Abstract
Context:
This review discusses the range of clinical presentations seen with poisonings by the major toxic alcohols--methanol, ethylene glycol, and isopropyl alcohol. It outlines a straightforward diagnostic strategy and discusses in detail the current treatment recommendations.
Evidence acquisition:
The authors conducted a literature search of primary and secondary sources related to the topic. For treatment recommendations, search restrictions included articles published between 2008 and 2019. For background information, search restrictions included articles written from 1990 – present.
Results:
This review discusses in detail how the diagnosis can be made via clinical signs, symptoms, and laboratory values as well as the most recent treatment recommendations. This paper will also discuss the limitations of the emergency department workup and how the absence of particular laboratory findings does not necessarily rule out the diagnosis.
Conclusion:
Poisoning with methanol, ethylene glycol, and isopropanol present diagnostic and therapeutic challenges to emergency physicians. Toxic alcohol poisonings lead to an elevated osmolar gap and, with the exception of Isopropanol, a metabolic acidosis. In order for the timely initiation of life-saving treatment, emergency physicians need a solid understanding of the pathophysiology, clinical presentation, laboratory workup, and treatment.
Keywords: Alcohols, Ethylene Glycol, Isopropanol, Methanol, Patient Care Management, Poisoning
Context
Poisonings with methanol, ethylene glycol, and isopropanol—commonly referred to as the toxic alcohols—often present the emergency physician with a major diagnostic challenge. The identity of the ingested substance is frequently a mystery on presentation. Patients with an intentional ingestion, either for recreation or with suicidal intent, may be less than forthcoming. Young patients may not be able to identify the substance. Patients may be in significant distress or comatose and unable to give any useful history. In these cases, the clinician must rely upon the nature of the presentation and the presence of metabolic derangements—and must always keep a high index of suspicion for toxic alcohol poisoning.
Direct assays for the toxic alcohols are seldom available. The American College of Clinical Toxicology states that for toxic alcohol levels to be clinically useful, they must be resulted within two hours of being drawn (1). The gold standard test for the determination of serum toxic alcohol levels, however, is gas chromatography, which the vast majority of hospital labs do not have the capability of performing. This lab becomes a “send-out” and therefore useless in the acute setting.
Because toxic alcohol poisoning may cause potentially irreversible damage in a time-dependent fashion, prompt diagnosis and treatment are crucial (2). Though we lack immediate testing for the toxic alcohols, there are useful laboratory clues that can help clinicians quickly zero in on the most likely toxic agent. Those clues include the osmol gap, the anion gap, and the patient’s acid-base status. This article will review the pathophysiology of these intoxications, the clinical presentations, the laboratory workup, and the treatment of toxic alcohol ingestions. This paper will also discuss the limitations of the emergency department workup and how the absence of particular laboratory findings does not necessarily rule out the diagnosis.
Evidence acquisition
The authors conducted a literature search of primary and secondary sources related to the topic. For treatment recommendations, search restrictions included articles published between 2008 and 2019. For background information, search restrictions included articles written from 1990 – present.
Results
Toxic alcohols are found in many readily available household and industrial products. Methanol (“wood alcohol”) is a major component of windshield washer fluid, many industrial solvents, and may also be ingested as a recreational intoxicant sometimes mislabeled “moonshine”. Ethylene glycol is a chief component of antifreeze (3). Isopropanol, widely known as rubbing alcohol, is a common antiseptic (4). Although fatalities from toxic alcohol ingestions are relatively rare in the United States (< 30 per year), delayed diagnosis and treatment are the main reasons for poor outcomes (5). It cannot be over-stressed that early identification and treatment can significantly reduce morbidity and mortality.
