Abstract
Increased stretch tolerance can contribute to improved range of motion (ROM). Since menthol-based topical analgesics (TopAnalg) suppress pain, they may increase stretch thresholds improving ROM. Other modalities such as transcutaneous electrical nerve stimulation and rolling have demonstrated decreased pain sensitivity in the contralateral limb. The purpose of this study was to investigate the effects of a TopAnalg on active and passive ROM of the treated and contralateral (untreated) leg. With a double blind, repeated measures design, 14 university students had a TopAnalg or a placebo gel applied to their hamstrings, rested for 20-min and then either performed static or dynamic stretching. Prior to gel application and after stretching, participants were tested for passive static, active and ballistic hip flexion ROM. Near significant greater ballistic hip flexion ROM for both legs (treated: p = 0.08; 3.6%; contralateral: p = 0.1; 1.6%) were observed with the TopAnalg. With dynamic stretching, ballistic hip flexion ROM of both limbs at post-test (p=0.01-0.007; 3.3-4.2%) and post-10 minutes (p = 0.06-0.01; 2.7-4.1%) decreased with the placebo, whereas there were no significant reductions with the TopAnalg. There was a near significant higher active hip flexion ROM (stretched leg: p = 0.05; 4.6%), and significantly higher ballistic hip flexion ROM (p = 0.04-0.05; 3.4-3.5%) with static versus dynamic stretching for both legs. In conclusion, TopAnalg can increase hip flexion ROM of the treated and contralateral limbs. Secondly, static stretching contributed to greater ballistic ROM in both the stretched and non-stretched contralateral limbs. Hence, TopAnalg may be used to enhance flexibility training with rehabilitation or highly trained athletes.
Key points.
A menthol based topical analgesic can increase ballistic hip flexion ROM of the treated and untreated contralateral limbs.
Static stretching contributed to greater ballistic ROM in both the stretched and non-stretched contralateral limbs.
Menthol based topical analgesic-induced increases in ROM may be of benefit for athletes and other individuals who seek to increase ROM, however caution should be exercised with individuals inexperienced in moving through a full ROM.
Key words: Menthol, flexibility, static stretching, dynamic stretching, crossover
Introduction
Increased range of motion (ROM) with stretching has been attributed to neural, mechanical, and psycho-physiological factors. Neural factors that can increase ROM include neural responses such as muscle spindle reflexive disfacilitation (decreased firing of nuclear bag and nuclear chain fibres), stretch-induced pre-synaptic inhibition, reciprocal inhibition, as well as small to trivial contributions from recurrent (Renshaw cells) and autogenic (Golgi tendon organs) inhibition (Behm, 2018b; Behm et al., 2016a; Behm and Chaouachi, 2011). Mechanical alterations contributing to improved flexibility can include thixotropic effects (shear stress and temperature-induced decreases in visco-elasticity), increased tissue compliance (decreased stiffness) and changes in fascicle angle, length and rotation (Behm, 2018b; Behm et al., 2016a; Behm and Chaouachi, 2011; Blazevich et al., 2012). Psycho-physiological factors refer to an increased stretch tolerance that allows the individual to accommodate greater stretch-induced discomfort or pain and thus force the joint through a greater ROM (Magnusson et al., 1996; 1997).
Menthol based topical analgesics are reported to work as counterirritants to decrease pain sensation (Galeotti et al., 2002; Macpherson et al., 2006). Menthol inhibits transient receptor potential ion channels such as TRPA1 that evoke thermal and pain sensations (Macpherson et al., 2006). In addition, menthol inhibits neuronal membrane Ca++ channel currents enhancing analgesic properties (Galeotti et al., 2002). In contrast, Yosipovitch et al. (1996) indicated that thermal pain thresholds were unaltered after menthol and alcohol application. Menthol has also been reported to provide a significant reduction in vascular conductance within 1 and 10 minutes of application (Olive et al., 2010). In addition, topical menthol can have a rapid effect on reducing ipsilateral and contralateral arterial blood flow (Topp et al., 2011b). It is unknown whether menthol-based pain or discomfort inhibition and decreased vascular conductance or blood flow would contribute to a greater stretch tolerance and hence greater ROM.
There is extensive evidence that the unilateral application of interventions such as muscle fatigue, stretching, and rolling can induce increased fatigue (Halperin et al., 2014a;b; 2015; Kawamoto et al., 2014), ROM (Behm et al., 2016b; Chaouachi et al., 2017) and pain thresholds (decreased pain sensitivity) (Aboodarda et al., 2015; Cavanaugh et al., 2017) in contralateral limbs, respectively. Since fatigue, stretching, rolling, and menthol based topical menthols can influence contralateral actions (i.e. fatigue, ROM, blood flow) and sensations (i.e. pain threshold), would unilateral application of a menthol based topical analgesic have global pain relief and augment the stretch threshold increasing ROM of the treated and untreated limbs.
