Abstract
Background and objective
Tumor size is an important prognostic factor in cancers. This study aims at investigating the interaction between gender status and tumor size to evaluate cancer-specific survival (CSS) in hepatocellular carcinoma (HCC).
Methods
In this study, we searched Surveillance, Epidemiology, and End Results (SEER) population-based data and identified 38,368 patients diagnosed with HCC between 1988 and 2012. Patients diagnosed between 1998 and 2007 were distributed into a training set (n = 19279), and the rest were assigned as a SEER validation set (n = 19089). Definition of cut-off value of tumor size stratified by gender was determined by the “X-Tile” program. The five-year CSS data were found. Long-term survival outcomes and risk factors were analyzed by the Kaplan–Meier methods and the multivariable Cox regression models.
Results
There were significant differences among these different tumor size subgroups with regards to five-year CSS (p < 0.001). When applying cutoff points of 38 mm and 75 mm tumor size in men, and 38 mm and 55 mm in women, the most significant difference was observed by the X-Tile program, respectively (p < 0.001). The five-year CSS was 27.5% for women and 25.7% for men in the training set, and 33.9% for women and 31.1% for men in the validating set (p < 0.001). Further analysis showed that this significant difference existed in localized, regional, and distant-stage patients.
Conclusions
These results demonstrated that women with HCC appeared to exhibit better survival rates than men. The sex-related discrepancies should be emphasized, particularly for HCC patients with 39 to 75 mm tumors.
Keywords: Hepatocellular carcinoma, gender, prognosis, SEER, tumor size
Key summary
- 1. Summarize the established knowledge on this subject:
- Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third leading cause of cancer-related death globally.
- HCC displays a markedly sex disparity and mainly affects men more than women.
- 2. What are the significant and/or new findings of this study?
- Tumor size is an important prognostic factor in HCC.
- Women with HCC appear to have better survival rates than men.
- Patients with 39 to 75 mm tumors have different prognoses in different genders.
Introduction
Liver cancer (including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma as well as other rare types) was predicted to be the sixth most common malignancy and the fourth leading cause of cancer death in 2018.1 HCC is closely associated with a history of chronic hepatitis caused by hepatitis B or C virus (HBV or HCV).2–4 Over the last few years, trends in HCC have shown significant increases.5 In 2013, an estimated 7920 women and 22,720 men in the United States (US) were diagnosed with primary liver cancer.6 This malignancy displays a significant gender disparity and mainly affects men more than women.7,8 The male-to-female ratio averages between 2:1 and >4:1 in different analyses.9 Recently, the role of estrogen has gained considerable attention, and it has also provided new insight into HCC development.10 In addition, cirrhosis progress to HCC is thought to occur more frequently in men and postmenopausal women, suggesting that sex hormones might play an important role in the gender difference in the incidence of HCC.11
Tumor size is a known independent prognostic factor for HCC.12–14 Hence, most staging systems are based on tumor size, and increased tumor size is closely correlated with decreased cancer-specific survival (CSS).15 In particular, tumor size correlates with survival in men and women may be different. Given the growing importance of sex-related discrepancies in tumor size, we used date from the Surveillance, Epidemiology, and End Results (SEER) program to identify the impact of gender and tumor size on HCC prognosis.
Materials and methods
Patients
The SEER database provides information on 18 population-based cancer statistics in an effort to reduce the cancer burden among the US population. According to the Site Recode Classifications, we extracted cases of invasive liver cancer (C22.0) from the SEER database. These cases were diagnosed between 1988 and 2012. Then, we expanded morphology codes for liver cancer. Those patients without histological type, incomplete staging or follow-up were excluded. Meanwhile, we also assessed variables for these patients including age, race, sex, stage, histologic type, tumor grade and size, as well as CSS rates between age 18 and 85 years at diagnosis. The primary end point of the study was five-year CSS rate. We obtained access to the SEER database public data file numbered 10504-November 2014.
Statistical analyses
Data are expressed as mean ± SEM, or as absolute number or percentage for categorical variables.
