Abstract
Background
The Faroe Islands currently have the highest recorded inflammatory bowel disease (IBD) incidence in the world.
Objective
This study investigated environmental risk factors for IBD in the Faroese population.
Methods
Environmental exposure data including lifestyle risk factors and neurotoxicants collected for over 30 years were retrieved from the Children's Health and the Environment in the Faroes (CHEF) cohorts including mainly mother–child pairs, with exposure data collected from pregnant mothers. For lifestyle risk factors, the incidence of IBD and ulcerative colitis (UC) was calculated as the rate ratio (RR) with 95% confidence intervals (CI) in exposed versus non-exposed persons. For neurotoxicants RR was calculated for persons with high versus low exposure.
Results
Six cohorts included 5698 persons with complete follow-up data and at least one exposure, and 37 were diagnosed with IBD. For pilot whale/blubber, the RR was 1.02 (95% CI, 0.48–2.18); RR of 1.01 for fish (95% CI, 0.35–2.91); and of the pollutants studied, a statistical significantly increased risk was found for 1,1,1,-trichloro-2,2-bis-(p-chlorophenyl) ethane (p,p'-DDT); RR 3.04 (95% CI, 1.12–8.30). RRs were 1.96 (95% CI, 1.03–3.73) for smoking and 1.10 (95% CI, 0.55–2.19) for alcohol intake.
Conclusion
The high IBD incidence is unlikely to be caused by special dietary habits or by environmental pollutants.
Keywords: Inflammatory bowel diseases, Faroe Islands, environmental risk factors
Key summary
The incidence of IBD is increasing worldwide
The Faroe Islands currently have the highest recorded IBD incidence in the world
Environmental factors play a key role
We have previously reported on the importance of environmental factors in the development of IBD and especially of UC in Faroese migrants to Denmark
Main findings of our study
To our knowledge, this is the first study to assess associations between several environmental toxicants and IBD
The special Faroese dietary habits and the environmental pollutants in this food is unlikely to have caused the record high IBD incidence
Statistically significant associations were found between smoking and IBD and for one environmental toxicant (p,p'-DDT)
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory and incurable condition in the gastrointestinal tract of unknown aetiology. There are two main subgroups of IBD; ulcerative colitis (UC) and Crohn's disease (CD). Currently, IBD incidence is increasing worldwide, and IBD is now considered to be a global disease.1 The Faroe Islands in the North Atlantic is no exception. This small country with 50,000 inhabitants was in 2010 identified in the European Epi-IBD inception cohort study to have the highest IBD incidence in the world with an age standardised (European Standard Population, ESP) incidence rate of 81.5 per 100,000,2 and the incidence has increased over time.3
The risk of IBD is known to have both genetic and environmental components, as it is an abnormal and dysregulated immune response occurring in genetically susceptible individuals. Although multiple susceptibility genes have been identified, genetics does not explain the drastic increase in the incidence of IBD.4 A hereditary predisposition for IBD may exist in the genetically homogeneous and isolated Faroese population, where genetically inherited diseases such as glycogen storage disease type IIIA,5 carnitine transporter deficiency6 and mitochondrial encephalomyopathy7 are frequent.
On environmental factors, it is known that children of parents, who migrate from a country with a low IBD prevalence to one with a high prevalence, adopt the IBD risk of their new home country.8 The reverse situation is present when Faroese people migrate to Denmark, where the IBD incidence is lower than in the Faroe Islands. First-generation Faroese immigrants to Denmark had a higher IBD incidence than Danes and the excess risk derived solely from UC and was restricted to the first 10 years of residence in Denmark, indicating the importance of environmental factors in the development of IBD and especially of UC.9
The evidence on modifiable environmental factors is inconclusive. Therefore it is difficult to undertake preventive measures and no clear guidance is available from the gastroenterology societies.10 Substantial data on environmental risk factors have been collected for more than 30 years within the Children's Health and the Environment in the Faroes (CHEF) project, established to assess impact on health of pollutants in the marine diet.11,12 On this background, the CHEF project enabled a unique search for environmental risk factors for IBD in this remotely located, high-risk population.
