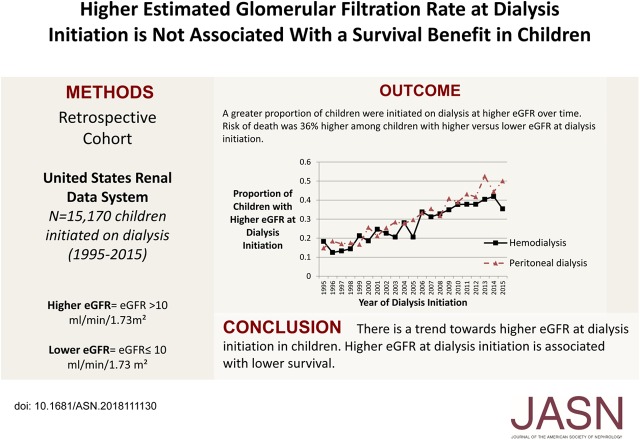
Significance Statement
Although observational studies suggest that dialysis initiation at higher levels of eGFR is not associated with survival benefit in adults with ESRD, the issue is not well studied in children. In a retrospective cohort study of the timing of dialysis initiation and survival in children who (according to the US Renal Data System) began dialysis in 1995–2015, the authors found a trend toward increased initiation of dialysis at higher eGFR over that period. Higher eGFR at dialysis initiation was associated with an increased risk of death, particularly for children who initiated treatment with hemodialysis rather than peritoneal dialysis. These findings may have important implications for the care of children with ESRD; a more concerted effort to delay dialysis initiation in asymptomatic children may reduce exposure to dialysis.
Keywords: pediatric nephrology, ESRD, dialysis
Visual Abstract
Abstract
Background
Study findings suggest that initiating dialysis at a higher eGFR level in adults with ESRD does not improve survival. It is less clear whether starting dialysis at a higher eGFR is associated with a survival benefit in children with CKD.
Methods
To investigate this issue, we performed a retrospective cohort study of pediatric patients aged 1–18 years who, according to the US Renal Data System, started dialysis between 1995 and 2015. The primary predictor was eGFR at the time of dialysis initiation, categorized as higher (eGFR>10 ml/min per 1.73 m2) versus lower eGFR (eGFR≤10 ml/min per 1.73 m2).
Results
Of 15,170 children, 4327 (29%) had a higher eGFR (median eGFR, 12.8 ml/min per 1.73 m2) at dialysis initiation. Compared with children with a lower eGFR (median eGFR, 6.5 ml/min per 1.73 m2), those with a higher eGFR at dialysis initiation were more often white, girls, underweight or obese, and more likely to have GN as the cause of ESRD. The risk of death was 1.36 times higher (95% confidence interval, 1.24 to 1.50) among children with a higher (versus lower) eGFR at dialysis initiation. The association between timing of dialysis and survival differed by treatment modality—hemodialysis versus peritoneal dialysis (P<0.001 for interaction)—and was stronger among children initially treated with hemodialysis (hazard ratio, 1.56, 95% confidence interval, 1.39 to 1.75; versus hazard ratio, 1.07, 95% confidence interval, 0.91 to 1.25; respectively).
Conclusions
In children with ESRD, a higher eGFR at dialysis initiation is associated with lower survival, particularly among children whose initial treatment modality is hemodialysis.
There is considerable practice variation with regard to the timing of dialysis initiation for adults and children with ESRD.1–3 Over time, eGFR at dialysis initiation among adults with ESRD has increased.4,5 Specifically, adults with ESRD in the United States were initiated on dialysis at an average eGFR that was 2.7 ml/min per 1.73 m2 higher in 2007 compared with 1997,4 although there is evidence that this trend toward dialysis initiation at higher eGFR levels in adults has reversed since 2010.6
The trend toward earlier initiation of dialysis is concerning given that observational studies in adults with ESRD have not shown a survival benefit to starting dialysis at higher levels of eGFR.7–11 The Initiating Dialysis Early and Late (IDEAL) trial in adult patients with CKD, which randomized patients to earlier (defined as an eGFR of 10–14 ml/min per 1.73 m2) versus later (defined as an eGFR of 5–7 ml/min per 1.73 m2) initiation of dialysis, did not find a survival benefit to the planned initiation of dialysis at higher eGFR.12
Fewer studies have focused on how the timing of dialysis initiation in children with CKD has changed over time, and the extent to which the timing of dialysis initiation is associated with mortality. A previous study in children found that higher eGFR at dialysis initiation (defined as >15 ml/min per 1.73 m2) was associated with lower risk of hospitalization for hypertension and pulmonary edema.13 The objectives of this study were to describe temporal trends in the timing of dialysis initiation by treatment modality, to determine whether initiation of dialysis at higher eGFR is associated with a survival benefit in children with ESRD registered in the US Renal Data System (USRDS), and to evaluate whether this association differs depending on initial dialysis treatment modality.
