Abstract
Background:
A growing body of evidence suggests that nonfunctioning and subclinical cortisol secreting adrenal incidentalomas (AIs) are associated with several components of metabolic syndrome resulting in increased cardiometabolic risk. The long-term metabolic outcome of these AIs is largely unknown and their most appropriate management remains controversial.
Objectives:
To undertake a systematic review of the prevalence of cardiometabolic abnormalities in nonfunctioning and subclinical cortisol secreting AIs and long-term outcome of conservative treatment and adrenalectomy.
Methods:
MEDLINE, Cochrane Controlled Trials Register, and EMBASE were searched for relevant studies and systematic review was performed. National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies was used to assess the risk of bias in the studies.
Results:
Of the 65 studies screened, 18 (10 retrospective, 5 prospective, 2 cross-sectional studies, and 1 randomized controlled trial) were included in the systematic review. Prevalence of hypertension (HTN), impaired glucose metabolism, dyslipidaemia, and raised body mass index (BMI) was higher in subclinical cortisol secreting AIs as compared with nonfunctioning AIs. Surgical intervention had a beneficial effect on blood pressure, glucometabolic control, and obesity in patients with subclinical Cushing's syndrome. The results for lipid metabolism were equivocal. There was no significant improvement in cardiometabolic risk factors after adrenalectomy in nonfunctioning AIs. The quality of evidence was found to be low to moderate.
Conclusions:
The systematic review demonstrated increased prevalence of components of metabolic syndrome in patients with subclinical cortisol secreting and nonfunctioning AIs. A beneficial role of adrenalectomy on HTN, glucometabolic control, and BMI was observed in patients with subclinical cortisol secreting AIs.
Keywords: Adrenal incidentalomas, metabolic syndrome, subclinical Cushing's syndrome, systematic review
INTRODUCTION
Adrenal incidentalomas (AIs) are defined as adrenal mass lesions >1 cm in diameter, discovered incidentally during investigation for conditions unrelated to adrenal disease. These incidentally discovered adrenal masses may be hormonally active or nonfunctioning and malignant or benign; the need for any treatment depends on the nature of the mass.
A growing body of evidence, both clinical and experimental, indicates that hormone-secreting AIs are associated with several components of metabolic syndrome, such as hypertension (HTN), atherogenic dyslipidemia, increased thrombogenicity, impaired glucose tolerance, insulin resistance, fatty liver disease, and abdominal obesity, through the effects of excessive adrenal hormones on various metabolic pathways.[1,2,3] A similar association has been found in subclinical autonomous cortisol-secreting AIs and, paradoxically, nonfunctioning AIs, adding a new dimension to the clinical management and follow-up of these patients.[4]
It has been suggested that patients with AI meet the criteria for the metabolic syndrome and that hyperinsulinemia is a major factor promoting tumor growth.[5] According to the modified National Cholesterol Education Program criteria,[6] the presence of any three of the five factors is required for a diagnosis of metabolic syndrome: abdominal obesity, hypertriglyceridemia (triglycerides ≥1.7 mmol/L); low high-density lipoprotein (HDL) (cholesterol ≤1.03 mmol/L for men and ≤1.29 mmol/L for women); elevated blood pressure (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg or current use of antihypertensive drugs); impaired fasting glucose (fasting plasma glucose ≥ 5.6 mmol/L).
Subclinical Cushing's syndrome (SCS) is the most frequent hormonal abnormality detected in patients with AI with wide variation in prevalence (5–47%) and the issue of subtle cortisol secretion by AIs is among the most controversial issues in endocrine practice. It has been hypothesized that subtle cortisol overproduction in subjects with AI may impact on carbohydrate metabolism.[7] Comparison of groups of patients has generally revealed modest increases in body mass index (BMI), HTN, reduced insulin sensitivity, glucose intolerance or frank diabetes, adverse cardiovascular risk profile, and osteopenia/osteoporosis in patients with SCS compared to controls; however, most of the studies suffer to a degree from referral bias.[8,9]
Nonfunctioning AIs paradoxically have been recently implicated to predispose to metabolic syndrome and increasing evidence supports an association between nonfunctioning AIs and obesity, HTN, hyperglycemia, dyslipidemia, and insulin resistance.[1,7,10,11,12,13] This relationship appears to be theoretically inexplicable, as nonfunctioning AIs usually remain inactive; the cause–effect relationship and the underlying mechanisms have not been conclusively delineated and remain to be further elucidated.
Improvement in certain metabolic parameters has also been observed after unilateral adrenalectomy in patients with AIs. The aim of this systematic review is to advance the understanding of the association between nonfunctioning and subclinical autonomous cortisol secreting AIs and components of metabolic syndrome and the long-term clinical implications including surgical outcome, in order to appropriately address the management issues and to formulate suggestions for future research.
METHODS
Search strategy
A systematic search was performed for English language articles using MEDLINE, Cochrane Controlled Trials Register (1960–2005), and EMBASE (1991–2005). The literature was searched for the period from each database's earliest inception up to June 2018. In addition, the reference lists of the retrieved articles were examined to identify additional eligible studies. Combinations of keywords were used including “adrenal incidentalomas” or “adrenal mass,” “subclinical Cushing's syndrome” or “preclinical Cushing's syndrome” or “autonomous cortisol secretion”, “nonfunctioning adrenal incidentalomas” in combination with “metabolic syndrome”, or “cardiometabolic” or “cardiovascular risk”, or “insulin resistance” in the title or the abstract. The review was conducted in line with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement.[14]
Inclusion and exclusion criteria
Original retrospective, prospective, or cross-sectional studies which analyzed patients with nonfunctioning and/or subclinical cortisol secreting AIs based on their biochemical profile reporting at least two components of metabolic syndrome (diabetes, impaired glucose tolerance, fasting hyperinsulinemia, dyslipidemia, HTN, and obesity/central adiposity) and the results of adrenalectomy and/or conservative management on these outcomes were included.
Studies without biochemically confirmed subclinical hypercortisolism and studies reporting only preoperative data or insufficient postoperative data were excluded from the systematic review, as were case reports and series including fewer than ten operated patients. Data quoted as unpublished or derived from abstracts were not used in the systematic review. The patients in the studies were classified into two groups: nonfunctioning AIs and subclinical cortisol secreting AIs. Nonfunctioning AI was defined as a subgroup of AI where the possibility of profound or even subclinical adrenal hormone excess was ruled out based on a comprehensive endocrine evaluation. Subclinical cortisol secreting AI was defined as a subgroup of AI with an abnormal response to standard tests of hypothalamic–pituitary–adrenal (HPA) axis function.
A predesigned data extraction form was used to collect data from the eligible studies. All variables were listed for which data were sought and information was extracted from each study including: 1) first author's last name, country, study design, number of participants; 2) age range and gender of study participants; 3) endocrine tests performed for autonomous cortisol secretion; 4) AI category: subclinical autonomous cortisol secreting or non-functioning; 5) outcome measures including, a) prevalence of components of metabolic syndrome in subclinical cortisol secreting AIs and nonfunctioning AIs, b) cardiometabolic outcomes of conservative management and adrenalectomy.
