Significance Statement
The NaCl cotransporter NCC in the kidney distal convoluted tubule (DCT) regulates urinary NaCl excretion and BP. The long-term effects of the mineralocorticoid aldosterone on modulating NaCl reabsorption via NCC are well established, and their importance illustrated by the effects of NCC-targeting diuretics and the salt-wasting observed in Gitelman syndrome. In this study the authors demonstrate that aldosterone also has rapid effects on the DCT, partly via the membrane receptors EGFR and GPR30. Signaling from these receptors affect NCC activity so that when aldosterone is released in response to hypovolemia, aldosterone rapidly increases NaCl reabsorption to help restore blood volume.
Keywords: mineralocorticoid, SPAK, systems biology, cotransporter, epidermal growth factor receptor, Na transport
Visual Abstract
Abstract
Background
The NaCl cotransporter NCC in the kidney distal convoluted tubule (DCT) regulates urinary NaCl excretion and BP. Aldosterone increases NaCl reabsorption via NCC over the long-term by altering gene expression. But the acute effects of aldosterone in the DCT are less well understood.
Methods
Proteomics, bioinformatics, and cell biology approaches were combined with animal models and gene-targeted mice.
Results
Aldosterone significantly increases NCC activity within minutes in vivo or ex vivo. These effects were independent of transcription and translation, but were absent in the presence of high potassium. In vitro, aldosterone rapidly increased intracellular cAMP and inositol phosphate accumulation, and altered phosphorylation of various kinases/kinase substrates within the MAPK/ERK, PI3K/AKT, and cAMP/PKA pathways. Inhibiting GPR30, a membrane-associated receptor, limited aldosterone’s effects on NCC activity ex vivo, and NCC phosphorylation was reduced in GPR30 knockout mice. Phosphoproteomics, network analysis, and in vitro studies determined that aldosterone activates EGFR-dependent signaling. The EGFR immunolocalized to the DCT and EGFR tyrosine kinase inhibition decreased NCC activity ex vivo and in vivo.
Conclusions
Aldosterone acutely activates NCC to modulate renal NaCl excretion.
Aldosterone modulates body sodium (Na+) and potassium (K+) balance by regulating renal Na+ reabsorption and K+ secretion. Aldosterone primarily mediates its effects on the aldosterone-sensitive distal nephron,1 comprising the late distal convoluted tubule (DCT), connecting tubule, and collecting duct. The classic mechanism for aldosterone actions involves the binding of aldosterone to the mineralocorticoid receptor (MR), translocation of the MR to the nucleus, and subsequent MR-mediated alterations in gene transcription of various ion-transporting proteins such as the epithelial Na+ channel ENaC.2 These so called “genomic” effects influence Na+ and K+ transport within 2–3 hours and play an important role for electrolyte balance and the maintenance of BP. Additionally, aldosterone can rapidly cause subcellular redistribution of ENaC to the plasma membrane and increase amiloride-sensitive Na+ currents within minutes.3–5
Aldosterone also has the capacity for rapid (within seconds or minutes) “nongenomic” effects on target cells, which occur in concurrence with alterations in cytosolic Ca2+ levels (via PLC/DAG/IP3 pathway), or cAMP-mediated signaling.2 Whether these rapid effects are mediated via the MR alone,6 by interaction of the MR with the EGF receptor (EGFR), IGF1R, PDGFR, AT-1, or GPR30 (also known as GPER17) or via direct activation of alternative membrane-bound receptors are controversial.2 For example, although aldosterone can increase intracellular cAMP and Ca2+ levels in MR-deficient mice8 and noncell-permeable aldosterone analogs can trigger PKC signaling or ERK activation,9,10 the existence of a plasma membrane-bound receptor that responds consistently to aldosterone is hotly debated.7,11,12 However, one such candidate, GPR30, is functionally expressed in kidney epithelial cells and responds to aldosterone stimulation.13,14
Although chronic aldosterone administration increases abundance and phosphorylation (activation) of the thiazide-sensitive NaCl cotransporter NCC15–18 in cells of the late DCT, it is unknown whether these effects are direct. Furthermore, although in a timeframe of 12–24 hours aldosterone can activate NCC in a SPAK-dependent manner,19 whether aldosterone exerts acute (within seconds or minutes) effects in DCT cells has never been examined. The aim of this study was to investigate whether aldosterone can have rapid effects on DCT cells to regulate NCC and to identify the potential signaling mediators behind this effect. The major finding is that within minutes, aldosterone is able to activate a variety of different signaling cascades in DCT cells that ultimately results in increased NCC activity. The results demonstrate for the first time an important new role of nongenomic aldosterone actions in the DCT that is likely to have major implications for the rapid regulation of NaCl transport and, consequently, BP regulation.
Methods
Extensive methods are provided in Supplemental Appendix 1.
Antibodies
The majority of utilized antibodies were commercial rabbit monoclonal antibodies, the details of which are listed in Supplemental Appendix 1. Specificity of the commercial antibodies was indicated by the fact that they either gave a single unique band on an immunoblot corresponding to the target protein’s predicted molecular weight, or the most prominent band on the immunoblot was at the target protein’s predicted molecular weight (with no other bands of similar size). Other antibodies were SPAK and pSPAK,20 and pNCC-T58.20
Ca2+ and cAMP Measurements
The mouse kidney DCT cell line (mpkDCT) has been characterized previously.21,22 Cells were routinely cultured at 37°C in 5% CO2 in mpkDCT medium (DMEM/F12- media [Invitrogen] containing 60 nM sodium selenite, 5 μg/ml transferrin, 2 mM glutamine, 1 nM triiodothyronine, 10 ng/ml EGF, 5 μg/ml insulin, 20 mM D-glucose, and 20 mM HEPES; pH 7.4). For Ca2+ assays, cells were cultured in black Visiplates (Wallac) until confluent, with the last 24 hours in pure phenol red free DMEM-F12 media. Cells were washed with HBSS before incubating in loading dye solution (5 µM Fluo-4, 2.5 mM probenecid, 1× PowerLoad in HBSS) for 30 minutes at 37°C. Cells were washed in assay buffer (HBSS containing 2.5 mM probenecid) and overlaid with 200 µl assay buffer. Fluorescence was measured using an EnSpire plate reader (PerkinElmer, Waltham, MA), with an excitation wavelength of 495 nm and an emission wavelength of 510 nm continuously for 2 minutes (background fluorescence), and for 10 minutes after addition of 50 µl of agonist (to reach final concentrations as indicated) or control solution. For cAMP assay experiments, cells were grown on a semipermeable filter support (Transwell; Corning) until a fully confluent polarized monolayer was formed (transepithelial resistance >5 kΩ.cm2). Cells were stimulated as indicated for 30 minutes, lysed, and intracellular cAMP levels were measured using a cAMP enzyme immunoassay kit (Enzo) according to manufacturer’s instructions. All measurements were carried out in triplicates on at least five separate days.
Inositol Phosphate Measurements
Inositol phosphate (IP) accumulation was measured using a scintillation proximity–based inositol-phosphate accumulation assay.23 In brief, mpkDCT cells were seeded at 35,000 cells/well in 100 µl mpkDCT medium in 24-well filter plates and incubated for 24 hours at 37°C with 0.5 µCi of myo-[3H]inositol (PerkinElmer). Medium without myo-[3H]inositol was added to the basolateral compartment. The following day, cells were washed twice in pure DMEM-F12 and incubated in pure DMEM-F12 supplemented with 10 mM LiCl at 37°C for 30 minutes in the presence of diluent (DMSO), aldosterone (1 nM), or the activator of phospholipase C m-3M3FBS (Tocris) as a positive control. Cells were incubated in 10 mM formic acid for 60 minutes before 35 µl from each well was transferred to a 96-well plate, 1 mg of yttrium silicate SPA beads (SPA-Ysi, RPNQ; PerkinElmer) was added, and the plate mixed for 30 minutes by high-speed agitation. Beads were centrifuged (5 minutes at 400×g) and [3H]IP-binding was measured on a TopCount NXT (PerkinElmer).