We used different ways to analyze and evaluate different targets: the chi-squared (χ2) test for evaluating the relationship between gender categories and clinicopathological parameters, the Student t-test for comparing continuous variables, the Kaplan–Meier method for generating survival curves, the log-rank test for analyzing differences between the curves, and the Cox proportional-hazards model for assessing survival outcomes and risk factors. P values ≤0.05 were considered significant. All statistical analyses were performed using the statistical software package SPSS for Windows, version 17 (SPSS, Inc).
Results
Patient characteristics
In total, 19,279 eligible patients with HCC between 1988 and 2007 were enrolled. A total of 14,524 (70.9%) were men and 4755 (29.1%) were women. The median follow-up period was 29 months. Between 2008 and 2012, a total of 19,089 HCC patients were diagnosed from the SEER data and selected as a validation set. Patient demographics and pathological features are summarized in Table 1.
Table 1.
Characteristics of patients from SEER database by gender.
| No. (%) of patients |
||||
|---|---|---|---|---|
| Total | Men | Women | ||
| Characteristic | n = 19279 | n = 14524 | n = 4755 | p value |
| Media follow-up (mo) | 29 | 28 | 31 | |
| (IQR) | 4–40 | 3–38 | 4–45 | |
| Years of diagnosis | <0.001 | |||
| 1988–1992 | 781 | 557 | 224 | |
| 1993–1997 | 1883 | 1328 | 555 | |
| 1998–2002 | 5230 | 3873 | 1357 | |
| 2003–2007 | 11,385 | 8766 | 2619 | |
| Age | 0.193 | |||
| ≤45 | 1408 | 1081 | 327 | |
| >45 | 17,871 | 13,443 | 4428 | |
| Race | <0.001 | |||
| Caucasian | 12,005 | 9170 | 2835 | |
| African American | 2116 | 1593 | 523 | |
| Othera | 5158 | 3761 | 1397 | |
| Pathological grading | 0.04 | |||
| High/Moderate | 6558 | 4927 | 1631 | |
| Poor/UD | 1940 | 1420 | 520 | |
| Unknown | 10,781 | 8177 | 2604 | |
| Stage | <0.001 | |||
| Localized | 10,366 | 7629 | 2737 | |
| Regional | 5712 | 4479 | 1233 | |
| Distant | 2415 | 1828 | 587 | |
| Unstaged | 786 | 588 | 198 | |
| Marital status | <0.001 | |||
| Married | 11,351 | 9121 | 2230 | |
| Never married | 3157 | 2525 | 632 | |
| Divorced/Separated | 2349 | 1769 | 580 | |
| Widowed | 1865 | 692 | 1173 | |
| Unknown | 557 | 417 | 140 | |
| Tumor size | 0.298 | |||
| 0–38 mm | 6218 | 4648 | 1570 | |
| 39–54 mm | 3851 | 2936 | 915 | |
| 55–75 mm | 3306 | 2473 | 833 | |
| ≥76 mm | 5904 | 4467 | 1437 | |
IQR: interquartile range; mo: months; SEER: Surveillance, Epidemiology, and End Results; UD: undifferentiated.
Including other (American Indian/Alaska Native, Asian/Pacific Islander) and unknown.