Methodological quality and risk of bias assessment
NIH quality assessment tool for observational cohort and cross-sectional studies was used to assess the risk of bias. It is based on the key concepts for evaluating the internal validity of a study; the critical assessments are made separately and are divided into 14 set of questions.[15]
RESULTS
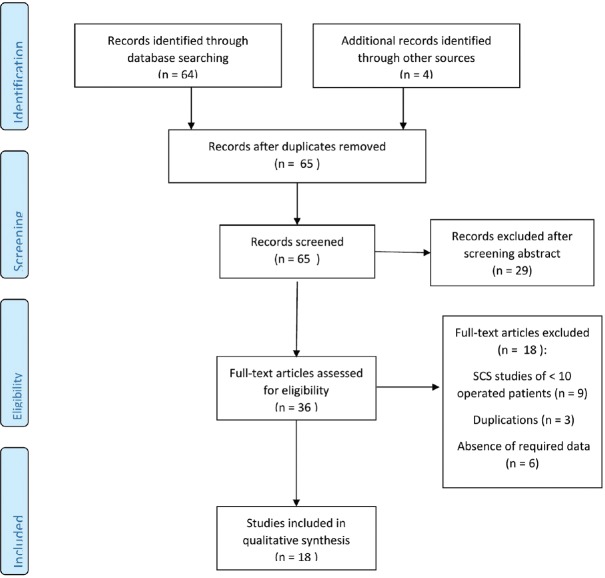
The literature search yielded 65 publications in total. After screening abstracts for relevance, 36 full-text articles were assessed for eligibility and after applying the inclusion/exclusion criteria, 18 studies were included in the systematic review. Quantitative meta-analysis was not performed owing to significant clinical heterogeneity among the studies. A flow chart of this process is presented in Figure 1.
Figure 1.
PRISMA flowchart of study selection process
A total of 15 cohort studies (5 prospective and 10 retrospective), 1 randomized controlled trial (RCT), and 2 cross-sectional studies were included in the systematic review. Studies were mostly from European centers; 9 of the studies originated from Italy,[2,16,17,18,19,20,21,22,23] 4 from Japan,[24,25,26,27] 2 from Greece,[28,29] and 1 each from Turkey,[30] Hungary,[31] and Korea.[32] The studies were published between 2002 and 2017. They involved in total 1772 patients with AI. Of these, 1028 (58%) had nonfunctioning AI, while 500 (28.2%) had SCS. The main characteristics and demographics of the studies and outcome definitions are presented in Tables 1 and 2, respectively.
Table 1.
Study characteristics and baseline demographics
| Study | Study size (n) | Study design | Country of origin | Age of participants (years) | Sex of participants | Types of AI and number | Duration of follow up | Cardiometabolic outcomes assessed |
|---|---|---|---|---|---|---|---|---|
| Giordano et al. 2010[18] | 118 | Cohort prospective | Italy | 62.4±0.9 | 77 F 47 M |
NFA 10 SCS 16 |
1-10 years (median 3 years) | Obese/overweight HTN dyslipidemia IFG/IGT/T2DM |
| Vassilatou et al. 2009[29] | 77 | Cohort prospective | Greece | 57.1±10.9 | 55 F 22 M |
NFA 57 SCS 20 |
12-154 months (median 60 months) | Obese/overweight hypertension T2DM |
| Di Dalmazi et al. 2012[17] | 348 | Cross sectional | Italy | 60.3±10.9 NFA 67.3±8.3 SCS |
127 F 76 M NFA 11 F 8 M SCS |
NFA 20 SCS 19 Intermediate phenotypes 126 |
No follow up | HTN DM CHD Stroke |
| Erbil et al. 2006[30] | 39 | Cohort prospective | Turkey | 57±24 SCS 43±12 CS |
10 F 1 M SCS 26 F 2 M CS |
SCS 1 CS 28 |
1 year post op (details NA) | Obesit HTN T2DM Dyslipidaemia |
| Toniato et al. 2009[23] | 45 | Randomised prospective | Italy | 63±4.1 SCS operated 64±1.8 SCS conservative |
11 F 12 M SCS operated 12 F 10 M SCS conservative |
SCS operated 23 SCS Conservative 22 |
Mean 7.7 years (range 2-17 years) | HTN DM Obesity Hypercholesterolemia |
| Chiodini et al. 2010[16] | 108 | Cohort retrospective | Italy | 54.8±11.6- SCS operated 64.4±10.1 SCS conservative 57.1±10.9 NFA operated 61.1±9.8 NFA conservative |
33 F 8 M SCS 47 F 20 F NFA |
SCS 41 NFA 67 | At least 18 months | HTN DM Obesity Dyslipidaemia |
| Morelli et al. 2014[20] | 206 | Cohort retrospective | Italy | 58.5±10.1 NFA 62.2±11 SCS |
119 F 48 M NFA 25 F 14 M SCS |
NFA 167 SCS 39 |
At least 5 years | HTN DM Obesity dyslipidemia |
| Iacobone et al. 2012[19] | 35 | Cohort prospective | Italy | Median 57 (range 36-78) SCS operated 58 (range 39-75) SCS conservative |
12 M 8 F SCS operated 8 M 7 F SCS conservative |
SCS operated 20 SCS conservative 15 |
Mean 54±34 months SCS operated 56±37 months SCS conservative |
HTN DM dyslipidemia Abnormal BMI (overweight and obese) |
| Maehana et al. 2012[25] | 73 | Cohort prospective | Japan | Mean 60 (range 32-81) NFA 56 (range 31-72) SCS |
35 M 25 F NFA 5 M 8 F SCS |
NFA 60 SCS 13 |
Median 51.2 months (range 6.0-171.9 months) NFA 11.7 months SCS | HTN DM dyslipidemia |
| Perysinakis et al. 2013[28] | 29 | Cohort retrospective | Greece | Mean 54 (range 31-68) | 8 M 21 F |
SCS operated 29 | Mean 77 months | HTN DM obesity |
| Raffaeli et al. 2017[22] | 29 | Cohort retrospective | Italy | 57.3±12.5 (range 30-76) | 7 M 22 F |
SCS operated 29 (23 followed up post op) | 51.0±24.0 months (range 11-90 months) | HTN DM obesity |
| Kawate et al. 2014[24] | 27 | Cohort retrospective | Japan | 55.3±9.4 SCS operated 66.3±8.8 SCS conservative | 2 M 13 F SCS operated 6 M 6 F SCS conservative |
SCS operated 15 SCS conservative 12 |
Median 5.3 years | HTN DM obesity dyslipiemia |
| Miyazato et al. 2011[26] | 114 | Cohort retrospective | Japan | Median 58 (range 54-77) SCS 46 (range 20-65) CS |
21 M 34 F SCS 8 M 51 F CS |
SCS 55 CS 59 |
Median 2.9 years range 1-16 years | HTN DM BMI Dyslipidemia |
| Petramala et al. 2017[21] | 70 | Cohort retrospective | Italy | 61.9±8.4 | 52 M 18 F |
SCS operated 26 SCS conservative 44 |
Mean 12 months (range 9-15 months) | HTN Obesity DM Metabolic syndrome |
| Sereg et al. 2009[31] | 125 | Cohort retrospective cohort | Hungary | 51.8±9.9 | 29 M 96 F |
NFA operated 47 NFA conservative 78 |
Mean 9.1 years (range 5-16 years) | HTN DM/IGT Obesity Dyslipidemia |
| Tsuiki et al. 2008[27] | 20 | Cohort retrospective | Japan | Median 59.7 (range 43-74) | 6 M 14 F |
SCS operated 10 SCS conservative 12 |
7-19 months (average 13±3.8 months) SCS operated 15-69 months (average 27±15.2 months) SCS conservative |
HTN Impaired glucose metabolism Dyslipidemia Obesity |
| Terzolo et al. 2002[2] | 41 | Cross sectional | Italy | Mean 54.0±10.7 (range 19-69) | NA | NFA 29 SCS 12 |
No follow up | HTN Impaired glucose metabolism Dyslipidaemia Raised BMI |
| Kim et al. 2014[32] | 268 | Cohort retrospective | Korea | Mean 55.8±15.8 (range 15-90) | 138M 130 F |
NFA 218 SCS 19 Other AIs 31 |
No follow up | HTN DM BMI Hypercholesterolemia |
SCS: Subclinical cushing’s syndrome, CS: Cushing’s syndrome, NFA: Nonfunctioning adrenal adenoma, DM: Diabetes mellitus, IFG: Impaired fasting glucose, IGT: Impaired glucose tolerance, HTN: Hypertension, BMI: Body mass index, CHD: Coronary heart disease, NA: Not available, AIs: Adrenal incidentalomas
Table 2.