Immunoblotting Experiments
mpkDCT cells were grown on filter plates in mpkDCT media until transepithelial resistance >5 kΩ.cm2. The evening before experiments, cells were cultured in pure phenol red free DMEM-F12 media. Cells were treated with agonists/antagonists or relevant control solutions as indicated for 30 minutes at 37°C. For G-36 or erlotinib antagonist experiments, cells were preincubated with G-36 or pure media for 20 minutes before subsequent stimulation. Standard procedures were utilized for SDS-PAGE and immunoblotting. For detection of GPR30 and EGFR in native DCT cells, protein lysates from DCT cells isolated from parvalbumin-GFP mice were used as previously described.24 Extensive details are provided in Supplemental Appendix 1. EGFP purity (DCT cell purity) in the sample was 94%.
Immunohistochemistry and Confocal Microscopy Analysis
Archived paraffin-embedded male mouse kidney tissue was processed and immunolabeled for light or confocal laser scanning microscopy as previously described.25
RT-PCR
RNA purification and RT-PCR was performed on 1 µg RNA as previously described.26 Archived cDNA from male mouse kidney cortex was used as a positive control. Primer sequences are in Supplemental Appendix 1.
Mouse Cortical Tubule Suspensions
Mouse cortical tubule suspensions from male C57BL/6J mice were prepared as previously described.22 Equal volumes of the suspensions were plated into 24-well tissue culture plates and preincubated in pure DMEM-F12 media for 2 hours, 30 minutes at 37°C and 5% CO2. For erlotinib and G36 studies, the antagonists were added to the suspensions for the last 30 minutes of preincubation. Suspensions were treated using antagonists, aldosterone (1 nM), or relevant controls as indicated, for 30 minutes at 37°C and 5% CO2. In some studies, 5 µM actinomycin D and 100 µM cycloheximide (Sigma-Aldrich) were included throughout the 2 hours, 30 minutes preincubation and stimulation steps. At the end of the experiment media was removed and cells lysed in Laemmli sample buffer containing DTT (15 mg/ml), sonicated, and heated at 65°C for 15 minutes before immunoblotting. For high K+ experiments, KCl was added to the media where indicated, to make the final K+ concentration 8 mM.
Acute Aldosterone Effects in Mice
All animal protocols complied with the European community guidelines for the use of experimental animals, and were approved and performed under a license issued by the Danish Ministry of Justice (Dyreforsøgstilsynet). Male C57BL/6J mice (approximately 30 g body wt) were administered 50 µl of an aldosterone solution (43 µM in DMSO) by intramuscular injection. This resulted in a three- to five-fold increase in plasma aldosterone levels (Figure 1, Supplemental Figure 1). After 30 or 60 minutes, animals were anesthetized using isoflurane and blood sampled from the retro-orbital plexus. Kidneys were removed and processed for immunoblotting as previously described.20
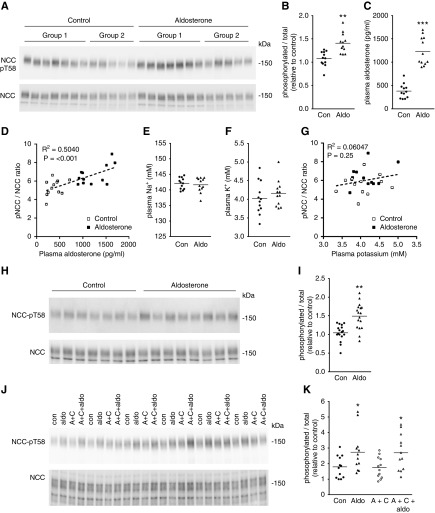
Figure 1.
Aldosterone rapidly modulates NCC activity in native DCT cells. (A) Mice were injected with aldosterone and after 60 minutes the phosphorylation levels of NCC at T58 (NCC-pT58, surrogate marker of NCC activation) was assessed by immunoblotting. Groups 1 and 2 refer to independent experimental cohorts. (B) Summary of data (n=12) from two independent experiments. Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. (C) Plasma aldosterone levels. (D) A significant positive correlation exists between plasma aldosterone concentration and the NCC-pT58/total NCC ratio. (E) Plasma Na+ levels are not significantly different. (F) Plasma K+ levels are not significantly different. (G) No significant correlation was observed between plasma K+ levels and the NCC-pT58/total NCC ratio after 60 minutes of aldosterone treatment. (H) Cortical tubule suspensions were treated with aldosterone (1 nM) for 30 minutes and NCC-pT58 levels were assessed by immunoblotting. (I) Summary of data (n=17/group). (J) Cortical tubule suspensions were treated for 150 minutes with actinomycin D and cycloheximide (A+C) where indicated, before treatment with aldosterone (1 nM) for 30 minutes. NCC-pT58 levels were assessed by immunoblotting. (K) Summary of data. Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. *P<0.05; **P<0.01; ***P<0.001 relative to control. Aldo, aldosterone; Con, control.
In Vivo Role of EGFR and GPR30
In study 1, 10-week-old male FVB mice (Janvier Labs, Le Genest-Saint-Isle, France) were used and their bladder emptied at the beginning of the experiment. Mice received either gefitinib (0.8 mg/g body wt; Selleckchem) or vehicle (20% PEG300) by oral gavage (250 µl). The experimental period was 3 hours, during which the mice were housed in their usual cages. Spot urine was collected during the last 1-hour period. Blood was removed from the vena cava under isoflurane anesthesia into lithium heparin tubes, the animals euthanized and the kidneys collected and snap frozen in liquid nitrogen. Immunoblotting samples were prepared as previously described.27 For study 2, 10-week-old female FVB mice were used and the experimental period was 1 or 2 hours. For study 3, 10-week-old male FVB mice were used. After 1 hour of treatment with gefitinib or vehicle, mice were administered 50 µl of an aldosterone solution (43 µM aldosterone) or vehicle by intramuscular injection. The experiment was terminated 1 hour later by decapitation and trunk blood was collected. Generation of the GPR30−/− mice has been previously described.28 Archived kidneys isolated from 3-month-old female GPR30−/− and wild-type control mice were used.29 Both groups were subject to ovariectomy 4 weeks before tissue was collected. All animal experiments were approved by the Ethics Committees for Animal Research at Gothenburg University and Karolinska Institutet.
SILAC Labeling, LC-MS/MS Sample Preparation and Running Conditions, and LC-MS/MS Data Analysis
SILAC labeling and LC-MS/MS experiments were performed in a similar manner to our previous study.24 Extensive details are provided in Supplemental Appendix 1. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE30 partner repository with the dataset identifier PXD010032.
Bioinformatics
Regulated phosphorylation motifs were predicted using standard settings in Motif-X (http://motif-x.med.harvard.edu/motif-x.html). Gene Ontology term analysis was carried out using app ClueGo v2.5.1 in Cytoscape. Proteins (UniProt accessions used) with significant changes in phosphorylation levels were subjected to phosphorylation core analysis using Ingenuity Pathway Analysis (Qiagen) to generate functional pathways.
Statistical Analyses
Immunoblotting and second messenger data are expressed as mean±SEM. For two groups, data meeting the statistical assumptions of normality were assessed using an unpaired t test. Comparisons of more than two groups were performed using a one-way ANOVA followed by Tukey or Dunnett multiple comparison tests. Significance was considered as P<0.05.