Identification of cutoff points of tumor size stratified by gender
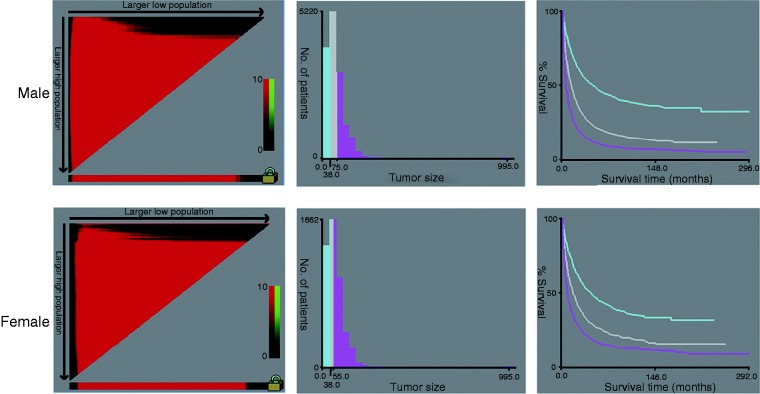
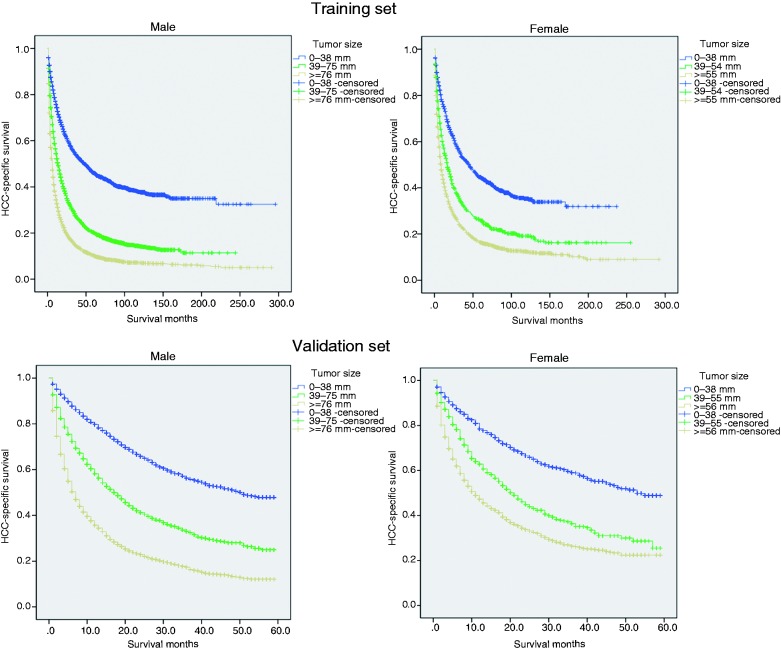
By using the X-Tile program, our results showed that the maximum chi-squared points of 2173.432 and 454.299 were achieved when applying 38 mm and 75 mm tumor size as the optimal cutoff point in men (survival rate 45.9%, 20.2% and 10.2% for patients with 0–38 mm, 39–75 mm and ≥76 mm tumors, respectively; p < 0.001); and 38 mm and 55 mm in women (survival rate 44.5%, 25.8% and 16.3% for patients with 0–38 mm, 39–55 mm and ≥56 mm tumors, respectively; p < 0.001) (Figures 1 and 2(a)). By using the abovementioned cutoff points, we can divide the HCC patients into three subgroups in terms of five-year CSS.
Figure 1.
X-tile analysis of survival data from the SEER registry. X-tile analysis was done on patient data from the SEER registry, equally divided into training and validation sets. The optimal cut-point highlighted by the black circle in the left panels (A) is shown on a histogram of the entire cohort (middle panels) (B), and a Kaplan-Meier plot (right panels) (C). P values were determined by using the cut-point defined in the training set and applying it to the validation set.
Figure 2.
Survival curves in HCC patients according to different tumor size groups. a. 0-38 mm tumors versus 39-75 mm tumors versus ≥76 mm tumors in the training set, P<0.001; 0-38 mm tumors versus 39-55 mm tumors versus ≥56 mm tumors in the training set, P<0.001; b. 0-38 mm tumors versus 39-75 mm tumors versus ≥76 mm tumors in the validation set, P<0.001; 0-38 mm tumors versus 39-55 mm tumors versus ≥56 mm tumors in the validation set, P<0.001;
HCC: hepatocellular carcinoma.
