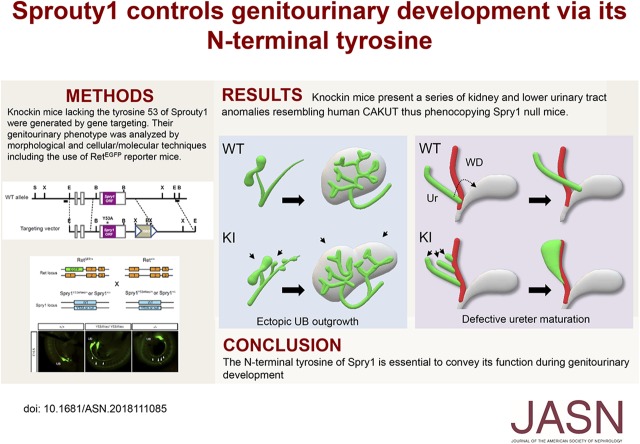
Significance Statement
Sprouty1 is a critical regulator of genitourinary development that ensures only one kidney forms on each side of the embryo. The molecular mechanisms that Sprouty1 uses to do this are largely unknown. The authors show that removing a single tyrosine from Sprouty1 in mice is enough to inactivate its function during genitourinary development. Knockin mice lacking this tyrosine develop supernumerary kidneys, megaureter, and vesicoureteral reflux, the same defects seen in Sprouty1 knockout mice. These findings shed light on the elusive mechanisms of action of Sprouty proteins and provide a valuable tool to investigate the developmental origin of human congenital anomalies of kidney and lower urinary tract.
Keywords: cell signaling, genetics and development, kidney development, pediatric nephrology, ureteric bud, vesico-ureteral reflux
Visual Abstract
Abstract
Background
Studies in mice suggest that perturbations of the GDNF-Ret signaling pathway are a major genetic cause of congenital anomalies of the kidney and urinary tract (CAKUT). Mutations in Sprouty1, an intracellular Ret inhibitor, results in supernumerary kidneys, megaureters, and hydronephrosis in mice. But the underlying molecular mechanisms involved and which structural domains are essential for Sprouty1 function are a matter of controversy, partly because studies have so far relied on ectopic overexpression of the gene in cell lines. A conserved N-terminal tyrosine has been frequently, but not always, identified as critical for the function of Sprouty1 in vitro.
Methods
We generated Sprouty1 knockin mice bearing a tyrosine-to-alanine substitution in position 53, corresponding to the conserved N-terminal tyrosine of Sprouty1. We characterized the development of the genitourinary systems in these mice via different methods, including the use of reporter mice expressing EGFP from the Ret locus, and whole-mount cytokeratin staining.
Results
Mice lacking this tyrosine grow ectopic ureteric buds that will ultimately form supernumerary kidneys, a phenotype indistinguishable to that of Sprouty1 knockout mice. Sprouty1 knockin mice also present megaureters and vesicoureteral reflux, caused by failure of ureters to separate from Wolffian ducts and migrate to their definitive position.
Conclusions
Tyrosine 53 is absolutely necessary for Sprouty1 function during genitourinary development in mice.