Methods
Study Population
We performed a retrospective cohort study of children (1–18 years of age) who, according to the USRDS, started RRT between 1995 and 2015. The USRDS is a national registry that tracks administrative and outcomes data on all patients with ESRD in the United States. We identified all children who started hemodialysis (HD) or peritoneal dialysis (PD) for the first time between January 1, 1995 and December 31, 2015. We excluded patients with prior dialysis exposure, prior kidney transplants, those who received preemptive transplants, and those with missing serum creatinine or height data (necessary to calculate eGFR) from our study. We excluded children <1 year of age because the GFR estimating equations have not been well validated in this age group.14 We also excluded those children who were lost to follow-up or recovered kidney function within the first 90 days after the dialysis start date to ensure that our cohort included children requiring chronic dialysis.
Data on treatment modality, modality change, loss to follow-up, and renal recovery were obtained from the RXHIST modality file.
Primary Predictor and Outcome Determination
eGFR at the start of dialysis was determined using the bedside Schwartz equation([0.413× height]/serum creatinine concentration).14 We defined “higher eGFR” as an eGFR>10 ml/min per 1.73 m2 at the time of dialysis initiation based on definitions used in a prior study of this issue.12 We defined “lower eGFR” as an eGFR≤10 ml/min per 1.73 m2 at the time of dialysis initiation. Height and serum creatinine were extracted from the Center for Medicare and Medicaid (CMS) Medical Evidence Report (CMS-2728) submitted around the time of dialysis initiation (MEDEVID file) and Patients file.
Dates of dialysis initiation and death were obtained from the USRDS Patients file. Throughout this study, follow-up was censored at time of death, renal recovery, loss to follow-up, or December 31, 2015.
Covariate Ascertainment
Data on age at dialysis initiation, sex, race, ethnicity, cause of ESRD, serum albumin, serum hemoglobin, body mass index (BMI), presence of hypertension, presence of cardiovascular disease (stroke, heart failure, coronary artery disease, peripheral vascular disease, and other cardiac disease), presence of other comorbidities (cancer, diabetes, immobility, tobacco, alcohol or drug use, and chronic obstructive pulmonary disease), patient zip code, insurance status, date of transplantation, and cause of death were extracted from the CMS Medical Evidence Report (CMS-2728) submitted around the time of dialysis initiation (MEDEVID file) and Patients file.
BMI values were age- and sex-standardized to z-scores using the 2000 Centers for Disease Control (CDC) standards for children in the United States.15 We defined underweight as BMI<5th percentile for age (corresponding to a z-score <−1.64) and obese as BMI≥95th percentile for age (corresponding to a z-score ≥1.64) according to the CDC criteria.
The zip code for the dialysis center was obtained from the Facility file and the distance from the patient’s zip code to the dialysis center was calculated as the geodetic distance in miles between the centroid of each zip-code location using a Statistical Analysis System (SAS) function (“ZIPCITYDISTANCE”).
Statistical Analyses
To evaluate differences in patient characteristics by timing of dialysis initiation (higher eGFR versus lower eGFR), we compared continuous variables with the t test or the Wilcoxon rank-sum test (for non-normally distributed data), and categoric data using chi-squared tests.
Temporal Trends in Timing of Dialysis Initiation
We explored temporal trends in the timing of dialysis initiation among children who met our inclusion criteria. We tested whether these trends were statistically significant using calendar year as the predictor and eGFR as the outcome of interest in a linear regression model adjusted for patient age, sex, race/ethnicity, BMI category, cause of ESRD, presence of hypertension, and dialysis type.
Association Between Timing of Dialysis Initiation and Survival
Next, we examined the association between eGFR at dialysis initiation (primary predictor) and time to death (primary outcome). Survival estimates were obtained according to the Kaplan–Meier approach. For our primary analysis evaluating the association between timing of dialysis initiation and survival, we used adjusted Cox models. Potential confounders of the association between dialysis timing and survival were selected a priori by the investigators. We adjusted for the following covariates: sex, race/ethnicity, cause of ESRD, BMI category, presence of hypertension, and dialysis type as categoric variables; and age and calendar year of dialysis initiation as continuous variables. We also adjusted for transplant as a time-varying covariate, accounting for the duration of time each patient spent with a functional first transplant.
Secondary Analyses
To ascertain whether nonlinearity in the association between timing of dialysis initiation and survival was present, in sensitivity analysis, we redefined our primary predictor using categories of eGFR at time of dialysis initiation as follows: eGFR of <5 ml/min per 1.73 m2, eGFR of 5 to <10 ml/min per 1.73 m2, eGFR of 10 to ≤15 ml/min per 1.73 m2, and eGFR of >15 ml/min per 1.73 m2. We then repeated our analysis using adjusted Cox models as described above.
