Abstract
Background
The management of inflammatory bowel disease in patients who have previously undergone liver transplantation can be a clinical challenge. There are serious concerns among physicians regarding the use of biologics for treating such immuno-compromised patients.
Objective
We performed a systematic review on vedolizumab therapy in transplant recipients to assess its safety.
Methods
PubMed/Embase/Scopus were searched up to November 2018 to identify papers regarding liver transplant recipients and therapy with vedolizumab. Primary outcomes were adverse events. Secondary outcomes were liver transplant and inflammatory bowel disease outcomes.
Results
Eight studies (31 patients) were included. Nine out of 31 patients experienced an infection within a mean follow-up time ranging from 5–20 months. No malignancies were reported. Inflammatory bowel disease clinical response was experienced by 20/26 patients. Abnormalities in liver tests were recorded in 2/22 patients.
Conclusion
Vedolizumab may be considered safe for treating inflammatory bowel disease in liver transplant recipients. Caution is recommended for patients with an unstable liver graft function.
Keywords: Liver transplantation, inflammatory bowel disease, primary sclerosing cholangitis, vedolizumab, adverse events
Background
The therapeutic management of inflammatory bowel disease (IBD) in patients who had previously undergone a liver transplant (LT) can be a real clinical challenge because of the increased risk of infectious events in transplant recipients.1
These patients are not anecdotal as around 5% of IBD patients develop primary sclerosing cholangitis (PSC).2 This is a chronic fibro-inflammatory disorder which lacks effective treatment and can result in end-stage liver disease and need for a liver transplantation.2
The natural course of the IBD after liver transplantation is still unpredictable. In a systematic review by Singh et al.,3 39% of patients showed no significant change in IBD course compared with their pre-liver transplantation IBD activity. Otherwise, one-third of patients may have a paradoxical worsening of their bowel symptoms in spite of immunosuppressive therapy.
Nevertheless, there are serious concerns among physicians regarding the use of biologic agents for treating immuno-compromised patients such as LT recipients. Anti-tumour necrosis factor (TNF) α agents, have been associated with an increased incidence of either de novo or reactivated systemic infections.4 Evidence regarding the efficacy and safety of these biologic agents is very scarce in LT recipients, although in a recent systematic review by Westerouen van Meeteren et al.,5 they are apparently comparable to those of general post-LT patients.
Even less evidence is available for vedolizumab, a humanized monoclonal antibody targeting the alpha4-beta7 integrin, preventing leukocyte trafficking into the gastrointestinal district.6 Its selective mechanism may explain the very low incidence of bacterial, mycotic and viral infections reported in clinical trials and real-life studies.7 This may suggest vedolizumab as an attractive option for active IBD in patients who have previously undergone LT.
To assess the safety of anti-integrin therapy in transplant recipients, we performed a systematic review, pooling data from the individual initial experiences in this field.
Methods
The methods of our analysis and inclusion criteria were based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.8 Our protocol was registered with PROSPERO (www.crd.york.ac.uk/prospero/) on January 2019, after searching for ongoing systematic reviews (protocol number: CRD42019120929).
Data sources and search strategy
A comprehensive literature search was performed by using PubMed, SCOPUS, and EMBASE (up to 30 November 2018) to identify papers, letters and abstracts, regarding LT recipients and anti-integrin therapy with vedolizumab. We supplemented the electronic searches by manual screening of references of both the included studies and review articles.
We identified studies using the following medical subject headings (MeSH) and keywords including: ‘liver transplantation’ and ‘Vedolizumab’.
The Medline search strategy was: (‘Vedolizumab’[Supplementary Concept] OR ‘Vedolizumab’[All Fields]) AND (‘liver transplantation’[MeSH Terms] OR (‘liver’[All Fields] AND ‘transplantation’[All Fields]) OR ‘liver transplantation’[All Fields]).
Outcome assesment
Primary outcomes were adverse events such as infections and malignancies on a per patient basis. Mortality related to infections and malignancies was also reported. The secondary outcomes were liver transplant outcomes defined as rejection, graft loss, liver-related mortality and IBD treatment outcomes defined as clinical response and remission.
Inclusion and exclusion criteria
We considered for inclusion all clinical studies on liver transplant recipients treated with vedolizumab for IBD. Studies not reporting the adverse events rate were excluded. Prospective and retrospective studies, published as English-language papers, letters or abstracts, were considered.