Results
Aldosterone Rapidly Modulates NCC Activity in Native DCT Cells
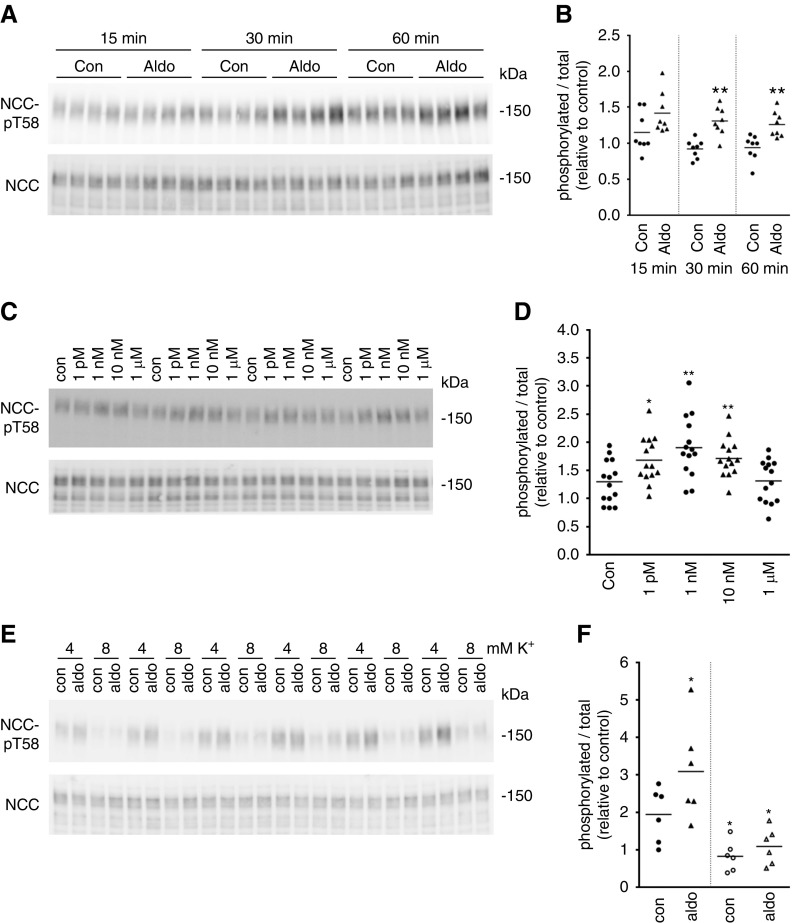
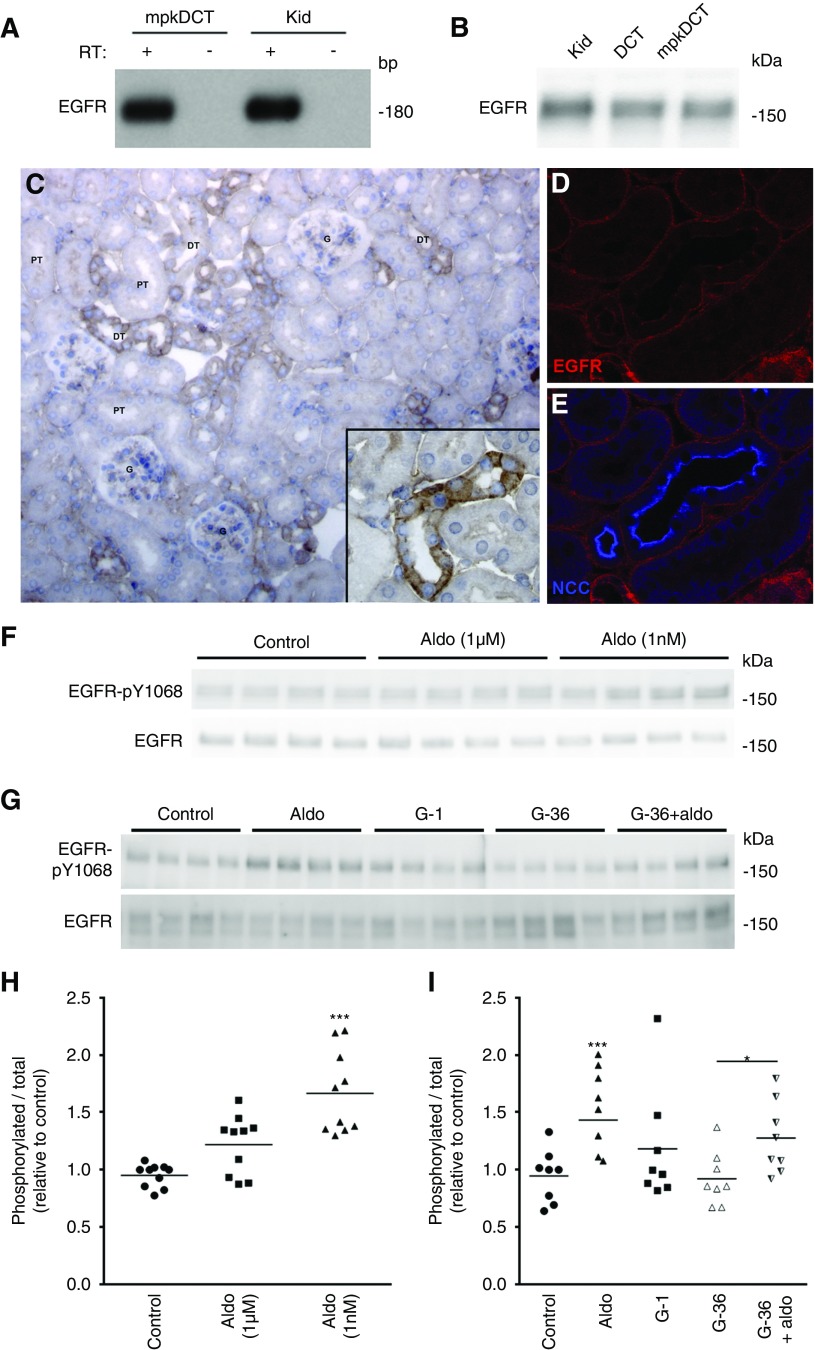
Kidneys were isolated from mice 60 minutes after a single intramuscular injection of aldosterone, and the phosphorylation levels of NCC at an activating site (T58) were examined (Figure 1A). Relative to total NCC, the levels of pT58-NCC were significantly increased with aldosterone (Figure 1B), with a positive and significant correlation between pT58-NCC and plasma aldosterone concentrations (Figure 1, C and D). These changes in pT58-NCC levels occurred independently of alterations in plasma Na+ or K+ levels (Figure 1, E–G). Similar observations were apparent in another cohort of mice 30 minutes after aldosterone injection (Supplemental Figure 1). To study the isolated effects of aldosterone, ex vivo cortical tubule suspensions were isolated from mouse kidney and incubated with aldosterone (1 nM) for 30 minutes. pT58-NCC levels normalized for total NCC levels were significantly elevated in the treated group relative to controls (Figure 1, H–I). Similar effects of aldosterone (1 nM) were observed in cortical tubule suspension experiments performed in the presence of the transcriptional inhibitor actinomycin D and the protein translation inhibitor cycloheximide (Figure 1, J and K), excluding a genomic role of aldosterone in the activation of NCC. Additional cortical tubule experiments determined that the effect of aldosterone on pT58-NCC levels occurred within 15 minutes (Figure 2, A and B), and that lower aldosterone concentrations (1 pM–10 nM) had a greater effect on pT58-NCC than 1 μM (Figure 2, C and D). However, the effects of aldosterone were not observed when similar cortical tubule experiments were performed in high K+ (8 mM)-containing media (Figure 2, E and F).
Figure 2.
Aldosterone effects on NCC activity in ex vivo cortical tubule suspensions are time and concentration dependent but absent in high K+ media. (A) Cortical tubule suspensions from male mice were treated with aldosterone (1 nM) for various times and NCC and NCC-pT58 levels were assessed by immunoblotting. (B) Summary of data (n=8/group). *: 0.01<P<0.05; **: 0.001<P<0.01 relative to control. (C) Cortical tubule suspensions from male mice were treated with various aldosterone concentrations (1 pM–1 μM) for 30 minutes and NCC-pT58 levels were assessed by immunoblotting. (D) Summary of data (n=14/group). *: 0.01<P<0.05; **: 0.001<P<0.01 relative to time-matched control. (E) Cortical tubule suspensions from male mice were preincubated in media containing 8 mM K+ before incubation with aldosterone (1 nM) for 30 minutes and NCC-pT58 levels were assessed by immunoblotting. (F) Summary of data (n=6/group). *: 0.01<P<0.05 relative to 4 mM control conditions. All data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. Aldo, aldosterone; Con, control.