Because prior studies have suggested that the association between timing of dialysis initiation and survival depends on the treatment modality used,10,16 we stratified our analysis by whether the initial treatment modality was HD versus PD. We did not require a minimum amount of time on either HD or PD for definition of the initial treatment modality because we examined the number of children who switched modalities within the first 30 days of dialysis initiation and found that only 148 patients changed from PD to HD in the first month after dialysis initiation and 295 patients changed from HD to PD (total n=443 patients, 3% of the cohort). Thus, we did not account for these modality changes given the small number of patients in whom this occurred. We tested for interaction between timing of dialysis initiation (higher versus lower eGFR) and treatment modality in models adjusted for the same covariates as described above.
In sensitivity analyses, we restricted our analysis to children who had serum creatinine measurement dates within a narrow time range relative to the date of dialysis initiation. We examined the following restricted groups: those with serum creatinine measurements dated 30 days before to 7 days after the date of dialysis initiation, and those with creatinine measurements dated within 14 days before the date of dialysis initiation.
Given that serum creatinine measurements started being standardized in 2006 using an isotope dilution mass spectrometry standardized assay,17 and given that the bedside Schwartz equation was validated using this standardization,14 we felt that it was also important to examine whether our findings were consistent during this more contemporary time period (compared with the overall time period). We therefore repeated our Cox models in a sensitivity analysis where we only included children who initiated dialysis between 2006 and 2015. We adjusted for the same covariates as described previously. We tested for interaction between the cohort (1995–2005 versus 2006–2015) on timing of dialysis initiation for the outcome of death.
Lastly, to isolate the relationship between timing of dialysis initiation and survival during chronic dialysis therapy, we conducted an additional analysis in which we censored children at the time of transplant. We adjusted for the same covariates in our primary models as described above, with the exception of transplant.
The Committee on Human Research of the University of California, San Francisco does not consider the scope of this work to be human subjects research. With the exception of conversion of BMI into standardized BMI z-scores, which was performed using a SAS tool provided by the CDC,15 and the distance between patient and dialysis center zip code, which was determined using SAS 9.4, STATA 13 (StataCorp, College Station, TX) was used for the conduct of all analyses.
Results
Study Cohort
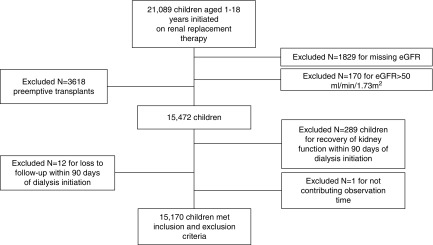
A total of 21,089 children (1–18 years of age) were initiated on RRT during the specified time period; 1999 potential participants were excluded (Figure 1) because height and/or serum creatinine data were missing (n=1829) or eGFR was >50 ml/min per 1.73 m2 and thus height and/or serum creatinine entries may have been erroneous (n=170). A total of 3618 children were excluded because they received a pre-emptive kidney transplant. Also, 289 children were excluded because they recovered renal function in the first 90 days after dialysis initiation, and 12 children were additionally excluded because they were lost to follow-up within the first 90 days after dialysis initiation. One additional child was excluded because they did not contribute any observation time (study entry date was the same as the censor date). Thus, a total of 15,170 children who were treated with dialysis were included for analysis (Figure 1).
Figure 1.
Derivation of the cohort results in 15,170 children being included in the study. Chart shows the inclusion and exclusion criteria for the cohort included in our analysis.
Of the entire cohort, 29.0% (n=4327) had higher eGFR at dialysis initiation. The median eGFR for those with higher eGFR at dialysis initiation was 12.8 (interquartile range [IQR], 11.1–16.0) ml/min per 1.73 m2. The median eGFR for those with lower eGFR at dialysis initiation was 6.5 (IQR, 4.8–8.0) ml/min per 1.73 m2. Table 1 shows the characteristics of those who were initiated on dialysis at higher versus lower eGFR. Children with higher eGFR at dialysis initiation were more often in the youngest age category, girls, non-Hispanic white, had GN as the cause of ESRD, and lived farther from the dialysis center. Additionally, those children with higher eGFR at dialysis initiation had lower serum albumin, higher hemoglobin, more often had abnormal BMI, and more often had hypertension.
Table 1.