Selection process
Two reviewers (MS and FC) independently performed the selection process. Full reports were obtained for all titles meeting the inclusion criteria. Full reports were retrieved even in case of any uncertainty. Then the review authors decided whether to include the retrieved studies by screening the full reports. Disagreements were resolved by collegial discussion. Neither of the review authors was blinded to journal titles/study authors/institutions. In the case of multiple articles for a single series, we used the latest publication.
Data extraction
Using standardized forms, two reviewers (MS and FC) extracted data independently. Discrepancies were resolved by discussion. Two arbitrators (AA and SD) resolved unresolved disagreements. The following data were extracted for each study: the study design and location, the study population and patient characteristics (average age, gender, IBD entity), mean time between liver transplantation and start of treatment with vedolizumab, previous biologic therapy, previous infections, immunosuppressive strategy, mean follow-up period, number of patients with infections, number of patients with malignancies, number of patients with recurrent PSC, number of patients with either clinical response and remission or none of them and number of patients with mucosal healing.
Quality assessment
We used the modified Newcastle-Ottawa Scale (NOS)9 for quality assessment. Two reviewers (MS and FC) assessed quality measures for included studies and disagreements were adjudicated by discussion of all the authors.
Results
Study characteristics and quality
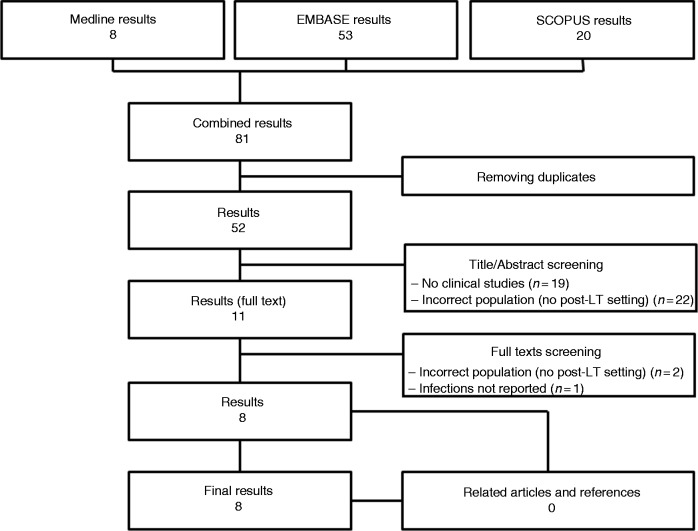
A total of 81 publications were initially identified (Figure 1). After the first review process of all the titles, 11 articles were considered as full text. Of these, eight studies matched the inclusion/exclusion criteria and were finally included.10–17 All studies were published between 2016–2018. The study origins were as follows: six studies were from Europe (25 patients), and the remains were from Australia (five patients) and from Argentina (one patient). All the studies were single-centre experiences.
Figure 1.
Flow chart of the study selection process. LT: liver transplant.
In total, 31 cases of patients treated with vedolizumab after LT were reported, most of whom (27/31) were suffering from ulcerative colitis. A new onset IBD was diagnosed in eight patients post-transplantation.
The mean age range across the studies was 20.0–47.5 years, most of the patients being male (23/31). The most common reason for LT was primary sclerosing cholangitis (29/31). Other reasons were autoimmune sclerosing cholangitis (one patient) and hepatitis C virus chronic infection (one patient). Anti-rejection immunosuppressive regimens are detailed in Table 1.
Table 1.
Anti-rejection immunosuppressive regimens.
| Reference | Number of patients | FK506/CSP | AZA/MMF | Steroid | Singlea | Doublea |
|---|---|---|---|---|---|---|
| Hartery 201610 | 5 | 5 | 2 | 2 | 3 | 2 |
| Lim 201611 | 5 | 4 | 2 | 3 | 2 | 2 |
| Wright 201714 | 10 | 10 | 4 | 2 | 6 | 4 |
| Olmedo-Martin 201713 | 2 | 2 | 0 | 0 | 2 | 0 |
| Mumtaz 201612 | 1 | 1 | 1 | 1 | 0 | 1 |
| Peverelle 201815 | 5 | NA | NA | NA | NA | NA |
| Drastich 201816 | 2 | NA | NA | NA | NA | NA |
| Daffra 201717 | 1 | NA | NA | NA | NA | NA |
AZA: azathioprine; CSP: ciclosporine; FK506: tacrolimus; MMF: mycophenolate.