Materials and methods
Children's Health and the Environment in the Faroes (CHEF) project
In 1985, Grandjean and Weihe investigated women aged 20 to 50 years in a small Faroese fishing settlement. The aim was to measure mercury exposure from marine food in blood samples. Due to the findings of increased mercury concentrations in these women, a larger nationwide study of mothers and their newborn children was initiated, marking the beginning of the CHEF project.13 In total, four mother–child cohorts have been established since 1986 and one cohort of pregnant women. The project has been carried out at the Department of Occupational Medicine and Public Health within the Faroese Hospital System, and the project currently includes over 2,500 Faroese mother–child pairs. In the present observational and prospective study, the study population consisted of the four mother–child cohorts, and grandmothers and fathers14 that were recruited within the last mother–child cohort. Furthermore, the cohort of only pregnant women12 was included alongside one additional cohort investigating semen quality in young Faroese men, born between 1981 and 1984, and their mothers (Table 1).15
Table 1.
Overview of the Faroese cohorts included in this study.
| Cohort name | Recruitment period | Percentage of all births | Entry date | Children | Adults | Total |
|---|---|---|---|---|---|---|
| Cohort 1a | March 1986–1987 (end of) | 75% | Child's birthdate | 1001 | 980 | 1981 |
| Cohort 2a | March 1994–March 1995 | 64% | Child's birthdate | 189 | 189 | 378 |
| Cohort 3b | Nov. 1997–March 2000 | 60% | Child's birthdate | 654 | 638 | 1292 |
| Cohort 4c | Oct. 2000–Sept 2001 | 23%e | 1 April 2001 | - | 166 | 166 |
| Cohort 5a | Oct. 2007–April 2009 | 73% | Child's birthdate | 488 | 504 | 992 |
| - Fathers from cohort 5 d | Feb. 2009–Feb. 2010 | Child's birthdate | - | 282 | 282 | |
| - Maternal grandmothers from cohort 5 d | Oct. 2007–April 2009 | Child's birthdate | - | 337 | 337 | |
| - Paternal grandmothers from cohort 5d | Oct. 2007–April 2009 | Child's birthdate | - | 201 | 201 | |
| Young men | Feb. 2007–Feb. 2009 | 1 Feb. 2008 | 263 | 207 | 470 | |
| Totalf | 2595 | 3504 | 6099 | |||
| Unique recordsg | 5773 | |||||
Cohort 1, 2 and 5 included singleton births only.
Cohort 3 included twin births also.
Cohort 4 recruited pregnant women only.
Cohort 5 enrolled fathers alongside maternal and paternal grandmothers.
Cohort 4 did not intend to include all births within the period, thus the lower percentage.
A given person could be included more than one time.
A given person included only first time.
In the Faroe Islands, hunting of pilot whale for human consumption is a tradition going far back in time. The pilot whale is a toothed whale and thus a carnivorous marine mammal eating squid and fish, and in modern times contaminated with high levels of pollutants accumulated in the marine food chain.16 The measured environmental toxicants include methylmercury (MeHg), persistent organic pollutants (POPs) including polychlorinated biphenyls (PCBs) and perfluorinated compounds (PFCs).
The first mother–child pairs were recruited in 1986–87, the last pairs, and the young men and their mothers, in 2007–09. The cohorts represented up to 75% of all Faroese births in the recruitment periods (Table 1). Number of participants by gender and cohort is provided in supplementary Tables 1 (any registration) and 2 (first registration only). The CHEF project has gathered extensive exposure information from questionnaires answered by the mothers at recruitment during pregnancy or shortly after delivery and biological samples including umbilical cord blood, whole blood, maternal hair and breast milk.
Data on environmental exposures in the Faroe Islands
In the present study, we included exposure data on smoking, alcohol consumption and dietary intake of pilot whale/blubber and fish retrieved from self-reported questionnaires that were filled in at recruitment, though the timing of the exposure status varied across cohorts. For mothers, their exposure status refers to exposure during pregnancy; for children their exposure in uterus; for fathers their exposure shortly before the pregnancy of their partners. For grandmothers their exposure for smoking and alcohol consumption refers to the time prior to their pregnancy with the now pregnant daughter/son's pregnant partner, while their intake of traditional Faroese foods refers to exposure during pregnancy. For the young men their exposure is at the time of recruitment; and for mothers of young men their exposure prior to their pregnancy for smoking and alcohol consumption, while intake of traditional Faroese foods refers to exposure during pregnancy. The phrasing of the exposure questions varied slightly across the six cohorts, and in order to standardize the data, all answers were dichotomized as yes/high or no/low to usage/intake (Table 2).