Aldosterone Rapidly Increases cAMP and IP Levels and Phosphorylation of Kinases/Kinase Substrates within the MAPK/ERK, PI3K/AKT, and cAMP/PKA Signaling Pathways
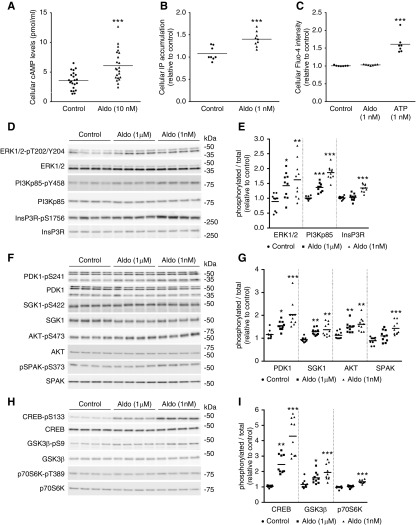
Although in our experience mpkDCT cells do not reliably express NCC, they are a good model to study signaling mechanisms in the DCT.22 The levels of three major intracellular second messengers in mpkDCT cells after 30 minutes of aldosterone treatment are shown in Figure 3. Intracellular cAMP levels were consistently increased by approximately 50% after aldosterone treatment (Figure 3A). Levels of total cellular IP were significantly increased after 30 minutes of aldosterone treatment (Figure 3B), suggesting aldosterone can activate an IP3-Ca2+ signaling pathway. However, within a similar timeframe no measurable differences in total cellular Ca2+ levels were observed (Figure 3C).
Figure 3.
Aldosterone rapidly increases cAMP and IP levels and induces phosphorylation of various kinases/kinase substrates within the MAPK/ERK, PI3K/AKT, and cAMP/PKA signaling pathways. (A) Intracellular cAMP levels were significantly increased in mpkDCT cells stimulated with aldosterone for 30 minutes. (B) Total cellular IP levels were significantly raised in mpkDCT cells stimulated with aldosterone for 30 minutes. (C) No significant differences in total intracellular Ca2+ levels were detectable after 30 minutes aldosterone treatment. ATP was used as a positive control. (D) mpkDCT cells were treated with aldosterone for 30 minutes and protein homogenates examined by immunoblotting with antibodies targeting phosphorylation sites of ERK1/2, PI3K, and InsP3R. (E) Summary of data from three independent experiments (n=10/group). (F) Immunoblotting with antibodies targeting phosphorylation sites of PDK1, SGK1, AKT, and SPAK. (G) Summary of data from three independent experiments (n=10/group). (H) Immunoblotting with antibodies targeting phosphorylation sites of CREB, GSK3β, and p70S6K. (I) Summary of data from three independent experiments (n=10/group). Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. *P<0.05; **P<0.01, ***P<0.001 relative to individual control group. Aldo, aldosterone; Con, control.
Alterations in intracellular cAMP and IP levels suggest that aldosterone signaling involves a variety of different pathways e.g., phosphoinositide 3-kinase (PI3K), PLC, PKC, and PKA.2 To examine this further, mpkDCT cells were treated with aldosterone (1 µM and 1 nM) for 30 minutes and phosphorylation levels (as an indicator of activity) relative to total protein abundances of various components of these major pathways were examined. Phosphorylation levels of ERK1/2 were increased (Figure 3, D and E), and in line with increased IP levels, phosphorylation of the inositol 1,4,5-triphosphate receptor (InsP3R) was increased at S1756 (Figure 3, D and E), a site known to modulate sensitivity of the receptor to IP3.31 Phosphorylation at Y458 on the p85 regulatory subunit, a site that has previously been reported to track with the activation of the PI3K complex,32 was also greatly increased by aldosterone (Figure 3, D and E). Combined, these data suggest that the rapid effects of aldosterone in mpkDCT cells involve activation of the MAPK/ERK pathway and the PI3K pathway. In line with this, aldosterone increased phosphorylation levels of two downstream targets of PI3K,33 namely PDK S241 and AKT S473 (Figure 3, F and G). Furthermore, aldosterone increased phosphorylation of SGK1 at S422 (Figure 3, F and G), which may be a converging node between the PKA pathway, likely activated because of the increased cAMP levels and the PDK pathway. PKA activation and convergence of PDK/PKA pathway are further suggested by increased phosphorylation of SPAK on S373 within its regulatory domain34 (Figure 3, F and G) and S133 of CREB (Figure 3, H and I). Supporting activation of AKT are increased levels of GSK3β phosphorylation at S9 (Figure 3, H and I). In addition, a small increase in phosphorylation of P70S6K at T389 (Figure 3, H and I) suggests that AKT-mediated activation of mTORC1 is also involved in the acute response of mpkDCT cells to aldosterone. Interestingly, in line with the ex vivo effects of aldosterone on pT58-NCC, for the majority of signaling molecules examined, a greater response to the lower aldosterone concentration (1 nM) was observed. This concentration was used in subsequent studies.
GPR30 Partially Mediates the Rapid Effects of Aldosterone in mpkDCT Cells
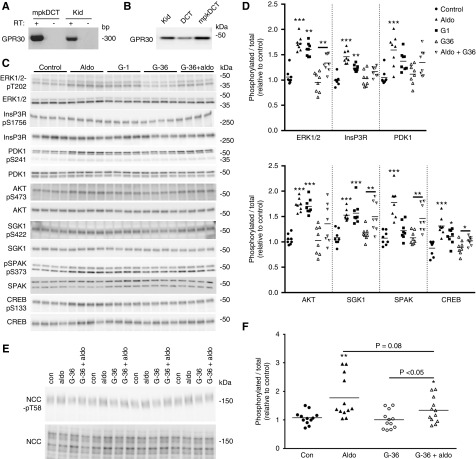
Rapid aldosterone effects are attributed to a variety of different receptors, including GPR30.7,35–38 RT-PCR confirmed GPR30 expression in mouse kidney and mpkDCT cells (Figure 4A). GPR30 was also detected in ex vivo mouse DCT cells purified by FACS (Figure 4B). To functionally address the role of GPR30, mpkDCT cells were treated for 30 minutes with aldosterone, the GPR30 agonist G-1, or aldosterone in the presence of the GPR30 antagonist G-36 (Figure 4C). Treatment with G-1 significantly increased the phosphorylation status of ERK1/2, InsP3R, AKT, CREB, and SGK (Figure 4D). In the presence of G-36, aldosterone was unable to significantly increase phosphorylation of InsP3R, PDK1, AKT, and CREB (Figure 4D). In the presence of G-36, phosphorylation of ERK1/2, SGK1, SPAK, and CREB was significantly increased by aldosterone, but the relative phosphorylation levels were attenuated versus aldosterone-only treatment (Figure 4D). Some of the effects of G-1 on mpkDCT cells may be due to altered cAMP levels, but the responses were highly variable (Supplemental Figure 2). Furthermore, in cortical tubule suspensions, the effects of aldosterone (1 nM) on pT58-NCC levels were partially blunted in the presence of G-36 (Figure 4, E and F). Together this ex vivo and in vitro data indicate that although some of the acute effects of aldosterone to activate NCC appear to be mediated via GPR30, by itself it cannot fully account for the signaling networks activated by aldosterone. Supporting a role of GPR30 in modulating NCC activity in vivo, the levels of phosphorylated NCC were significantly reduced in ovariectomized (both GPR307 and NCC39 respond to estradiol) GPR30 knockout mice (Supplemental Figure 3).
Figure 4.