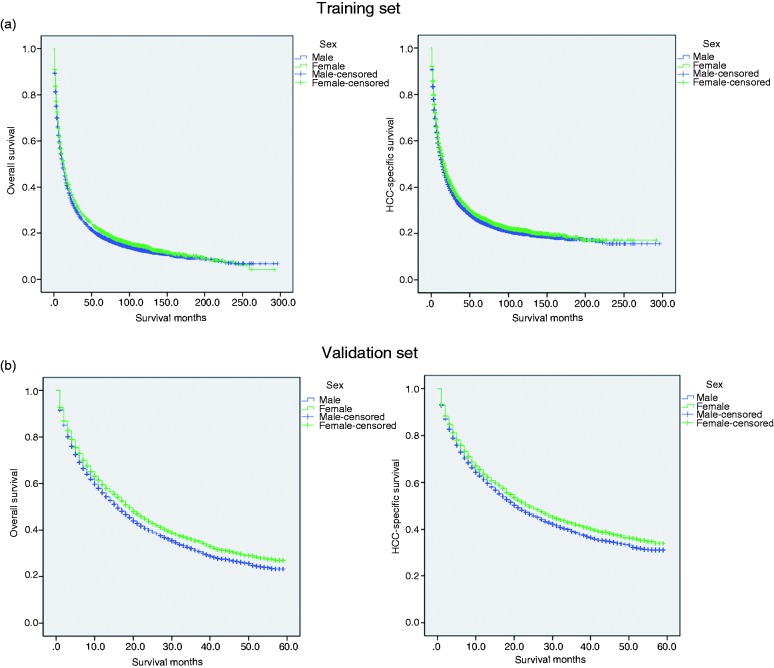
Effect of gender on CSS
The five-year overall (p < 0.001) and CSS (p = 0.002) rates were 19.1% and 25.7% in men; and 21.4% and 27.5% in women (Figure 3(a)). Additionally, in men (p = 0.002), univariate analysis found that larger tumor size, the widowed group, higher stage, poor/undifferentiated tumor grade, African American race, age older than 45 years, an early year of diagnosis as well as being men were considered significant risk factors. Additionally, the following eight factors were found to be independent prognostic factors when multivariate analysis with Cox regression was performed (Table 2): (female, HR 0.871, 95% confidence interval (CI) 0.836–0.908), year of diagnosis (1993–1997, HR 0.987, 95% CI 0.900–1.083; 1998–2002, HR 0.918, 95% CI 0.844–1.999; 2003–2007, HR 0.814, 95% CI 0.750–0.883), age (>45, HR 1.432, 95% CI 1.337–1.534), race (African American, HR 1.126, 95% CI 1.067–1.188; others, HR 0.847, 95% CI 0.814–0.882), pathological grading (poor/undifferentiated, HR 1.468, 95% CI 1.383–1.559; unknown, HR 1.588, 95% CI 1.528–1.651), stage (regional, HR 1.652, 95% CI 1.588–1.718; distant, HR 2.541, 95% CI 2.411–2.677; unstaged, HR 1.781, 95% CI 1.639–1.936), marital status (never married, HR, 1.129, 95% CI, 1.075–1.185; divorced/separated, HR, 1.126, 95% CI, 1.067–1.188; widowed, HR, 1.351, 95% CI, 1.274–1.434; unknown, HR 1.127, 95% CI 1.019–1.246) and tumor size (39–54 mm, HR 1.651, 95% CI 1.569–1.737; 55–75 mm, HR 1.996, 95% CI 1.894–2.104; ≥ 76 mm, HR 2.481, 95% CI 2.367–2.601).
Figure 3.
Survival curves in HCC patients according to different gender status. The overall survival. Males versus females in the training set, P<0.001; The cancer-specific survival. Males versus females in the training set, P=0.002; b. The overall survival. Males versus females in the validation set, P<0.001; The cancer-specific survival. Males versus females in the validation set, P<0.001.
HCC: hepatocellular carcinoma.
Table 2.