Characteristics of children with higher eGFR compared with lower eGFR at dialysis initiation
| Characteristics | Higher eGFR at Dialysis Initiation (n=4327) | Lower eGFR at Dialysis Initiation (n=10,843) | P Value |
|---|---|---|---|
| eGFR (ml/min per 1.73 m2), median (IQR) | 12.8 (11.1–16.0) | 6.5 (4.8–8.0) | <0.001 |
| Median age in years (IQR) | 14 (9–16) | 14 (10–17) | <0.001 |
| Age category, N (%) | <0.001 | ||
| Age 1 to <5 yr | 572 (13.2) | 928 (8.6) | |
| Age 5 to <13 yr | 1261 (29.1) | 2937 (27.1) | |
| Age ≥13 yr | 2494 (57.6) | 6978 (64.4) | |
| Girls, N (%) | 2173 (50.2) | 4850 (44.7) | <0.001 |
| Race/ethnicity, N (%) | <0.001 | ||
| Non-Hispanic white | 1945 (45.0) | 4082 (37.6) | |
| Non-Hispanic black | 1023 (23.6) | 3046 (28.1) | |
| Hispanic | 1107 (25.6) | 3108 (28.7) | |
| Asian | 164 (3.8) | 437 (4.0) | |
| Other/unknown | 88 (2.0) | 170 (1.6) | |
| Primary cause of ESRD, N (%) | <0.001 | ||
| GN | 2012 (46.5) | 4484 (41.3) | |
| CAKUT | 1219 (28.2) | 3232 (29.8) | |
| Diabetes | 26 (0.6) | 71 (0.7) | |
| Hypertension | 121 (2.8) | 526 (4.8) | |
| Malignancy | 59 (1.3) | 71 (0.7) | |
| Other or unknown | 890 (20.6) | 2459 (22.7) | |
| BMI category by z-score, N (%) | 0.01 | ||
| <5th% (underweight) | 542 (12.5) | 1231 (11.4) | |
| 5th%–94th% (normal weight) | 2757 (63.7) | 7180 (66.2) | |
| ≥95th% (obese) | 1028 (23.8) | 2432 (22.4) | |
| Mean serum albumin (g/dl), (SD)a | 3.2 (1.1) | 3.3 (1.3) | <0.001 |
| Median hemoglobin (g/dl), (IQR)b | 9.9 (8.6, 11.2) | 9.1 (7.7, 10.6) | <0.001 |
| Comorbidity, N (%) | |||
| Hypertensionc | 2002 (47.0) | 4763 (44.7) | 0.01 |
| Cardiovascular diseased | 279 (6.5) | 504 (4.7) | <0.001 |
| Other comorbiditiese | 339 (7.8) | 492 (4.5) | <0.001 |
| Distance between patient and dialysis center (miles), median (IQR) | 15.1 (6.3, 45.0) | 14.1 (5.8, 41.0) | <0.001 |
| Insurance, % (N) | |||
| Medicaid | 2369 (54.8) | 5305 (48.9) | <0.001 |
| None | 147 (3.4) | 999 (9.2) | <0.001 |
| PD modality, N (%)f | 1805 (42.2) | 4179 (39.2) | 0.001 |
CAKUT, congenital anomalies of the kidney and urinary tract.
Missing in n=2231.
Missing in n=1019.
Missing in n=266.
Cardiovascular disease includes stroke, heart failure, coronary artery disease, peripheral vascular disease, and other cardiac disease.
Other comorbidities include cancer, diabetes, immobility, tobacco, alcohol or drug use, and chronic obstructive pulmonary disease.
Missing in n=239.
Temporal Trends in Timing of Dialysis Initiation
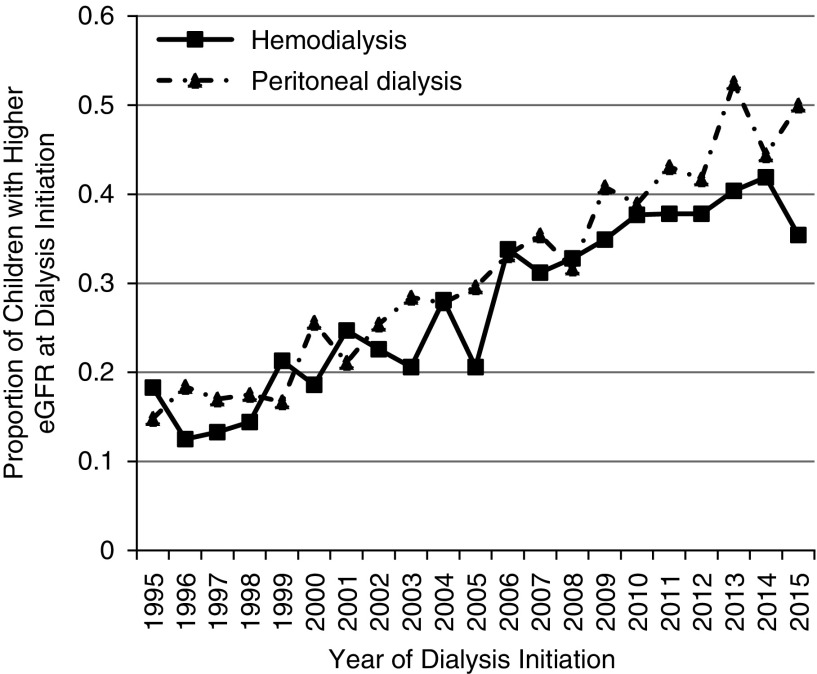
There was a statistically significant trend toward dialysis initiation at higher eGFR over time (Figure 2), with an annual 0.18 ml/min per 1.73 m2 increase in eGFR in our adjusted model (95% confidence interval [95% CI], 0.17 to 0.19; P<0.001). This trend did not differ by treatment modality (HD versus PD, P=0.10 for interaction). The number of children with higher eGFR at dialysis initiation increased substantially from 1995 (16.6% of children with higher eGFR at dialysis initiation) to 2015 (40.7% of children with higher eGFR at dialysis initiation).