As there have been no studies to the authors’ knowledge that have examined the local and global effects of a menthol based topical analgesic on ROM, the objective of the present study was to administer a menthol based topical analgesic unilaterally and observe the effects upon the passive static, active and ballistic ROM of the treated and contralateral untreated hip flexion ROM. Based on the aforementioned literature demonstrating non-local effects, it was hypothesized that the unilateral application of a menthol based topical analgesic would enhance stretch tolerance and increase ROM in the treated and untreated limbs. A further objective was to detect possible sex ROM differences due to the application of a menthol based topical analgesic.
Methods
Participants
A priori statistical power analysis (G Power: Universitat Dusseldorf) was conducted to determine the minimum number of participants needed to achieve an alpha of 0.05, power of 0.8 and effect size of 0.5. The analysis of ROM with the Behm et al. (2016b) and Chaouachi et al. (2017) studies indicated that sample sizes of 8 and 12 respectively were needed. Hence, 14 participants consisting of seven males (21.7 ± 1.5 years, 1.80 ± 0.07 m, 84.2 ± 8.1 kg, BMI: 25.9 ± 2.3) and seven females (20.5 ± 1.3 years, 1.65 ± 0.06 m, 67.6 ± 16.9 kg, BMI: 25.2 ± 1.8) were recruited from the undergraduate student population, with an age range of 18-24 years. Participants were excluded if: (a) they had any pre-existing health conditions, such as cardiovascular problems or any musculoskeletal injuries and (b) they were unable to properly perform the stretches involved in the study. Prior to testing, all participants were provided with information regarding the nature of the study, the requirements upon participation in each data collecting session (i.e. attendance to all four sessions upon commitment to study, warming up for 5 minutes on the cycle ergometer prior to testing) and each participant completed an informed consent form. The study approved by the institution’s Interdisciplinary Committee on Ethics in Human Research (20192235-HK) and was conducted according to the Declaration of Helsinki.
Experimental design
Fourteen participants were tested for passive static, active and ballistic hip flexion ROM of both legs, pre- and post-interventions. The double blind, repeated measures (crossover) experimental design included the random allocation of four conditions: 1. Placebo with static stretching (SS), 2. Placebo with dynamic stretching (DS), 3. Menthol based topical analgesic with SS, 4. Menthol based topical analgesic with DS. After the pre-test measures, the menthol based topical analgesic or placebo gel was applied unilaterally to the right hamstrings by the researcher with latex free gloves, followed by a 15-minute rest period to achieve the full effects of the topical analgesic (Johar et al., 2012; Topp et al., 2011a;2011b). The gel was applied to the leg (no prior preparation such as shaving, abrading or alcohol cleansing) superficially without massage. After the 15 minute waiting period, a dynamic warm-up was employed, which consisted of a five-minute cycling period on a cycle ergometer at 70 rpm/Kp. Following this warm-up, either unilateral SS (three repetitions of 30-seconds with 30-second rest between repetitions) or DS (three repetitions of 30 kicks at 1Hz with a 30-second rest between repetitions) of the treated (right leg: placebo or topical analgesic) leg was performed. Post-intervention tests of both legs were performed immediately thereafter (post-test) and 10 minutes after the intervention (post-10-min). Both legs were tested to determine the local and non-local (global or crossover) effects of the unilateral interventions (topical analgesic vs. placebo and SS vs DS).
Menthol based topical analgesic versus placebo gel intervention
A volume of 5 ml of menthol based topical analgesic gel (Biofreeze: Performance Health Inc: Akron Ohio) or placebo gel was applied unilaterally to the entire length and breadth of the right hamstrings. The placebo gel supplied by Performance Health Inc was formulated to look, and smell similar, as well as have a similar gel composition. Whether the gel applied to the hamstrings was a placebo or topical analgesic was blinded to all participants and researchers. To alleviate bias, participants were informed that one of the objectives of the research was to evaluate two different types of topical analgesic gels.
Stretching interventions
The SS intervention consisted of three repetitions of a 30-second static hold with a 30-second rest between repetitions with the right leg. The SS was performed supine on a mat with the researcher moving the participant’s right leg to a maximum point of hip flexion discomfort. The dynamic stretching intervention consisted of 3 repetitions of 30 kicks at 1Hz with a 30-second rest between repetitions with the right leg. Participants were informed to perform the hip flexion kicks relatively quickly but under control (not ballistic) and to their perceived maximum ROM.
