Abstract
Background:
Despite precautions, surgical procedures carry risk of infection. Radiation-protective lead aprons worn by operating personnel are a potential source of bacterial contamination and have not been fully evaluated.
Aim/objective:
To evaluate lead aprons as a source of bacterial contamination, identify organisms most commonly found on this source, and devise a method with which to lower the risk of contamination.
Methods:
In this basic science study, 20 randomly selected lead X-ray aprons were swabbed at three time points. The experimental treatment was with a hospital-grade disinfectant wipe. The samples were assessed for bacterial growth via traditional plating methods and mass spectrometry. Plates were graded on a scale of 0 to 4+ based on the number of quadrants with growth. Growth on one quadrant or more was considered contaminated.
Findings/results:
Bacteria were initially detected via IBIS on a majority of the aprons (32/40), most commonly Staphylococcus epidermidis and Propionibacterium acnes. Virulent organisms cultured were Methicillin-resistant Staphylococcus epidermidis (MRSE), Neisseria, Streptococcus viridans and pseudomonas. MRSE were detected on 5/20 of the samples. Immediately after treatment, the majority of aprons showed less bacterial contamination (0/20 standard culture positive; 13/20 IBIS positive) with some recurrence at the 6-h time point (2/20 standard culture positive, 16/20 IBIS positive). All MRSE detected initially was eradicated.
Discussion:
Lead X-ray aprons worn in the operating room harbour bacteria. Disinfecting before use may prevent the introduction of virulent organisms to patients. Our proposed method of sanitising with a disinfectant wipe is quick and effective.
Keywords: Lead apron, X-ray, IBIS, FISH, biofilm, MRSE, disinfectant, wipe, surgical site infection
Background
The risk of wound infection is present with surgical intervention. Various precautions are taken to diminish bacterial load in the operating room (OR) in an effort to decrease the likelihood of surgical site infection (SSI). The use of intraoperative fluoroscopy in the OR has increased as X-ray technology is routinely used for a variety of orthopaedic procedures (Theocharopoulos et al., 2008) and, as such, radiation protective lead X-ray aprons are frequently utilised during surgery. One potential source of bacterial inoculation is the lead X-ray apron worn over clothing by surgeons, nurses and other OR staff members.
The cost of infection can be high and can lead to prolonged hospitalisation, increased morbidity, compromised final outcome, and higher financial and emotional cost to the patient (Emohare et al., 2014; Malizos, 2017; Parisi et al., 2017). Although most SSIs are caused by the patient’s endogenous bacterial flora, OR personnel are also a source of bacterial contamination (Salassa and Swiontkowski, 2014). In an effort to reduce contamination, the number of personnel in the OR is often limited. Other proposals to reduce bacterial contamination include wearing long sleeves during skin preparation (Markel et al., 2018), using disposable impermeable gowns (Bellchambers et al., 1999), and double gloving by the surgical personnel (Tanner and Parkinson, 2006).
The use of lead X-ray aprons for protection from radiation is a standard precaution for both personnel as well as patients in the OR (Matiyahu et al., 1993). The aprons are often shared among personnel and patients, and those participating directly in the surgery wear these lead X-ray aprons beneath their sterile surgical gowns. Over the years, advances in occlusive clothing for operating personnel have further reduced infection rates, but no surgical gown is completely impenetrable to bacteria (Bible et al., 2009; Lankester et al., 2002). Thus, bacteria present on the lead X-ray aprons could serve as a source of bacterial inoculation to the patient; proper sanitising of these gowns may be another opportunity to reduce bacterial contamination in the OR.
Aim/objective
Our aim in this study was to evaluate these lead aprons as a source of bacterial contamination, identify the organisms most commonly found on this source and devise a method with which to lower the risk of contamination. Our study was facilitated by recent microbiological advances in which traditional culture methods are supplemented with polymerase chain reaction (PCR)-mass spectrographic (IBIS) technology and with confocal microscope visualisation of bacteria using the fluorescence in situ hybridisation (FISH) technique. To the best of our knowledge, no other study investigating lead garment contamination has utilised IBIS mass spectrometry technology or the FISH technique.