Definitions of clinical outcomes
| Obesity definition | HTN definition (mmHg) | DM definition | Dyslipidemia definition | Others | |
|---|---|---|---|---|---|
| Giordano et al.[18] | Overweight/obesity: BMI >25 kg/m2 | BP >125/80 | IGT: 2 h post OGTT glucose 140-200 mg/dl Diabetes: fasting glucose 126 mg/dl |
Triglycerides >150 mg/dl Total cholesterol >240 mg/dl |
|
| Vassilatou et al.[29] | NA | NA | NA | NA | |
| Di Dalmazi et al.[17] | Obesity not in outcome | SBP ≥140 and/or DBP ≥90 measured on at least 2 separate occasions and/or antihypertensive treatment | T2DM: OGTT as per the ADA position statement[33] and/or treatment with antidiabetic drugs | Total cholesterol ≥200 mg/dl and/or Triglycerides ≥150 mg/dl |
|
| Erbil et al.[30] | Obesity: BMI >30 kg/m2 | BP >130/85 or antihypertensive treatment | IFG: fasting glucose >110 mg/dl DM: fasting glucose >126 mg/dl or treatment with antidiabetic drugs | Triglycerides >150 mg/dl HDL <40 mg/dl in men HDL <50 mg/dl in women | |
| Toniato et al.[23] | Overweight: BMI 25-30 kg/m2 Obese: BMI >30 kg/m2 | SBP >150 and DBP >90 | IFG: fasting glucose >110 mg/dl DM: fasting glucose >126 mg/dl or treatment with antidiabetic drugs |
Triglycerides >150 mg/dl HDL <40 mg/dl in men HDL <50 mg/dl in women |
|
| Morelli et al.[20] | NA | SBP ≥140 and/or DBP ≥90 and/or antihypertensive treatment | WHO criteria[34] | Triglycerides ≥150 mg/dl or HDL <40 mg/dl in men HDL <50 mg/dl in women or on anti-dyslipidemic treatment |
|
| Iacobone et al.[19] | Overweight: BMI 25-29.9 kg/m2
Obese: BMI ≥30 kg/m2 |
SBP ≥140 DBP ≥90 or antihypertensive treatment |
IGT: FPG >110 mg/dl DM: FPG >126 mg/dl or treatment with antidiabetic drugs |
Triglycerides ≥150 mg/dl HDL <40 mg/dl in men HDL <50 mg/dl in women Patients on anti-dyslipidemic treatment |
|
| Maehana et al.[25] | NA | NA | NA | NA | |
| Perysinakis et al.[28] | Obesity: BMI ≥30 kg/m2 | SBP ≥135 DBP ≥85 or antihypertensive treatment |
DM: WHO criteria of FPG >126 or treatment with antidiabetic drugs |
||
| Raffaelli et al.[22] | NA | NA | NA | NA | |
| Kawate et al.[24] | Obesity: BMI≥25 kg/m2 | SBP ≥140 DBP ≥90 or antihypertensive treatment |
DM: FPG ≥126 mg/dl and/or random glucose≥200 mg/dl and/or HbA1c ≥6.5% and/or treatment with antidiabetic drugs IGT: FBS ≥110 mg/dl and/or random glucose 140-199 mg/dl |
Total cholesterol ≥220 mg/dl and/or LDL ≥140 mg/dl and/or HDL <40 mg/dl and/or Triglycerides ≥150 mg/dl Treatment with lipid-lowering medication |
|
| Miyazato et al.[26] | Obesity: BMI ≥25 kg/m2 | SBP >150 DBP >90 |
IGT: FPG ≥110 mg/dl DM: FPG ≥126 mg/dl or treatment with antidiabetic drugs |
Total cholesterol 220 mg/dl, Triglycerides ≥172 mg/dl or statin use |
|
| Petramala et al.[21] | NA | NA | NA | NA | Metabolic syndrome defined by ATP III-NCEP criteria[35] |
| Sereg et al.[31] | NA | BP persistently >140/90 mmHg or antihypertensive treatment | Previous definitive diagnosis of DM and treatment with antidiabetic drugs DM: OGTT FPG >7.0 mmol/l, glucose response after 120 min ≥11.1 mmol/l IGT: OGTT glucose response after 120 min 7.8-11.0 mmol/l |
Total cholesterol >5.2 mmol/l LDL >2.6 mmol/l or Triglycerides >1.7 mmol/l or treatment with lipid-lowering medication | |
| Tsuiki et al.[27] | Obesity: BMI ≥25 kg/m2 | SBP >140 and/or DBP >90 | DM: FPG >126 mg/dl or 2 h plasma glucose >200 mg/dl IGT: FPG 110-125 and/or 2 h plasma glucose 140-199 mg/dl on 75 g OGTT |
Total cholesterol >220 mg/dl | |
| Terzolo et al.[2] | No criteria; absolute values of BMI | No criteria; absolute values of SBP and DBP | No criteria; absolute values of 2 hour glucose and insulin sensitivity index | No criteria; absolute values of Triglycerides, total and HDL cholesterol | |
| Kim et al.[32] | No criteria; absolute values of BMI | Prior diagnosis of HTN or antihypertensive treatment SBP ≥140 or DBP ≥90 |
Prior diagnosis of DM or treatment with oral hypoglycaemic agents FPG ≥126 mg/dl or HbA1c ≥6.5% |
No criteria; absolute values of total cholesterol |
OGTT: Oral glucose tolerance test, IGT: Impaired glucose tolerance, FPG: Fasting plasma glucose, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, ADA: American diabetes association, WHO: World health organisation NA: Not available
Diagnostic criteria or definition of SCS were heterogeneous in the studies, as presented in Table 3. They were mostly based on high morning cortisol concentrations after dexamethasone suppression test (DST) combined with at least one other test of HPA axis function.
Table 3.