Congenital anomalies of the kidney and urinary tract (CAKUT) is a group of diseases affecting approximately 1 of 500 of human births, and the main cause of pediatric CKD. CAKUT includes a broad spectrum of developmental defects including renal agenesis, hydronephrosis, vesicoureteral junction obstruction, megaureter, and vesicoureteral reflux.1
Development of definitive (metanephric) kidneys begins around embryonic day 10.5 (E10.5) with an outgrowth of the Wolffian duct (WD) termed the ureteric bud (UB), which will repeatedly branch to ultimately generate the ureter and the collecting duct system of the kidney. Both the emergence and ulterior branching of the UB are triggered by signals emanating from the receptor tyrosine kinase Ret, which is expressed in the UB epithelium. Ret becomes activated upon binding to its ligand glial cell line–derived neurotrophic factor (GDNF), secreted by the adjacent metanephric mesenchyme. Knockout mice lacking GDNF, Ret, or its coreceptor GDNF family receptor α-1 (GFRα1) fail to generate the UB and consequently die at birth due to renal agenesis.2 Later during development, ureters detach from the WD and migrate to their final position at the bladder in a process known as ureter maturation. This remodeling process involves apoptosis of the portion of the WD between the bladder wall and the ureter, known as the common nephric duct (cnd), as well as migration of the distal tip of ureters to their definitive location at the bladder wall.3,4 Defective ureter maturation leads to vesicoureteral junction obstruction, hydronephrosis, hydroureter, or vesicoureteral reflux. Interestingly, the GDNF-GFRα1-Ret signaling axis is also a major genetic pathway governing ureter maturation.5
The Sprouty family of genes is composed of four members in mammals (Spry1–4), orthologous to a single Drosophila melanogaster gene (dSpry). The dSpry gene product is a 63-kDa protein containing a cysteine-rich domain (CRD) in its carboxy terminus which is conserved across species.6 Outside the CRD, there is little sequence homology between Sprouty family members, except for a short stretch of amino acids surrounding a conserved tyrosine (tyrosine 53 in Spry1), and a serine-rich domain present in all four mammalian Sprouty proteins but poorly conserved in dSpry.7
Genetic experiments in mice clearly establish that Sprouty family members are negative regulators of signaling by Ret during development. Thus, deletion of Spry2 leads to hyperplasia of the enteric nervous system as a result of excessive Ret signaling,8 whereas Spry1 antagonizes Ret signaling during kidney morphogenesis.9,10 Mice deficient in Sprouty1 grow more than one UB per side of the embryo, thus giving rise to supernumerary kidneys that fuse together into a single anatomic unit. At birth, these animals also present massively dilated ureters (megaureters), multiple cystic cavities inside the kidney parenchyma, and dilation of kidney tubules (hydronephrosis). Epistasis experiments reveal that the underlying cause of such phenotype is excessive activation of the GDNF-GFRα1-Ret signaling axis.9,11,12
Both the molecular mechanisms and the structural domains critical for Sprouty function are far from being understood. The CRD has been shown to be important for homo- and hetero-oligomerization of Spry family members, as well as for translocation to the plasma membrane upon growth factor stimulation. A short stretch of amino acids within the CRD that binds to Raf-1 have also been shown to be important for Sprouty function. Srpy1 and Spry2 also bind to caveolin-1 through a conserved arginine located on their carboxy termini.7,13,14 Finally, the role of the conserved tyrosine in the amino-terminal (N-terminus) of Spry family members (tyrosine 53 in Spry1) is controversial. Thus, mutation of this tyrosine prevents the ability of Spry proteins to inhibit signaling by receptor tyrosine kinases in some models,15–17 but fails to do so in others.18 Although these discrepancies may reflect context-specific mechanisms of action of Sprouty proteins, they could also be a consequence of the experimental setting used in these experiments, which mainly consist of ectopic overexpression in cell lines.
To begin to address this question we have generated knockin mice bearing a tyrosine-to-alanine mutation in residue 53 of Spry1. Analysis of Spry1 knockin mice indicate that this tyrosine is absolutely required for Spry1 function during genitourinary (GU) development, because knockin mice faithfully phenocopy the CAKUT observed in Spry1 knockout mice. Mice lacking this tyrosine present numerous ectopic UBs along the WD that will ultimately generate supernumerary kidneys. Ureter maturation is also defective in these animals because ureters fail to detach from the WD and migrate to their final position, leading to vesicoureteral reflux. Finally, a series of internal genitalia malformations, likely secondary to ureter maturation defects, are also present in Spry1 knockin mice.
