | 0.56–1.27 |
| Specialized transport | 1.24 | 0.82–1.88 |
| Admission outside office hours | 0.79 | 0.53–1.17 |
| Season | ||
| Winter | Ref | |
| Spring | 1.29 | 0.74–2.25 |
| Summer | 1.53 | 0.87–2.70 |
| Autumn | 0.84 | 0.49–1.43 |
| Chronic conditions | ||
| No chronic condition | Ref | |
| CCC | 0.99 | 0.62–1.59 |
| NCCC | 0.53 | 0.19–1.45 |
| Diagnose subgroups | ||
| Trauma | Ref | |
| Cardiovascular | 0.91 | 0.30–2.77 |
| Neurological | 1.06 | 0.48–2.33 |
| Respiratory | 0.85 | 0.39–1.87 |
| Renal | 0.70 | 0..36–1.36 |
| Gastrointestinal | 0.61 | 0.05–7.79 |
| Post procedure | 0.77 | 0.42–1.44 |
| Miscellaneous | 1.38 | 0.54–3.52 |
| Glasgow coma scale at admission | 0.91 | 0.87–0.96 |
NCCC non-complex chronic condition, CCC complex chronic condition
Results from the unadjusted ORs are shown in Additional file 1: Table S3
Discussion
In this large retrospective cohort study in high-risk PICU patients, complex chronic conditions were not associated with mortality.
This is different compared to our previous study looking into low-risk admissions, where CCCs were associated with increased mortality [13]. In a general PICU-population, without risk stratification, a similar association was found [4]. Although some CCCs (for example: leukemia, hypoplastic left heart syndrome) are incorporated in the PIM2, the majority of CCCs is not part of the risk models. Having a chronic disease is often not reflected in physiological values and therefore not shown as a higher mortality risk. CCCs can be very heterogeneous. Some CCCs might be associated with death in the PICU (e.g. a patient with a complex heart disorder) while other CCCs are not lethal but may have impact on other outcome parameters like functional outcome. Furthermore, it’s possible that some patients with CCCs may be refused PICU admission and thus not contribute to the overall PICU mortality. We did not investigate this and therefore this statement is conjecture. In true high-risk patients other factors like the GCS have a clearer influence on mortality for patients with CCCs.
Our study has several limitations. First, an arbitrary choice was made for the definition of high-risk patients, using a combination of PIM2 and PRISM scores with a certain cut-off point. Both models use different predictors and different time windows to calculate their scores and do not give the same result. Because in the Dutch PICE registry both models are used and no model is superior to another, we used a combination of both models. Using only one model instead of a combination might underestimate a cohort of high-risk patients. Only a minority had a mortality risk of > 30% in both models. Mean predicted mortality was higher according to PRISM compared to PIM2. However, if only PRISM model had been used to detect high-risk patients, roughly a third of the high-risk cohort would not have been detected.
Third, an older version of the PRISM was used, dating from 1988 [10]. If the original PRISM model would have been used without recalibration, the predicted mortality would have been overestimated. However, because the PRISM was recalibrated to fit, it is a good predictor of mortality [15].
Fourth, no factors which are part of the PIM2/PRISM models were used for the multivariable logistic regression analysis, with the exception of the GCS at admission. The GCS at admission is not incorporated in the PIM2 model but is indirectly part of the PRISM score as a dichotomous variable. If the GCS within the first 24 h is less than 8, the PRISM score increases. However, a mild decrease in GCS such as GCS between 8 and 10 does not increase PRISM score, although there might be a serious neurological condition. We found a significant and clinically important lower GCS in non-survivors. This difference could not be explained by cardiac arrest patients. Therefore we decided to add the GCS as a continuous variable in the analysis.
Conclusions
Complex chronic conditions are not associated with mortality in PICU patients with a high predicted mortality-risk, in contrast to low-risk PICU patients. We recommend to explore the role of CCCs in (PICU) patients with different risk profiles further. Higher Glasgow coma scale at PICU admission was associated with lower mortality.
Additional file
Table S3. Variables associated with mortality survival in the high-risk group. (DOCX 12 kb)
Acknowledgements
Members of SKIC (Dutch collaborative PICU research network) were as follows:
Dick van Waardenburg, MD, PhD, department of pediatric intensive care, Academic Hospital Maastricht, the Netherlands.
Nicolette A. van Dam, MD, department of pediatric intensive care, Leiden University Medical Center, Leiden, the Netherlands.
Nicolaas J. Jansen, MD, PhD, department of pediatric intensive care, University Medical Center Utrecht, Utrecht, the Netherlands.
Marc van Heerde, MD, PhD, department of pediatric intensive care, VU University Medical Center, Amsterdam, the Netherlands.
Matthijs de Hoog, MD, PhD, department of pediatric intensive care, Erasmus University Medical Center – Sophia Children’s Hospital, Rotterdam, the Netherlands.
Martin Kneyber, MD, PhD, FCCM, department of paediatrics, division of pediatric critical care medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Maaike Riedijk, MD, PhD, department of pediatric intensive care, Academic Medical Center, Amsterdam, the Netherlands.