Figure 2.
There was a trend toward dialysis initiation at higher eGFR over time for both dialysis modalities. This figure shows the proportion of children initiated on dialysis at higher eGFR (eGFR>10 ml/min per 1.73 m2) by calendar year of initiation and dialysis type.
Association Between Timing of Dialysis Initiation and Survival
Median observation time was 6.1 (IQR, 2.4–10.9) years for those with higher eGFR at dialysis initiation as compared with 9.6 (IQR, 4.8–14.4) years for those with lower eGFR (P<0.001). A total of 2421 patients died during the observation period. The unadjusted 1-year survival for those with higher eGFR at dialysis initiation was 95.6% compared with 97.7% in those with lower eGFR at dialysis initiation. The most common reported cause of death was from cardiovascular causes (32.0%), followed by infectious causes (14.7%); the cause was listed as unknown for 15.6% of the cohort.
In our unadjusted model, the risk of mortality was 30% higher (95% CI, 1.19 to 1.42; P<0.001) for those with higher versus lower eGFR at dialysis initiation. In our adjusted model, the risk of mortality was 36% higher (95% CI, 1.24 to 1.50; P<0.001) for those with higher versus lower eGFR at dialysis initiation (Table 2).
Table 2.
Adjusted hazards of death for the overall cohort and in analysis restricted to the year 2006–2015
| Characteristics | Adjusted HR (95% CI) | P Value |
|---|---|---|
| Years 1995–2015 | ||
| All patients (n=14,696)a | 1.36 (1.24 to 1.50) | <0.001 |
| Patients initiated on HD (n=8794) | 1.56 (1.39 to 1.75) | <0.001 |
| Patients initiated on PD (n=5902) | 1.07 (0.91 to 1.25) | 0.44 |
| Years 2006–2015 | ||
| All patients (n=6757)b | 1.34 (1.11 to 1.62) | 0.002 |
| Patients initiated on HD (n=4151) | 1.68 (1.33 to 2.12) | <0.001 |
| Patients initiated on PD (n=2606) | 0.86 (0.62 to 1.20) | 0.37 |
A total of 474 persons missing from adjusted analysis due to missing covariate data.
A total of 217 persons missing from adjusted analysis due to missing covariate data.
Secondary Analyses
When we categorized eGFR with finer divisions, 2986 children had an eGFR of <5 ml/min per 1.73 m2, 7857 children had an eGFR of 5 to <10 ml/min per 1.73 m2, 3028 children had an eGFR of 10 to ≤15 ml/min per 1.73 m2, and 1299 children had an eGFR of >15 ml/min per 1.73 m2 at the time of dialysis initiation. In our adjusted survival analysis with the aforementioned categories of eGFR as the predictor, we found that compared with the reference group (eGFR<5 ml/min per 1.73 m2), there was an increasing hazard of death with higher categories of eGFR. Specifically, for an eGFR of 5 to <10 ml/min per 1.73 m2, the HR was 1.32 (95% CI, 1.18 to 1.47); for an eGFR of 10 to ≤15 ml/min per 1.73 m2, the HR was 1.57 (95% CI, 1.37 to 1.80); and for an eGFR of >15 ml/min per 1.73 m2, the HR was 1.94 (95% CI, 1.64 to 2.30).
When we stratified the cohort by initial treatment modality, we found that 59.9% (n=8947) were started on HD and 40.1% (n=5984) were started on PD as their initial treatment modality. The association between eGFR at dialysis initiation and mortality risk was more pronounced among children treated with HD (HR, 1.56; 95% CI, 1.39 to 1.75). Although children with higher eGFR at dialysis initiation who were treated with PD also had higher mortality risk (HR, 1.07; 95% CI, 0.91 to 1.25), the association was less robust compared with children receiving HD (P<0.001 for interaction) and did not reach statistical significance (Table 2).
In sensitivity analyses, we evaluated the distribution of recorded serum creatinine measurement dates relative to dialysis start dates in the entire cohort and found that the median difference between the dialysis start date and date of reported serum creatinine measurement was 1 day (IQR, 0–7 days). We then restricted our analysis to children who had a recorded serum creatinine date within 30 days before to 7 days after the dialysis start date. In this restricted analysis, 10,782 children were included for the adjusted analysis and the risk of death was 1.39 (95% CI, 1.25 to 1.55; P<0.001) times higher for children with higher eGFR at dialysis initiation. Next, we further restricted our analysis to those with a recorded creatinine date within 14 days before the dialysis start date. With this additional restriction, 4686 children were included for study and the risk of death was 1.28 (95% CI, 1.09 to 1.52; P=0.004) times higher for children with higher eGFR at dialysis initiation.