Univariate and multivariate survival analysis for evaluating the influence of tumor size on cause-specific survival in the SEER database.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Variable | Five-year HCSS (%) | Log rank χ2 test | p | HR (95% CI) | p |
| Sex | 9.830 | 0.002 | <0.001 | ||
| Male | 25.7% | Ref | |||
| Female | 27.5% | 0.871 (0.836–0.908) | |||
| Years of diagnosis | 309.139 | <0.001 | <0.001 | ||
| 1988–1992 | 14.5% | Ref | |||
| 1993–1997 | 18.1% | 0.987 (0.900–1.083) | 0.787 | ||
| 1998–2002 | 23.9% | 0.918 (0.844–1.999) | 0.046 | ||
| 2003–2007 | 29.3% | 0.814 (0.750–0.883) | <0.001 | ||
| Age | 37.673 | <0.001 | <0.001 | ||
| ≤45 | 33.0% | Ref | |||
| >45 | 25.6% | 1.432 (1.337–1.534) | <0.001 | ||
| Race | 104.520 | <0.001 | <0.001 | ||
| Caucasian | 26.0% | Ref | |||
| African American | 18.4% | 1.126 (1.067–1.188) | <0.001 | ||
| Othera | 29.5% | 0.847 (0.814–0.882) | <0.001 | ||
| Pathological grading | 823.558 | <0.001 | <0.001 | ||
| High/Moderate | 37.7% | Ref | |||
| Poor/UD | 20.8% | 1.468 (1.383–1.559) | <0.001 | ||
| Unknown | 19.6% | 1.588 (1.528–1.651) | <0.001 | ||
| Stage | 3291.011 | <0.001 | <0.001 | ||
| Localized | 37.9% | Ref | |||
| Regional | 15.2% | 1.652 (1.588–1.718) | <0.001 | ||
| Distant | 0.04% | 2.541 (2.411–2.677) | <0.001 | ||
| Unstaged | 10.7% | 1.781 (1.639–1.936) | <0.001 | ||
| Marital status | 165.343 | <0.001 | <0.001 | ||
| Married | 29.0% | Ref | |||
| Never married | 22.9% | 1.129 (1.075–1.185) | <0.001 | ||
| Divorced/Separated | 24.5% | 1.126 (1.067–1.188) | <0.001 | ||
| Widowed | 16.1% | 1.351 (1.274–1.434) | <0.001 | ||
| Unknown | 24.2% | 1.127 (1.019–1.246) | 0.02 | ||
| Tumor size (mm) | 2654.377 | <0.001 | <0.001 | ||
| 0–38 mm | 45.6% | Ref | |||
| 39–54 mm | 23.5% | 1.651 (1.569–1.737) | <0.001 | ||
| 55–75 mm | 17.1% | 1.996 (1.894–2.104) | <0.001 | ||
| ≥76 mm | 11.6% | 2.481 (2.367–2.601) | <0.001 | ||
CI: confidence interval; HCSS: hepatocellular carcinoma–specific survival; HR: hazard ratio; SEER: Surveillance, Epidemiology, and End Results; UD: undifferentiated.
Including other (American Indian/Alaska Native, Asian/Pacific Islander) and unknown.
P values were adjusted for years of diagnosis, sex, age, race, pathological grading, stage, marital status and tumor size as covariates between the two groups.
Interaction by gender in the training set
As illustrated in Table 3, in the male group, the HRs for hepatocellular carcinoma–specific mortality (HCSM) gradually increased with increasing tumor size (0–38 mm, HR 0.592, 95% CI 0.558–0.628, p < 0.001; 55–75 mm, HR 1.214, 95% CI 1.141–1.291, p < 0.001; ≥ 76 mm, HR 1.567, 95% CI 1.484–1.656, p < 0.001), as in the female group (0–38 mm, HR 0.647, 95% CI 0.584–0.718, p < 0.001; 55–75 mm, HR 1.223, 95% CI 1.096–1.364, p < 0.001; ≥ 76 mm, HR 1.366, 95% CI 1.236–1.510, p < 0.001). The differences were not apparent in tumors smaller than 75 mm between the male and female groups; however, in patients with ≥76 mm tumors, women seemed to have a lower HCSM compare with males (HR 1.567 vs 1.366).
Table 3.
Pairwise comparisons between different combinations of tumor size and gender relative to HCSM.
| Gender |
||||
|---|---|---|---|---|
| Male |
Female |
|||
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Tumor size (mm) | <0.001 | <0.001 | ||
| 0–38 | 0.592 (0.558–0.628) | <0.001 | 0.647 (0.584–0.718) | <0.001 |
| 39–54 | 1 | <0.001 | 1 | <0.001 |
| 55–75 | 1.214 (1.141–1.291) | <0.001 | 1.223 (1.096–1.364) | <0.001 |
| ≥76 mm | 1.567 (1.484–1.656) | <0.001 | 1.366 (1.236–1.510) | <0.001 |
CI: confidence interval; HR: hazard ratio; HCSM: hepatocellular carcinoma–specific mortality.