Testing measures
In each pre-test, the participant was required to perform 2-3 repetitions of each ROM test depending on whether the recorded values were within 5% of each other. If the measures exceeded a 5% difference a third measure was conducted. An electronic goniometer (Technical Services, Memorial University of Newfoundland, St. John’s Newfoundland, Canada) was used to measure passive static, active and ballistic hip flexion ROM of both legs. The order of testing for each leg was randomized. To ensure the leg was in a fully extended position throughout the testing, a knee brace was worn on the tested limb. Passive ROM measures were obtained with the aid of the researcher lifting (elevating) the extended leg to flex the hip to the point of maximal discomfort as indicated by the participant (Figure 1a). Active ROM was achieved by having the participant actively lift the braced and extended leg to maximum height at a moderate angular velocity of their own volition (typical duration to achieve full active ROM would have been two seconds) (Figure 1b). Ballistic ROM was achieved by asking the participant to kick the leg with as much force and velocity as possible to achieve a maximum ROM (Figure 1b). The participants stood on the contralateral leg when performing the ROM tests. Whereas the pre-test involved 2-3 stretches, only a single stretch for each passive static, active and ballistic ROM was employed post-intervention.
Figure 1.
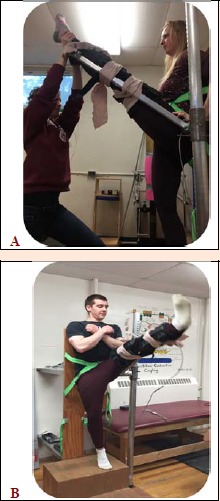
(A) Photo of researcher-assisted static passive ROM with knee brace to maintain knee extension. (B) Photo of dynamic range of motion test with knee brace to maintain knee extension. The movements were similar for both active dynamic and ballistic ROM however, active dynamic was performed slowly and under more control while the ballistic was performed with an explosive contraction.
Statistical analysis
Statistical analyses were completed using the SPSS software (Version 24.0, SPSS, Inc. Chicago, IL). The assumption of normality (Mauchly’s test) and sphericity (Shapiro-Wilk test) were tested for all dependent variables and if a violation was noted, the corrected values for non-sphericity with Greenhouse-Geisser were reported. A 3 way repeated measures ANOVA utilizing two sexes x four conditions (Placebo with SS, Placebo with DS, Topical Analgesic with SS and Topical Analgesic with DS) x three testing times (pre-test, post-test, post-10-min) was utilized to analyze ROM. In the event of significant main effects or interactions, planned pairwise comparisons were made using the Bonferroni method to test for differences. The level of significance was set at p < 0.05 and all results are expressed as mean ± SD. Effect size (d) magnitude of change were calculated for interactions and reported as trivial (<0.2), small (0.2-0.49), medium (0.5-0.79) or large (≥0.8) effect sizes (d) (Cohen, 1988). Inter-session reliability responses were assessed with Cronbach's alpha intraclass correlation coefficient (ICC).
Results
Reliability
Reliability measures as evaluated with ICCs illustrate moderate to strong testing consistency (Table 1).
Table 1.
Diurnal reliability (pre-tests performed on separate days) as assessed with Intraclass Correlation Coefficients (ICC).
| Static Stretching Intervention Right leg | Static Stretching Intervention Contralateral (Left) leg | Dynamic Stretching Intervention Right leg | Dynamic Stretching Intervention Contralateral (Left) leg | |
|---|---|---|---|---|
| Passive Static Range of Motion | 0.67 | 0.63 | 0.80 | 0.81 |
| Active Range of Motion | 0.86 | 0.86 | 0.81 | 0.87 |
| Ballistic Range of Motion | 0.60 | 0.70 | 0.83 | 0.83 |
Topical analgesic effects
There were no significant main effects or interactions for sex effects evident within the analysis. The three way ANOVA displayed a main effect for treatment with near significant, large to moderate magnitude, greater ballistic hip flexion ROM for both the treated (right leg: p = 0.08; 3.6%; d = 0.83) and the contralateral (p = 0.1; 1.6%; d = 0.56) legs with the unilateral (right leg) application of the topical analgesic, respectively. Furthermore, with the dynamic stretching intervention of the right leg, interactions indicated significant, small magnitude, decreases in ballistic hip flexion ROM at post-test (p = 0.01; 3.3%; d = 0.25) and post-10 minutes (p = 0.05; 2.7%; d = 0.29) with the placebo condition, whereas there were no significant reductions with the topical analgesic application (Figure 2). Similarly, testing of the left leg following dynamic stretching of the right leg, also showed significant, small magnitude, decreases in ballistic ROM with the placebo condition of 4.2% (p = 0.007; d = 0.30) and 4.1% (p = 0.01; d = 0.21) at post-test and post-10 minutes respectively, whereas there were no significant left leg impairments with the topical analgesic treatment (of the right leg) condition (Figure 2).