In an additional sensitivity analysis, when we restricted our analysis to a more contemporary period (2006–2015), 6974 children met our inclusion criteria. Of these, 37.5% (n=2615) had higher eGFR at dialysis initiation, and 38.5% (n=2625) had PD as their initial treatment modality. Unadjusted 1-year survival estimates were 96.5% for those with higher eGFR versus 98.3% for those with lower eGFR at dialysis initiation. In this sensitivity analysis restricted to a more contemporary time period, the point estimate for the risk of death comparing those with higher versus lower eGFR at dialysis initiation was similar to our primary analysis and reached statistical significance (HR 1.34; 95% CI, 1.11 to 1.62; P=0.002). There was also no statistically significant difference in survival among those starting PD at higher eGFR versus lower eGFR (Table 2), which was consistent with the results of our primary analysis. There was no significant interaction between time period (before or after 2006) and eGFR at dialysis initiation for the outcome of mortality (P=0.69 for interaction).
Median time to transplant (for those who underwent transplantation) was similar at 1.22 (IQR, 0.62–2.32) years for those with higher eGFR at dialysis initiation and 1.27 (IQR, 0.61–2.46) for those with lower eGFR at dialysis initiation (P=0.18). About 20% of the entire cohort underwent transplant within 6 months of starting dialysis.
In additional analysis censoring observations at the time of kidney transplantation, the median observation time was 1.45 (IQR, 0.66–3.16) years for those with lower eGFR and 1.31 (IQR, 0.62–2.67) years for those with higher eGFR at dialysis initiation (P≤0.001). In our adjusted model censoring follow-up at transplantation, the risk of mortality was 43% higher for those with higher versus lower eGFR at dialysis initiation (95% CI, 1.27 to 1.62; P<0.001), which did not differ substantially from findings in our primary analysis.
Discussion
Few studies have examined whether timing of dialysis initiation in children has changed over time and whether there may be any survival benefit associated with dialysis initiation at higher eGFR in children. In this study, we found that the number of children with higher eGFR at dialysis initiation has increased steadily from approximately 17% in 1995 to 41% in 2015. We also found that dialysis initiation at higher eGFR was not associated with a survival benefit in children. In fact, in children who began RRT with HD as their initial treatment modality, there was a 56% higher risk of death in those with higher eGFR (versus lower eGFR) at dialysis initiation. Notably, the association between higher eGFR at dialysis initiation and higher risk of death was attenuated for children who began RRT with PD rather than HD and did not reach statistical significance.
The trend toward dialysis initiation at higher eGFR over time in children may reflect changes to published guidelines, which suggested incorporation of eGFR as a criterion for determining when to start dialysis in 1997.18,19 Although a similar trend toward dialysis initiation at higher eGFR has been observed in adults with ESRD,20 there are data to suggest that this trend has been reversing in more recent years,6 likely due to trial-grade evidence showing that dialysis initiation at higher eGFR was not beneficial for adults with ESRD.12 In contrast, our data do not support the stabilization or reversal of this trend in children over the same time frame.
Our findings are largely consistent with a recently published study by Okuda et al.21 which also focused on the association between eGFR and risk of death among children starting RRT according to the USRDS, but our study provides additional information. In the study by Okuda et al.,21 follow-up was censored at transplantation and therefore the focus was on mortality risk during the dialysis phase of illness. However, children who die during dialysis may be less representative of the broader population of children who develop ESRD because the vast majority of children will survive to transplantation and receive transplantation as the preferred treatment modality. In contrast to Okuda et al.’s study, our study examines the association between timing of dialysis initiation and mortality risk accounting for transplant as a time-varying covariate (as opposed to a censoring event) in our primary analysis. Thus, we were able to evaluate the longer-term association between timing of dialysis initiation and patient survival, including deaths that may have occurred after transplantation. Additionally, our study focuses on the presence or absence of effect modification by initial dialysis treatment modality, which we believe to be clinically important because our findings were especially noteworthy in children who started RRT with HD (versus PD).
Our finding that survival was not improved with dialysis initiation at higher eGFR in children is also consistent with reports in the adult population. The only randomized study evaluating dialysis initiation at higher versus lower eGFR did not find a survival benefit to starting dialysis at higher eGFR, although the actual difference in eGFR between the two groups at the start of dialysis was smaller than intended.12 Observational studies among adults have also shown higher mortality for adults who were started on dialysis at higher eGFR.7–11 Similar to our findings, the association between higher eGFR at dialysis initiation and mortality differed by dialysis modality in adults, with the association being more notable among patients on HD.10 One potential explanation for the modification of the association between higher eGFR at dialysis initiation and mortality by dialysis type may be the accelerated loss of residual renal function that is seen with HD as compared with PD, which has been shown to associate with worse survival.22–24 In addition, longer exposure to dialysis may lead to accumulation of more cardiovascular risk factors (e.g., worsening left ventricular hypertrophy)25 and increase the risk for infections (e.g., related to dialysis access)26 which may increase the risk of death. We also speculate that other patient-specific factors, such as adherence to therapy, may contribute both to the decision to start dialysis at higher eGFR and the lower survival of children who started dialysis earlier (due to poorer compliance with therapy).