GPR30 is expressed in mouse kidney DCT cells and partly mediates the rapid effects of aldosterone. (A) GPR30 is detected in mpkDCT or mouse kidney (Kid) samples by RT-PCR. (B) GPR30 is detected in protein homogenates from mouse kidney, mpkDCT cells or DCT cells purified from mouse kidney (DCT) by immunoblotting. (C) mpkDCT cells were treated for 30 minutes with aldosterone, the GPR30-specific agonist G-1 or aldosterone (1 nM) in the presence of the GPR30 antagonist G-36 and phosphorylation levels of various signaling proteins examined by immunoblotting. (D) Summary of data (n=8/group). Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. *P<0.05; **P<0.01, ***P<0.001. (E) Cortical tubule suspensions were treated for 30 minutes with G-36 where indicated before treatment with aldosterone (1 nM) for 30 minutes. NCC-pT58 levels were assessed by immunoblotting. (F) Summary of data. Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. *P<0.05; **P<0.01, ***P<0.001 relative to individual control group. Aldo, aldosterone, Con, control.
Systems-Level Analysis of Rapid Aldosterone-Mediated Signaling
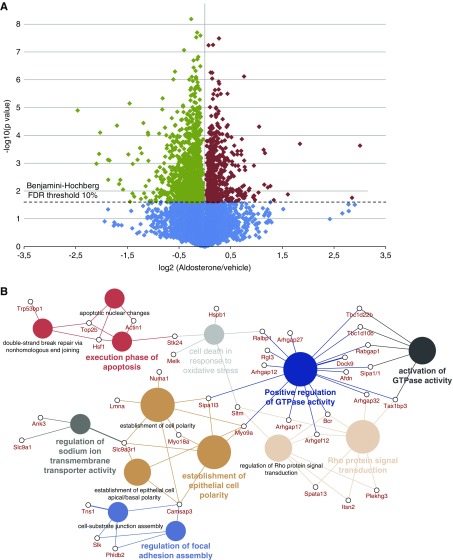
To uncover alternative pathways relaying the rapid aldosterone effects, a quantitative phosphoproteomics strategy was used to investigate the global cell signaling changes in mpkDCT cells (workflow in Supplemental Figure 4). By combining the LC-MS/MS data from four biologic replicates, 4723 unique protein groups were identified in mpkDCT cells (Supplemental Table 1). A total of 11,979 unique phosphorylation sites were identified on 1513 different proteins (Supplemental Table 2), of which 4432 sites were confidently assigned with a phosphoRS40 score >80. Of these, 3275 sites have a CPhos41 site conservation score >0.9, suggestive of biologic and functional importance. The distribution of phosphopeptides are represented as a volcano plot in Figure 5A. Phosphopeptides with large changes (log2 ratios all >0.75 or all <−0.75) were also retained for further analysis. In total, 602 phosphorylation sites significantly increased in abundance and 1178 sites were decreased significantly upon aldosterone treatment (Supplemental Table 2). Several of these sites are targets of the MAPK/ERK, cAMP/PKA, or PI3K/AKT signaling pathways. A similar proline-directed motif was suggested using Motif-X42,43 (Supplemental Figure 5A) or Icelogo (Supplemental Figure 5B)44 for the aldosterone-regulated phosphorylation sites. Gene Ontology term analysis of the proteins with regulated phosphorylation sites identified a predominance of proteins involved in biologic processes such as nucleic acid metabolic processes, cytoskeleton organization, and transcription or translation (Supplemental Figure 6A). Gene Ontology term analysis of proteins with greatly changed phosphorylation sites (log2 fold change >0.5 or <−0.5) suggest aldosterone actions are linked to establishment of epithelial polarity, signal transduction, and the regulation of Na+ ion transmembrane transporter activity (Supplemental Figure 6B). This analysis was further presented as a CluePedia45 view showing enriched genes that belong to the same biologic process groups or shared between processes (Figure 5B). Ingenuity Pathway Analysis using the regulated phosphorylation sites identified nine complex interlinked networks (Supplemental Figure 7A). The EGFR was predicted from the analysis of all complex networks to be an activated upstream signaling molecule (Supplemental Figure 7B). When EGFR was added to the top four predicted networks, it became a central network node connecting key kinases and subsequent substrates (Supplemental Figures 8 and 9).
Figure 5.
Systems-level analysis of rapid aldosterone-mediated signaling. mpkDCT cells were treated with aldosterone (1 nM) for 30 minutes and global phosphorylation levels determined using SILAC-based LC-MS/MS. (A) Volcano plot of phosphopeptide quantification from four independent experiments. y-axis indicates −log10 (P value) whereas the x-axis indicates the base2 logarithmic value of the mean peptide abundance ratio (aldosterone versus vehicle). Horizontal dashed line represents the Benjamini–Hochberg FDR threshold of 10% used for statistical significance. Phosphopeptides significantly increased are shown in red, and those decreased are shown in green. (B) CluePedia view of the Gene Ontology term analysis using regulated phosphopeptides with log2 ratios >0.5 or <−0.5. Genes belong to biologic process groups or shared between processes are shown.
EGFR Is Localized to Mouse DCT and Is Involved in the Rapid Aldosterone Response
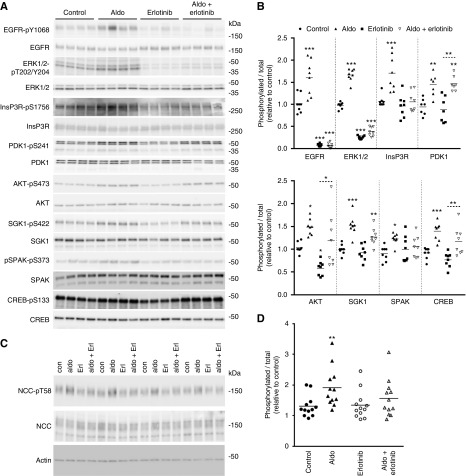
EGFR was identified in mpkDCT cells, mouse kidney and ex vivo FACS-purified mouse DCT cells (Figure 6, A and B). Immunohistochemistry on mouse kidney localized the EGFR to the majority of renal tubules, including the distal tubule (Figure 6C). Double-labeling with antibodies against NCC confirmed localization of the EGFR in the DCT (Figure 6, D and E), where it resides predominantly in the basolateral membrane domain. In mpkDCT cells treated with aldosterone for 30 minutes, phosphorylation of the EGFR at Y1068 (active site) was significantly increased (Figure 6, F and H). However, in the presence of G-36, aldosterone-induced phosphorylation of the EGFR was attenuated (Figure 6, G and I), suggesting some involvement of GPR30 in the aldosterone-induced activation of EGFR. Furthermore, when similar experiments were performed with dexamethasone in the cell culture media (limiting aldosterone selectivity for the MR), several of the effects of aldosterone, including increased phosphorylation of the EGFR, were diminished (Supplemental Figure 10). This suggests that the MR is involved in transactivation of the EGFR,46 or there is some crosstalk with the glucocorticoid receptor.47 To further assess the role of the EGFR, mpkDCT cells were treated either with aldosterone (1 nM) or aldosterone plus the EGFR inhibitor erlotinib (100 nM) for 30 minutes, and phosphorylation levels of various signaling components examined. As seen previously (Figure 3), aldosterone alone significantly increased phosphorylation of the EGFR, ERK1/2, InsP3R, PDK1, AKT, SGK, SPAK, and CREB (Figure 7). Erlotinib treatment alone suppressed phosphorylation of the EGFR, ERK1/2, and AKT, confirming inhibition of EGFR-dependent signaling. In the presence of erlotinib, aldosterone was unable to significantly increase phosphorylation of the EGFR, ERK1/2, InsP3R, SGK1, and SPAK. Furthermore, in cortical tubule suspensions, the effects of aldosterone (1 nM) to increase pT58-NCC levels were absent in the presence of erlotinib (Figure 7, C and D). Together these data indicate that the EGFR is involved in rapid aldosterone effects in DCT cells.
Figure 6.