Methods
Mice
All animal use was approved by the Animal Care Committee of the University of Lleida in accordance with the national and regional guidelines. Mice were maintained in a 12-hour light/dark cycle, and food and water was provided ad libitum. Mice knockout for Spry1 (Spry1tm1.1Jdli) is described elsewhere.9 Ret enhanced green fluorescent protein (EGFP) mice (Rettm13.1Jmi)19 were a gift from Dr. Sanjay Jain (Washington University, St Louis). Both knockin and knockout mice used in this article were on a mixed 50% C57BL/6 and 50% 129Sv genetic background.
Generation of Spry1Y53ANeo Knockin Mice
The targeting vector consisted of an EcoRI fragment of genomic DNA of approximately 11.5 Kb and included the three exons of Spry1, cloned into pBluescript.9 A change of two nucleotides (TAC to GGC) in the third exon was introduced, converting tyrosine 53 into alanine. A neomycin-resistant cassette flanked by flippase recognition target sites was inserted into the BclI site for positive selection. For negative selection, a thymidine kinase cassette driven by the phosphoglycerate kinase promoter was cloned into the SalI site of pBluescript. This vector was linearized with AdhI, and E14 embryonic stem (ES) cells were electroporated and selected in the presence of 0.250 mg/ml G418 plus 2 µM ganciclovir for 8 days. A total of 508 resistant clones were isolated and grown in 96-well plates. The presence of correctly recombined clones was screened by PCR using primers 396F (5′- GTATAGGAACTTCTGATCATGATGGC-3′) and 409R (5′-CACCTAACAAAACCACAAGCTTAAGACC-3′), and a mixture of 30U Taq:1U Pfx (ThermoFisher). Correctly recombined clones yielded an approximately 4.5-kb band that was resolved in 0.7% agarose gels. Genomic DNA from Spry1 knockout mice was used as a positive control because it was constructed using the same strategy. PCR-positive clones were confirmed by Southern blot. Briefly, genomic DNA was digested overnight with either XbaI (5′ arm) or BglII (3′ arm). Membranes were hybridized with digoxigenin-labeled probes generated by PCR using the PCR DIG Labeling Mix (catalog no. 1 835 289 910; Roche) as per manufacturer’s instructions, along with primers 390F (5′-ACACAATGGAGGCATTTAAGCAGAA-3′) and 391R (5′-ACGGTTATGTGTGGAATTACATGCA-3′) for the 5′ arm probe, and 392F (5′-CTAACCATTGAGCTACCTCTCCAGCC-3′) and 393R (5′-GCTGTGACATAACCCCATGACCAAGG-3′) for the 3′ arm probe. Blots were developed on x-ray films using CDP-Star (Roche) as a substrate. Two ES clones, 4AD4 and 6DA1, were independently injected to obtain two lines of mice bearing the mutation. The phenotypes of these mice were indistinguishable from each other. Most of the results were obtained with the 4AD4 clone. Spry1Y53ANeo mice were genotyped by PCR using primers 429F (5′-TATCAGCCAAGGGTTTCACTACGAG-3′), 430R (5′-TCTAAGAGCACCTCAGAAAGCCAGA-3′), and 431R (5′-CTACCGGTGGATGTGGAATGTGT-3′). After removal of the Neo cassette, Spry1Y53A mice were genotyped using primers 429F and 430R.
Western Blot and Immunoprecipitation
Protein extracts from mouse tissues were obtained by mechanical lysis at 4°C with nonidet P-40 (NP-40) buffer (1% NP-40, 150 mM sodium chloride, 50 mM HEPES, 1 mM EDTA, and 1 mM EGTA) supplemented with Protease Inhibitor Cocktail (Roche) and phosphatase inhibitors (1 mM sodium orthovanadate, 10 mM sodium fluoride, and 50 mM β-glycerophosphate). Western blot was performed as described.20 For immunoprecipitation, cells were lysed with NP-40 buffer supplemented with protease and phosphatase inhibitors as above. Protein was immunoprecipitated overnight at 4°C with the indicated antibodies plus Protein A/G Magnetic Beads (Pierce). Antibodies used were as follows: Anti-Spry1 (catalog no. 13013 for Western blot and catalog no. 12993 for immunoprecipitation; Cell Signaling), anti–β-actin (sc-1616; Santa Cruz), anti-phosphotyrosine (catalog no. 05–321; Millipore), anti-p53 (catalog no. sc-126; Santa Cruz), anti-p21Cip1 (catalog no. 05–345; Millipore), anti-p19Arf (catalog no. ab80; Abcam), and anti-Spry2 (catalog no. S1444; Sigma).