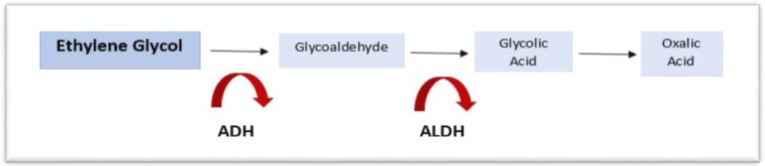
The toxicity of methanol and ethylene glycol arises primarily from highly toxic intermediate metabolites generated by the action of alcohol dehydrogenase (ADH), the key enzyme in their breakdown. The inhibition of ADH, therefore, becomes a crucial step in treatment (5). ADH catalyzes the first oxidation of methanol and ethylene glycol to formaldehyde and glycoaldehyde respectively. These compounds undergo further oxidation by aldehyde dehydrogenase (ALDH) to form carboxylic acid metabolites. Methanol is ultimately metabolized to formic acid, while ethylene glycol is metabolized to glycolic acid and oxalic acid (Figures 1, 2). These metabolic byproducts are potent organic acids that generate a high anion gap metabolic acidosis. They are also responsible, as further discussed below, for other significant toxic effects (1).
Figure 1:
The metabolic pathway of Methanol
Figure 2:
The metabolic pathway of Ethylene Glycol
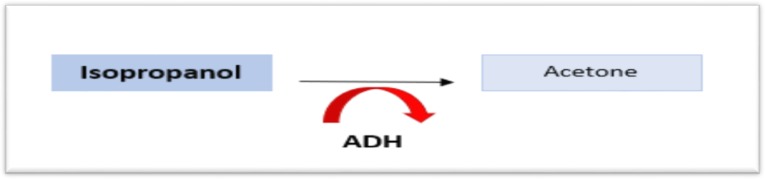
Although methanol and ethylene glycol both produce CNS depression, isopropanol generates the most profound degree (1). Isopropanol is rapidly absorbed and inebriates much like ethanol. It is directly converted to acetone by alcohol dehydrogenase, and acetone itself is a CNS depressant that leads to further sedation (Figure 3). Ingestions are sometime due to an attempt by an individual to replace ethanol when it is unavailable. It does not undergo further metabolism beyond acetone, which is eliminated renally and via the lungs. Acetone, however, does not induce a metabolic acidosis (1).
Figure 3:
The metabolic pathway of Isopropanol
Methanol and ethylene glycol, therefore, will produce an increased osmol gap and a metabolic acidosis. Isopropanol, on the other hand, yields only an increased osmolar gap without metabolic acidosis. The clinician must keep in mind, however, that the presence of a metabolic acidosis does not completely preclude isopropanol ingestion; concomitant hypotension or the co-ingestion of additional agents may upset the acid/base balance and cloud the picture. One thing remains true: low molecular weight substances in the serum, such as these alcohols, ethanol included, will raise the measured serum osmolality above the calculated osmolality, producing an osmol gap (2, 6). Table 1 shows stepwise approach to calculating the Osmol Gap.
Table 1:
Stepwise approach to calculating the Osmol Gap
| 1 Determining the osmol gap begins with obtaining a measured serum osmolality from the laboratory. The normal measured serum osmolality is typically between 285 – 290 mOsm/L | |
| 2. The calculated serum osmolality is then determined by the following equation:
| |
| 3. When attempting to determine the osmolar gap, it is crucial to also check an ethanol level. Ethanol, like the other alcohols, is a low molecular weight substance that will also increase the osmolar gap, altering the results. | |
| 4. Calculation of ethanol’s contribution to the serum osmolality is achieved with the following equation:
| |
| 5. The osmol gap is measured as the difference between the calculated serum osmolality (OSM calc) and the measured osmolality (OSM meas). The difference between the two should be less than or equal to 20
| |
| 6. Intoxication with methanol or ethylene glycol produce a high anion gap metabolic acidosis. The anion gap can be calculated with the following formula:
| |
| 7. It is important to remember, isopropanol poisoning will cause an elevated osmol gap withouta metabolic acidosis, making this calculation important in differentiating between possible intoxicants. |