All results were adjusted using Cox proportional hazards models for years of diagnosis, age, race, pathological grading, stage, marital status and tumor size.
Subgroup analysis for evaluating the effect of tumor size based on different stages
The univariate analysis showed large tumor size exhibited decreased five-year CSS rates across several subgroups. Multivariate Cox regression analyses were performed at different stages and the results showed tumor size was validated as an independent predictor of survival for localized stage (0–38 mm, HR 0.561, 95% CI 0.524–0.600; 55–75 mm, HR 1.177, 95% CI 1.090–1.272; ≥ 76 mm, HR 1.625, 95% CI 1.511–1.747), regional stage (0–38 mm, HR 0.629, 95% CI 0.572–0.692; 55–75 mm, HR 1.255, 95% CI 1.143–1.378; ≥ 76 mm, HR 1.538, 95% CI 1.419–1.667) and distant stage (0–38 mm, HR 0.880, 95% CI 0.749–1.034; 55–75 mm, HR 1.102, 95% CI 0.950–1.279; ≥ 76 mm, HR 1.263, 95% CI 1.111–1.436) (Table 4).
Table 4.
Univariate and multivariate analyses for evaluating tumor size influencing CSS in HCC based on different cancer stage.
| Variable | Five-year CCS (%) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Log rank χ2 test | p | HR (95% CI) | p | ||
| Localized | |||||
| Tumor size (mm) | 1080.240 | <0.001 | <0.001 | ||
| 0–38 | 53.3% | 0.561 (0.524–0.600) | <0.001 | ||
| 39–54 | 31.4% | 1 | |||
| 55–75 | 26.6% | 1.177 (1.090–1.272) | <0.001 | ||
| ≥76 mm | 20.2% | 1.625 (1.511–1.747) | <0.001 | ||
| Regional | |||||
| Tumor size (mm) | 489.865 | <0.001 | <0.001 | ||
| 0–38 | 31.9% | 0.629 (0.572–0.692) | <0.001 | ||
| 39–54 | 15.4% | 1 | |||
| 55–75 | 8.8% | 1.255 (1.143–1.378) | <0.001 | ||
| ≥76 mm | 8.1% | 1.538 (1.419–1.667) | <0.001 | ||
| Distant | |||||
| Tumor size (mm) | 34.039 | <0.001 | <0.001 | ||
| 0–38 | 6.6% | 0.880 (0.749–1.034) | 0.121 | ||
| 39–54 | 4.7% | 1 | |||
| 55–75 | 2.1% | 1.102 (0.950–1.279) | 0.2 | ||
| ≥76 mm | 3.8% | 1.263 (1.111–1.436) | <.001 | ||
CI: confidence interval; CSS: cancer-specific survival; HCC: hepatocellular carcinoma; HR: hazard ratio; NI: not included in multivariate survival analysis.
P values were adjusted for years of diagnosis, sex, age, race, pathological grading, marital status and tumor size as covariates between the two groups.
Validation of outcomes
The external validation was performed in another SEER validation set. By using the optimal cutoff value, the validation patients could also be divided into three subsets in terms of five-year CSS rates (Figure 2(b)). The five-year overall (p < 0.001) and CSS (p < 0.001) rates were 23.3% and 31.1% in men, and 27.0% and 33.9% in women (Figure 3(b)). Multivariate analysis showed gender was an independent prognostic factor for HCC, validating that women with HCC had better survival (Table S1). Meanwhile, small tumor size groups also had survival benefits across several subgroups (Tables S2 and S3).