Single and double immunosuppressant therapies were considered irrespectively the steroid assumption.
Three out of 31 patients underwent the liver transplant while receiving vedolizumab without interruption. The remaining patients started receiving vedolizumab up to 159.6 months after LT. Across the four studies reporting vedolizumab dosage (18 patients), the initial dosing was 300 mg at weeks 0, 2 and 6 then every eight weeks thereafter (standard protocol). Three out of 18 patients needed an optimized maintenance regimen (300 mg of vedolizumab every four weeks).
Overall, 15/32 patients (seven studies) received anti-TNF agents before anti-integrin treatment, either pre- or post-LT. The average NOS of the studies was 4.3 (range 3–6). An overview of studies and baseline characteristics is given in Supplementary Material Tables 1 and 2.
Primary outcomes
Seven out of 31 patients experienced an infectious event after a mean time of vedolizumab exposure of 11.4 months (range 5–20 months). In particular, one out of the five patients included in the series by Peverelle et al. experienced an episode of Clostridium difficile (CD) colitis treated successfully with oral antibiotics.15 The study by Wright et al. reported a total of 11 infective adverse events experienced by five patients.14 Four cases of cholangitis, four episodes of CD colitis, two empyemas, and one case of pneumonia occurred. Drastich et al. reported one patient experiencing mild infective events.16 The infection type was not specified. No fungal nor viral systemic infections were reported. No deaths occurred during the period of vedolizumab exposure, but intensive care monitoring was needed in one case of cholangitis. Infectious events are briefly reported in Table 2. No malignancies were reported.
Table 2.
Infections.
| Reference | Number of patients | Mean VDZ exposure time (months) | Infectionsa (patients) | Infections (events) | Infections |
|---|---|---|---|---|---|
| Hartery 201610 | 5 | 6.8 | 0 | 0 | \ |
| Lim 201611 | 5 | 8.1 | 0 | 0 | \ |
| Wright 201714 | 10 | 13.1 | 5 | 11 | Pneumonia (1), empyema (2), CD (4), cholangitis(4) |
| Olmedo-Martin 201713 | 2 | 12.0 | 0 | 0 | \ |
| Mumtaz 201612 | 1 | 8.0 | 0 | 0 | \ |
| Peverelle 201815 | 5 | 20.0 | 1 | 1 | CD (1) |
| Drastich 201816 | 2 | 6.0 | 1 | NA | NA |
| Daffra 201717 | 1 | 5.0 | 0 | 0 | \ |
CD: Clostridium difficile; NA: not available; VDZ: vedolizumab.
Number of patients who had at least one infection.
Secondary outcomes
IBD treatment outcomes were reported in eight series (27 patients). Clinical response was experienced by 21 of 27 patients after a mean follow-up time ranging from 5–20 months. Clinical remission coupled with mucosal healing was reported in 13 cases. Among the six patients who did not achieve clinical response, four were referred for surgery (Supplementary Material Table 3).
LT outcomes were provided by four studies. Among the 22 patients included, abnormalities in liver biochemistry were recorded in two patients. In both, liver disease had recurred before vedolizumab initiation. There was a single case of acute cellular rejection with complete recovery once tacrolimus dose was optimized. Two cases of re-transplant were reported. In both, liver function had already deteriorated before vedolizumab initiation because of recurrent PSC and previous episode of drug-induced liver injury related to anti-TNF agents.
No cases of liver-related mortality were reported.
Discussion
This systematic review of the available literature provides data on the safety of vedolizumab therapy in LT recipients.
Currently, there are limited data on using biological therapies in this setting. Further, data are mainly reported in the form of single-centre studies. Hence this systematic review provides the first comprehensive point of view on this evolving scenario.
A recent meta-analysis has investigated safety of anti-TNF agents in this setting. The study by Westerouen van Meeteren et al. found no significant increased in the rate of serious infection in 52 LT recipients during exposure to anti-TNF agents5 when compared to non-exposed LT recipients.