Definitions of subclinical Cushing’s syndrome
| Study | Criteria for SCS diagnosis |
|---|---|
| Giordano et al.[18] | Absence of clinical signs or symptoms of cortisol excess, and Post 1 mg overnight DST cortisol ≥1.8 µg/dl (50 nmol/l) plus one other abnormal test of HPA axis: post LDDST cortisol ≥1.8 µg/dl, low ACTH, elevated midnight salivary cortisol, high UFC |
| Vassilatou et al. [29] | Absence of clinical signs of cortisol excess, and Post LDDST cortisol ≥50 nmol/l plus at least one other abnormal test of HPA axis: low ACTH, abnormal cortisol rhythm (plasma cortisol at 24·00: 8·00% ratio >50%), high UFC, low for age DHEAS |
| Di Dalmazi et al. [17] | Absence of clinical signs or symptoms specific to overt CS, and For SCS- post 1 mg overnight DST cortisol >138 nmol/l with no other additional tests For intermediate phenotypes- overnight DST cortisol 50-138 nmol/l plus one other abnormal test (low ACTH and high 24 h UFC) |
| Erbil et al. [30] | No symptoms directly attributed to the disease, and Both LDDST and HDDST failed to suppress cortisol <3 µg/dl |
| Toniato et al.[23] | No clinical signs of hormone excess, and 1 mg overnight DST >2.5 µg/dl and 1 other HPA axis functional alteration |
| Morelli et al.[20] | Absence of signs of overt hypercortisolism, and Post 1 mg overnight DST cortisol levels >5 µg/dL (138 nmol/L) or 2 of the 3 abnormalities of HPA axis: suppressed ACTH, increased UFC, and 1 mg-DST cortisol levels >3.0 µg/dL (83 nmol/L) |
| Iacobone et al.[19] | Absence of clinical features of CS, and Post 1 mg overnight DST cortisol >5 µg/dL, morning ACTH suppressed and increased 24 h UFC |
| Maehana et al.[25] | Absence of clinical features of CS, and Normal basal cortisol, post 1 mg ODST cortisol >3 µg/dl plus one of: suppressed ACTH, loss of cortisol circadian rhythm, low DHEAS, or unilateral visualisation on adrenocortical scintigraphy |
| Perysinakis et al.[28] | Absence of clinical signs of cortisol excess, and Post LDDST Cortisol ≥1.8 µg/dl plus at least one other abnormal test of HPA axis: suppressed ACTH, raised 24 hours UFC, midnight cortisol/morning cortisol percent ratio >50%. |
| Raffaelli et al.[22] | Absence of clinical features of overt CS, and Post 1 mg overnight DST cortisol >18 ng/mL plus at least 2 criteria of the following: suppressed basal ACTH levels, high UFC, altered circadian cortisol rhythm & unilateral uptake on adrenocortical scintigraphy |
| Kawate et al.[24] | Absence of typical CS features, and Post 1 mg overnight DST cortisol >1.8 µg/dl, low morning ACTH, increased uptake on adrenal scintigraphy, no diurnal changes in cortisol level and low DHEAS. |
| Miyazato et al.[26] | Diagnosed as SCS by the endocrinology team, criteria not mentioned |
| Petramala et al.[21] | Absent overt clinical signs of hypercortisolism, and 2 or more HPA axis function test abnormality: High UFC, morning cortisol >1.8 µg/dl after 1 mg overnight DST, morning ACTH levels suppressed |
| Tsuiki et al.[27] | Lack of characteristic features of CS, and Normal basal cortisol, cortisol >3 µg/dl after 1 mg overnight DST and cortisol >1 µg/dl after 8 mg overnight DST plus at least one of the additional criteria: suppressed basal ACTH levels, altered circadian cortisol rhythm, decreased DHEAS & unilateral uptake on adrenocortical scintigraphy |
| Terzolo et al.[2] | Absence of classical clinical features of CS, and Two of the following criteria: Elevated UFC, failure of cortisol to suppress to <138 nmol/l after 1 mg overnight DST, suppressed ACTH concentrations, and disturbed cortisol circadian rhythm |
| Kim et al.[32] | Lack of specific symptoms or signs of CS, and Elevated 24 hUFC and low morning ACTH (LDDST to increase diagnostic specificity of CS, but not a criteria for SCS in the study) |
CS: Cushing’s syndrome, ACTH: Adrenocorticotropic hormone, SCS: Subclinical Cushing’s syndrome, UFC: Urinary free cortisol, DST: Dexamethasone suppression test, DHEAS: Dehydroepiandrosterone sulfate, LDDST: Low dose dexamethasone suppression test
All the studies included in the systematic review had patients with subclinical cortisol secreting AIs and half of the studies had patients with nonfunctioning AIs. The clinical characteristics at baseline and outcome on follow-up in patients with SCS are presented in Table 4.
Table 4.
SCS and cardiometabolic outcomes
| Study | Patients with SCS (n) | Adrenalectomy (n) | Conservative management (n) | Metabolic parameters at baseline | Metabolic parameters at follow up | ||
|---|---|---|---|---|---|---|---|
| Adrenalectomy | Conservative | Adrenalectomy | Conservative | ||||
| Toniato et al.[23] | 45 | 23 | 22 | DM 8 (34.8%) HTN 18 (78.3%) Obesity 6 (26.1%) Hypercholesterolemia 8 (34.8%) |
DM 6 (27.3%) HTN 15 (68.2%) Obesity 6 (27.3%) Hypercholesterolemia 7 (31.8%) |
HTN improved 7 (38.9%), normalised 5 (27.8%) P=0.046 DM improved 3 (37.5%), normalised 2 (25%) P=0.619 Hypercholesterolemia normalised 3 (37.5%) P=0.619 BMI normalised 3 (50%) P value NA |
DM worsened 2 (9%) HTN worsened 5 (22.7%) Hypercholesterolemia worsened 3 (13.6%) Obesity NA |
| Chiodini et al.[16] | 41 | 16 | 25 | Obesity 12 (48%) HTN 14 (56%) DM 7 (28%) Dyslipidaemia 12 (48%) |
Excluded from systematic review | Obesity P=0.05 HTN P<0.001 DM P<0.001 Dyslipidaemia P=0.05 |
Excluded from systematic review |
| Iacobone et al.[19] | 35 | 15 | 20 | Abnormal BMI (Obese + overweight) 15 (75%) HTN 15 (75%) DM & IGT 10 (50%) Dyslipidaemia 10 (50%) |
Abnormal BMI (Obese + overweight) 12 (80%) HTN 12 (80%) DM & IGT 6 (40%) Dyslipidaemia 7 (46.7%) |
BMI P=0.19 HTN P=0.002 DM & IGT P=0.032 Dyslipidaemia P=0.47 |
BMI worsened 3/12 (25%) HTN worsened 3/12 (25%) DM & IGT worsened 2/6 (33%) Dyslipidaemia no change |
| Kawate et al.[24] | 27 | 15 | 12 | HTN 10 (67%) DM 5 (33%) Dyslipidaemia 8 (53%) Obesity 5 (33%) |
HTN 6 (50%) DM 5 (42%) Dyslipidaemia 10 (83%) Obesity 5 (42%) |
Improvement: HTN 6 (67%) DM or IGT 3 (60%) Dyslipidaemia 1 (13%) P values NA |
Improvement: HTN 0 (0%) DM or IGT 1 (20%) Dyslipidaemia 1 (10%) Worsening: HTN 4 (67%) DM or IGT 2 (40%) Dyslipidaemia 2 (20%) |
| Petramala et al.[21] | 70 | 26 | 44 | HTN 85% DM 38% Obesity 53.8% Metabolic syndrome 54% Low HDL 27% High Triglycerides 34% |
HTN 63.1% DM 25% Obesity 33% Metabolic syndrome 39% Low HDL 11% High Triglyceride 34% |
HTN 58.82% P<0.05 DM 23.5% P=NS Obesity 24.5% P<0.05 Metabolic syndrome 23% P<0.05 Low HDL 11% P=NS High Triglycerides 27% P=NS |
HTN 72.5% DM 38.5% Obesity 42.7% Metabolic syndrome 45% Low HDL 13% High Triglycerides 38% |