Figure 2.
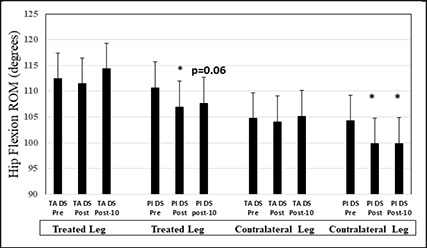
Means and standard deviations are depicted for the interactive effect of the treatment interventions and dynamic stretching on ballistic range of motion (ROM). Asterisks indicate significant decrease from pre-test, whereas p=0.06 represents the p value for the placebo post-10 minute test. Acronyms: TA: topical analgesic, DS: dynamic stretching intervention, Pl: Placebo
Stretching intervention effects
A main effect for the stretching intervention illustrated a near significant, small magnitude, higher active hip flexion ROM (p = 0.07; 4.6%; d = 0.31), and significantly, small magnitude, higher ballistic hip flexion ROM (p = 0.04; 3.4%; d = 0.35) with the static stretching versus dynamic stretching intervention for the right leg (stretch intervention leg). Similarly, with the contralateral (left, non- stretched) leg, there was evidence that unilateral (right leg) static stretching induced an overall, small magnitude, 3.5% (p = 0.05; d = 0.32) significantly greater hip flexion ballistic ROM in the non-stretched joint (Figure 3).
Figure 3.
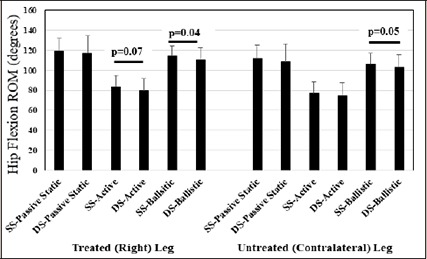
Means and standard deviations are depicted for main effects for the stretching intervention with testing of passive static range of motion (ROM), active ROM and ballistic ROM. SS-: Static stretching intervention; DS-: Dynamic stretching intervention.
Main effects for time
A main effect for time showed that static stretching of the right (treated and stretched) leg significantly (p = 0.05) improved by a small magnitude, passive static hip flexion ROM by 2.5% (d = 0.22) and 2.4% (d = 0.20) at post-test and post-10 minutes. In contrast, the active ROM of the right leg significantly (p = 0.03) decreased by a trivial magnitude, 3.2% (d = 0.18) and 2.6% (d = 0.15) at post-test and post-10 minutes respectively, while the ballistic ROM of the right leg (p = 0.03) also diminished 2.2 (d = 0.22) and 1.7% (d = 0.18) at post-test and post-10 minutes respectively. Similar results were observed with the contralateral (left) hip flexion ballistic ROM (no treatment and no stretching) as there was significant (p = 0.007), small and trivial magnitude, reductions in ballistic hip flexion ROM of 2.1% (d = 0.21) and 2.0% (d = 0.19) at post-test and post-10 minutes respectively (Figure 4).
Figure 4.
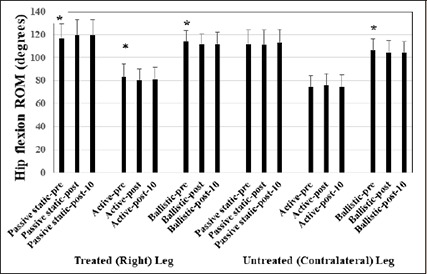
Means and standard deviations are depicted for main effects for time with testing of passive static range of motion (ROM), active ROM and ballistic ROM at pre-test, post-test, and post-10-minutes.
Discussion
The most important findings in the present study were that near significant and significant main effects and interactions provided evidence that a menthol based topical analgesic can increase ballistic hip flexion ROM of the treated and untreated contralateral limbs. Secondly, there was also evidence that static stretching contributed to greater ballistic ROM in both the stretched and non-stretched contralateral limbs. Finally, while static stretching improved passive static ROM over the testing period, active and ballistic stretching ROM was impaired.