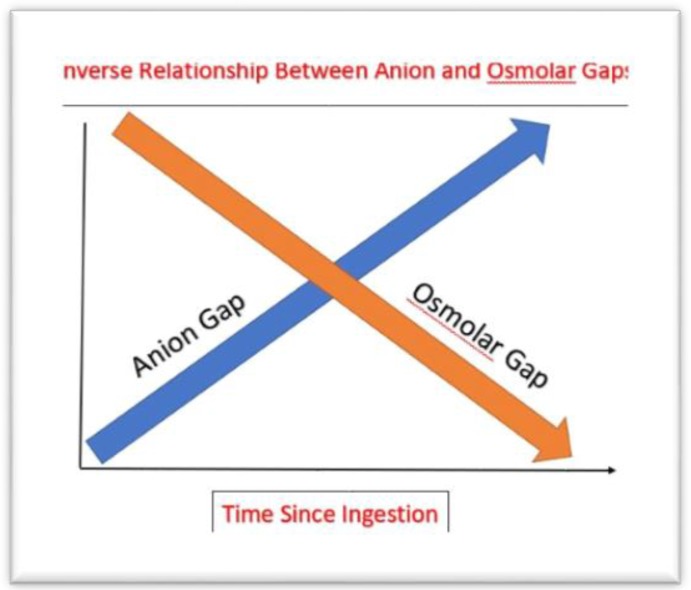
The anion gap is neither sensitive nor specific for diagnosing toxic alcohol poisoning. Soon after ingestion, the patient may manifest symptoms of toxic alcohol ingestion without an anion gap metabolic acidosis. The metabolic acidosis increases as the toxic alcohols are metabolized to organic acids (formate, oxalate). This creates an inverse relationship between the serum anion gap and osmol gap making the values time-dependent from ingestion, which is often unknown (Figure 4). In addition, the process of metabolism of toxic alcohols to organic acids can be slow. The half-life of methanol to formic acid is approximately 6 – 18 hours with acidosis developing as late as 20 hours post ingestion (7).
Figure 4:
The inverse relationship between anion and osmol gaps
Like the anion gap, the osmol gap has low sensitivity and specificity for toxic alcohol poisoning. The range of “normal” for the osmol gap varies, typically see as < 10 to < 20 mOsm/L, depending upon source. The differential diagnosis for elevated osmol gap is wide as any osmotically active substance present in the blood can lead to higher than normal serum osmolality. Important, alternative etiologies include ketoacidosis (diabetic, alcoholic), acute renal failure, chronic renal disease, lactic acidosis, mannitol, and shock (8). These disorders usually have an osmol gap ≤ 15 – 20 mOsm/L. Therefore, an osmol gap > 20 mOsm/L is suspicious for the accumulation of toxic alcohols, especially if there is no other identified source. The absence of an osmol gap does not exclude toxic alcohol poisoning because blood-alcohol levels that are sufficient to cause clinical abnormalities may not markedly elevate the osmol gap (2).
Methanol
Methanol is a colorless, volatile liquid at room temperature, possessing only a faint, sweet odor. Intoxications are rare (1000–2000 cases per year, about 1% of all significant poisonings) and occur primarily through the intentional or unintentional ingestion of methanol containing liquids including windshield washer fluid or antifreeze (2). Methanol has been used as an ethanol replacement and can be found in some products called “moonshine”. (Classic “moonshine” is a high-concentration ethanol solution distilled from fermented corn). Methanol is rapidly absorbed following ingestion and is metabolized by ADH. Toxicity has been reported with as little as 15 ml of 40% methanol solution, which is its approximate concentration in -30-degree F windshield washer fluid (2). Peak levels generally occur within 60–90 minutes (1).
Methanol is processed in the body via zero-order kinetics in which a constant amount of drug is eliminated per unit time independent of the total body load. The estimated elimination of methanol is about 8.5 mg/dl per hour. ADH’s affinity for ethanol is 10 times greater than its affinity for methanol, making ethanol an effective competitive inhibitor that diminishes the conversion of methanol to its toxic metabolites (1).
Formaldehyde and formic acid, the toxic metabolites of methanol metabolism, are extremely damaging to both the central nervous system and the gastrointestinal (GI) tract and generate a profound metabolic acidosis. Nausea, vomiting, and abdominal pain are common symptoms (9). With significant methanol poisonings the serum bicarbonate level can be extremely low. The optic nerve is especially susceptible to damage by formic acid, and visual disturbances occur in 29 – 72% of cases (10). These visual disturbances range from initial blurriness, to photophobia, altered visual fields and, if left untreated, eventual blindness (9).