The EGFR is localized to the DCT and activated by aldosterone. (A) EGFR is detected in mpkDCT or mouse kidney (Kid) samples by RT-PCR. (B) EGFR is detected by immunoblotting in protein homogenates from mouse kidney, mpkDCT cells or DCT cells purified from mouse kidney (DCT). (C) Immunohistochemistry of EGFR in mouse kidney sections. Staining is strongest in segments morphologically resembling distal tubules (DT) and is weaker in proximal tubules (PT). (D and E) Confocal images of mouse kidney sections immunolabeled for EGFR and colocalized with the DCT expressed protein NCC. (F) In mpkDCT cells treated with aldosterone for 30 minutes, phosphorylation of the EGFR at Y1068 (active site) was significantly increased. (G) In the presence of G-36, aldosterone had a reduced effect on EGFR phosphorylation levels. (H and I) Summary of data. Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. *P<0.05; ***P<0.001 relative to individual control group. Aldo, aldosterone; Con, control.
Figure 7.
The EGFR partially mediates the acute effects of aldosterone. (A) mpkDCT cells were treated with aldosterone (1 nM) or aldosterone plus the EGFR inhibitor erlotinib (100 nM) for 30 minutes and phosphorylation levels of various signaling proteins examined by immunoblotting. (B) Summary of data (n=8/group). Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. (C) Cortical tubule suspensions were treated for 30 minutes with erlotinib where indicated before treatment with aldosterone (1 nM) for 30 minutes. NCC-pT58 levels were assessed by immunoblotting. (D) Summary of data. Data are presented as phosphorylated levels relative to total protein levels and normalized relative to control conditions. *P<0.05; **P<0.01, ***P<0.001 relative to individual control group. Aldo, aldosterone; Con, control.
The EGFR Regulates NCC Activity In Vivo
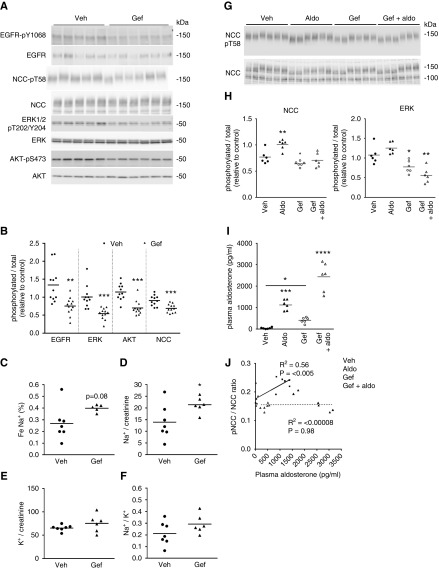
To examine if the EGFR plays a role for modulation of NCC activity in vivo, mice were treated for 3 hours with either the EGFR antagonist gefitinib or vehicle. Phosphorylation levels of the EGFR were significantly decreased after gefitinib treatment (Figure 8, A and B), confirming inhibition of the receptor. Concurrently ERK1/2, AKT, and NCC phosphorylation levels were decreased (Figure 8, A and B). These changes in NCC phosphorylation subsequent to EGFR inhibition correlated with a significantly raised urinary Na+-to-creatinine excretion and a marked trend for higher fractional excretion of Na+ (Figure 8, C–F). Similar observations on pT58-NCC levels were apparent in mice treated for 1 or 2 hours with gefitinib (Supplemental Figure 11). To further explore a role of the EGFR, mice were treated with either gefitinib or vehicle for 1 hour, plus either aldosterone or vehicle for 1 hour. Relative to vehicle, aldosterone significantly increased NCC-pT58 levels, but this was blunted by gefitinib (Figure 8, G and H), despite the fact that aldosterone levels in this group of mice were highest (Figure 8I). A significant positive correlation was observed between plasma aldosterone concentration and the NCC-pT58/total NCC ratio only in the absence of gefitinib (Figure 8J).
Figure 8.
In vivo inhibition of the EGFR reduces NCC activity and attenuates the effects of aldosterone on NCC activity. (A) Mice were treated for 3 hours with either the EGFR antagonist gefitinib (Gef) or vehicle and phosphorylation levels of various signaling proteins in the kidney examined by immunoblotting. (B) Summary of data (n=11/group). Data are presented as phosphorylated levels relative to total protein levels and normalized relative to vehicle. Phosphorylation levels of NCC at T58 were significantly reduced alongside phosphorylation of the EGFR, ERK1/2, and AKT. (C) Spot urine collected during the last 1-hour period (n=5–7/group) of EGFR inhibition demonstrated increased fractional excretion of Na+. (D) Significantly higher urinary Na+/creatinine excretion. (E) Urinary K+/creatinine excretion. (F) Urinary Na+ excretion relative to K+. *P<0.05; **P<0.01, ***P<0.001 relative to control. (G) Mice were treated with either the EGFR antagonist gefitinib (Gef) or vehicle (Veh) for 2 hours. One hour before the experiment was terminated animals were injected with aldosterone (aldo). Phosphorylation levels of NCC at T58 (NCC-pT58, surrogate marker of NCC activation) were assessed by immunoblotting. (H) Summary of NCC data (n=6). Data are presented as phosphorylated levels relative to total protein levels and normalized relative to vehicle. Aldosterone significantly increased NCC-pT58 levels, but this was limited by gefitinib. Similar samples were assessed for ERK phosphorylation as an indirect indication of successful EGFR inhibition. Summary data are shown. (I) Plasma aldosterone levels. (J) A significant positive correlation exists between plasma aldosterone concentration and the NCC-pT58/total NCC ratio in the absence of gefitinib (solid correlation line). In contrast, there was no correlation in the presence of gefitinib (dashed correlation line). *: 0.01<P<0.05; **: 0.001<P<0.01; ****: 0.0001<P<0.001; relative to vehicle group.
Discussion
The genomic actions of aldosterone to alter renal Na+ absorption and K+ secretion play an essential role in maintaining overall body Na+ and K+ balance. Although these aldosterone-induced genomic effects can occur within 60 minutes of aldosterone administration,48 the alterations in second messengers, the redistribution of ENaC, and increases in amiloride-sensitive Na+ currents that occur within seconds to minutes of aldosterone exposure cannot be explained by these mechanisms.3–5 Thus, other rapid aldosterone-mediated signaling mechanisms must exist. Given the important role of NCC in relaying aldosterone-dependent effects, we sought to determine whether aldosterone exerts acute modulation of this cotransporter and the signaling networks employed. A unique finding from this study is that within minutes, aldosterone increases the levels of intracellular cAMP and IP and activates several proteins in mpkDCT cells, including ERK1/2, SGK, AKT, PDK1, and SPAK, which have well established roles in modulating Na+ transport in the kidney. This ultimately leads to activation of the NaCl cotransporter NCC, a finding confirmed ex vivo and in vivo. Furthermore, altered phosphorylation of a variety of different transcription factors and translation initiation factors suggests that these early aldosterone effects act in concert with additional, but later onset genomic effects to modulate transcriptional and translational regulation in DCT cells. A simplified hypothetical signaling pathway for rapid aldosterone effects is illustrated in Figure 9.
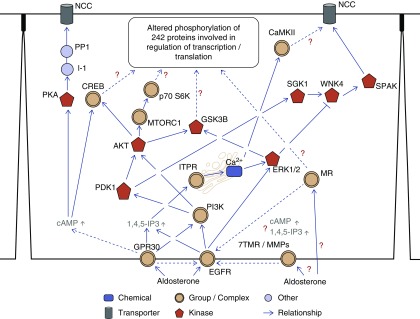
Figure 9.
Summary of potential rapid signaling mechanisms of aldosterone in the DCT. The nonbiased proteomics data were combined with data from immunoblotting experiments and current literature to generate a simplified hypothetical signaling pathway for rapid aldosterone effects in DCT cells. GPR30 and EGFR are involved in mediating some of the rapid effects of aldosterone, but a role for the MR, the other seven transmembrane receptors (7TMR), or matrix metalloproteinases (MMPs) requires further investigation. Dashed lines or question marks indicate potential pathways of aldosterone signaling that need further investigation.