Subcellular Fractionation and Palmitoylation Assay
Subcellular fractionation was performed using the Subcellular Protein Fractionation Kit for Cultured Cells from Pierce, following the manufacturer’s instructions. Antibodies used to confirm purity of the fractions included anti–caveolin-1 (catalog no. 610406; BD), anti– glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) (catalog no. 8245; Abcam), anti–SP-1 (catalog no. sc-17824; Santa Cruz), and anti-vimentin (catalog no. 550513; BD). Palmitoylation assays were performed using the acyl-resin-assisted capture pull-down technique exactly as described.21 Briefly, after blocking free thiols with methyl methanethiosulfonate (Sigma), lysates were split in half and palmitoyl thioester linkages were cleaved using hydroxylamine, or left intact. Newly liberated thiols were captured with thiopropyl Sepharose (Sigma) pull-down, and resolved by SDS-PAGE.
Immunofluorescence and Immunohistochemistry
Frozen sections were blocked with blocking buffer (4% BSA, 1% Triton X-100, 100 mM glycine in PBS) for 1 hour at room temperature and incubated with either FITC–Dolichos biflorus agglutinin (DBA) (Vector) or Biotin-Lotus tetragonolobus agglutinin (LTA) (Vector) diluted in blocking buffer for an additional hour at room temperature. Slides containing Biotin-LTA were then incubated with DyLight 594–labeled streptavidin (Jackson Immunoresearch) for 1 hour at room temperature. For uroplakin staining, sections were blocked as above and incubated overnight at 4°C with a rabbit polyclonal antibody (AUM 745) generously provided by Dr. Tung-Tien Sun (New York University). The next day, sections were incubated with Dylight 549–labeled secondary antibodies (Jackson). For Wilms’ Tumor 1 (DAKO) immunohistochemistry, paraffin sections were dewaxed and rehydrated using a xylene/ethanol gradient followed by antigen retrieval (95°C for 20 minutes in Tris/EDTA buffer, pH 9) using a PTLink apparatus (DAKO). Staining was performed by an Autostainer device (DAKO). Sections were counterstained with hematoxylin.
Quantitative RT-PCR
Total RNA was extracted using TRIZOL reagent (ThermoFisher). RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher) as per manufacturer’s instructions. Quantitative RT-PCR reactions were performed by means of the SYBR green method, using a 2× Master Mix qPCR Low ROX Kit (PCR Biosystems). The 2−ΔΔCt method was used, normalizing to actin expression. Reverse transcription-minus and blank reactions were included in all experiments. We used the following primers: actin, 616F: 5′-TTCTTTGCAGCTCCTTCGTT-3′ and 617R: 5′- ATGGAGGGGAATACAGCCC-3′; Spry1, 471F: 5′-CTCTGCGGGCTAAGGAGC-3′ and 472R: 5′-ACGCCGGCTGATCTTGC-3′; and Spry2, 620F: 5′-AGAGGATTCAAGGGAGAGGG-3′ and 621R: 5′-AGAGGATTCAAGGGAGAGGG-3′.