| Tsuiki et al.[27] | 20 | 10 | 12 | HTN 6 (60%) Impaired glucose metabolism 9 (90%) Dyslipidaemia 9 (90%) Obesity 3 (30%) |
HTN 4 (33%) Impaired glucose metabolism 6 (50%) Dyslipidaemia 6 (50%) Obesity 3 (25%) |
Improvement: HTN 5 Impaired glucose metabolism 2 Dyslipidaemia 6 Obesity 0 Prognosis of cardiovascular risks significantly better in the operated than the non-operated group P<0.001 |
Improvement: none HTN: 1 worsened; 1 developed HTN Impaired glucose metabolism: 1 worsened, 2 developed impaired glucose metabolism Dyslipidaemia: none worsened, 2 developed dyslipidaemia Obesity: none worsened, 2 developed obesity |
| Maehana et al.[25] | 13 | 12 | 1 (refused) | HTN 7 (58.3%) DM 2 (16.6%) Dyslipidaemia 3 (25%) |
- | Improvement: HTN 5/7 (71.4%) DM 2/2 (100%) Dyslipidaemia 2/3 (66.7%) |
- |
| Perysinakis et al.[28] | 29 | 29 | 0 | BMI >27 14 (48.3%) HTN 17 (58.6%) DM 12 (41.4%) |
- | Improvement: BMI 6/14 (42.9%) HTN 12/17 (70.6%) DM|/IGT 5/12 (41.7%) |
- |
| Raffaelli et al.[22] | 27 | 27 | 0 | BMI 28.0±3.2 (24-37) SBP 138.8±13.1 (120-160) DBP 87.7±8.0 (75-100) Fasting glucose 132.7±17.8 (105-168) |
- | Improvement: BMI P=0.75 SBP P=0.07 DBP P=0.41 DM P<0.05 |
- |
| Miyazato et al.[26] | 55 | 55 | 0 | HTN 33 (60%) DM 17 (30.9%) Dyslipidaemia 5 (9.1%) BMI >25 20 (36.3%) |
- | Improvement: HTN 6/33 (18.2%) P<0.001 DM 3/17 (17.7%) P=0.001 Dyslipidaemia 0 P=0.292 |
- |
| Erbil et al.[30] | 11 | 11 | 0 | Obesity - 5 (55%) HTN 7 (63%) DM 4 (36%) Hypercholesterolemia 4 (36%) Hypertriglyceridemia 3 (27%) |
- | Improvement: Obesity 3 (60%) P=0.127 HTN 5 (71%) P=0.03 DM 2 (33%) P=0.338 Hypercholesterolemia 3 (75%) P=0.127 Hypertriglyceridemia 1 (33%) P=0.611 |
- |
| Vassilatou et al.[29] | 20 | 0 | 20 | - | Mean BMI 30.1±4.6 HTN 15 (75%) DM 4 (20%) |
BMI NS HTN 16 (80%) DM 5 (25%) |
|
| Morelli et al.[20] | 39 | 0 | 39 | - | Obesity 13 (33.3%) HTN 26 (66.7%) DM 13 (33.3%) Dyslipidaemia 21 (53.8%) |
- | Obesity 18 (46.2%) HTN 34 (87.2%) P<0.05 DM 17 (43.6%) Dyslipidaemia 27 (69.2%) |
Out of 18 studies included in the review, 9 had patients with nonfunctioning AIs, 3 of the studies had no follow-up data, so they were only used for comparison between SCS and nonfunctioning AIs at baseline.[2,17,32] The clinical characteristics at baseline and outcome on follow-up in patients with nonfunctioning AIs are presented in Table 5.
Table 5.
Nonfunctioning AIs and cardiometabolic outcomes
| Study | Patients with non-functioning AI (n) | Adrenalectomy (n) | Conservative management (n) | Metabolic parameters at baseline | Metabolic parameters at follow up | ||
|---|---|---|---|---|---|---|---|
| Adrenalectomy | Conservative | Adrenalectomy | Conservative | ||||
| Giordano et al.[18] | 102 | 0 | 102 | Obese/overweight 57 (55%) HTN 60 (58%) Dyslipidaemia 24 (23%) IFG/IGT/DM 15 (15%) |
Obese/overweight 57 (55%) HTN 60 (58%) Dyslipidaemia 27 (26%) IFG/IGT/DM 22 (21%) |
||
| Vassilatou et al.[29] | 57 | 0 | 57 | Mean BMI 30.1±4.6 HTN 32 (56%) DM 13 (22.8%) |
BMI no significant change HTN 36 (63%) DM 15 (26.3%) |
||
| Chiodini et al.[16] | 67 | 30 | 37 | Obesity 8 (26.7%) HTN 16 (53.3%) DM 3 (14.3%) Dyslipidaemia 9 (30%) |
Improvement: Obesity 3 (10%) HTN 9 (30%) DM 3 (10%) Dyslipidaemia 8 (26.7%) Worsening: Obesity 6 (20%) HTN 4 (13.3%) DM 1 (3.3%) Dyslipidaemia 3 (10%) |
||
| Morelli et al.[20] | 167 | 0 | 167 | Obesity 46 (27.5%) HTN 90 (53.9%) DM 28 (16.8%) Dyslipidaemia 70 (41.9%) |
Obesity 54 (32.3%) HTN 105 (62.9%) DM 37 (22.2%) Dyslipidaemia 90 (53.9%) P<0.05 for Dyslipidaemia |
||
| Maehana et al.[25] | 60 | 21 | 39 | HTN 5 (23.8%) DM 2 (9.5%) Dyslipidaemia 1 (4.7%) |
Improvement: HTN 1/5 (20%) DM 0/2 (0%) Dyslipidaemia 0/1 (0%) P values NA |
||
| Sereg et al.[31] | 113 | 43 | 70 | Obesity 27 (63%) HTN 37 (86%) DM 4 (9%) IGT 15 (35%) Dyslipidaemia 24 (56%) |
Obesity 29 (41%) HTN 56 (80%) DM 19 (27%) IGT 11 (16%) Dyslipidaemia 42 (60%) |
Obesity 26 (60%) HTN 38 (88%) DM 18 (42%) IGT 7 (16%) Dyslipidaemia 39 (91%) Worsening: Dyslipidaemia P<0.0001 DM P<0.001 IGT P<0.05 Rest P=NS |
Obesity 30 (43%) HTN 63 (90%) DM 36 (51%) IGT 4 (6%) Dyslipidaemia 54 (77%) Worsening: Dyslipidaemia P<0.05 DM P<0.05 Rest P=NS |
Six studies compared the prevalence of cardiometabolic risk factors in patients with nonfunctioning AIs and subclinical cortisol secreting AIs.[2,16,17,18,29,32] The comparison is presented in Table 6.
Table 6.
Comparison of prevalence of cardiometabolic risk factors between SCS and NFAI
| Number of patients (n) | Obesity/overweight | HTN | DM | Dyslipidaemia | Others | |
|---|---|---|---|---|---|---|
| Giordano et al.[18] | NFAI 102 SCS 16 |
P=NS | P=NS | P=NS | P=0.033 | |
| Vassilatou et al.[29] | NFAI 57 SCS 20 |
P=0.98 (NS) | P=0.10 (NS) | P=0.9 (NS) | NA | |
| Di Dalmazi et al.[17] | NFAI 203 SCS 19 |
Not checked | NS | P<0.01 | Not checked | CHD: P<0.01 Stroke: P=NS |
| Kim et al.[32] | NFAI 218 SCS 19 |
P=0.031 | P=0.047 | P=0.034 | Total cholesterol: P=0.048 LDL, HDL, Triglycerides: P=NS |
|
| Terzolo et al.[2] | NFAI 29 SCS 12 |
P=NS | P=NS | 2 h glucose: P=0.04 Insulin sensitivity index: P=0.0001 |
Triglycerides: P=0.006 Total cholesterol, HDL: P=NS |
|
| Chiodini et al.[16] | ||||||
| Operated SCS vs operated NFAI | NFAI 30 SCS 25 |
P<0.01 | P<0.01 | P<0.01 | ||
| Non operated SCS vs non operated NFAI | NFAI 37 SCS 16 |
P=NS | P=NS | P=0.05 [worsened glycaemic control for SCS] | P=NS |
SCS: Subclinical Cushing’s syndrome, CS: Cushing’s syndrome, NFAI: Nonfunctioning adrenal incidentaloma, HTN: Hypertension, CHD: Coronary heart disease, DM: Diabetes mellitus, OGTT: Oral glucose tolerance test, IGT: Impaired glucose tolerance, FPG: Fasting plasma glucose, SBP: Systolic blood pressure, DBP: Diastolic blood press, BMI: Body mass index, ADA: American Diabetes Association, WHO: World Health Organisation, NS: Not significant, NA: Not available
The systematic review demonstrated higher prevalence of HTN, impaired glucose metabolism, dyslipidemia, and raised BMI in patients with subclinical cortisol secreting AIs as compared to patients with nonfunctioning AI. In patients with SCS surgical intervention had a beneficial effect on blood pressure, glucometabolic control, and obesity when compared with conservative management. The results for lipid metabolism were equivocal. There was no significant improvement in cardiometabolic risk factors after adrenalectomy in patients with nonfunctioning AIs. Summary of the results is presented in Table 7.