Table 2.
Definition of exposure status for smoking, alcohol consumption and intake of pilot whale/blubber and fish.
| Cohort members | Smoking |
Alcohol |
Pilot whale/blubberb |
Fish |
||||
|---|---|---|---|---|---|---|---|---|
| Exposed answered Yes to | Non-exposed answered No to | Exposed answered Yes to | Non-exposed answered No to | Exposed ate more than one | Non-exposed ate less than one | Exposed ate more than one | Non-exposed ate less than one | |
| Mothers in cohort 1,a 2,a 3,a 4, 5a | Smoking during pregnancy | Alcohol consumption during pregnancy | Dinner per month during pregnancy | Dinner per week during pregnancy | ||||
| Fathers in cohort 5 | Smoking shortly before pregnancy of their partner | Alcohol consumption shortly before pregnancy of their partner | Dinner per year at recruitment | Dinner per week at recruitment | ||||
| Maternal and paternal grandmothers in cohort 5 and mothers of young men | Smoking shortly before pregnancy | Alcohol consumption shortly before pregnancy | Dinner per year during pregnancy | Dinner per week during pregnancy | ||||
| Young men | Current smoking | Current alcohol consumption | Dinner per year at recruitment | Dinner per week at recruitment | ||||
Exposure status of the children in cohorts 1, 2, 3 and 5 equates to that of the mothers as questionnaires were answered during pregnancy.
For the analysis the different time scales were standardized.
Furthermore, data were included on toxicants measured in the blood samples taken at the time of recruitment: POPs (PCBs: 1,1-dichloro-2,2-bis(p-chlorophenyl) ethane (p,p'-DDE); 1,1,1,-trichloro-2,2-bis-(p-chlorophenyl) ethane (p,p'-DDT); β-hexachlorocyclohexane (β-HCH)); perfluorooctanoic acid (PFOA); perfluorooctane sulfonate (PFOS); perfluorononanoic acid (PFNA); perfluorodecanoic acid (PFDA); perfluorohexane sulfonate (PFHxS); perfluorooctane sulphonamide (Total FOSA). MeHg was measured in hair and blood samples. In order to ensure that potential differences in the results were not due to the measurement method, both measurements were included. Because PCB is a mixture of several congeners, the ΣPCB was calculated as the sum of PCB-138, PCB-153 and PCB-180 multiplied by 2, as the sum of these represent close to 50% of the total PCB concentration.17
The cohorts were followed up via the Faroese National Population Register from the date of their first recruitment (entry date) until date of emigration, death, diagnosis of IBD or 31 May 2017 (end of follow-up). Incident cases of IBD among the cohort participants were identified within the nationwide IBD database established in the Faroese IBD study.3 All cases were clinically characterized according to the Montreal classification.18 For the purpose of the current study, data were updated until end of follow-up in cooperation with the Medical Centre at the National Hospital of the Faroe Islands. Definition and classification of IBD cases in the Faroese IBD study have been described previously.3
Analysis
For a given exposure, we estimated the incidence of IBD among exposed persons as compared with the incidence of IBD among non-exposed. A given cohort member was included in the analysis from her/his date of first recruitment into CHEF, and followed up until date of IBD diagnosis, death, emigration or end of the study. A re-immigrated person was re-included when she/he returned to the Faroe Islands. The rate ratio (RR) and the 95% confidence interval (95% CI) of IBD incidence and of UC incidence between exposed and non-exposed cohort members were estimated with Poisson regression controlling for age (0–19, 20–39, 40–59, 60–79, 80+) and calendar period (before 1 January 2000 and after). Gender was not controlled for, as the IBD incidence is identical for men and women in the Faroe Islands.3 Data on the environmental toxicants were divided into tertiles with RR and 95% CI calculated as comparison of the incidence between the highest and the lowest tertiles. Geometric mean and interquartile range for each exposure group were calculated. SAS software version 9.4 and R version 3.4.3 were used for the statistical analysis.