Discussion
Despite the prognosis of HCC patients improving recently because of advances in early diagnosis and treatment, the incidence is still increasing.16 A more comprehensive understanding of cancer biology could increase our knowledge of HCC and individualized treatments. Regardless of predisposing factors, there is a remarkable sex disparity in HCC incidence, with a solid predominance for men.7 Epidemiological studies showed that elevated testosterone levels in male HBsAg carriers were correlated with the increased risk of HCC.17,18 Meanwhile, antiandrogen agents can prevent HCC development in male rodents.19 In addition to the tumor-promoting activity of androgen, estrogen in women seems to protect them from hepatocarcinogenesis. Postmenopausal females are at high risk of HCC, as are early-oophorectomy patients.20
Tumor size is also a known risk factor for poor survival of HCC.13,21 Both the Barcelona Clinic Liver Cancer classification and the American Joint Committee on Cancer staging system include tumor size as an important variable. However, the cutoff value for tumor size varies among different staging systems and the prognostic significance of tumor size in patients with HCC is controversial.12,14 Pawlik et al. reported that tumor size could predict vascular invasion and histologic grade.22 However, Zhang and colleagues found tumor size did not affect long-term survival of solitary HCC without macroscopic vascular invasion.23 Owing to the sex-related discrepancies, the application of the proper tumor size cutoff value stratified by gender in HCC patients in predicting the survival rates has been a controversial issue.
In this study, we analyzed the SEER data of 19,279 HCC patients and identified 38 mm and 75 mm tumor size as the optimal cutoff value in male patients, and 38 mm and 55 mm in female patients. The five-year CSS rates were 45.9%, 20.2% and 10.2% in the 0–38 mm, 39–75 mm and ≥76 mm tumors in men, and 44.5%, 25.8% and 16.3% in the 0–38 mm, 39–55 mm and ≥56 mm tumors in women. Among these patients, regardless of gender, 0–38 mm tumors always exhibited the best survival whereas ≥76 mm tumors had the worst survival in male and female patients, indicating that 39–75 mm tumors was essentially heterogeneous. Meanwhile, a piecewise relationship between large tumor size and HCSM was observed. In patients with ≥76 mm tumors, women had a lower HCSM compared with men, which was consistent with epidemiology results that estrogen protects women from HCC. Moreover, female patients with HCC had certain clinicopathological features and prognostic factors different from those in male patients. The percentage of patients with HCC at high/moderate grade was 33.9% in men compared with 34.3% in women. In addition, the female patients also had a higher percentage of localized stage compared with males (57.5% vs 52.5%). Further subgroup analysis demonstrated that HCC patients with 0–38 mm tumors exhibited an increased five-year CSS across several subgroups. These results were further validated in another validation set.
Our results might affect clinical practice. As a result of the sex-related discrepancies, patients with 39 to 75 mm tumors have different prognoses in different genders. These HCC patients should be treated with a personalized treatment strategy, especially those HCC patients with 39 to 75 mm tumors.
This population-based surveillance study also has several limitations. First, HCC predisposing factors such as hepatitis virus and steatohepatitis were not included in the SEER database. Second, despite being based on a large, multicenter study population, individual subgroups became small after stratifying by tumor size, yielding limited statistical power. Third, the SEER database is retrospective rather than prospective, which might introduce unaccounted biases and affect the analysis. Fourth, although we observed that men and women have different cutoff points of tumor size, a real and precise association between tumor size and CSS in different genders needs to be further confirmed. Despite these limitations, the SEER database provides the opportunity to analyze a large number of patients with significant follow-up, rendering our results more convincing.
In conclusion, our study demonstrated that women appeared to have better survival rates than men in HCC, which correlated with more favorable tumor characteristics. Sex-related discrepancies should be emphasized and salvage therapy should be individualized, particularly in patients with 39 to 75 mm tumors.
Supplemental Material
Supplemental Material for Differences in the prognostic value of tumor size on hepatocellular cancer-specific survival stratified by gender in a SEER population-based study by Wenjie Zhang, Kangpeng Jin, Fei Wang, Guangyan Zhangyuan, Weiwei Yu, Yang Liu, Haitian Zhang, Ping Zhang and Beicheng Sun in United European Gastroenterology Journal
Acknowledgments
We would like to thank the SEER database for its open access.
Author contributions
W.Z., P.Z. and B.S.: conceptualization, methodology, validation, investigation, writing–original draft, writing–review and editing, and visualization. W.Z., K.J. and B.S.: data curation and writing–review and editing. W.Z., G.Z. and W.Y formal analysis, investigation, and writing–review and editing. Y.L.: investigation, resources, writing–original draft, and writing–review and editing. WJZ, PZ and B.S.: formal analysis, writing–original draft, writing–review and editing, visualization, and supervision. K.J. and G.Z.: investigation, resources, writing–original draft, and writing–review and editing. W.Y. and Y.L.: formal analysis, investigation, resources, and writing–review and editing. H.Z., P.Z and B.S.: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, and supervision. B.S. is Yangtze River Scholars Distinguished Professor.