Table 7.
Summary of results
| Subclinical cortisol secreting AI | Non-functioning AI | |
|---|---|---|
| Baseline prevalence (range) | ||
| DM/IGT | 16.6-90% | 9-27% |
| HTN | 33-85% | 24-86% |
| Dyslipidaemia | 9-90% | 5-60% |
| Obesity/overweight | 25-80% | 27-63% |
| Number of patients who had surgery | Range 10-55 Mean 22 | Range 21-43 |
| Significant improvement in surgically treated group (P<0.05) | ||
| DM/IGT | More than half of the studies | None; significant worsening in one study |
| HTN | Most studies | None |
| Dyslipidemia | Few studies | None; significant worsening in one study |
| Obesity/overweight | more than one third of the studies | None |
| Significant improvement in conservatively managed group (P<0.05) | ||
| DM/IGT | None | None; significant worsening in one-quarter of the studies |
| HTN | None; significant worsening in one study | None |
| Dyslipidemia | None | None; significant worsening in half of the studies |
| Obesity/overweight | None | None |
Quality assessment and risk of bias
Most of the studies included in the systematic review were observational cohort studies, two were cross-sectional studies, and there was a single Randomized controlled trial (RCT). The methodological quality of the studies was therefore assessed using the NIH quality assessment tool for observational cohort and cross-sectional studies.[15]
In view of inclusion of only a single RCT which was available on the review subject, it was suspected that the quality of evidence would be very low or poor. However, the assessment using the tool specifically designed for observational cohort and cross-sectional studies revealed that the quality of evidence was low to moderate or “fair” as per the NIH tool.
The risk of bias was mainly due to lack of confirmed presence of exposure prior to the outcomes; lack of sample size justification; heterogeneity between studies for diagnostic protocols, definitions of outcome and duration of follow-up; lack of blinded outcome assessments; and absence of adjustment for confounders. The results of quality assessment of individual studies as per NIH quality assessment tool are presented in Table 8.
Table 8.
Results of NIH quality assessment for observational cohort and cross-sectional studies
| Criteria | Yes | No | Other (CD, NR, NA)* |
|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Iacoboe et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. | Kawate et al. | |
| 2. Was the study population clearly specified and defined? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuki et al., Vassilatou et al. | ||
| 3. Was the participation rate of eligible persons at least 50%? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. | NR: Iacobone et al. | |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaell et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. | ||
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Tsuiki et al., Vassilatou et al. | CD: Toniato et al. | |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Chiodini et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. | CD: Di Dalmazi et al. | |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Di Dalmazi et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Raffaell et al., Sereg et al., Terzolo et al., Toniato et al., Vassilatou et al. | Chiodini et al., Erbil et al., Petramala et al., Tsuiki et al. | |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Morelli et al.(for CVE), Di Dalmazi et al. | Chiodini et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. | |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Morelli et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. | NR: Miyazato et al. | |
| 10. Was the exposure(s) assessed more than once over time? | Chiodini et al., Giordano et al., Iacobone et al., Maehana et al., Morelli et al., Sereg et al., Toniato et al., Vassilatou et al. | Erbil et al., Tsuiki et al. | NR: Kawate et al., Miyazato et al., Perysinakis et al., Petramala et al., Raffaelli et al. NA: Di Dalmazi et al., Kim et al., Terzolo et al. |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Chiodini et al., Di Dalmazi et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Miyazato et al., Morelli et al., Perysinakis et al., Sereg et al., Toniato et al., Tsuiki et al. | Maehana et al., Petramala et al., Raffaelli et al., Terzolo et al., Vassilatou et al. | |
| 12. Were the outcome assessors blinded to the exposure status of participants? | NR: Chiodini et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Maehana et al., Miyazato et al., Perysinakis et al., Petramala et al., Morelli et al., Raffaelli et al., Sereg et al., Toniato et al., Tsuiki et al., Vassilatou et al. NA: Di Dalmazi et al., Kim et al., Terzolo et al. | ||
| 13. Was loss to follow-up after baseline 20% or less? | Chiodini et al., Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Maehana et al., Miyazato et al., Morelli et al., Perysinakis et al., Petramala et al., Sereg et al., Toniato et al., Tsuiki et al., Vassilatou et al. | Raffaelli et al. | NA: Di Dalmazi et al., Kim et al., Terzolo et al. |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Chiodini et al., Di Dalmazi et al., Morelli et al. | Erbil et al., Giordano et al., Iacobone et al., Kawate et al., Kim et al., Maehana et al., Miyazato et al., Perysinakis et al., Petramala et al., Raffaelli et al., Sereg et al., Terzolo et al., Toniato et al., Tsuiki et al., Vassilatou et al. |
CD: Cannot determine, NA: Not applicable, NR: Not reported
DISCUSSION
Systematic review of studies with heterogeneous and limited published data suggest increased prevalence of components of metabolic syndrome in subclinical cortisol secreting and nonfunctioning AIs; a beneficial role of adrenalectomy on HTN, glucometabolic control, and BMI was observed in patients with subclinical cortisol secreting AIs.
For SCS, baseline prevalence of abnormal glucose metabolism ranged from 16.6% to 90%, HTN from 33 to 85%, high BMI from 25 to 80%, and dyslipidemia from 9 to 90% in the included studies. There were lesser number of studies on nonfunctioning AIs in the present review and the baseline prevalence of abnormal glucose metabolism ranged from 9% to 27%, HTN from 24% to 86%, high BMI from 27% to 63%, and dyslipidaemia from 5% to 60%. One study also looked into the prevalence of cardiovascular events and showed increased prevalence in patients with SCS[20] and another study reported increased prevalence of coronary heart disease and stroke in SCS.[17]
Studies assessing surgical outcome in SCS patients showed significant improvement in blood pressure from baseline in most of the studies, improvement in glucose metabolism in more than half, and improved BMI in more than a third of the studies. In the study by Petramala et al.,[21] where outcome on metabolic syndrome (as per ATP III criteria)[35] was studied, there was significant improvement in metabolic syndrome following adrenalectomy in SCS patients. For the conservatively managed SCS group, only a single study showed a significant worsening of HTN.
There was no significant improvement in any of the cardiometabolic parameters in patients with nonfunctioning AIs who underwent adrenalectomy. One study[31] showed significant worsening in diabetes and dyslipidemia. For the conservatively managed patients with nonfunctioning AIs, follow-up data showed significant worsening of diabetes in a quarter of the included studies and worsening of dyslipidemia in half of them. There was no significant worsening of the other metabolic parameters in any of the studies.
The summary of results is presented in Table 7.
When the prevalence of cardiometabolic risk factors was compared between patients with nonfunctioning and subclinical cortisol secreting AIs, parameters of glucose metabolism and dyslipidemia were significantly worse for patients with SCS in most of the studies. However, there was no significant difference between the two groups for HTN and BMI. When the operated groups of nonfunctioning AI and SCS were compared in the study by Chiodini et al., there was significant improvement in diabetes, HTN, and obesity in surgically treated SCS patients.[16] Comparison of prevalence of cardiometabolic risk factors between SCS and nonfunctioning AI is presented in Table 6.