Methods
Swabbing procedure
X-ray aprons are stored on several racks stationed between dedicated orthopaedic OR suites at our institution. Twenty X-ray aprons were randomly selected. In order to serve as a control, each apron was swabbed before any cleansing treatment. To serve as a negative control, six sterile scrub gowns were also swabbed. All X-ray aprons were then treated with a branded hospital-grade disinfectant wipe that is 55% alcohol based (Super Sani-Cloth Germicidal Wipe, PDI Healthcare, Orangeburg, NY, USA) and again swabbed immediately after cleaning (T0). The gowns were placed in a sealed orthopaedic OR for 6 h (Figure 1). Each of the gowns was swabbed again at the end of the 6-h incubation period (T6).
Figure 1.
The lead X-ray gowns incubating in the sealed OR.
Three separate areas on each of the aprons were swabbed – once on each shoulder pad and once on the upper left area near the pocket line. Sterile technique was observed and each of the culture swabs were initially placed in a sterile saline solution. The swabs were gently tapped to remove excess fluid before sampling the X-ray aprons. Traditional culture methods were supplemented with IBIS mass spectrometry technology (Ibis T-5000- IBIS Inc. Carlsbad, CA, USA) and with the FISH technique in which bacteria were directly visualised (Costerton et al., 2011; Ecker et al., 2008). Swab samples were taken for the traditional plating methods as well as for IBIS.
Standard cultures
Swabs were streaked on one quadrant of a Colombia blood agar, which is a non-selective medium used for culturing both Gram-positive and Gram-negative organisms. Subsequent dilutions were achieved by streaking the remaining three quadrants in succession, making sure to use a new sterile and disposable loop for each streaking manoeuvre. The plates were incubated at 37 °C for 48 h, after which they were evaluated for the presence of bacterial colonies. The plates were then graded on a scale of 0 to 4+ based on the number of quadrants on each plate that showed positive growth. Any growth pattern of one or higher was considered contaminated.
IBIS analysis. Each of the samples were placed through the IBIS machine, which utilises PCR and mass spectrometry technology to identify all bacterial species present in the sample as previously described (Ecker et al., 2008). FISH was performed on a subset of lead apron outer coat and stitching material as previously described (Nistico et al., 2011). Briefly, fixed samples were treated with a solution of 0.1 mg of lysozyme/mL (Sigma) in 0.1 M Tris-HCl and 0.05 M Na2EDTA and incubated at 37 °C for 2 h as an additional permeabilisation step for the improved detection of Gram-positive bacteria. Fixed, permeabilised samples were then incubated in an ethanol series of 80 and 100% for 3 min each and FISH was performed with Staphylococcus species-specific fluorescent 16S rRNA probe (Integrated DNA Technologies, Inc, Coralville, IA, USA), conjugated with the sulfoindocyanine dye Cy3. Each material section was incubated with probe-specific formamide and salt concentrations and then immersed in washing buffer with the probe-specific salt concentration (Nistico et al., 2011). Samples were rinsed in sterile MilliQ water and observed with confocal laser scanning microscopy (CLSM).
CLSM
CLSM was performed as previously described (Nistico et al., 2011). Briefly, after staining, samples were mounted in a 35-mm petri plate and imaged with a Leica DM RXE microscope attached to a TCS SP2 AOBS confocal system (Leica Microsystems, Exton, PA, USA) using either a ×63 water immersion lens (NA 1.2) or a ×10 dry objective lens for low-power mapping. Images were collected and analysed by using the Leica LCS software and Imaris software (Bitplane, St. Paul, MN, USA).
Statistics
Chi-square tests were performed on both the standard culture and IBIS data to determine if the disinfectant wipe intervention made a significant difference in bacterial growth and detection. Differences were considered significant for P < 0.05.
Findings/results
Bacterial contamination was observed on all the X-ray aprons analysed by the IBIS (20/20) and the majority of those cultured with traditional plating methods (19/20). A wide range of pathogens were present. The most common organisms were skin flora, such as Staphylococcus epidermidis and Propionibacterium acnes (P. acnes), which were present on 18/20 aprons analysed by the IBIS and 18/20 aprons cultured via standard culture methods (Figure 2) at T0. Other more virulent organisms included Methicillin-resistant Staphylococcus epidermidis (MRSE), Niesseria meningitidis and Pseudomonas species. Before treatment, four of the aprons analysed by the IBIS and one of the aprons analysed by standard culture had MRSE present before treatment (Table 1). None of the six sterile scrub gowns that were used as negative controls showed the presence of bacteria either by standard culture methods or by the IBIS technology.
Figure 2.