There was evidence for a positive effect of a menthol based analgesic on ballistic hip flexion ROM. The application of a menthol based topical analgesic on the hamstrings induced large and moderate magnitude, overall improvements in ballistic ROM in treated and contralateral (untreated) legs respectively (main effect for treatment). Furthermore, the topical analgesic counteracted the significant, small magnitude impairments in ballistic ROM induced by dynamic stretching in the treated and contralateral (untreated) legs respectively. There are no other studies in the literature that have examined the effects of a menthol based topical analgesic on flexibility nor its crossover effects.
First suggested by Magnusson (Magnusson et al., 1996;1997) and supported in many other stretch-related studies (Behm, 2018b; Behm et al., 2016a; Behm and Chaouachi, 2011; Kay and Blazevich, 2012); the concept of stretch tolerance has been advocated as a significant contributing mechanism for acute and chronic increases in ROM. A meta-analysis by Freitas et al. (2017) examined 26 stretch training studies (durations ranged from 3-8 weeks with a weekly stretching duration of approximately 20 minutes) and reported that on average, the stretch training programs did not modify musculotendinous properties. Therefore, they purported that the enhanced extensibility must have been related to a greater stretch tolerance. Behm et al. (2016b) suggested that increased stretch tolerance would be a primary mechanism with the increased ROM of the shoulders after stretching the hip adductors and similarly the increased ROM of the hip flexors after stretching the shoulders. A similar mechanism was expounded by Chaouachi et al. (2017), with the improved ROM of the contralateral hip flexors following unilateral stretching of the hamstrings. Increased stretch tolerance could be related to changes in nociceptive sensitivity (Marchettini, 1993) that permits an accommodation of greater stretching-related discomfort or pain and a higher stretching intensity.
Since menthol based topical analgesics act as counterirritants to decrease pain sensation (Galeotti et al., 2002; Macpherson et al., 2006), they could contribute to an increased stretch tolerance. Menthol inhibits transient receptor potential ion channels such as TRPA1 that evoke thermal and pain sensations (Macpherson et al., 2006) and inhibit neuronal membrane Ca++ channel currents enhancing analgesic properties (Galeotti et al., 2002). Hence with less discomfort or pain, the individuals might have been able to push themselves to greater ROM.
This augmentation of ROM was seen in both the treated and untreated, contralateral legs. This crossover effect of a non-stretched contralateral leg would suggest that the increased stretch or pain tolerance had global consequences. Sigurdsson and Maixner (1994) suggested that the pain inhibition effects of noxious counterirritants can produce secondary hyperalgesia. Two foam rolling studies demonstrated that rolling the contralateral plantar flexors reduced muscle tender point pain (Aboodarda et al., 2015) and evoked tetanic stimulation pain (Cavanaugh et al., 2017) in the tested calf. The authors proposed a global pain modulatory system that might be related to the gate control theory of pain (Melzack and Wall, 1965; Moayedi and Davis, 2013) or the diffuse noxious inhibitory control model (Mense, 2000). With the gate control theory of pain, endogenous analgesia can result from descending signals to the opioid receptors, which would inhibit pain with serotonergic and noradrenergic neurons (Pud et al., 2009). Diffuse noxious inhibitory control is activated by nociceptive stimuli from a non-local tissue. The non-local receptor activity is transmitted to multi-receptive, wide dynamic range convergent neurons in the cortical subnucleus reticularis dorsalis where it inhibits pain transmission monoaminergically (Mense, 2000; Pud et al., 2009; Sigurdsson and Maixner, 1994) reducing pain perception not only locally but also at non-local sites (Pud et al., 2009; Sigurdsson and Maixner, 1994). However, in this study, this effect manifested small to large magnitude improvements with ballistic ROM only.
The lack of topical analgesic effect on active ROM may be related to the substantially smaller ROM. Whereas, the overall ballistic ROM values were 114.5° ± 10.3 and 110.4° ± 11.5 for topical analgesic and placebo respectively, the active ROM averaged 82.4° ± 10.4 and 80.9° ± 12.5 respectively. The active ROM was limited not only by the extensibility of the hamstrings musculotendinous units, but also the force output or strength of the hip flexor muscles (i.e. quadriceps, iliopsoas). Since the active ROM was approximately 300 less than ballistic ROM, the effect of the topical analgesic on hamstrings stretch-related discomfort would not have played a major role.