The mechanisms by which aldosterone elicits rapid signaling responses in target cells are evolving. Because rapid effects occur using noncell-permeable aldosterone analogs,9,10 in the presence of transcriptional and translation inhibitors49 or in MR knockout mice,8 they are, at least in part, mediated by a plasma membrane-bound receptor. The increased total cellular IP and cAMP levels in mpkDCT cells within minutes of aldosterone application also suggests that a membrane-bound receptor is playing a role in the DCT. GPR30,7 which can signal via cAMP or IP3 and responds to aldosterone stimulation,13,14 was detected in both mpkDCT cells and the DCT in vivo, making this a possible candidate. The EGFR, which can be transactivated after stimulation of GPR30 or the MR,46 was also expressed in the DCT. Supporting a role of the EGFR and GPR30, in mpkDCT cells aldosterone acutely altered phosphorylation of various kinases/kinase substrates within the MAPK/ERK, PI3K/AKT, or cAMP/PKA signaling pathways, including ERK, PI3Kp85, PDK1, SGK1, AKT, CREB, and GSK3β, which are known to be modulated by GPR30 and/or EGFR-mediated signaling. A role of GPR30 was further strengthened by the inhibition of aldosterone-mediated InsP3R, PDK1, and AKT phosphorylation in the presence of the GPR30 antagonist G-36, whereas the EGFR antagonist erlotinib inhibited aldosterone-mediated increases in ERK1/2, InsP3R, SGK1, and SPAK phosphorylation. These data suggest that aldosterone-mediated signaling via the EGFR and GPR30 are not mutually exclusive, and some signaling pathways may be activated by one, or both receptors simultaneously. Alternatively, presuming the antagonists fully inhibit EGFR or GPR30-dependent signaling, these results indicate that GPR30 and/or EGFR alone are not fully accountable for the acute aldosterone effects in mpkDCT cells and that other mediators of rapid aldosterone effects in these cells must exist. The existence of alternative membrane receptors that may relay aldosterone effects, including IGF1R and PDGFR6, were identified in our nonbiased signaling networks generated from phosphoproteomics data. Nonetheless, as the aldosterone effects on phosphorylated NCC are reduced after inhibition of GPR30 or the EGFR in ex vivo cortical tubule suspensions, and pT58-NCC levels are reduced after genetic or pharmacologic inhibition of GPR30 or the EGFR in vivo, these receptor-dependent pathways are a novel mechanism by which NaCl transport is regulated in the DCT. Further studies, potentially including the generation of mice with DCT-specific deletion of the EGFR,50 are required to further assess the contributions of these receptors in the acute aldosterone response.
Another observation is that the largest changes in signaling pathways occurred at lower concentrations of aldosterone, suggesting that the acute actions of aldosterone to activate NCC occur within the normal physiological range of aldosterone.51–53 Why the higher concentration of aldosterone has a reduced response is unclear, but a similar biphasic effect on NCC activity occurs with angiotensin II.54 Another point of note is that although aldosterone consistently increased SPAK phosphorylation, a key mediator of NCC activation, these effects are relatively modest. This suggests that other SPAK-independent mechanisms may be involved, such as alterations in the rate of NCC dephosphorylation.55
The effects of aldosterone on NCC were initially assumed to be MR-dependent. However, recent studies indicate that the MR may be dispensable for the long-term effects of aldosterone on NCC56,57 and changes in NCC have instead been attributed to changes in plasma K+.58–60 Our studies demonstrate that rapidly aldosterone can increase phosphorylated NCC levels, independently of total NCC levels, in ex vivo cortical tubule suspensions or in vivo. This increase in phosphorylated NCC in mouse kidney was not positively correlated with plasma [K+] under these conditions. However, in cortical tubule suspensions pT58-NCC levels were significantly reduced in the presence of high [K+] and significant effects of short-term aldosterone treatment on phosphorylated NCC were inhibited. Together, this adds a new dimension to both the mechanisms and the time-course within which aldosterone affects the DCT to modulate NCC activity. When the DCT encounters aldosterone released because of hypovolemia, its main function is to rapidly increase NaCl reabsorption to help restore blood volume. Thus, it is of physiologic benefit that the positive effects of aldosterone on NCC activity occur within minutes. Conversely, when the DCT encounters aldosterone and high K+ concentrations in hyperkalemic states, the effects of high K+ appear to “overrule” the acute effects of aldosterone and reduce NCC activity, promoting K+ secretion in downstream portions of the renal tubule.61,62
In conclusion, we have identified novel signaling pathways by which aldosterone can exert rapid effects in the DCT. This study identifies an important new role of nongenomic aldosterone actions in the DCT that are likely to have major implications for modulation of NaCl transport and ultimately BP regulation.
Disclosures
None.
Funding
Funding (to Fenton and Dimke) is provided by the Novo Nordisk Foundation (including Fabrikant Vilhelm Pedersen og Hustrus Mindelegat), the Lundbeck Foundation, and the Danish Medical Research Council. Fenton is further supported by the Leducq Foundation. Wu is supported by an EU Horizon 2020 Marie Skłodowska-Curie Individual Fellowship (project no. 705682) and the Danish Medical Research Council (reference no. 6110-00118B). Lundberg is supported by the Swedish Research Council (2016-02427) and the Swedish Cancer Foundation (CAN 2016/423). Rieg is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK110621).
Supplementary Material
Acknowledgments
We would like to acknowledge the technical assistance of Inger-Merete Paulsen, Helle Høyer, Tina Drejer, Ahmed Abduljabar, Lene Bundgaard Andersen, and Christian Westberg. Marleen Kortenoeven is thanked for FACS isolation of DCT cells.
Fenton made the conception and initial design of the work. Cheng, Wu, Olesen, Poulsen, Peng, Olde, Leeb-Lundberg, Pisitkun, Dimke, Rieg, Esteva-Font, and Fenton acquired data, analyzed and interpreted data, or developed new reagents. Cheng and Fenton drafted the manuscript. All authors critically revised the manuscript for intellectual content, approved the final version of the manuscript, and agree to be accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101025/-/DCSupplemental.
Supplemental Figure 1. Aldosterone rapidly modulates NCC activity in native DCT cells.
Supplemental Figure 2. Effects of G-1 and G-36 on cAMP levels in mpkDCT cells.
Supplemental Figure 3. Phosphorylated NCC levels are reduced in GPR30 knockout mice.
Supplemental Figure 4. Schematic overview of the experimental phosphoproteomics workflow.
Supplemental Figure 5. Information based sequence logo of the up- and downregulated phosphorylation motifs following aldosterone stimulation of mpkDCT cells.
Supplemental Figure 6. GO-term biological processes analysis by Clue-Go.
Supplemental Figure 7. IPA pathway analysis of aldosterone signaling events in mpkDCT cells.
Supplemental Figure 8. Interaction Network Analysis of the top 4 networks demonstrates that aldosterone stimulation mediates a highly interlinked signaling network.
Supplemental Figure 9. Interaction Network Analysis of the top 4 networks demonstrates that aldosterone stimulation mediates a highly interlinked signaling network.
Supplemental Figure 10. The MR may be involved in the rapid aldosterone mediated transactivation of the EGFR.
Supplemental Figure 11. Inhibition of the EGFR in vivo acutely reduces NCC activity.