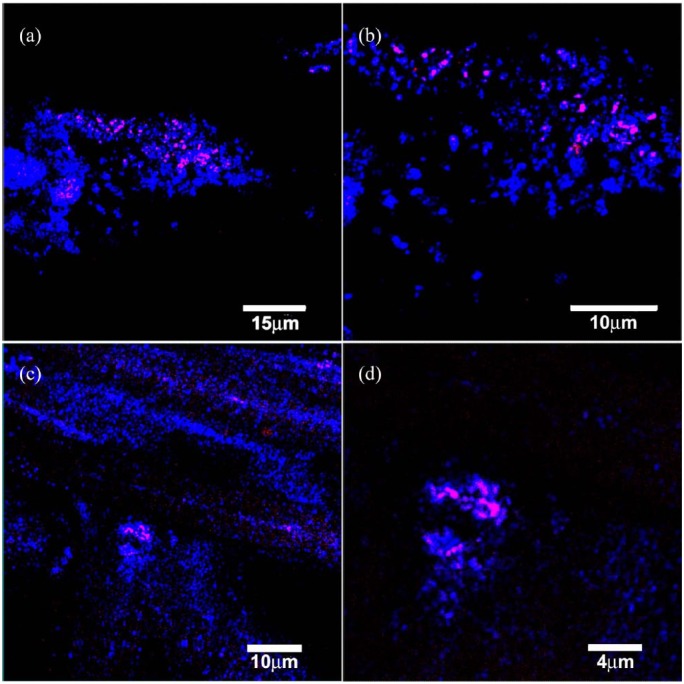
FISH image showing the presence of bacterial cells in a biofilm from the different aspects of the lead X-ray aprons. The FISH probe was a Staphylococcus probe. Red/pink are the bacteria, while blue is the reflected light from the sample. (a) Lead apron outer coat material. (b) Zoom of (a). (c) Stitches material from the apron from the x. Although there appear to be only a few cells (n = 10) in the field of view, this equates to approximately 105 CFU/cm2. EPS, extracellular polymeric substance. Legend in bottom right of each panel.
Table 1.
Organisms found on the lead X-ray aprons at all three time points.
| Before treatment | Time 0 h | Time 6 h | |
|---|---|---|---|
| IBIS | Propionibacterium acnes (17) | Pseudomonas sp. (1) | Streptococcus sp. (6) |
| Staphylococcus epidermidis (7) | Enterococcus faecalis (4) | Enterococcus faecalis (5) | |
| MRSE (4) | Staphylococcus sp. (3) | Staphylococcus epidermidis (4) | |
| Listeria sp. (4) | Listeria sp. (2) | Neisseria meningitides (3) | |
| Staphylococcus sp. (3) | Staphylococcus species (3) | ||
| Streptococcus sp. (2) | |||
| Corynebacterium sp. (2) | |||
| Enterococcus faecalis (2) | |||
| Clostridium beijerinckii (2) | |||
| Culture | Coagulase negative Staphylococcus (18) | Coagulase negative Staphylococcus (2) | |
| Diptheroids (12) | |||
| Micrococcus sp. (12) | |||
| MRSE (1) | |||
| Bacillus sp. (1) | |||
| Streptococcus viridans (1) | |||
| Neisseria sp. (1) |
Numbers in parentheses refer to number of bacterial detections.
MRSE, methicillin-resistant Staphylococcus epidermidis; Sp., species.
After treatment with the disinfectant wipe, overall bacteria contamination was significantly decreased. Immediately after treatment, all aprons showed significantly less bacterial contamination (0/20 showed contamination on the standard culture plates while 13/20 showed contamination via the IBIS), with some recurrence at the 6-h mark (2/20 for standard culture plates, 16/20 for IBIS). Most of the growth at T6 was Staphylococcus epidermidis and P. acnes. All five of the X-ray aprons that grew MRSE in the pre-treatment groups showed complete eradication of the organism after treatment with the disinfectant wipe. There was no recurrence of the MRSE at T6. Overall, there was significantly less bacterial contamination on the gowns at T0 (IBIS P < 0.012, standard culture P < 0.001), while some gowns demonstrated recurrent contamination at T6 (Figure 3).
Figure 3.