Ethics
This is a register-based study approved by the Faroese Committee on Biomedical Research Ethics and the Faroese Data Inspection Agency, J. nr. 16/00037. No contact was made to the participating cohort members, IBD patients or relatives. All cohort members signed an informed consent in order to participate in any of the investigations conducted at the Department of Occupational Medicine and Public Health.
Results
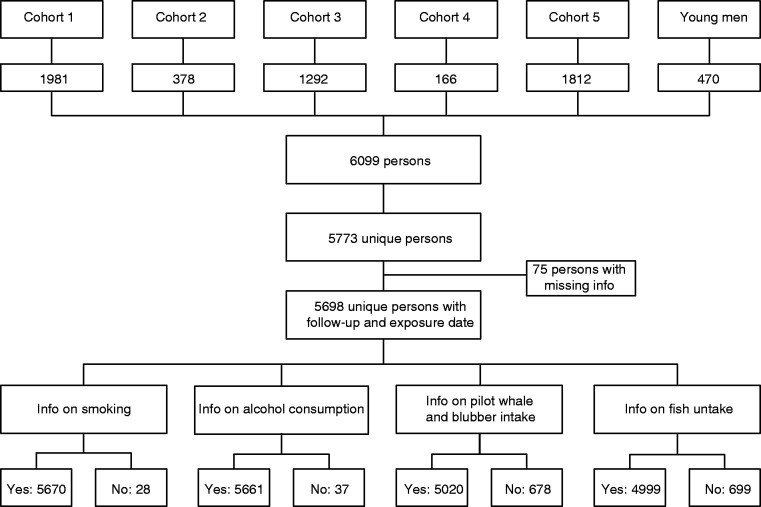
In total, the six cohorts included 6099 records of which 5773 were unique persons with 5698 having complete follow-up information on migration and death and data on at least one type of exposure (Figure 1). During the follow-up period, 37 individuals within the age range of 11–67 years were diagnosed with IBD, of which 32 had UC and 5 had CD. None of the CD cases had perianal disease. The Montreal classifications of the IBD cases are shown in supplementary Table 3.
Figure 1.
Flow chart of number of participants in the cohorts with information on smoking, alcohol consumption and food intake.
The six cohorts contributed a total of 96,507.6 person-years (PY). The RRs and 95% CIs for the associations between the environmental risk factors and the incidence of IBD and UC are listed in Table 3. Only smoking was statistically significantly associated with IBD. Exposure data on smoking were complete for 5670 persons. Comparison of the 1935 smokers (33,132.2 PY) with 19 cases of IBD with the 3735 non-smokers (63,072.6 PY) with 18 cases of IBD resulted in a RR of 1.96 (95% CI, 1.03–3.73).
Table 3.
Number of persons (N), person-years (PY), IBD and UC (cases) by exposure status; rate ratios (RRs) and 95% confidence intervals (95% CIs) for exposed persons as compared with non-exposed persons.
| Exposure | Exposed persons |
Non-exposed persons |
Total |
RR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | PY | Cases | N | PY | Cases | N | PY | Cases | ||
| Smoking and IBD | 1935 | 33,132.2 | 19 | 3735 | 63,072.6 | 18 | 5670 a | 96,204.8 | 37 | 1.96 (1.03–3.73) |
| Smoking and UC | 1935 | 33,138.2 | 16 | 3735 | 63,084.7 | 16 | 5670 a | 96,222.9 | 32 | 1.85 (0.92–3.70) |
| Alcohol and IBD | 1759 | 27,287.8 | 12 | 3902 | 68,804.0 | 25 | 5661 b | 96,091.8 | 37 | 1.10 (0.55–2.19) |
| Alcohol and UC | 1759 | 27,287.8 | 12 | 3902 | 68,821.9 | 20 | 5661 b | 96,109.7 | 32 | 1.37 (0.67–2.80) |
| Pilot whale/blubber and IBD | 3456 | 63,253.5 | 27 | 1564 | 21,801.2 | 9 | 5020 c | 85,054.7 | 36 | 1.02 (0.48–2.18) |
| Pilot whale/blubber and UC | 3456 | 63,268.4 | 23 | 1564 | 21,801.2 | 9 | 5020 c | 85,069.6 | 32 | 0.86 (0.39–1.87) |
| Fish and IBD | 4195 | 76,652.1 | 32 | 804 | 8115.5 | 4 | 4999 d | 84,767.6 | 36 | 1.01 (0.35–2.91) |
| Fish and UC | 4195 | 76,658.1 | 29 | 804 | 8124.5 | 3 | 4999 d | 84,782.6 | 32 | 1.24 (0.37–4.14) |
Missing data for:
28 persons;
37 persons;
678 persons;
699 persons.