In contrast, the passive static ROM was similar to ballistic ROM with 117.9° ± 14.4 and 119.3° ± 15.1 for the topical analgesic and placebo conditions respectively and yet there was no significant topical analgesic effect on passive static ROM. The passive ROM was achieved with the aid of the researcher rather than an active contraction of the hip flexors as with active and ballistic ROM. Hence with ballistic ROM, the ROM was constrained by the propulsive force of the hip flexors, which may have influenced to a greater degree, the tolerable stretch limit of the participants. Furthermore, with the passive static ROM, the Hawthorne effect could have played a role whereby the participants may have consciously or subconsciously tried to please the researcher and surpassed their typical zone of (dis)comfort (Sedgwick and Greenwood, 2015; Ulmer, 1976). Therefore, if the researcher surpassed the participant’s typical level of discomfort, the researcher may have inadvertently exceeded the stretch tolerance, minimizing the effect of the topical analgesic.
A second major finding was that unilateral static stretching contributed to small magnitude, greater ballistic ROM in both the stretched and non-stretched contralateral limbs (3.4-4.6%; d = 0.31-0.35). It has been reported that static stretching may have a greater impact on increasing muscle compliance (decreasing stiffness) (Behm, 2018a; Behm et al., 2016a; Blazevich et al., 2012; Kay and Blazevich, 2012; Kay et al., 2015). As mentioned previously, the limits of the active ROM were approximately 300 less than the ballistic ROM and thus muscle compliance issues would not have impacted active ROM to a substantial degree. Secondly as already mentioned, the passive static and active ROM would have been constrained by the researcher and the hip flexor muscle force output respectively. While the ballistic ROM would also be constrained by hip flexor muscle force output, the force-velocity relationship indicates that forces decrease at higher velocities (Pertuzon and Bouisset, 1973; Thorstensson et al., 1976; Trafton, 1951). Thus potential hip flexor muscle forces would have been less than the slower active ROM forces or the researcher assisted passive static ROM. Therefore in attempting to achieve maximal ballistic ROM (only 3-90 difference between passive static and ballistic ROM), with a reduced force output, the advantage of increased musculotendinous compliance with static stretching may have permitted a greater ballistic ROM.
The main effects for time illustrated that while static stretching improved passive static ROM over the testing period, active and ballistic stretching ROM were impaired. While at first glance this finding may be perplexing, it must be remembered that the main effect for time combines dynamic and static stretching as well as topical analgesic and placebo conditions. Whereas, the static stretching intervention was conducted while lying supine with a researcher performing the stretching movements, the dynamic stretching intervention was performed while standing erect and performing 3 sets of 30 active contractions. Furthermore, when tested, the participants had the tested leg strapped to the electronic goniometer and thus had to stand on one leg while tested. The combination of active contractions during both the dynamic stretch intervention and the testing could have induced some fatigue and negatively impacted the ROM test that necessitated active contractions (active and ballistic ROM).
While the menthol based topical analgesic effect augmenting stretch tolerance to increase ROM could be of benefit for athletes and others interested in improving joint flexibility, there must be some caution exercised. Individuals who are not accustomed to moving their limbs through a full range of joint motion, or individuals with compromised muscle and tendon strength (rehabilitation) could possibly stretch beyond their tensile limit leading to muscle or tendon strain. Hence, surpassing the normal stretch tolerance should only be attempted by trained and experienced individuals.
While including both sexes could be considered a strength of the study, a limitation would be that there were only seven participants for each sex. Thus, the lack of significant sex-related findings may be related to a lack of statistical power for this factor.
Conclusion
In conclusion, evidence is provided that a menthol based topical analgesic can increase ballistic hip flexion ROM of the treated and untreated contralateral limbs. The likely mechanism is that the menthol based topical analgesic induced a higher stretch tolerance both locally and globally permitting a greater tolerance to the levels of discomfort associated with maximum perceived ballistic ROM. Furthermore, static stretching may have increased muscle compliance under the lower force output conditions of a high velocity ballistic contraction contributing to a greater ballistic ROM in both the stretched and non-stretched contralateral limbs. These findings may be used in rehabilitation with pain sensitive individuals who find the discomfort limiting their attempts extend their ROM. These findings may also apply to extreme flexibility athletes such as gymnasts, dancers and figure skaters who need extensive flexibility and the application of a menthol based topical analgesic may allow them to extend a little farther during flexibility training than under normal circumstances.
Acknowledgements
The study comply with the current laws of the country in which were performed. The authors report no conflict of interest.