Clinical symptoms of methanol toxicity usually develop over 6–24 hours. This time course can be delayed if ethanol is co-ingested.10 Later in the course, a common triad of symptoms will usually be noticed (1):
Visual disturbances (12–24 hours)
GI bleeding, pancreatitis
Metabolic acidosis
The mainstay of treatment for methanol poisoning is twofold: 1) the prevention of metabolism; and 2) elimination from the body.10 Regarding the first factor, the American Academy of Clinical Toxicology (AACT) recommends that ethanol or fomepizole be given to block the metabolism of methanol based on the following set of criteria (11):
Plasma methanol concentration > 20 mg/dl
Recent history of ingestion of methanol with serum osmol gap > 10 mOsm/L
- Strong clinical suspicion of methanol poisoning with at least two of the following:
- arterial pH < 7.3
- serum HCO3 < 20 mEq/L
- osmol gap > 20 mOsm/L
Because ADH has a much greater affinity for ethanol than for methanol, it will preferentially bind to ethanol if available. This will disrupt the conversion of methanol to formaldehyde and formic acid. Maintaining an adequate and steady ethanol level in the blood (100–150 mg/dL) is critical for this method to be successful (1).
The use of intravenous ethanol in the treatment of methanol poisoning has advantages and disadvantages. Ethanol is cheap, effective, and readily available. Conversely, administration of intravenous ethanol requires frequent monitoring of serum levels, risk for hypoglycemia, CNS depression, and need for close monitoring, preferably in an intensive care unit.10 Ethanol is also not an FDA approved method of treatment.
Fomepizole is a competitive inhibitor of ADH that received FDA approval for the treatment of methanol poisoning in 2000 (2, 11). It is more commonly used now in the United States than ethanol. In 2015, 90–94% of cases of methanol poisoning were treated with fomepizole, versus 5–6% treated with ethanol (10).
ADH has an affinity for fomepizole up to 8000 times greater than that for ethanol. Fomepizole is effective at low concentrations, has a minimal side effect profile, and does not require frequent levels to be checked or close monitoring in the ICU (11). Initially, the cost of fomepizole was a limiting factor to its widespread adoption. The development of generic fomepizole has made treatment costs comparable to ethanol and have solidified it as the treatment of choice (5, 10).
Since the development of fomepizole, there are mixed feelings among toxicologists concerning the use of hemodialysis in the removal of formic acid in methanol poisoning. A 2002 study by Kerns, et al. found little impact on the half-life of formic acid and concluded that, with low serum methanol levels, dialysis may provide little benefit (12, 13). Fomepizole has, in some cases, eliminated the need for emergent dialysis in patients with improving metabolic acidosis and a serum methanol concentration < 50 mg/dL, allowing it to be delayed until hours after admission (2).
The Extracorporeal Treatments in Poisonings Workgroup (EXTRIP) has presented clear recommendations for the use of hemodialysis in severe methanol poisoning (14):
Coma, Seizures, New Vision Deficits
Metabolic acidosis (blood pH ≤ 7.15)
Persistent metabolic acidosis despite adequate supportive measures and antidotes
Serum anion gap higher than 24 mmol/L
- Serum methanol concentration:
- greater than 70 mg/dL in the context of fomepizole therapy
- greater than 60 mg/dL in the context of ethanol treatment
- greater than 50 mg/dL in the absence of an alcohol dehydrogenase blocker
Renal Failure
Folate has also been shown to accelerate the conversion of formic acid to carbon dioxide and water, aiding in its elimination from the body (1, 9). Given folate’s low side effect profile, its administration should be considered in all methanol poisoned patients. The administration of base is recommended to treat the metabolic acidosis as it also aids in the renal removal of formic acid (2). Gastric decontamination with activated charcoal has limited, if any, value because methanol is rapidly absorbed within 30 minutes of ingestion. Gastric lavage is contraindicated for this same reason (1).