Table 4 illustrates the RRs and 95% CIs for the associations between the environmental toxicants and the incidence of IBD and UC. The only toxicant statistically significantly associated with IBD was p,p'-DDT. In total, 1721 persons had high exposure and 1712 had low exposure to this pollutant with a RR of 3.04 (95% CI, 1.12–8.30). We tested also the association for p,p'-DDT and UC; RR of 3.60 (95% CI, 1.20–10.82). No statistically significant association was found for other compounds on the risk of UC (data not shown). Not all environmental toxicants were measured in every cohort.
Table 4.
Geometric mean (GM), interquartile range (IQR), number of persons (N), person-years (PY) and IBD (cases) by toxicological compound; rate ratios (RRs) and 95% confidence intervals (95% CIs) for persons with high exposure vs. low.
| Exposure | GM |
IQR (25%–75%) |
N |
PY |
Cases |
RR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | ||
| ΣPCBa | 0.270 | 2.063 | 0.20–0.42 | 1.45–2.61 | 1715 | 1561 | 27,991.9 | 28,145.2 | 9 | 11 | 1.27 (0.52–3.07) |
| p,p′-DDEa | 0.093 | 1.062 | 0.07–0.16 | 0.71–1.41 | 1706 | 1584 | 27,165.8 | 29,671.1 | 9 | 9 | 0.97 (0.38–2.44) |
| p,p′-DDTa | 0.002 | 0.064 | 0.002–0.003 | 0.036–0.096 | 1712 | 1721 | 27,365.6 | 38,146.7 | 5 | 18 | 3.04 (1.12–8.30) |
| β-HCHa | 0.004 | 0.045 | 0.003–0.006 | 0.03–0.07 | 1691 | 1680 | 34,649.8 | 31,043.8 | 13 | 10 | 0.88 (0.38–1.99) |
| PFOAb | 0.948 | 4.422 | 0.76–1.34 | 3.55–4.98 | 1457 | 1386 | 26,048.6 | 26,979.7 | 11 | 7 | 0.60 (0.23–1.56) |
| PFOSb | 2.332 | 26.88 | 1.93–2.90 | 21.90–32.24 | 1494 | 1304 | 37,074.7 | 20,369.9 | 15 | 3 | 0.30 (0.08–1.07) |
| PFNAb | 0.209 | 1.099 | 0.21–0.21 | 0.79–1.38 | 1815 | 1212 | 45,552.9 | 14,396.5 | 18 | 5 | 0.62 (0.22–1.76) |
| PFDAb | 0.196 | 0.453 | 0.21–0.21 | 0.34–0.53 | 2373 | 1222 | 52,524.5 | 15,634.7 | 19 | 5 | 0.70 (0.25–1.95) |
| PFHxSb | 0.184 | 0.722 | 0.20–0.21 | 0.49–0.94 | 1819 | 845 | 37,274.4 | 14,277.2 | 16 | 6 | 0.80 (0.31–2.07) |
| Total FOSAb | 0.297 | 1.832 | 0.23–0.41 | 1.22–2.20 | 606 | 638 | 15,380.5 | 15,947.1 | 5 | 4 | 0.76 (0.20–2.84) |
| MeHg (blood)b | 2.142 | 39.59 | 1.62–3.34 | 28.15–53.05 | 1038 | 1275 | 9755.7 | 31,927.3 | 6 | 10 | 0.84 (0.29–2.40) |
| MeHg (hair)c | 0.636 | 6.931 | 0.47–1.00 | 4.87–9.05 | 1829 | 1769 | 19,887.7 | 40,029.7 | 11 | 15 | 0.81 (0.36–1.79) |
Toxicants were measured in:
µg/g lipid;
µg/L;
µg/g hair.