According to our review, seven of the 31 patients exposed to Vedolizumab experienced infectious events. In particular, three patients were admitted for CD infection. Although the colitis was successfully managed with oral antibiotics in all of the cases, one patient developed acute cellular rejection in the setting of immunosuppressant dose reduction. Clinical and biochemical parameters were restored once the original tacrolimus dose was resumed.
Since CD colitis is one of the most frequent IBD-related infections, it might be hasty to blame vedolizumab for these events. Indeed, a low frequency of CD infections has been reported in vedolizumab trials.3 Furthermore, two of the three patients with CD infections had already suffered from CD-related colitis prior to vedolizumab initiation, emphasizing the intrinsic susceptibility of this subgroup of patient to develop such infections. Thus it is not surprising to find a similar incidence of CD infection among the 52 patients exposed to anti-TNFs in the meta-analysis by Westerouen van Meeteren et al.5
Just as IBD patients are predisposed to develop intestinal superinfection (i.e. CD colitis), so LT recipients are at high risk of biliary complications,1 even more so in cases of PSC recurrence. Hence, as expected, another important cause of morbidity across the included reports were cholangitis. Of note, in the study by Wright et al., three of the four episodes of cholangitis occurred in patients with pre-existing percutaneous transhepatic catheter (PTC) tubes. Both the mechanism specifically targeting the intestine and the independent risk of developing infections of such patients, make the role of vedolizumab probably irrelevant.
Gut selectivity, despite being one of the signature features of vedolizumab, may still be a reason of concern. In fact the PSC-IBD phenotype typically results in an increased risk of colorectal cancer and a weakened local immune vigilance might potentially be a drawback in this context. Among the included patients no malignancies were reported, but the limited follow-up prevents any definitive conclusion.
Another interesting field to be investigated in the post-LT setting is the interaction between vedolizumab pharmacodynamics and PSC developments. Normally, mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1) is expressed in the digestive tract endothelium. It is involved in the recruitment of lymphocytes expressing integrin α4β7, the target of vedolizumab. In PSC patients, MAdCAM-1 is also expressed by hepatic endothelium, encouraging the recruitment of immune cells. Thus, vedolizumab may be theoretically considered as a promising therapy for the treatment of PSC. However, two recent studies18,19 investigated the possible role of vedolizumab in treating PSC, but none of them found any improvement in liver biochemistry or disease course. Moreover, a randomised controlled trial evaluating the efficacy of vedolizumab for PSC treatment has been stopped prematurely. On the other hand since vedolizumab in IBD patients is associated with clinical and endoscopic response, it is not surprising that the included studies were consistent in reporting a favourable efficacy profile. Data on liver-related outcomes are limited in our review. Indeed, across the two studies providing such data two out of 22 patients had abnormalities in liver biochemistry for recurrent PSC, suggesting the need of ad-hoc studies pursuing the issue. Nevertheless, the study by Wright et al. reported that two re-transplant were needed in LT recipients while being on vedolizumab.14 The reasons for a second transplant were one case of recurrent PSC and one episode of drug-induced liver injury related to anti-TNF agents. The authors did not consider the two cases of graft loss as related to vedolizumab and both patients remained on biologic therapy after the new LT. Interestingly, previous articles focusing on IBD patients who underwent LT for PSC showed a rate of recurrences of up to 50% within five years,20 with a re-transplantation rate of up to 14.6%.21 In our opinion, the setting of patients with an unstable graft function has not been sufficiently investigated to speculate on the role of vedolizumab in those cases. Indeed, the included series reported cases with an optimal control of graft function, preventing any definitive conclusion on vedolizumab safety when the immune balance is still compromised.
The main limitation of our study was the small sample size, especially if compared with previous studies reporting safety outcomes in standard IBD patients. However, LT recipients are invariably excluded from trials assessing efficacy and safety of IBD treatments, giving value to the included reports.
Another drawback which needs to be reported is the high risk of temporal bias, as the time between LT and vedolizumab exposure among the included patients was highly heterogeneous. We think that one of the key points in term of safety is the assessment of the optimal timing for vedolizumab initiation following LT, a point that we were unable to assess in our analyses and deserves future consideration.
Finally, although we compared our results with previous available findings in IBD patients in post-LT setting, there is a lack of head-to-head comparative studies between LT recipients undergoing vedolizumab therapy for IBD and patients undergoing standard immunosuppressive therapy alone, for anti-rejection purposes. However, our study may be a reassuring background on which to design such comparative studies.