Abbreviations
- ANZPIC
Australian and New Zealand Paediatric Intensive Care Registries
- CCCs
Complex chronic conditions
- CGS
Glasgow coma scale
- NCCC
Non-complex chronic condition
- PICE registry
Dutch pediatric intensive care registry (‘Pediatrische Intensive Care Evaluatie’)
- PICU
Pediatric intensive care unit
- PIM
Paediatric Index of Mortality
- PRISM
Pediatric Risk of Mortality
Authors’ contributions
CV conceptualized and designed the study, acquired the data, carried out the analyses, drafted and revised the initial manuscript and approved the final manuscript as submitted. NW and IV acquired the data, assisted with the interpretation of the data and revised the manuscript and approved the final manuscript. JH, JL, JvdH and MvdB conceptualized and designed the study, supervised the data collection, critically reviewed the manuscript and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Availability of data and materials
The data that support the findings of this study were used under license from the national PICU database containing data from all pediatric intensive care departments in the Netherlands (‘PICE registry’) for the current study, and so are not publicly available. Limited data are available from the author on reasonable request.
Ethics approval and consent to participate
The Institutional Review Board approved the study and waived the need for informed consent (Commissie Mensgebonden Onderzoek Radboudumc; 2017–3848).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This work was performed at the department of intensive care, Radboud university medical center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carin W. Verlaat, Phone: +31(0)24-3617273, Email: carin.verlaat@radboudumc.nl
SKIC (Dutch collaborative PICU research network):
Dick van Waardenburg, Nicolette A. van Dam, Nicolaas J. Jansen, Marc van Heerde, Matthijs de Hoog, Martin Kneyber, and Maaike Riedijk
References
- 1.ANZPIC-registry: Report of the Australian and New Zealand Paediatric intensive care registry 2013. In: https://www.anzics.com.au/annual-reports/. 2013.
- 2.ANZPIC-registry: ANZPICR Annual Report 2014. In. https://www.anzics.com.au/annual-reports/. 2014.
- 3.ANZPIC-registry: ANZPICR Annual Report 2015. In. https://www.anzics.com.au/annual-reports/. 2015.
- 4.Edwards JD, Houtrow AJ, Vasilevskis EE, Rehm RS, Markovitz BP, Graham RJ, Dudley RA. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay*. Crit Care Med. 2012;40(7):2196–2203. doi: 10.1097/CCM.0b013e31824e68cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards JD, Lucas AR, Boscardin WJ, Dudley RA. Repeated critical illness and unplanned readmissions within 1 year to PICUs. Crit Care Med. 2017;45(8):1276–1284. doi: 10.1097/CCM.0000000000002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington state, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 7.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shann F, Pearson G, Slater A, Wilkinson K. Paediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med. 1997;23(2):201–207. doi: 10.1007/s001340050317. [DOI] [PubMed] [Google Scholar]
- 9.Slater A, Shann F, Pearson G. Paediatric index of mortality study G: PIM2: a revised version of the Paediatric index of mortality. Intensive Care Med. 2003;29(2):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 10.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Pollack MM, Holubkov R, Funai T, Dean JM, Berger JT, Wessel DL, Meert K, Berg RA, Newth CJ, Harrison RE, et al. The pediatric risk of mortality score: update 2015. Pediatr Crit Care Med. 2016;17(1):2–9. doi: 10.1097/PCC.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verlaat CW, Visser IH, Wubben N, Hazelzet JA, Lemson J, van Waardenburg D, van der Heide D, van Dam NA, Jansen NJ, van Heerde M, et al. Factors associated with mortality in low-risk pediatric critical care patients in the Netherlands. Pediatr Crit Care Med. 2017;18(4):e155–e161. doi: 10.1097/PCC.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 14.Eulmesekian PG. Low-risk pediatric critical care patients, are they really a different population? Pediatr Crit Care Med. 2017;18(4):390–391. doi: 10.1097/PCC.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 15.Visser IH, Hazelzet JA, Albers MJ, Verlaat CW, Hogenbirk K, van Woensel JB, van Heerde M, van Waardenburg DA, Jansen NJ, Steyerberg EW. Mortality prediction models for pediatric intensive care: comparison of overall and subgroup specific performance. Intensive Care Med. 2013;39(5):942–950. doi: 10.1007/s00134-013-2857-4. [DOI] [PubMed] [Google Scholar]
- 16.Atkins DL, Everson-Stewart S, Sears GK, Daya M, Osmond MH, Warden CR, Berg RA. Resuscitation outcomes consortium I: epidemiology and outcomes from out-of-hospital cardiac arrest in children: the resuscitation outcomes consortium Epistry-cardiac arrest. Circulation. 2009;119(11):1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Browning B, Pemberton VL, Page K, et al. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376(4):318–329. doi: 10.1056/NEJMoa1610493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slater A, Shann F, McEniery J, Group AS The ANZPIC registry diagnostic codes: a system for coding reasons for admitting children to intensive care. Intensive Care Med. 2003;29(2):271–277. doi: 10.1007/s00134-002-1600-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3. Variables associated with mortality survival in the high-risk group. (DOCX 12 kb)
Data Availability Statement
The data that support the findings of this study were used under license from the national PICU database containing data from all pediatric intensive care departments in the Netherlands (‘PICE registry’) for the current study, and so are not publicly available. Limited data are available from the author on reasonable request.