Whole-Mount Immunofluorescence
For EGFP fluorescence, the GU system of embryos of the indicated ages was dissected and directly photographed under a Nikon SMZ18 fluorescence stereoscope coupled to a Nikon DS-Ri2 camera. For whole-mount cytokeratin staining, embryos were fixed overnight at 4°C in 4% paraformaldehyde. Their GU systems were dissected the next day and blocked in blocking buffer (4% BSA, 1% Triton X-100,100 mM glycine, 0.2% sodium azide in PBS) overnight at 4°C with gentle agitation. GU systems were then incubated with a 1:100 dilution of anti-cytokeratin antibody (DSHB, TROMA-I) in blocking buffer for 3–5 days at 4°C. GU systems were then washed three times in PBS 1% Triton X-100 for 2 hours each at room temperature, and incubated with fluorescently labeled secondary antibodies (Jackson Immunoresearch) in blocking buffer overnight at 4°C. Tissues were cleared in 1:2 benzyl alcohol/benzyl benzoate after being dehydrated through a graded series of methanol. Specimens were placed on coverslips and imaged using an Olympus Fluoview FV1000 confocal laser scanning microscope.
Generation and Validation of Phospho-Y53 Spry1–Specific Antibody
The anti–phospho-Y53 Spry1 antibody was produced by Abyntek (Derio, Spain). Rabbits were immunized with the peptide GSNEpYTEGPSVARRPAPRC conjugated to keyhole limpet hemocyanin, and antiserum was affinity purified using the same phosphopeptide. We further purified phospho-antibodies by adsorbing them against the corresponding nonphosphopeptide attached to AminoLink Resin (ThermoFisher). 293T cells were transfected with plasmids coding for wild-type or Y53A hemagglutinin-tagged mouse Spry1 using the calcium phosphate method. Before lysis, cells were stimulated or not with 50 ng/ml fibroblast growth factor (FGF) (Preprotech) for 10 minutes. Lysates were subjected to Western blot using the pY53 Spry1–purified antibody diluted 1:1000, antibodies to hemagglutinin (catalog no. 00000001186742300; Sigma), phospho–extracellular signal–regulated kinase (ERK) (catalog no. 675502; BioLegend), or actin (catalog no. sc-1616; Santa Cruz).
Results
Generation of Spry1Y53ANeo Knockin Mice
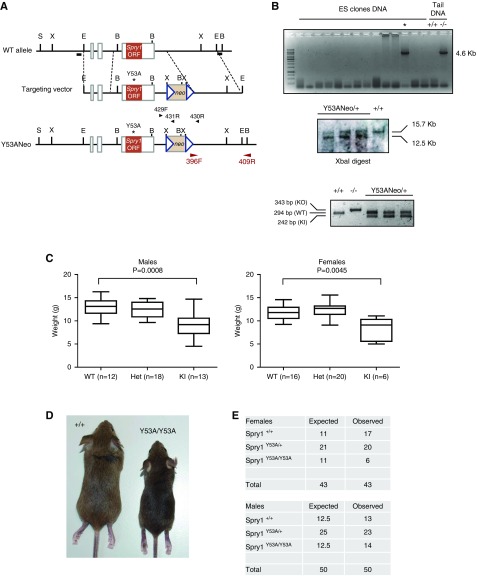
To explore the role of Spry1 tyrosine 53 in vivo, we generated knockin mice bearing a tyrosine-to-alanine mutation in this residue. We followed the same strategy used to generate Spry1 knockout mice,9 but the targeting vector lacked loxP sites flanking the Spry1 single coding exon and contained a single nucleotide change generating a Y53A amino acid substitution instead (Figure 1A). ES cells were electroporated with the linearized construct and G418-resistant clones were screened for homologous recombination by PCR as described in the Methods section (Figure 1B, top panel). Targeted insertion of both homology arms in PCR-positive clones was confirmed by Southern blot (Figure 1B, middle panel). The presence of the point mutation was confirmed by sequencing. Two independent ES clones, 4AD4 and 6DA1, were used to generate heterozygous knockin mice bearing the Y53A mutation plus the Neo cassette (Spry1Y53ANeo/+). We next crossed Spry1Y53ANeo/+ animals to obtain knockin mice and examined their offspring. Spry1Y53ANeo/Y53ANeo were born at the expected Mendelian proportions (data not shown). At weaning, knockin mice were slightly smaller than wild-type littermates (Figure 1, C and D) and the number of homozygous mutant females recovered was less than expected, although this trend was not statistically significant (P=0.249 by chi-squared test) (Figure 1E).