Discussion
The incidence of IBD in the Faroe Islands is the highest in the world, and a strong influence of environmental factors on disease risk has been demonstrated in Faroese migrants to Denmark. Therefore, further studies on environmental exposures in Faroese inhabitants prior to the emergence of IBD are important. The present study did not indicate the high risk of IBD in the Faroe Islands to derive from the intake of traditional Faroese food in the form of pilot whale/blubber and fish. Neither did we find clear associations between the measured toxicants in seafood and IBD. Only one compound (p,p'-DDT) was statistically significantly associated with an increased risk of IBD and UC, but with the many measured associations this could be a chance finding. For toxicants, most risk estimates were actually below 1, and one could speculate that this could be an immunosuppressive effect, in line with the prior finding of a reduced antibody response to childhood vaccines in children exposed to PCBs.19
Alcohol consumption was not associated with the risk of IBD and/or UC. The risk of IBD was, however, statistically significantly elevated in smokers, a tendency that remained for UC only, though based on small numbers. Data were too scarce for separate analysis of CD.
Although the Faroe Islands are located in Northern Europe, this country differs from the rest of Europe in terms of its traditional food of pilot whale and blubber. Evidence suggests that a diet high in fruits, vegetables, fibre and omega-3 fatty acids is associated with a lower IBD risk.10 It is noteworthy to mention that pilot whale, blubber and fish to various extents contain omega-3-fatty acids, which may in part explain why these foods did not increase the risk of IBD.11 The northern latitude of the Faroe Islands and thus the low exposure to sunlight is believed to result in lower levels of vitamin D, although similar levels have been found in Denmark, Norway and Finland.20 Vitamin D deficiency is common in IBD patients and considered an important component in the development of IBD.21 In the present study we did not include data on vitamin D status, previously reported from cohort 3 and 5.22 The high intake of seafood in the studied cohorts may compensate for the low sunlight exposure. Nevertheless, since January 2018, Faroese milk with 0.5% fat has been enriched with vitamin D.
To our knowledge, this is the first study assessing the associations between environmental toxicants and IBD. An association between PFOA and UC has been reported from an occupationally exposed cohort,23,24 but we did not see this association. A possible explanation for the discrepancy between our study and the US study is the lower exposure in the Faroese cohorts. Another explanation is differences in assessment of exposure: Steenland et al.23 combined occupational and residential serum estimates, while exposure in the Faroese cohorts was measured directly from each individual, showing current exposure, not cumulative or estimated exposure. In our study, only p,p'-DDT was associated with IBD and UC. As no other study has investigated p,p'-DDT and IBD, this positive finding needs replication. However, the lack of associations between environmental toxicants and IBD was not surprising, as we found no association between intake of seafood and IBD.
It was surprising that intake of traditional food was not associated with an increased risk of IBD. The Faroese immigrants to Denmark had an excess IBD incidence during their first 10 years in Denmark, but thereafter their incidence resembled that of Danes.9 One could have expected this change to coincide with a decreased intake of the traditional Faroese food. However, the IBD incidence in the Faroe Islands increased from the 1960s to the 2010s,3 which is a period where the Faroese diet became increasingly westernized. Taking the combined evidence into account, it seems reasonable to hypothesize that the high incidence of IBD in the Faroe Islands derives from recent dietary or other habits not found in Denmark, but at present we are unable to pinpoint specific factors.
The lack of an association between alcohol consumption and the risk of IBD was confirmed in the recently reported European prospective cohort study (EPIC).25 A meta-analysis on beverage consumption also found no association between alcohol intake and the risk of UC,26 but the role of alcohol in the aetiology of IBD is inconclusive and widely debated, as some studies found an increased IBD risk in relation to alcohol consumption.27
The association between smoking and IBD is complex. CD is four-fold more frequent in current smokers than in never smokers, and former smokers have an increased prevalence of CD. Smoking cessation is recommended to CD patients, as continued smoking often complicates disease course. Smoking has, on the other hand, a protective effect on the development of UC, and smoking cessation in UC will induce a more severe disease course.10 The majority of the studies on smoking and IBD are case–control studies, where recall- and reporting biases cannot be excluded. The evidence on the long-term effect of smoking on CD and UC is limited, but one prospective study from the Nurses Health Study found an increased risk of UC among former smokers with a hazard ratio of 1.56 (95% CI, 1.26–1.93).28 When we combined their hazard ratios for current and former smokers, the estimate was 1.32, well within the 95% confidence interval of our result for smoking; RR 1.85 (95% CI, 0.92–3.70). Further prospective cohort data on smoking and risk of UC are highly warranted. The Faroe Islands continue to have the highest percentage of daily smokers in the Nordic countries at 20% for men and 24% for women.29 No free of charge tobacco cessation courses are available in the country.