Declaration of conflicting interests
None declared.
Funding
This work was supported by grants from the National Key Research and Development Program of China (grant number: 2016YFC0905900 to B.S.); the State Key Program of National Natural Science Foundation (grant number: 81430062 to B.S.); Innovative Research Groups of National Natural Science Foundation (grant number: 81521004 to B.S.); The National Natural Science Foundation of China (grant number: 81702344 to W.Z.), and the TianQing Liver Disease Research Fund (grant number: TQGB20180095 to W.Z.).
Ethics approval
Approval from the ethical board for this study was not required because of the public nature of all the data.
Informed consent
This study did not involve personal identifying information or interact with human individuals. Therefore, informed consent was not required.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Huang YT, Jen CL, Yang HI, et al. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol 2011; 29: 3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Q, Fan J, Yang XR, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: A large-scale, multicentre study. Lancet Oncol 2012; 13: 817–826. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Sun B. Impact of age on the survival of patients with liver cancer: An analysis of 27,255 patients in the SEER database. Oncotarget 2015; 6: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 2011; 103: 714–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 7.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45: 529–538. [DOI] [PubMed] [Google Scholar]
- 8.Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: The roles of sex hormones. Oncology 2010; 78(Suppl 1): 172–179. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–2576. [DOI] [PubMed] [Google Scholar]
- 10.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317: 121–124. [DOI] [PubMed] [Google Scholar]
- 11.Rogers AB, Theve EJ, Feng Y, et al. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res 2007; 67: 11536–11546. [DOI] [PubMed] [Google Scholar]
- 12.Nathan H, Schulick RD, Choti MA, et al. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg 2009; 249: 799–805. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Wang X, Jiang R, et al. Effect of tumor size on cancer-specific survival in small hepatocellular carcinoma. Mayo Clin Proc 2015; 90: 1187–1195. [DOI] [PubMed] [Google Scholar]
- 14.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002; 20: 1527–1536. [DOI] [PubMed] [Google Scholar]
- 15.Boffa DJ, Greene FL. Reacting to changes in staging designations in the 7th edition of the AJCC staging manual. Ann Surg Oncol 2011; 18: 1–3. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed F, Perz JF, Kwong S, et al. National trends and disparities in the incidence of hepatocellular carcinoma, 1998–2003. Prev Chronic Dis 2008; 5: A74. [PMC free article] [PubMed] [Google Scholar]
- 17.Yu MW, Yang YC, Yang SY, et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: A nested case-control study among men. J Natl Cancer Inst 2001; 93: 1644–1651. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Sakai H, Hashizume M, et al. Serum testosterone:estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res 2000; 60: 5106–5110. [PubMed] [Google Scholar]
- 19.Toh YC. Effect of neonatal castration on liver tumor induction by N-2-fluorenylacetamide in suckling BALB/c mice. Carcinogenesis 1981; 2: 1219–1221. [DOI] [PubMed] [Google Scholar]
- 20.Yu MW, Chang HC, Chang SC, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology 2003; 38: 1393–1400. [DOI] [PubMed] [Google Scholar]
- 21.Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: Single-institution experience with 2558 patients. J Gastrointest Surg 2015; 19: 1281–1290. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005; 11: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Yuan SX, Dai SY, et al. Tumor size does not independently affect long-term survival after curative resection of solitary hepatocellular carcinoma without macroscopic vascular invasion. World J Surg 2014; 38: 947–957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Differences in the prognostic value of tumor size on hepatocellular cancer-specific survival stratified by gender in a SEER population-based study by Wenjie Zhang, Kangpeng Jin, Fei Wang, Guangyan Zhangyuan, Weiwei Yu, Yang Liu, Haitian Zhang, Ping Zhang and Beicheng Sun in United European Gastroenterology Journal