Ethylene Glycol
Ethylene glycol (EG) is a colorless, odorless liquid with a sweet taste. Most poisonings happen through oral ingestion of EG containing liquids, most commonly antifreeze. There are cases of EG poisonings from ethanol substitution, suicide attempts, and accidental ingestions (2, 15). EG intoxication is more common in the United States than methanol. A 2013 survey detailed a total of 5,956 exposures (2% of all significant poisonings), 2,314 of which required hospitalization, versus 1,578 methanol poisonings, 616 of which required inpatient treatment) (5). These numbers have remained reasonably stable year to year.
The mortality of EG poisonings varies greatly owing to the differences in amount ingested and time to treatment. The highest mortality is found in patients with the greatest degree of metabolic acidosis (pH < 7.1) and the longest time from exposure to initiation of treatment (> 10 hours.) (2). The lethal dosage of EG has been reported to be 1.4 – 1.5 mg/kg body weight (about 100 mL in a 70kg adult). Death has been shown at lower amounts, however, and survival at greater concentrations (2). Like methanol, EG is rapidly absorbed from the GI tract and peak serum concentrations are present within 1–2 hours of ingestion.
EG is metabolized in a 2-step process by ADH and aldehyde dehydrogenase with first-order kinetics. In this mechanism, a constant proportion of the substance is eliminated per unit time and the rate of elimination is directly proportional to the amount of substance present. EG is first metabolized by ADH to glycoaldehyde, then to glycolic acid and oxalic acid by aldehyde dehydrogenase. Glycolic acid is the primary metabolite responsible for the severe metabolic acidosis seen with EG intoxications. Glycolic acid inhibits cellular respiration, which favors anaerobic metabolism and the production of lactic acid.
Oxalic acid leads to the formation of calcium oxalate crystals. The accumulation of calcium oxalate crystals in the kidney may cause varying degrees of renal failure. The deposition of calcium oxalate in tissues, furthermore, can induce enough hypocalcemia to depresses cardiac output. Calcium oxalate will generally be detectable in the urine four to eight hours after ingestion (2). In the first 4–5 hours, envelope-shaped, dihydrate crystals will be seen. At 5–7 hours, a combination of dihydrate crystals and needle shaped monohydrate crystals will appear. At greater than 7 hours, only monohydrate crystals typically remain (2). Based upon clinical suspicion of EG poisoning, the examination of urine sediment by light microscopy can aid in the diagnosis. However, this finding is neither highly sensitive nor specific as there are a number of other causes of calcium oxalate crystal formation, and the absence of crystals does not rule out the diagnosis.
Some manufacturers of EG containing antifreeze add sodium fluorescein (SF) to the mixture for the purpose of allowing mechanics to visualize automotive coolant leaks during inspection (16). The examination of urine with a wood’s lamp has been suggested as a possible diagnostic tool in patients with suspected EG intoxication. There are several factors that decrease the usefulness of this testing adjunct. SF is present in a number of drugs, food products, and other toxins leading to false positive results (2). Also, not every brand of antifreeze adds SF to their product, creating false negatives. Additionally, SF has a short half-life of 4.25 hours making its absence in the urine unreliable, especially with an unknown time of ingestion (2, 16, 17).
There have been a number of studies examining the use of SF detection by wood’s lamp to aid in the diagnosis of EG poisoning. When testing physicians’ ability to detect SF by this method, a 2001 study by Wallace et al showed a mean sensitivity, specificity, and accuracy for detecting the presence of SF in urine to be 35%, 75%, and 48% respectively (18). An additional study in 2005 by Parsa et al showed that physicians in two separate groups were able to detect SF in urine under the influence of a Wood’s lamp in 80.7% (group 1) and 69.3% (group 2) of samples with poor inter-rater agreement (72.5%) (17). Given these data, physicians should be careful about confirming or excluding the diagnosis of EG toxicity based on this test, and it should never be used independently when making a decision about initiating treatment (2).
Clinically, patients with ethylene glycol intoxication present with the following triad:
Stage 1: Occurs 30 minutes to 12 hours after ingestion and is characterized by slurred speech, ataxia, anion gap-metabolic acidosis, proteinuria, hematuria, and CNS depression.