Biographies

Arielle WHALEN
Employment
Kinesiologist
Degree
Bachelor of Kinesiology Honours
Research interest
Exercise and rehabilitative sciences
E-mail: afw136@mun.ca

Kaitlyn FARRELL
Employment
Kinesiologist
Degree
Bachelor of Kinesiology Honours (Co-operative)
Research interest
Exercise and rehabilitative sciences
E-mail: kf8381@mun.ca

Stephanie ROBERTS
Employment
Kinesiologist
Degree
Bachelor of Kinesiology Honours (Co-operative)
Research interest
Exercise and rehabilitative sciences
E-mail: sor175@mun.ca

Hannah SMITH
Employment
Kinesiologist/Dance Instructor
Degree
Bachelor of Kinesiology Honours (Co-operative)
Research interest
Exercise and rehabilitative sciences
E-mail: hls253@mun.ca

David G. BEHM
Employment
University Research Professor
Degree
PhD
Research interest
Neuromuscular physiology and sport
E-mail: dbehm@mun.can
References
- Aboodarda S.J., Spence A.J., Button D.C. (2015) Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskeletal Disorders 16, 265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm D.G. (2018a) Neuromuscular Physiology, Exercise, and Training During Youth-The Year That Was 2017. Pediatric Exercise Science 30, 35-37. [DOI] [PubMed] [Google Scholar]
- Behm D.G. (2018b) The Science and Physiology of Flexibility and Stretching: Implications and Applications in Sport Performance and Health. London, UK: Routledge Publishers. [Google Scholar]
- Behm D.G., Blazevich A.J., Kay A.D., McHugh M. (2016a) Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: a systematic review. Applied Physiology Nutrition Metabolism 41, 1-11. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Cavanaugh T., Quigley P., Reid J.C., Nardi P.S., Marchetti P.H. (2016b) Acute bouts of upper and lower body static and dynamic stretching increase non-local joint range of motion. European Journal of Applied Physiology 116, 241-249. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Chaouachi A. (2011) A review of the acute effects of static and dynamic stretching on performance. European Journal of Applied Physiology 111, 2633-2651. [DOI] [PubMed] [Google Scholar]
- Blazevich A.J., Cannavan D., Waugh C.M., Fath F., Miller S.C., Kay A.D. (2012) Neuromuscular factors influencing the maximum stretch limit of the human plantar flexors. Journal of Applied Physiology 113, 1446-1455. [DOI] [PubMed] [Google Scholar]
- Cavanaugh M.T., Doweling A., Young J.D., Quigley P.J., Hodgson D.D., Whitten J.H., Reid J.C., Aboodarda S.J., Behm D.G. (2017) An acute session of roller massage prolongs voluntary torque development and diminishes evoked pain. European Journal of Applied Physiology 117, 109-117. [DOI] [PubMed] [Google Scholar]
- Chaouachi A., Padulo J., Kasmi S., Othmen A.B., Chatra M., Behm D.G. (2017) Unilateral static and dynamic hamstrings stretching increases contralateral hip flexion range of motion. Clinical Physiolology and Functional Imaging 37, 23-29. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioural sciences. Hillside N.J.: L. Erbraum Associates; pp. 11-84. [Google Scholar]
- Freitas S.R., Mendes B., Le Sant G., Andrade R.J., Nordez A., Milanovic Z. (2017) Can chronic stretching change the muscle-tendon mechanical properties? A review. Scandinavian Journal of Medicine Science and Sports 28, 794-806. [DOI] [PubMed] [Google Scholar]
- Galeotti N., Di Cesare Mannelli L., Mazzanti G., Bartolini A., Ghelardini C. (2002) Menthol: a natural analgesic compound. Neuroscience Letters 322, 145-8. [DOI] [PubMed] [Google Scholar]
- Halperin I., Aboodarda S.J., Behm D.G. (2014a) Knee extension fatigue attenuates repeated force production of the elbow flexors. European Journal of Sport Science 14, 823-829. [DOI] [PubMed] [Google Scholar]
- Halperin I., Chapman D.W., Behm D.G. (2015) Non-local muscle fatigue: effects and possible mechanisms. European Journal of Applied Physiology 115, 2031-48. [DOI] [PubMed] [Google Scholar]
- Halperin I., Copithorne D., Behm D.G. (2014b) Unilateral isometric muscle fatigue decreases force production and activation of contralateral knee extensors but not elbow flexors. Applied Physiology Nutrition Metabolism 39, 1338-1344. [DOI] [PubMed] [Google Scholar]