In conclusion, while awaiting more reliable data, our study suggests that vedolizumab may be considered as a safe alternative for treating moderate to severe IBD in LT recipients. However, caution is recommended for patients with an unstable liver graft function due to lack of data to support this indication in this complex setting.
Supplemental Material
Supplemental Material for Safety of vedolizumab in liver transplant recipients: A systematic review by Marco Spadaccini, Alessio Aghemo, Flavio Caprioli, Ana Lleo, Federica Invernizzi, Silvio Danese and Maria F Donato in United European Gastroenterology Journal
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357: 2601–2614. . [DOI] [PubMed] [Google Scholar]
- 2.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010; 51: 660–678. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Loftus EV, Jr, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol 2013; 108: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 4.Ali T, Kaitha S, Mahmood S, et al. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf 2013; 5: 79–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westerouen van Meeteren MJ, Hayee B, Inderson A, et al. Safety of anti-TNF treatment in liver transplant recipients: A systematic review and meta-analysis. J Crohns Colitis 2017; 11: 1146–1151. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut 2017; 66: 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (2011, accessed 25 November 2012).
- 10.Hartery K, O'Reilly S, Houlihan D, et al. Letter: Vedolizumab for the management of inflammatory bowel disease in patients after liver transplantation for primary sclerosing cholangitis. Aliment Pharmacol Ther 2017; 45: 376–378. [DOI] [PubMed] [Google Scholar]
- 11.Lim TY, Pavlidis P, Gulati S, et al. Vedolizumab in inflammatory bowel disease associated with autoimmune liver disease pre- and postliver transplantation: A case series. Inflamm Bowel Dis 2016; 22: E39–E40. [DOI] [PubMed] [Google Scholar]
- 12.Mumtaz S, Goh J, Hirschfield GM, et al. Evolving strategies to reduce colectomy rates in primary sclerosing cholangitis-inflammatory bowel disease: Clinical remission of corticosteroid refractory colitis post-liver transplant with Vedolizumab. Frontline Gastroenterol 2016; 7: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olmedo Martin RV, Amo Trillo V, Gonzalez Grande R, et al. Efficacy and safety of vedolizumab as a treatment option for moderate to severe refractory ulcerative colitis in two patients after liver transplant due to primary sclerosing cholangitis. Rev Esp Enferm Dig 2017; 109: 659–662. [DOI] [PubMed] [Google Scholar]
- 14.Wright AP, Fontana RJ, Stidham RW. Vedolizumab is safe and effective in moderate-to-severe inflammatory bowel disease following liver transplantation. Liver Transpl 2017; 23: 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peverelle M, Mills CD, Testro A, et al. Real world outcomes of vedolizumab for inflammatory bowel disease post liver transplantation. Gastroenterology 2018; 154: S845. [Google Scholar]
- 16.Drastich P, Brezina J, Bajer L, et al. Vedolizumab for UC after infliximab failure in PSC patients after OLTx: Two case reports. J Crohns Colitis 2018; 12: S358–S359. [Google Scholar]
- 17.Daffra PR, Etchevers MJ, Sobrero MJ, et al. Use of vedolizumab in a transplanted liver patient: A case report of the first experience in a liver transplant referral center in Argentina. J Crohns Colitis 2017; 11: S147. [Google Scholar]
- 18.Christensen B, Micic D, Gibson PR, et al. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment Pharmacol Ther 2018; 47: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tse CS, Loftus EV, Jr, Raffals LE, et al. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther 2018; 48: 190–195. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand T, Pannicke N, Dechene A, et al. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: A retrospective multicenter analysis. Liver Transpl 2016; 22: 42–52. [DOI] [PubMed] [Google Scholar]
- 21.Singh A, Fritze D, Mansouri M, et al. Characteristics and outcomes of liver transplantation for primary biliary cholangitis (PBC) in young patients: Analysis of the United Network for Organ Sharing Database. Transplantation. Epub before print 30 October 2018. DOI: 10.1097/TP.0000000000002501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Safety of vedolizumab in liver transplant recipients: A systematic review by Marco Spadaccini, Alessio Aghemo, Flavio Caprioli, Ana Lleo, Federica Invernizzi, Silvio Danese and Maria F Donato in United European Gastroenterology Journal