The reason for a higher prevalence of cardiometabolic risk factors in patients with subclinical cortisol secreting AIs may be subtle or intermittent autonomous cortisol hypersecretion and the outcomes might have been related not only to the degree but also on the duration of hypersecretion, as well as the sensitivity of each individual to cortisol excess. As most patients with AIs are of an age when HTN, diabetes, and obesity are highly prevalent, establishing whether these metabolic complications are truly related to excess cortisol in a patient with an AI is not clear-cut.
Results from studies on outcomes of adrenalectomy have been conflicting but in general, the data indicated an improvement in metabolic complications, especially in blood pressure and diabetes, in patients with subclinical cortisol secreting AI after unilateral adrenalectomy, and deterioration or no improvement in patients treated conservatively. Five studies in the review directly compared the difference between surgical and medical outcomes and assessed changes in cardiovascular risk factors in the long term[19,21,23,24,27] including one prospective randomized study.[23] Petramala et al. also investigated metabolic syndrome (as per ATP III criteria)[35] and the ambulatory BP (nondipper profile) and found a significant improvement in metabolic syndrome and nondipper profile in SCS group managed by adrenalectomy.[21]
There is no consensus on management of SCS associated with AIs and one major issue is that there is no formal agreement as to its definition. The overnight low-dose DST is favored as the most sensitive screening test in patients with AI based on pathophysiological reasoning, simplicity, and the fact that the test was incorporated in the diagnostic algorithms of most studies.[36] As patients lack the classical clinical features of Cushing's syndrome, it is difficult to determine the true value of the test. Diagnosing SCS by arbitrary cut-offs of cortisol secretion leads to unavoidable misclassifications in some patients. Therefore, additional tests in combination with the dexamethasone test are usually required to validate the biochemical diagnosis of hypercortisolism, although each has some limitations.
Increased prevalence of cardiometabolic risk factors was also observed in nonfunctioning AIs, although less than subclinical cortisol secreting AIs. It is speculated that the nonfunctioning AIs may not be entirely nonsecretory and may constitute an almost continuous spectrum of heterogeneous endocrine abnormalities. They may not produce adrenocortical hormones in sufficient amounts to cause clinically apparent disease or be detected biochemically, but they may have subtle qualitative alterations of steroidogenesis, which might adversely affect various metabolic pathways.[37,38]
The studies included in the current review did not reveal any significant impact of surgery on cardiometabolic morbidities in nonfunctioning AIs and there was significant worsening in diabetes and dyslipidemia in one study.[31] Some studies on nonfunctioning AIs suggest improvement in parameters of metabolic syndrome after surgical treatment;[11,12,39] however, they were not included in the review due to small number of operated patients. One of the likely explanations for this observation could be a potential misclassification of patients; for example, one study described that a significant proportion of patients with nonfunctioning AIs developed adrenal insufficiency after surgery, clearly suggesting that autonomous glucocorticoid production was not recognized preoperatively.[39] Overall, glucocorticoid production has been linked to HTN, abnormal glucose tolerance, and increased BMI.[40] Therefore, it is also possible that a temporary reduction of the glucocorticoid load caused by unilateral adrenalectomy could have contributed to a short-term improvement in blood pressure described in patients with nonfunctioning AIs.
Strengths and limitations
This systematic review has important strengths including an in-depth and comprehensive literature search, focused review questions, predefined inclusion and exclusion criteria, and use of standard quality assessment tool.
Limitations were heterogeneity in definitions of SCS in the studies with variable definitions of endpoints and outcomes and wide variations in length of follow-up. The length of time these metabolic risk factors were present was unknown. Groups also varied in the medical treatment for cardiovascular risk factors and it was unclear in the studies how aggressive the conservative management was in nonoperated patients. Subgroup analysis of age, gender, and size of the AI which might influence cardiovascular outcomes, was not possible, as individual variables were not consistently reported.
Future research directions
There is a clear need for prospective and appropriately powered studies to evaluate disease-specific and all-cause mortality and other hard clinical endpoints (i.e., myocardial infarction or stroke) to assess the potential cardiovascular morbidity associated with subclinical cortisol secreting AIs and nonfunctioning AIs and to substantiate whether surgical excision is beneficial in these patients. Such studies on subclinical cortisol secreting AI should also provide evidence for a suitable biochemical definition of autonomous cortisol secretion and help define the optimal investigation algorithm that balances the false-positive and negative endocrine test rates. Establishment of an international collaborative study group to develop a database of patients with clinically silent adrenal adenomas might be a useful step in this regard.
CONCLUSIONS
Available low-to-moderate-quality evidence obtained from heterogeneous studies in this systematic review with at least 12 months of follow-up suggests increased prevalence of components of metabolic syndrome in patients with subclinical cortisol secreting and nonfunctioning AIs. Screening for independent cardiometabolic risk factors in patients with subclinical cortisol secreting AI is recommended and careful evaluation and long-term follow-up are required. Adrenalectomy might be considered for patients with mild hypercortisolism when medical treatment fails and there is progression of cardiovascular risks. Patients with subclinical cortisol secreting AI who are not candidates for surgery should be followed up clinically to detect, treat, and control cardiovascular risk factors. Until more data are available, a flexible approach guided by clinical judgment is recommended. Detailed endocrine workup of nonfunctioning AIs should include the evaluation of components of metabolic syndrome to identify patients at high cardiometabolic risk and appropriate lifestyle changes and medical treatment should be advised. Surgical intervention in the absence of hormonal excess is not recommended at present and is an area requiring further research.
Given the increasing prevalence of subclinical cortisol secreting and nonfunctioning AIs, their associated cardiometabolic morbidities and the controversies surrounding their management, there is a clear need for further studies and randomized controlled trials to guide future recommendations for therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Peppa M, Boutati E, Koliaki C, Papaefstathiou N, Garoflos E, Economopoulos T, et al. Insulin resistance and metabolic syndrome in patients with nonfunctioning adrenal incidentalomas: A cause-effect relationship? Metabolism. 2010;59:1435–41. doi: 10.1016/j.metabol.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Terzolo M, Pia A, Ali A, Osella G, Reimondo G, Bovio S, et al. Adrenal incidentaloma: A new cause of the metabolic syndrome? J Clin Endocrinol Metab. 2002;87:998–1003. doi: 10.1210/jcem.87.3.8277. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Tang ZY, Wang WQ, Ning G. Metabolic syndrome inpatients with adrenocortical adenoma. Zhonghua Yi Xue Za Zhi. 2006;86:3397–400. [PubMed] [Google Scholar]
- 4.Peppa M, Koliaki C, Raptis SA. Adrenal incidentalomas and cardiometabolic morbidity: An emerging association with serious clinical implications. J Intern Med. 2010;268:555–66. doi: 10.1111/j.1365-2796.2010.02291.x. [DOI] [PubMed] [Google Scholar]
- 5.Reincke M, Fassnacht M, Vath S, Mora P, Allolio B. Adrenal incidentalomas: A manifestation of the metabolic syndrome? Endocr Res. 1996;22:757–61. doi: 10.1080/07435809609043773. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Real JM, Engel WR, Simo R, Salinas I, Webb SM. Study of glucose tolerance in consecutive patients harbouring incidental adrenal tumours. Study Group of Incidental Adrenal Adenoma. Clin Endocrinol. 1998;49:53–61. doi: 10.1046/j.1365-2265.1998.00437.x. [DOI] [PubMed] [Google Scholar]
- 8.Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69–82. doi: 10.1016/j.beem.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Abdelmannan D, Aron DC. Adrenal incidentalomas and subclinical Cushing's syndrome. Rev Endocr Metab Disord. 2010;11:135–40. doi: 10.1007/s11154-010-9141-5. [DOI] [PubMed] [Google Scholar]
- 10.Androulakis II, Kaltsas G, Piaditis G, Grossman AB. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41:552–60. doi: 10.1111/j.1365-2362.2010.02436.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernini G, Moretti A, Iacconi P, Miccoli P, Nami R, Lucani B, et al. Anthropometric, haemodynamic, humoral and hormonal evaluation in patients with incidental adrenocortical adenomas before and after surgery. Eur J Endocrinol. 2003;148:213–9. doi: 10.1530/eje.0.1480213. [DOI] [PubMed] [Google Scholar]
- 12.Midorikawa S, Sanada H, Hashimoto S, Suzuki T, Watanabe T. The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin Endocrinol. 2001;54:797–804. doi: 10.1046/j.1365-2265.2001.01274.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagnerova H, Dudasova D, Lazurova I. Hormonal and metabolic evaluation of adrenal incidentalomas. Neoplasma. 2009;56:521–5. doi: 10.4149/neo_2009_06_521. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011. [Last accessed on 2018 Sep 21]. Available from: http://handbook.cochrane.org/v5.0.0/chapter_21/21_4_assessment_of_study_quality_and_risk_of_bias.htm .