- Johar P., Grover V., Topp R., Behm D.G. (2012) A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. International Journal of Sports Physical Therapy 7, 314-322. [PMC free article] [PubMed] [Google Scholar]
- Kawamoto J.E., Aboodarda S.J., Behm D.G. (2014) Effect of differing intensities of fatiguing dynamic contractions on contralateral homologous muscle performance. Journal of Sports Science and Medicine 13, 836-845. [PMC free article] [PubMed] [Google Scholar]
- Kay A.D., Blazevich A.J. (2012) Effect of acute static stretch on maximal muscle performance: a systematic review. Medicine Science Sports and Exercise 44, 154-164. [DOI] [PubMed] [Google Scholar]
- Kay A.D., Husbands-Beasley J., Blazevich A.J. (2015) Effects of Contract-Relax, Static Stretching, and Isometric Contractions on Muscle-Tendon Mechanics. Medicine Science Sports and Exercise 47, 2181-2190. [DOI] [PubMed] [Google Scholar]
- Macpherson L.J., Hwang S.W., Miyamoto T., Dubin A.E., Patapoutian A., Story G.M. (2006) More than cool: promiscuous relationships of menthol and other sensory compounds. Molecular Cell Neuroscience. 32, 335-343. [DOI] [PubMed] [Google Scholar]
- Magnusson S.P., Simonsen E.B., Aagaard P., Boesen J., Johannsen F., Kjaer M. (1997) Determinants of musculoskeletal flexibility: viscoelastic properties, cross-sectional area, EMG and stretch tolerance. Scandinavian Journal of Medicine Science and Sports 7, 195-202. [DOI] [PubMed] [Google Scholar]
- Magnusson S.P., Simonsen E.B., Aagaard P., Sorensen H., Kjaer M. (1996) A mechanism for altered flexibility in human skeletal muscle. Journal of Physiology 497 (Pt 1), 291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchettini P. (1993) Muscle pain: animal and human experimental and clinical studies. Muscle and Nerve 16, 1033-9. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P.D. (1965) Pain mechanisms: a new theory. Science 150, 971-979. [DOI] [PubMed] [Google Scholar]
- Mense S. (2000) Neurobiological concepts of fibromyalgia--the possible role of descending spinal tracts. Scandinavian Journal of Rheumatology Suppl 113, 24-29. [DOI] [PubMed] [Google Scholar]
- Moayedi M., Davis K.D. (2013) Theories of pain: from specificity to gate control. Journal of Neurophysiology 109, 5-12. [DOI] [PubMed] [Google Scholar]
- Olive J.L., Hollis B., Mattson E., Topp R. (2010) Vascular conductance is reduced after menthol or cold application. Clinical Journal of Sports Medicine 20, 372-376. [DOI] [PubMed] [Google Scholar]
- Pertuzon E., Bouisset S. (1973) Instantaneous force-velocity relationship in human muscle. Medicine and Sport 8, 230-234. [Google Scholar]
- Pud D., Granovsky Y., Yarnitsky D. (2009) The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144, 16-19. [DOI] [PubMed] [Google Scholar]
- Sedgwick P., Greenwood N. (2015) Understanding the Hawthorne effect. BMJ 351, 672–678. [DOI] [PubMed] [Google Scholar]
- Sigurdsson A., Maixner W. (1994) Effects of experimental and clinical noxious counterirritants on pain perception. Pain 57, 265-275. [DOI] [PubMed] [Google Scholar]
- Thorstensson A., Grimby G., Karlsson J. (1976) Force-velocity relations and fiber composition in human knee extensor muscles. Journal of Applied Physiology 40, 12-16. [DOI] [PubMed] [Google Scholar]
- Topp R., Winchester L., Mink A.M., Kaufman J.S., Jacks D.E. (2011a) Comparison of the effects of ice and 3.5% menthol gel on blood flow and muscle strength of the lower arm. Journal of Sport Rehabilitation 20, 355-366. [DOI] [PubMed] [Google Scholar]
- Topp R., Winchester L.J., Schilero J., Jacks D. (2011b) Effect of topical menthol on ipsilateral and contralateral superficial blood flow following a bout of maximum voluntary muscle contraction. International Journal of Sports Physical Therapy 6, 83-91. [PMC free article] [PubMed] [Google Scholar]
- Trafton H. (1951) Force-velocity relationships. Research Quarterly 410-415. [Google Scholar]
- Ulmer F.C. (1976) The Hawthorne effect. Educational Directions For Dental Auxiliaries 1, 28-30. [PubMed] [Google Scholar]
- Yosipovitch G., Szolar C., Hui X.Y., Maibach H. (1996) Effect of topically applied menthol on thermal, pain and itch sensations and biophysical properties of the skin. Archives of Dermatological Research 288, 245-258. [DOI] [PubMed] [Google Scholar]