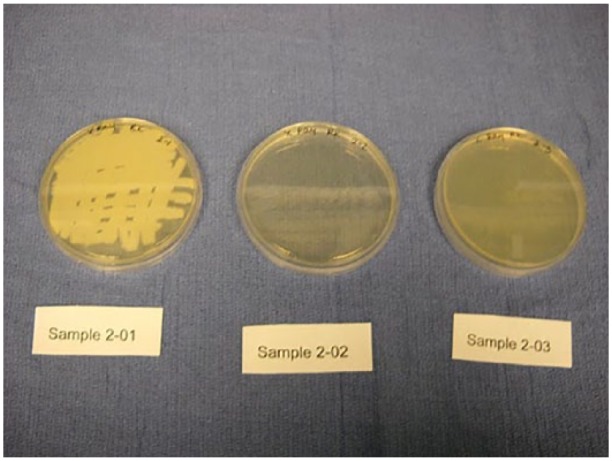
Streaked plates from lead X-ray gown 2. Sample 2-01 is before treatment. Sample 2-02 was collected immediately after treatment with a Sani-Cloth (T0) and sample 2-03 was collected after 6 h of incubation (T6).
Discussion
In our study, in addition to standard culture methods, PCR-mass spectrographic (IBIS) technology and confocal microscope visualisation of bacteria using the 16-S rRNA-based FISH technique were utilised to find that nearly all untreated gowns initially harboured bacteria, including virulent organisms. Other authors have also studied bacterial contamination on lead garments utilising standard microbiological methods. Feierabend and Siegel (2015) investigated the potential risk of infection from thyroid shields. Of the thyroid shields, 81% were contaminated before cleansing with a bleach-based disinfectant wipe. A significant reduction in bacteria occurred after cleaning. Grogan et al. (2011) looked at a single time point just before a weekly scheduled cleaning on lead garments worn in the OR. They reported that a weekly cleansing with a low-alcohol concentration wipe was enough to eradicate > 97% of bacteria. Differences from our results may be from a combination of variables including pre-cleansing conditions, type of disinfectants used and culture methods utilised.
We did observe discrepancies between the culture data and the IBIS. The IBIS picked up P. acnes, which culture did not because an extended anaerobic incubation was not performed. Alternately, the standard culture methods picked up dipthroids and micrococcus, but IBIS did not. Furthermore, it is not surprising that IBIS detected bacterial DNA even when the culture was negative since this could be DNA from inert bacteria. Although inconsistencies between culture and PCR methods have been previously reported in the literature (Swearingen et al., 2016), there was a general agreement that after cleansing with the disinfectant wipe there was a significant reduction of bacteria in both methods. Interestingly, a second run of IBIS and culture data were performed (n = 20) for quality assurance purposes one week after implementation of a new lead apron cleansing protocol at our institution, which detected few skin commensals from the IBIS data and no growth on standard culture at all three time points (Jain et al., (unpublished report) 2010) demonstrating a drastic reduction of bacterial load utilising this protocol.
There were several limitations to the study including the relatively small sample size of gowns tested (n = 20). Furthermore, since we did not study bacterial transmission through the surgical gown or to the surgical site, additional investigation is required to determine a correlation between incidence of SSI and treatment of X-ray aprons with a disinfectant wipe. Lastly, we only tested one type of high-level cleansing wipe comprising 55% alcohol. It is possible that other commercially available wipes with different active ingredient compositions would yield different results.
Our study demonstrates that lead-lined X-ray aprons that have been cleaned with the disinfectant wipe have significantly less bacteria in comparison to untreated gowns. Treating all lead X-ray aprons before entering the OR would lead to a decreased bacterial load. Our study showed mild recurrence of bacteria after 6 h. Based on this time point, it is our recommendation that each X-ray apron should be regularly treated with a disinfectant wipe, ideally before each surgical case. Further study is required to determine if this practice would indeed lead to fewer SSIs. Additionally, it may be of benefit to compare more than one type of hospital-grade wipe to determine if there are differences in efficacy between wipes. This basic science investigation utilising both standard culture methods and IBIS and FISH technology suggests that lead X-ray aprons are a potential source of contamination that can be treated with a regular sanitation policy using hospital-grade disinfectant wipes.
Acknowledgments
The authors thank Lauren M O’Keefe for her role in this investigation.