Taken together, our findings have important implications for clinical practice because we found that pediatric nephrologists are initiating children on dialysis with higher levels of kidney function during the contemporary time period, although our findings do not support the presence of any associated mortality benefit. Although we do not have granular data on the reasons why a child started dialysis, those children with higher eGFR at dialysis initiation could theoretically have reduced or avoided dialysis exposure altogether if they had been initiated on dialysis at lower eGFR. This is evidenced by the fact that 20% of those starting dialysis received a kidney transplant within 6 months of initiating RRT, which is similar to the time difference among those started on dialysis at higher versus lower eGFR in the IDEAL trial.12 In children, any dialysis exposure has been associated with worse survival compared with no dialysis exposure (i.e., preemptive transplantation),27 and other studies have demonstrated incrementally worse allograft outcomes with longer exposure to dialysis.28–30 In addition, preservation of access (and avoidance of tunneled-line placement, which is the most common initial type of HD access in children) could have long-term implications for children who tend to have excellent long-term survival31 and will likely need dialysis at some point during their lifetime. Thus, we speculate that deferring dialysis in asymptomatic children may allow for more transplants to be performed preemptively and for vascular access sites to be preserved.
The strengths of our study include the large cohort of pediatric patients with a high prevalence of important clinical outcomes. In addition, we believe that this is one of the first studies to examine the relationship between timing of dialysis initiation and survival in children with ESRD. Our study has a number of important limitations, however, given its observational nature. There is a potential for lead-time bias,32 given that those with higher eGFR at dialysis initiation would contribute longer observation time to our analysis. However, we believe that this would have biased our findings to underestimate the actual risk of death associated with dialysis initiation at higher eGFR, because those with lower eGFR at dialysis initiation would contribute less observation time. We also acknowledge that we do not have data on clinical management of children during the CKD phase of illness. Thus, it is possible that our study may be subject to survival bias,11 whereby children who were sicker may have died before dialysis was started and thus would have been excluded from analysis. In general, however, death rates are extremely low in children with CKD (unlike in adults); for example, in the CKD in Children Study, only six participants have died (out of nearly 900 participants and over more than a decade of follow-up).33 For this reason, we do not believe that death before ESRD is the main explanation for our findings.
We acknowledge that another important limitation to this study is the lack of information on patient symptoms and the indications for dialysis initiation. We found differences in clinical and demographic characteristics between children with higher versus lower eGFR at dialysis initiation which suggest that those who started dialysis at higher eGFR may have had greater severity of illness. Although we adjusted for these demographic and clinical characteristics in our models, residual confounding may still be present. We were also unable to provide data on other important clinical outcomes (such as nutrition, growth, and cognitive function) that may factor into the decision to initiate dialysis given limitations to the data available within the USRDS.
In conclusion, we found that there was no survival benefit to initiating dialysis at higher eGFR in children with kidney disease. In fact, initiation of dialysis at higher eGFR was associated with lower survival in children, especially if HD was the initial treatment modality. However, because of the limitations and potential for residual confounding in observational studies, confirmation of our findings with prospective trials in children with CKD should be considered. On the basis of our findings, we conclude that, although the decision to initiate dialysis should be based on the individual patient, consideration of delaying dialysis initiation in children who are asymptomatic may be prudent.
Disclosures
Dr. Johansen reports “other” from GlaxoSmithKline, outside the submitted work. Dr. Cabana, Dr. Warady, Dr. Johansen, Dr. Ku, and Dr. McCulloch report grants from NIH during the conduct of the study.
Funding
This publication was supported by NIH grants R01 DK115629 to Dr. Ku and Dr. Johansen and K24 DK085153 to Dr. Johansen. This publication was also supported by the National Center for Advancing Translational Sciences, NIH, through University of California, San Francisco Clinical and Translational Science Institute (UCSF-CTSI) grant number KL2 TR001870 to Dr. McCulloch and UCSF-CTSI grant number UL1 TR000004 to Dr. Grimes.
Acknowledgments
Dr. Winnicki and Dr. Ku designed the study with input from the other authors. Dr. Winnicki and Dr. Grimes analyzed the data. Dr. Winnicki wrote the first draft of the manuscript. All authors critically revised the manuscript. All authors approved the final version of the manuscript.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Time’s Up! Start Dialysis Later in Children,” on pages 1344–1345.