Figure 1.
Generation of Spry1Y53A knockin mice. (A) Targeting strategy used, including primers used for PCR screening and genotyping, and Southern blot probes (black horizontal bars). (B) A first screening was performed by using PCR using primers 396F and 409R (top panel). Tail DNA from Spry1−/− mice served as positive control. PCR-positive clones (asterisk) were confirmed by Southern blot (middle panel), and SpryY53ANeo/+ mice were genotyped using primers 429F, 430R, and 431R (lower panel). (C and D) Reduced weight of male and female knockin mice at weaning. P values were calculated by the Mann–Whitney test. (E) Allelic frequencies at weaning (3–4 weeks). S, SpeI; X, XbaI; E, EcoRI; B, BglII; Het, heterozygous; KI, knockin; KO, knockout; ORF, open reading frame; WT, wild type.
Tyrosine 53 is the Major Phosphotyrosine of Spry1
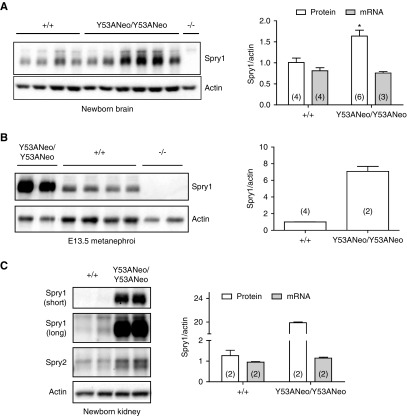
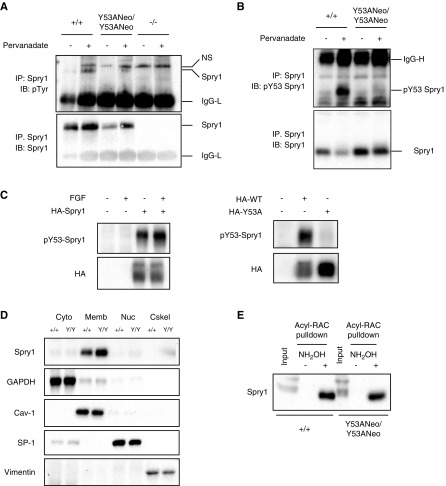
Immunoblot analysis revealed that Spry1 was present at slightly increased levels in brains from newborn Spry1Y53ANeo/Y53ANeo when compared with those from wild-type littermates (Figure 2A, left panel). Importantly, tissues from knockout mice confirmed the specificity of the Spry1 antibody used. These differences in protein levels were not caused by increased mRNA levels (Figure 2A, right panel). We next analyzed expression of Sprouty1 in E13.5 and newborn kidneys. At both ages, the amount of Sprouty1 was greatly increased in mutant mice (Figure 2, B and C). Again, mRNA levels were unchanged (Figure 2C, middle panel), indicating that regulation of Sprouty1 levels by tyrosine 53 is post-transcriptional. Interestingly, levels of Sprouty2 were modestly increased in kidneys from Spry1Y53ANeo/Y53ANeo mice. We next assessed the contribution of Spry1 tyrosine 53 to the global pattern of tyrosine phosphorylation of the protein. Dermal fibroblasts from newborn Spry1Y53ANeo/Y53ANeo mice and wild-type littermates were placed in culture and stimulated with sodium pervanadate. After lysis, Spry1 was immunoprecipitated and probed with anti-phosphotyrosine antibodies. Again, skin fibroblasts from Spry1 knockout mice served as negative control. As shown in Figure 3A, pervanadate caused a robust tyrosine phosphorylation of Spry1 that was severely reduced but not completely eliminated upon mutation of tyrosine 53. As expected, no band was found in immunoprecipitates from knockout cells. Thus, tyrosine 53 constitutes a major but not the only phosphorylatable tyrosine of Spry1. To confirm these observations, we generated a phospho-Y53 Spry1–specific antibody. As expected, sodium pervanadate induced a robust phosphorylation of Spry1 immunoprecipitated from fibroblasts from wild-type but not Spry1Y53ANeo/Y53ANeo mice (Figure 3B). Specificity of this new anti–phospho-Y53 Spry1 was confirmed using transfected 293T cells (Figure 3C). Finally, we wanted to confirm that mutation of tyrosine 53 does not result in mislocalization of Sprouty1. In agreement with previous studies,22 both wild-type and mutant Sprouty1 were localized to the membranous compartments of the cell (Figure 3D) and were palmitoylated (Figure 3E).