References
- 1.Terker AS, Ellison DH: Renal mineralocorticoid receptor and electrolyte homeostasis. Am J Physiol Regul Integr Comp Physiol 309: R1068–R1070, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong GS, Young MJ: Mineralocorticoid regulation of cell function: The role of rapid signalling and gene transcription pathways. J Mol Endocrinol 58: R33–R57, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Zhou ZH, Bubien JK: Nongenomic regulation of ENaC by aldosterone. Am J Physiol Cell Physiol 281: C1118–C1130, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Quinn S, Harvey BJ, Thomas W: Rapid aldosterone actions on epithelial sodium channel trafficking and cell proliferation. Steroids 81: 43–48, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Dooley R, Angibaud E, Yusef YR, Thomas W, Harvey BJ: Aldosterone-induced ENaC and basal Na+/K+-ATPase trafficking via protein kinase D1-phosphatidylinositol 4-kinaseIIIβ trans Golgi signalling in M1 cortical collecting duct cells. Mol Cell Endocrinol 372: 86–95, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Ruhs S, Nolze A, Hübschmann R, Grossmann C: 30 years of the mineralocorticoid receptor: Nongenomic effects via the mineralocorticoid receptor. J Endocrinol 234: T107–T124, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Feldman RD, Limbird LE: GPER (GPR30): A nongenomic receptor (GPCR) for steroid hormones with implications for cardiovascular disease and cancer. Annu Rev Pharmacol Toxicol 57: 567–584, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Haseroth K, Gerdes D, Berger S, Feuring M, Günther A, Herbst C, et al.: Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor-knockout mice. Biochem Biophys Res Commun 266: 257–261, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Ashton AW, Le TY, Gomez-Sanchez CE, Morel-Kopp MC, McWhinney B, Hudson A, et al.: Role of nongenomic signaling pathways activated by aldosterone during cardiac reperfusion injury. Mol Endocrinol 29: 1144–1155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Moëllic C, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, et al.: Early nongenomic events in aldosterone action in renal collecting duct cells: PKCalpha activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol 15: 1145–1160, 2004 [PubMed] [Google Scholar]
- 11.Feldman RD, Limbird LE: Copernicus revisited: Overturning ptolemy’s view of the GPER universe. Trends Endocrinol Metab 26: 592–594, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, Thomas P, et al.: Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol 382: 950–959, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Ren Y, D’Ambrosio MA, Garvin JL, Leung P, Kutskill K, Wang H, et al.: Aldosterone sensitizes connecting tubule glomerular feedback via the aldosterone receptor GPR30. Am J Physiol Renal Physiol 307: F427–F434, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmeister MV, Damkier HH, Christensen BM, Olde B, Fredrik Leeb-Lundberg LM, Fenton RA, et al.: 17β-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am J Physiol Renal Physiol 302: F358–F368, 2012 [DOI] [PubMed] [Google Scholar]
- 15.van der Lubbe N, Lim CH, Meima ME, van Veghel R, Rosenbaek LL, Mutig K, et al.: Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflugers Arch 463: 853–863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolley MJ, Wu A, Xu S, Gordon RD, Fenton RA, Stowasser M: In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol 28: 56–63, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulsen SB, Christensen BM: Long-term aldosterone administration increases renal Na+-Cl- cotransporter abundance in late distal convoluted tubule. Am J Physiol Renal Physiol 313: F756–F766, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Ko B, Mistry AC, Hanson L, Mallick R, Wynne BM, Thai TL, et al.: Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am J Physiol Renal Physiol 305: F645–F652, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA: Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, et al.: Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem 47: 757–768, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Wu Q, Kortenoeven MLA, Pisitkun T, Fenton RA: A systems level analysis of vasopressin-mediated signaling networks in kidney distal convoluted tubule cells. Sci Rep 5: 12829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandish PE, Hill LA, Zheng W, Scolnick EM: Scintillation proximity assay of inositol phosphates in cell extracts: High-throughput measurement of G-protein-coupled receptor activation. Anal Biochem 313: 311–318, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Moeller HB, Stevens DA, Sanchez-Hodge R, Childers G, Kortenoeven MLA, et al.: CHIP regulates aquaporin-2 quality control and body water homeostasis. J Am Soc Nephrol 29: 936–948, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller HB, Knepper MA, Fenton RA: Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaek LL, Kortenoeven ML, Aroankins TS, Fenton RA: Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289: 13347–13361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulsen SB, Kristensen TB, Brooks HL, Kohan DE, Rieg T, Fenton RA: Role of adenylyl cyclase 6 in the development of lithium-induced nephrogenic diabetes insipidus. JCI Insight 2: e91042, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, et al.: Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150: 687–698, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Windahl SH, Andersson N, Chagin AS, Mårtensson UE, Carlsten H, Olde B, et al.: The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab 296: E490–E496, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Vizcaíno JA, Csordas A, del-Toro N, Dianes JA, Griss J, Lavidas I, et al.: 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 44[D1]: D447–D456, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR: Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem 277: 39397–39400, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Warfel NA, Niederst M, Newton AC: Disruption of the interface between the pleckstrin homology (PH) and kinase domains of Akt protein is sufficient for hydrophobic motif site phosphorylation in the absence of mTORC2. J Biol Chem 286: 39122–39129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risso G, Blaustein M, Pozzi B, Mammi P, Srebrow A: Akt/PKB: One kinase, many modifications. Biochem J 468: 203–214, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Gagnon KB, Delpire E: On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl- cotransport activity. Am J Physiol Cell Physiol 299: C614–C620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, et al.: Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148: 3236–3245, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Filardo EJ: Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: A novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80: 231–238, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, et al.: GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 57: 442–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER: A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Rojas-Vega L, Reyes-Castro LA, Ramírez V, Bautista-Pérez R, Rafael C, Castañeda-Bueno M, et al.: Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am J Physiol Renal Physiol 308: F799–F808, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Taus T, Köcher T, Pichler P, Paschke C, Schmidt A, Henrich C, et al.: Universal and confident phosphorylation site localization using phosphoRS. J Proteome Res 10: 5354–5362, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Zhao B, Pisitkun T, Hoffert JD, Knepper MA, Saeed F: CPhos: A program to calculate and visualize evolutionarily conserved functional phosphorylation sites. Proteomics 12: 3299–3303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou MF, Schwartz D: Biological sequence motif discovery using motif-X. Curr Protoc Bioinformatics Chapter 13: Unit 13.15–13.24, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Schwartz D, Gygi SP: An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol 23: 1391–1398, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Colaert N, Helsens K, Martens L, Vandekerckhove J, Gevaert K: Improved visualization of protein consensus sequences by iceLogo. Nat Methods 6: 786–787, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Bindea G, Galon J, Mlecnik B: CluePedia cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 29: 661–663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermidorff MM, de Assis LV, Isoldi MC: Genomic and rapid effects of aldosterone: What we know and do not know thus far. Heart Fail Rev 22: 65–89, 2017 [DOI] [PubMed] [Google Scholar]
- 47.D’Uva G, Lauriola M: Towards the emerging crosstalk: ERBB family and steroid hormones. Semin Cell Dev Biol 50: 143–152, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Funder JW, Pearce PT, Smith R, Smith AI: Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988 [DOI] [PubMed] [Google Scholar]
- 49.Dooley R, Harvey BJ, Thomas W: Non-genomic actions of aldosterone: From receptors and signals to membrane targets. Mol Cell Endocrinol 350: 223–234, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al.: Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science 269: 230–234, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Kerstens MN, Kobold AC, Volmer M, Koerts J, Sluiter WJ, Dullaart RP: Reference values for aldosterone-renin ratios in normotensive individuals and effect of changes in dietary sodium consumption. Clin Chem 57: 1607–1611, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Baas SJ, Endert E, Fliers E, Prummel MF, Wiersinga WM: Establishment of reference values for endocrine tests. III: Primary aldosteronism. Neth J Med 61: 37–43, 2003 [PubMed] [Google Scholar]
- 53.Graudal NA, Galløe AM, Garred P: Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: A meta-analysis. JAMA 279: 1383–1391, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Ko B, Mistry A, Hanson L, Mallick R, Hoover RS: Mechanisms of angiotensin II stimulation of NCC are time-dependent in mDCT15 cells. Am J Physiol Renal Physiol 308: F720–F727, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, et al.: Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25: 511–522, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J: The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468: 849–858, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al.: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenbaek LL, Rizzo F, MacAulay N, Staub O, Fenton RA: Functional assessment of sodium chloride cotransporter NCC mutants in polarized mammalian epithelial cells. Am J Physiol Renal Physiol 313: F495–F504, 2017 [DOI] [PubMed] [Google Scholar]
- 60.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, et al.: Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and independent mechanisms. J Physiol 594: 6319–6331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadchouel J, Ellison DH, Gamba G: Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Welling PA: Roles and regulation of renal K channels. Annu Rev Physiol 78: 415–435, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.