Stage 2: Occurs 12 to 36 hours after ingestion and is characterized by symptoms including rapid respiration, cyanosis, and pulmonary edema.
Stage 3: Occurs 2 to 3 days after ingestion and is characterized by renal insufficiency including proteinuria, oliguria, and anuria (1).
The management strategy for EG poisoning is similar to that of methanol. Because its toxicity rests in the byproducts of metabolism (glycolic acid and oxalic acid), blocking this process is key. Fomepizole has a far greater affinity for ADH than does EG making it, again, a powerful competitive inhibitor of the enzyme. Ethanol can also be used to block ADH and is an effective therapy if fomepizole is not available. Gastric decontamination with charcoal is ineffective because of rapid absorption of EG via the GI tract (1). The American Academy of Clinical Toxicology (AACT) recommends that ethanol or fomepizole be given in the presence of the following clinical findings (2):
Ethylene glycol level > 20 mg/dl or a documented history of ingestion of potentially toxic amounts of ethylene glycol
Serum osmol gap > 10 mOsm/L
- Or a history or strong clinical suspicion of ethylene glycol poisoning and two of the following abnormalities:
- Arterial pH < 7.3
- Serum bicarbonate concentration < 20 mEq/L
- Osmol gap > 10 mOsm/L
- The presence of oxalate crystals in the urine
Hemodialysis has been shown to be effective at removing ethylene glycol and glycolic acid from the system but like methanol poisoning, the role of dialysis is controversial (2, 19). Three studies from 1986, 1998, and 2001 demonstrated the effectiveness of hemodialysis at removing both EG and glycolic acid with a mean clearance of about 145–230 ml/min (2). This is extremely important as glycolic acid is largely responsible for the metabolic acidosis seen in EG intoxication and its levels correlate with prognosis and mortality.
In contrast, investigations from 1999, 2001, and 2010 question the use of hemodialysis, especially in uncomplicated patients treated with fomepizole, in spite of high EG levels (20–22). In particular, the 1999 retrospective study by Borron et al looked at 11 patients, 8 of which were treated with fomepizole alone. The patients without renal failure recovered without the use of hemodialysis (20). The AACT recommends dialysis for the treatment of EG poisoning when any of the following clinical factors are present: 1) Persistent metabolic acidosis (pH <7.25) despite therapy; 2) renal failure; 3) Ethylene glycol levels > 50 mg/dl. Because the serum concentration of glycolic acid has been shown to be an important prognostic sign, hemodialysis is also recommended if concentrations are 8 to 10 mmol/L (23). Glycolic acid levels are not readily available in a timely fashion in many laboratories.
Isopropanol
Isopropanol is a common substance found in cleaning agents and also marked as “rubbing alcohol,” a household antiseptic. It is the active component in “alcohol swabs” used in modern medicine. The majority of isopropanol exposures are unintentional and occur most commonly in children less than 6 years old (4). Other exposures have also been reported in suicide attempts and as an ethanol replacement (10). Poison control data from 1997, 1999, and 2004 showed a total of 27,000 exposures making it more common than methanol and ethylene glycol. These exposures presented very low mortality (0.1% in 2004) (10).
Isopropanol is absorbed much faster than ethanol making it roughly two times as intoxicating. An isopropanol level of 100 mg/dL can be considered equivalent to an ethanol level of 200 mg/dL (1). It is a powerful CNS depressant—as is its metabolic product, acetone. Its rapid conversion via ADH makes the time from ingestion to symptom onset within 30 minutes (1). Patients with isopropanol intoxication present with headache, dizziness, miotic pupils, stupor, or coma. Isopropanol is a GI irritant and may cause abdominal pain, vomiting, diarrhea and hematemesis (1, 2).
Acetone is primarily eliminated through the renal system with some escaping via the lungs. Acetone produces a “fruity” odor on the breath as seen in DKA.1 Most commonly, the patient with isopropanol intoxication will present with an elevated osmolar gap without an acidosis (2). This is not always the case because high levels of isopropanol can cause hypotension leading to hypoperfusion and a lactic acidosis. The triad of normal acid-base status, hyperosmolality, and elevated acetone levels are very suggestive of the diagnosis (2).