- 16.Chiodini I, Morelli V, Salcuni A, Eller-Vainicher C, Torlontano M, Coletti F, et al. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J Clin Endocrinol Metab. 2010;95:2736–45. doi: 10.1210/jc.2009-2387. [DOI] [PubMed] [Google Scholar]
- 17.Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: A large cross-sectional study. Eur J Endocrinol. 2012;166:669–77. doi: 10.1530/EJE-11-1039. [DOI] [PubMed] [Google Scholar]
- 18.Giordano R, Marinazzo E, Berardelli R, Picu A, Maccario M, Ghigo E, et al. Long-term morphological, hormonal, and clinical follow-up in a single unit on 118 patients with adrenal incidentalomas. Eur J Endocrinol. 2010;162:779–85. doi: 10.1530/EJE-09-0957. [DOI] [PubMed] [Google Scholar]
- 19.Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Mondi I, et al. Adrenalectomy may improve cardiovascular and metabolic impairment and ameliorate quality of life in patients with adrenal incidentalomas and subclinical Cushing's syndrome. Surgery. 2012;152:991–7. doi: 10.1016/j.surg.2012.08.054. [DOI] [PubMed] [Google Scholar]
- 20.Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, et al. Long-term follow-up in adrenal incidentalomas: An Italian multicenter study. J Clin Endocrinol Metab. 2014;99:827–34. doi: 10.1210/jc.2013-3527. [DOI] [PubMed] [Google Scholar]
- 21.Petramala L, Cavallaro G, Galassi M, Marinelli C, Tonnarini G, Concistrè A, et al. Clinical benefits of unilateral adrenalectomy in patients with subclinical hypercortisolism due to adrenal incidentaloma: Results from a single center. High Blood Press Cardiovasc Prev. 2017;24:69–75. doi: 10.1007/s40292-017-0182-7. [DOI] [PubMed] [Google Scholar]
- 22.Raffaelli M, De Crea C, D'Amato G, Gallucci P, Lombardi CP, Bellantone R. Outcome of adrenalectomy for subclinical hypercortisolism and Cushing syndrome. Surgery. 2017;161:264–71. doi: 10.1016/j.surg.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 23.Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: A prospective randomized study. Ann Surg. 2009;249:388–91. doi: 10.1097/SLA.0b013e31819a47d2. [DOI] [PubMed] [Google Scholar]
- 24.Kawate H, Kohno M, Matsuda Y, Akehi Y, Tanabe M, Horiuchi T, et al. Long-term study of subclinical Cushing's syndrome shows high prevalence of extra-adrenal malignancy in patients with functioning bilateral adrenal tumors. Endocr J. 2014;61:1205–12. doi: 10.1507/endocrj.EJ14-0155. [DOI] [PubMed] [Google Scholar]
- 25.Maehana T, Tanaka T, Itoh N, Masumori N, Tsukamoto T. Clinical outcomes of surgical treatment and longitudinal non-surgical observation of patients with subclinical Cushing's syndrome and nonfunctioning adrenocortical adenoma. Indian J Urol. 2012;28:179–83. doi: 10.4103/0970-1591.98461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazato M, Ishidoya S, Satoh F, Morimoto R, Kaiho Y, Yamada S, et al. Surgical outcomes of laparoscopic adrenalectomy for patients with Cushing's and subclinical Cushing's syndrome: A single center experience. Int Urol Nephrol. 2011;43:975–81. doi: 10.1007/s11255-011-9950-9. [DOI] [PubMed] [Google Scholar]
- 27.Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K. Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing's syndrome. Endocr J. 2008;55:737–45. doi: 10.1507/endocrj.k07e-177. [DOI] [PubMed] [Google Scholar]
- 28.Perysinakis I, Marakaki C, Avlonitis S, Katseli A, Vassilatou E, Papanastasiou L, et al. Laparoscopic adrenalectomy in patients with subclinical Cushing syndrome. Surg Endosc. 2013;27:2145–8. doi: 10.1007/s00464-012-2730-5. [DOI] [PubMed] [Google Scholar]
- 29.Vassilatou E, Vryonidou A, Michalopoulou S, Manolis J, Caratzas J, Phenekos C, et al. Hormonal activity of adrenal incidentalomas: Results from a long-term follow-up study. Clin Endocrinol. 2009;70:674–9. doi: 10.1111/j.1365-2265.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- 30.Erbil Y, Ademoglu E, Ozbey N, Barbaros U, Yanik BT, Salmaslioǧlu A, et al. Evaluation of the cardiovascular risk in patients with subclinical Cushing syndrome before and after surgery. World J Surg. 2006;30:1665–71. doi: 10.1007/s00268-005-0681-x. [DOI] [PubMed] [Google Scholar]
- 31.Sereg M, Szappanos A, Toke J, Karlinger K, Feldman K, Kaszper E, et al. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: A long-term follow-up study. Eur J Endocrinol. 2009;160:647–55. doi: 10.1530/EJE-08-0707. [DOI] [PubMed] [Google Scholar]
- 32.Kim B, Chun A, Kim K, Jung C, Kang SK, Mok J, et al. Clinical characteristics and metabolic features of patients with adrenal incidentalomas with or without subclinical Cushing's syndrome. Endocrinol Metab. 2014;29:457–63. doi: 10.3803/EnM.2014.29.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 35.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection; evaluation; and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 36.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the study of adrenal tumors. Eur J Endocrinol. 2016;175:G1–34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 37.Maser-Gluth C, Reincke M, Allolio B, Schulze E. Metabolism of glucocorticoids and mineralocorticoids in patients with adrenal incidentalomas. Eur J Clin Invest. 2000;30:83–6. doi: 10.1046/j.1365-2362.2000.0300s3083.x. [DOI] [PubMed] [Google Scholar]
- 38.Sippel RS, Chen H. Subclinical Cushing's syndrome in adrenal incidentalomas. Surg Clin North Am. 2004;84:875–85. doi: 10.1016/j.suc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Dall'Asta C, Barbetta L, Libé R, Passini E, Ambrosi B. Coexistence of 21-hydroxylase and 11 beta-hydroxylase deficiency in adrenal incidentalomas and in subclinical Cushing's syndrome. Horm Res. 2002;57:192–6. doi: 10.1159/000058381. [DOI] [PubMed] [Google Scholar]
- 40.Crowley RK, Hughes B, Gray J, McCarthy T, Hughes S, Shackleton CH, et al. Longitudinal changes in glucocorticoid metabolism are associated with later development of adverse metabolic phenotype. Eur J Endocrinol. 2014;171:433–42. doi: 10.1530/EJE-14-0256. [DOI] [PubMed] [Google Scholar]