Strengths and limitations
We investigated risk factors for IBD in the largest data set on human environmental exposures available from the Faroe Islands with individual follow-up of cohort members for death and e/immigrations from the population register, and with individual linkage to the nationwide Faroese IBD database.3 From previous work we had established that Faroese re-immigrate repeatedly, hence the need for a precise measurement of person-years.9 The participants in this study can be regarded as representative of the general population as they were unselected and resided throughout the country, though individuals from the capital region were overrepresented. The only exception was cohort 2 where participants resided away from the capital area of Tórshavn.12
Data on lifestyle risk factors were derived from self-reported questionnaires. For 49% of the cohorts (mothers and fathers), exposure data referred to exposure at time of recruitment, but for 40% (children) the data referred to exposure before birth; and for the remaining 11% (grandmothers/fathers; mothers of young men) to time of pregnancy with their now adult daughter/son. While these time differences probably do not affect the data on dietary habits, they could have affected the smoking and alcohol data. The use of the mother's intake of traditional food during pregnancy as a proxy for the child's exposure is also a limitation of the study. Furthermore, it was not possible to control for various other environmental factors that the cohort members might have been exposed to in the Faroe Islands or abroad. A final limitation was the limited number of IBD cases due to the still relatively young age of the cohort members recruited as newborns as these had not yet reached the age at which IBD may develop. Exposure to environmental toxicants is commonly found in the Faroese population why it can be expected that no one is truly unexposed. We therefore compared individuals of the lowest exposure tertile with those in the highest (Table 4).
Conclusion
This study combined comprehensive data on marine environmental exposures with that of IBD incidence in the Faroe Islands. The study was undertaken within an ideal setting for investigating the impact of the environment on IBD risk in a location that has seen a dramatic increase in the incidence of IBD within the same decades as those in which the studied cohorts were recruited. The study showed that neither the special dietary habits in the form of pilot whale and blubber nor the environmental pollutants in this food were likely to explain the high incidence of IBD in the Faroe Islands.
Supplemental Material
Supplemental Material for Dietary risk factors for inflammatory bowel diseases in a high-risk population: Results from the Faroese IBD study by T Hammer, S Nymand Lophaven, K Rubek Nielsen, M Skaalum Petersen, P Munkholm, P Weihe, J Burisch and E Lynge in United European Gastroenterology Journal
Acknowledgements
TH would like to thank colleagues at the Department of Occupational Medicine and Public Health for their help and highly valued encouragements.
Author contributions
All authors contributed to this work. PW and MSP collected the data. TH and EL designed the study, obtained funding and drafted the manuscript. SNL performed the statistical analysis and contributed to the interpretation. TH, SNL, KRN, MSP, PM, PW, EL and JB critically revised and approved the final manuscript.
Conflict of interest
JB reports personal fees from AbbVie, personal fees from Janssen-Cilag, personal fees from Celgene, personal fees from MSD, personal fees from Pfizer, grants and personal fees from Takeda, outside the submitted work.
Ethics approval
This is a register-based study approved by the Faroese Committee on Biomedical Research Ethics and the Faroese Data Inspection Agency, J. nr. 16/00037. No contact was made to the participating cohort members, IBD patients or relatives.
Funding
This work was supported by the Faroese Research Council, the European Crohn’s and Colitis Organisation (ECCO), Beckett Foundation, the Danish Colitis–Crohn Patients Organisation (CCF) and Aage & Johanne Louis-Hansens Foundation (to TH).
Informed consent
All cohort members signed an informed consent in order to participate in any of the investigations conducted at the Department of Occupational Medicine and Public Health.