There are conflicting data with regard to blood/isopropanol levels and clinical outcomes. Some studies have suggested that levels of 150–200 mg/dL can lead to increased mortality. Other studies have demonstrated only levels greater than 400 mg/dL associated with poor outcomes (2). Management of isopropanol toxicity generally consists of supportive care. This includes airway management, IV fluids, and control of GI bleeding.1 Hemodialysis has proven effective at removing isopropanol. Dialysis is a reasonable consideration in patients with an elevated isopropanol level (> 200 mg/dL) and the presence of significant symptoms including coma and/or hypotension.
Discussion
The emergency physician must maintain a high index of suspicion for patients presenting with an unknown intoxication. This review discusses in detail how the diagnosis can be made via clinical signs, symptoms, and laboratory values as well as the most recent treatment recommendations. This paper will also discuss the limitations of the emergency department workup and how the absence of particular laboratory findings does not necessarily rule out the diagnosis.
In the United States, toxic alcohol poisonings are rare, but delays in treatment are associated with significant morbidity and mortality. This makes rapid laboratory assessment and diagnosis essential. Recognizing toxic alcohol poisoning is a challenging endeavor for the emergency physician. These patients typically receive their initial evaluation in the emergency department and may present with a host of nonspecific symptoms (abdominal pain, nausea, vomiting, headache, GI bleeding) and altered mental status, stupor, or coma. With the lack of an inexpensive and readily available diagnostic test to detect these intoxicants, a high index of suspicion should be maintained for any patient presenting with unexplained metabolic acidosis, acute renal failure, neurologic dysfunction, and an elevated osmol gap (2).
As discussed above, the measurements of anion gap and osmol gap are not sensitive nor specific enough to definitively diagnose or rule out toxic alcohol poisoning. Treatment with ethanol, fomepizole, and/or dialysis may need to be started before a definitive diagnosis has been made. A clear understanding of the toxic alcohol pathophysiology, patient presentation, the usefulness and limitations of the laboratory workup, and treatment options will expedite the diagnosis and improve patient outcomes.
Limitations
The information presented in this review article was garnered from a range of literature published over several decades and included the most current and relevant papers available. Important limitations in the diagnosis and treatment of toxic alcohol poisonings discussed in the paper include the accuracy and reliability of the osmol gap as there is a wide array of accepted “normal” ranges. In addition, there is an inverse relationship between the osmol gap and the anion gap making time of ingestion important in the interpretation of these results. Much of the workup for toxic alcohol poisoning is time-dependent from the time of ingestion and this is very often unknown. In addition, definitive assays for the detection of toxic alcohol ingestions are not immediately available at most institutions. Toxicology is a continually evolving field, however, and as new studies are reported, some of our conclusions may need revision.
Conclusions
Poisoning with methanol, ethylene glycol, and isopropanol present diagnostic and therapeutic challenges to emergency physicians. Toxic alcohol poisonings lead to an elevated osmolar gap and, with the exception of Isopropanol, a metabolic acidosis. In order for the timely initiation of life-saving treatment, emergency physicians need a solid understanding of the pathophysiology, clinical presentation, laboratory workup, and treatment.
Acknowledgements
None.
Footnotes
Authors’ contribution
All authors passed four criteria for authorship contribution based on recommendations of the International Committee of Medical Journal Editors.
Conflict of interest
None declared.
Funding
None declared.