Footnotes
Author Note: Rebecca A Rajfer is now affiliated to Department of Orthopaedic Surgery, Loma Linda University, Loma Linda, CA, USA.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Daniel T Altman  https://orcid.org/0000-0002-0926-1173
https://orcid.org/0000-0002-0926-1173
References
- Bellchambers J, Harris JM, Cullinan P, Gaya H, Pepper JR. (1999) A prospective study of wound infection in coronary artery surgery. European Journal of Cardiothoracic Surgery 15(1): 45–50. [DOI] [PubMed] [Google Scholar]
- Bible JE, Biswas D, Whang PG, Simpson AK, Grauer JN. (2009) Which regions of the operating gown should be considered most sterile? Clinical Orthopaedics and Related Research 467(3): 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Post JC, Ehrlich GD, Hu FZ, Kreft R, Nistico L, Kathju S, Stoodley P, Hall-Stoodley L, Maale G, James G, Sotereanos N, DeMeo P. (2011) New methods for the detection of orthopaedic and other biofilm infections. FEMS Immunology and Medical Microbiology 61(2): 133–140. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Sampath R, Massire C, Blyn LB, Hall TA, Eshoo MW, Hofstadler SA. (2008) Ibis T5000: a universal biosensor approach for microbiology. Nature Reviews Microbiology 6(7): 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW, Kang MM. (2014) Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine Journal 14(11): 2710–2715. [DOI] [PubMed] [Google Scholar]
- Feierabend S, Siegel G. (2015) Potential infection risk from thyroid radiation protection. Journal of Orthopaedic Trauma 29(1): 18–20. [DOI] [PubMed] [Google Scholar]
- Grogan BF, Cranston WC, Lopez DM, Furbee C, Murray CK, Hsu JR. and Skeletal Trauma Research Consortium. (2011) Do protective lead garments harbour harmful bacteria? Orthopedics 34(11): e765–767. [DOI] [PubMed] [Google Scholar]
- Jain S, Kreft R, Costerton JW. (2010) One-month follow-up IBIS data. (unpublished report) Allegheny Health Network, Pittsburgh, PA. May. [Google Scholar]
- Lankester BJ, Bartlett GE, Garneti N, Blom AW, Bowker KE, Bannister GC. (2002) Direct measurement of bacterial penetration through surgical gowns: a new method. Journal of Hospital Infection 50(4): 281–285. [DOI] [PubMed] [Google Scholar]
- Malizos KN. (2017) Global Forum: The Burden of Bone and Joint Infections. Journal of Bone and Joint Surgery. American Volume 99(5): e20. [DOI] [PubMed] [Google Scholar]
- Markel TA, Gormley T, Greeley D, Ostojic J, Wagner J. (2018) Wearing long sleeves while prepping a patient in the operating room decreases airborne contaminants. American Journal of Infection Control 46(4): 369–374. [DOI] [PubMed] [Google Scholar]
- Matiyayu A, Duffy RK, Goldhahn S, Joeris A, Richter PH, Gebhard F. (2017) The Great Unknown – A systematic literature review about risk associated with intraoperative imaging during orthopaedic procedures. Injury 48(8): 1727–1734. [DOI] [PubMed] [Google Scholar]
- Nistico L, Kreft R, Gieseke A, Coticchia JM, Burrows A, Khampang P, Liu Y, Kerschner JE, Post JC, Lonergan S, Sampath R, Hu FZ, Ehrlich GD, Stoodley P, Hall-Stoodley L. (2011) Adenoid reservoir for pathogenic biofilm bacteria. Journal of Clinical Microbiology 49(4): 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi TJ, Konopka JF, Bedair HS. (2017) What is the long-term economic societal effect of periprosthetic infections after THA? A Markov analysis. Clinical Orthopaedics and Related Research 475(7): 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salassa TE, Swiontkowski MF. (2014) Surgical attire in the operating room: Role in infection prevention. Journal of Bone and Joint Surgery. American Volume 96(17): 1485–1492. [DOI] [PubMed] [Google Scholar]
- Swearingen MC, DiBartola AC, Dusane D, Granger J, Stoodley P. (2016) 16S rRNA analysis provides evidence of biofilms on all components of three infected periprosthetic knees including permanent braided suture. Pathogens and Disease 74(7): ftw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J, Parkinson H. (2006) Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev 19: CD003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharopoulos N, Perisinakis K, Damilakis J, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. (2003) Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. Journal of Bone and Joint Surgery. American Volume 85-A(9): 1698–1703. [DOI] [PubMed] [Google Scholar]