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut 2014; 63: 588–597. [DOI] [PubMed] [Google Scholar]
- 3.Hammer T, Nielsen KR, Munkholm P, et al. The Faroese IBD study: incidence of inflammatory bowel disease across 54 years of population-based data. J Crohns Colitis 2016; 10: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol 2010; 6: 339–346. [PMC free article] [PubMed] [Google Scholar]
- 5.Santer R, Kinner M, Steuerwald U, et al. Molecular genetic basis and prevalence of glycogen storage disease type IIIA in the Faroe Islands. Eur J Hum Genet 2001; 9: 388–391. [DOI] [PubMed] [Google Scholar]
- 6.Poulsen SD, Lund AM, Christensen E, et al. [Carnitine transporter deficiency is a hereditary disease with a high incidence in the Faroe Islands]. Ugeskr Laeger 2012; 174: 1217–1219. [PubMed] [Google Scholar]
- 7.Ostergaard E, Hansen FJ, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain 2007; 130: 853–861. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 9.Hammer T, Lophaven SN, Nielsen KR, et al. Inflammatory bowel diseases in Faroese-born Danish residents and their offspring: further evidence of the dominant role of environmental factors in IBD development. Aliment Pharmacol Ther 2017; 45: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maaser C, Langholz E, Gordon H, et al. European Crohn's and Colitis organisation topical review on environmental factors in IBD. J Crohns Colitis 2017; 11: 905–920. [DOI] [PubMed] [Google Scholar]
- 11.Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19: 417–428. [DOI] [PubMed] [Google Scholar]
- 12.Weihe P, Grandjean P. Cohort studies of Faroese children concerning potential adverse health effects after the mothers' exposure to marine contaminants during pregnancy. Acta Vet Scand 2012; 54: S7. [Google Scholar]
- 13.Grandjean P, Weihe P, Jørgensen PJ, et al. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health 1992; 47: 185–195. [DOI] [PubMed] [Google Scholar]
- 14.Petersen MS, Halling J, Weihe P, et al. Spermatogenic capacity in fertile men with elevated exposure to polychlorinated biphenyls. Environ Res 2015; 138: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halling J, Petersen MS, Jørgensen N, et al. Semen quality and reproductive hormones in Faroese men: a cross-sectional population-based study of 481 men. BMJ Open 2013; 3: e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julshamn K, Andersen A, Ringdal O, et al. Trace elements intake in the Faroe Islands. I. Element levels in edible parts of pilot whales (Globicephalus meleanus). Sci Total Environ 1987; 65: 53–62. [DOI] [PubMed] [Google Scholar]
- 17.Grandjean P, Weihe P, Needham LL, et al. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res 1995; 71: 29–38. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann C, Grandjean P, Weihe P, et al. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med 2006; 3: e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalgård C, Petersen MS, Schmedes AV, et al. High latitude and marine diet: vitamin D status in elderly Faroese. Br J Nutr 2010; 104: 914–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chetcuti Zammit S, Ellul P, Girardin G, et al. Vitamin D deficiency in a European inflammatory disease inception cohort: an Epi-IBD study. Eur J Gastroenterol Hepatol 2018; 30: 1297–1303. [DOI] [PubMed] [Google Scholar]
- 22.Dalgård C, Petersen MS, Steuerwald U, et al. Umbilical cord serum 25-hydroxyvitamin D concentrations and relation to birthweight, head circumference and infant length at age 14 days. Paediatr Perinat Epidemiol 2016; 30: 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenland K, Zhao L, Winquist A, et al. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environ Health Perspect 2013; 121: 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steenland K, Zhao L, Winquist A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med 2015; 72: 373–380. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann MM, Hernandez V, Bernigau W, et al. No association of alcohol use and the risk of ulcerative colitis or Crohn's disease: data from a European Prospective cohort study (EPIC). Eur J Clin Nutr 2017; 71: 512–518. [DOI] [PubMed] [Google Scholar]
- 26.Nie JY, Zhao Q. Beverage consumption and the risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine 2017; 96: e9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang XY, Wei WB, Zeng LR, et al. Controversial role of alcohol in the development of inflammatory bowel diseases. Eur J Clin Nutr 2018; 72: 304. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol 2012; 107: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomesco. Health Statistics in the Nordic countries 2017. 2017. http://norden.diva-portal.org/smash/get/diva2:1148509/FULLTEXT05.pdf (accessed 10 October 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Dietary risk factors for inflammatory bowel diseases in a high-risk population: Results from the Faroese IBD study by T Hammer, S Nymand Lophaven, K Rubek Nielsen, M Skaalum Petersen, P Munkholm, P Weihe, J Burisch and E Lynge in United European Gastroenterology Journal