Reference
- 1.Williams R, Erickson T. Evaluating toxic alcohol poisoning in the emergency setting. Lab Med. 1998;29(2):102–8. [Google Scholar]
- 2.Kraut J, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol. 2008;3(1):208–25. [DOI] [PubMed] [Google Scholar]
- 3.Latus J, Kimmel M, Alscher M, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J. 2012;5(2):120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaughter R, Mason R, Beasley D, et al. Isopropanol poisoning. Clin Toxicol (Phila). 2014;52(5):470–8. [DOI] [PubMed] [Google Scholar]
- 5.McMartin K, Jacobsen D, Hovda K. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81(3):505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carstairs S, Suchard J, Smith T, Simon LV, Kalynych CJ, Shimada M, et al. Contribution of serum ethanol concentration to the osmol gap: a prospective volunteer study. Clin Toxicol (Phila). 2013;51(5):398–401. [DOI] [PubMed] [Google Scholar]
- 7.Kraut J, Madias N. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2(1):162–74. [DOI] [PubMed] [Google Scholar]
- 8.Marts L, Hsu D, Clardy P. Mind the Gap. Ann Am Thorac Soc. 2014;11(4):671–4. [DOI] [PubMed] [Google Scholar]
- 9.Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002;40(4):415–46. [DOI] [PubMed] [Google Scholar]
- 10.Kraut J, Mullins M. Toxic Alcohols. N Engl J Med. 2018;378(3):270–80. [DOI] [PubMed] [Google Scholar]
- 11.Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360(21):2216–23. [DOI] [PubMed] [Google Scholar]
- 12.Kerns W, 2nd, Tomaszewski C, McMartin K, Ford M, Brent J, META Study Group. Formate kinetics in methanol poisoning. J Toxicol Clin Toxicol. 2002;40(2):137–43. [DOI] [PubMed] [Google Scholar]
- 13.Hovda KE, Froyshov S, Gudmundsdottir H, Rudberg N, Jacobsen D. Fomepizole may change indication for hemodialysis in methanol poisoning: prospective study in seven cases. Clin Nephrol. 2005;64(3):190–7. [DOI] [PubMed] [Google Scholar]
- 14.Roberts DM, Yates C, Megarbane B, Winchester JF, Maclaren R, Gosselin S, et al. Recommendations for the role of extracorporeal treatments in the management of acute methanol poisoning: a systematic review and consensus statement. Crit Care Med. 2015;43(2):461–72. [DOI] [PubMed] [Google Scholar]
- 15.Karlson-Stiber C, Persson H. Ethylene-glycol poisoning: experiences from an epidemic in Sweden. J Toxicol Clin Toxicol. 1992;30(4):565–74. [DOI] [PubMed] [Google Scholar]
- 16.Winter M, Ellis M, Snodgrass W. Urine fluorescence using a Wood’s lamp to detect the antifreeze additive sodium fluorescein: A qualitative adjunctive test in suspected ethylene glycol ingestions. Ann Emerg Med. 1990;19(6):663–7. [DOI] [PubMed] [Google Scholar]
- 17.Parsa T, Cunningham SJ, Wall SP, Almo SC, Crain EF. The usefulness of urine fluorescence for suspected antifreeze ingestion in children. Am J Emerg Med. 2005;23(6):787–92. [DOI] [PubMed] [Google Scholar]
- 18.Wallace K, Suchard J, Curry S, Reagan C. Diagnostic use of physicians’ detection of urine fluorescence in a simulated ingestion of sodium fluorescein-containing antifreeze. Ann Emerg Med. 2001;38(1):49–54. [DOI] [PubMed] [Google Scholar]
- 19.Grabow P, Clay K, Sullivan J, Lepoff R. Organic Acids in Ethylene Glycol intoxication. Ann Int Med. 1986;105:16–20. [DOI] [PubMed] [Google Scholar]
- 20.Borron S, Megarbane B, Baud F. Fompeizole in the Treatment of uncomplicated ethylene glycol poisoning. Lancet. 1999;354(9181):831. [DOI] [PubMed] [Google Scholar]
- 21.Boyer E, Mejia M, Woolf A, Shannon M. Severe ethylene glycol ingestion treated without hemodialysis. Pediatrics. 2001;107(1):172–3. [DOI] [PubMed] [Google Scholar]
- 22.Megarbane B. Treatment of patients with ethylene glycol or methanol poisoning: focus on fomepizole. Open Access Emerg Med. 2010;2:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter W, Rutter P, Bush B, Pappas AA, Dunnington JE. Ethylene Glycol toxicity: the role of serum glycolic acid in hemodialysis. J Toxicol Clin Toxicol. 2001;39(6):607–15. [DOI] [PubMed] [Google Scholar]