References
- 1.Slinin Y, Guo H, Li S, Liu J, Morgan B, Ensrud K, et al.: Provider and care characteristics associated with timing of dialysis initiation. Clin J Am Soc Nephrol 9: 310–317, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dart AB, Zappitelli M, Sood MM, Alexander RT, Arora S, Erickson RL, et al.: Variation in estimated glomerular filtration rate at dialysis initiation in children. Pediatr Nephrol 32: 331–340, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Saban JA, Zappitelli M, Samuel SM, Sood MM, Alexander RT, Arora S, et al. : Perceptions of pediatric nephrologists regarding timing of dialysis initiation in children in Canada. Can J Kidney Health Dis 3: 31, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Hare AM, Choi AI, Boscardin WJ, Clinton WL, Zawadzki I, Hebert PL, et al.: Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med 171: 1663–1669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hare AM, Wong SP, Yu MK, Wynar B, Perkins M, Liu CF, et al.: Trends in the timing and clinical context of maintenance dialysis initiation. J Am Soc Nephrol 26: 1975–1981, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosansky SJ, Clark WF: Has the yearly increase in the renal replacement therapy population ended? J Am Soc Nephrol 24: 1367–1370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, et al.: Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol 14: 2305–2312, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, et al.: Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant 24: 3175–3182, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, et al.: Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 5: 1828–1835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, et al. : GFR at initiation of dialysis and mortality in CKD: A meta-analysis. Am J Kidney Dis 59: 829–840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crews DC, Scialla JJ, Boulware LE, Navaneethan SD, Nally JV Jr., Liu X, et al.: DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators : Comparative effectiveness of early versus conventional timing of dialysis initiation in advanced CKD. Am J Kidney Dis 63: 806–815, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al.: IDEAL Study : A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Atkinson MA, Oberai PC, Neu AM, Fivush BA, Parekh RS: Predictors and consequences of higher estimated glomerular filtration rate at dialysis initiation. Pediatr Nephrol 25: 1153–1161, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al.: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku E, Glidden DV, Hsu CY, Portale AA, Grimes B, Johansen KL: Association of body mass index with patient-centered outcomes in children with ESRD. J Am Soc Nephrol 27: 551–558, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain AK, Sontrop JM, Perl J, Blake PG, Clark WF, Moist LM: Timing of peritoneal dialysis initiation and mortality: Analysis of the Canadian organ replacement registry. Am J Kidney Dis 63: 798–805, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al.: National Kidney Disease Education Program Laboratory Working Group : Recommendations for improving serum creatinine measurement: A report from the laboratory working group of the national kidney disease education program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation: NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 30[Suppl 2]: S67–S136, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rosansky SJ, Clark WF, Eggers P, Glassock RJ: Initiation of dialysis at higher GFRs: Is the apparent rising tide of early dialysis harmful or helpful? Kidney Int 76: 257–261, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Okuda Y, Soohoo M, Tang Y, Obi Y, Laster M, Rhee CM, et al.: Estimated GFR at dialysis initiation and mortality in children and adolescents. Am J Kidney Dis 73: 797–805, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Feber J, Schärer K, Schaefer F, Míková M, Janda J: Residual renal function in children on haemodialysis and peritoneal dialysis therapy. Pediatr Nephrol 8: 579–583, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Bargman JM, Thorpe KE, Churchill DN; CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT; NECOSAD Study Group : Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 62: 1046–1053, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Curtis BM, Randell EW, Parfrey PS: Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5: 805–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camins BC: Prevention and treatment of hemodialysis-related bloodstream infections. Semin Dial 26: 476–481, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Amaral S, Sayed BA, Kutner N, Patzer RE: Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int 90: 1100–1108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier-Kriesche HU, Kaplan B: Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation 74: 1377–1381, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Mange KC, Joffe MM, Feldman HI: Dialysis prior to living donor kidney transplantation and rates of acute rejection. Nephrol Dial Transplant 18: 172–177, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Gill JS, Rose C, Joffres Y, Landsberg D, Gill J: Variation in dialysis exposure prior to nonpreemptive living donor kidney transplantation in the United States and its association with allograft outcomes. Am J Kidney Dis 71: 636–647, 2018 [DOI] [PubMed] [Google Scholar]
- 31.US Renal Data System : Chapter 7: ESRD among children, adolescents, and young adults. Am J Kidney Dis 71: S383–S416, 2018 [Google Scholar]
- 32.Janmaat CJ, van Diepen M, Krediet RT, Hemmelder MH, Dekker FW: Effect of glomerular filtration rate at dialysis initiation on survival in patients with advanced chronic kidney disease: What is the effect of lead-time bias? Clin Epidemiol 9: 217–230, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, et al.: Chronic Kidney Disease in Children (CKiD); Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) Study Investigators : Estimating time to ESRD in children with CKD. Am J Kidney Dis 71: 783–792, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]