Figure 2.
Expression of wild-type and mutant Sprouty1. (A) Levels of Spry1 from newborn brains of the indicated genotypes were measured by Western blot (left panel). Right panel, quantification of protein and mRNA levels from newborn brains of the indicated genotypes. Equal mRNA levels indicate that regulation of protein levels by tyrosine 53 is post-transcriptional. *P=0.11, Mann–Whitney test. (B) Protein levels of E13.5 kidneys from the indicated genotypes. Note the much higher levels in knockin metanephroi. (C) Sprouty1 protein and mRNA levels of newborn kidneys from the indicated genotypes. Short and long exposures of Sprouty1 immunoblots are shown to illustrate the difference between genotypes. Levels of Sprouty2 are included for comparison. The number in parentheses indicate sample number in all graphs.
Figure 3.
Characterization of Spry1 Y53A protein. (A, B) Spry1 was immunoprecipitated from pervanadate-stimulated skin fibroblasts of the depicted genotypes, and membranes probed with the indicated antibodies. (C) Validation of phospho-Y53 Spry1 antibody. 293T cells were transfected with empty vector or plasmids coding for hemagglutinin (HA)-tagged wild-type or Y53A mouse Spry1. Cells were stimulated or not with FGF for 10 minutes, lysed, and resolved by SDS-PAGE. Membranes were incubated with the indicated antibodies. Note that overexpression of Sprouty1 in 293T cells leads to constitutive phosphorylation of tyrosine 53. (D) Subcellular fractionation of skin fibroblast extracts indicates that both wild-type and Y53A Sprouty1 proteins localize to the membranous fraction of the cell. GAPDH, caveolin-1, SP-1, and vimentin are markers of cytosolic (Cyto), membranous (Memb), nuclear (Nuc), and cytoskeletal (Cskel) fractions, respectively. (E) Both wild-type and Y53A Sprouty1 are palmitoylated. The presence of a band in the hydroxylamine-treated lysates from skin fibroblasts indicates palmitoylation of the protein (see Methods for details). Acyl-RAC, acyl-resin-assisted capture; IgG-L, low mol wt Ig chains; IgG-H, high mol wt Ig chains; IB, immunoblot; IP, immunoprecipitate; NH2OH, hydroxylamine; NS, nonspecific band; p-, phosphorylated; WT, wild type.
Mutation of Tyrosine 53 of Spry1 Causes GU Defects Identical to Those Found in Spry1 Knockout Mice
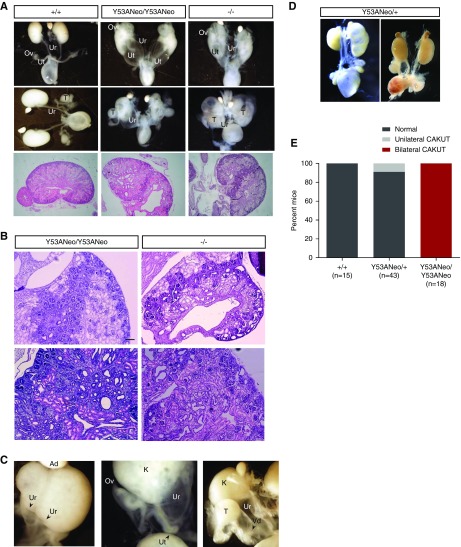
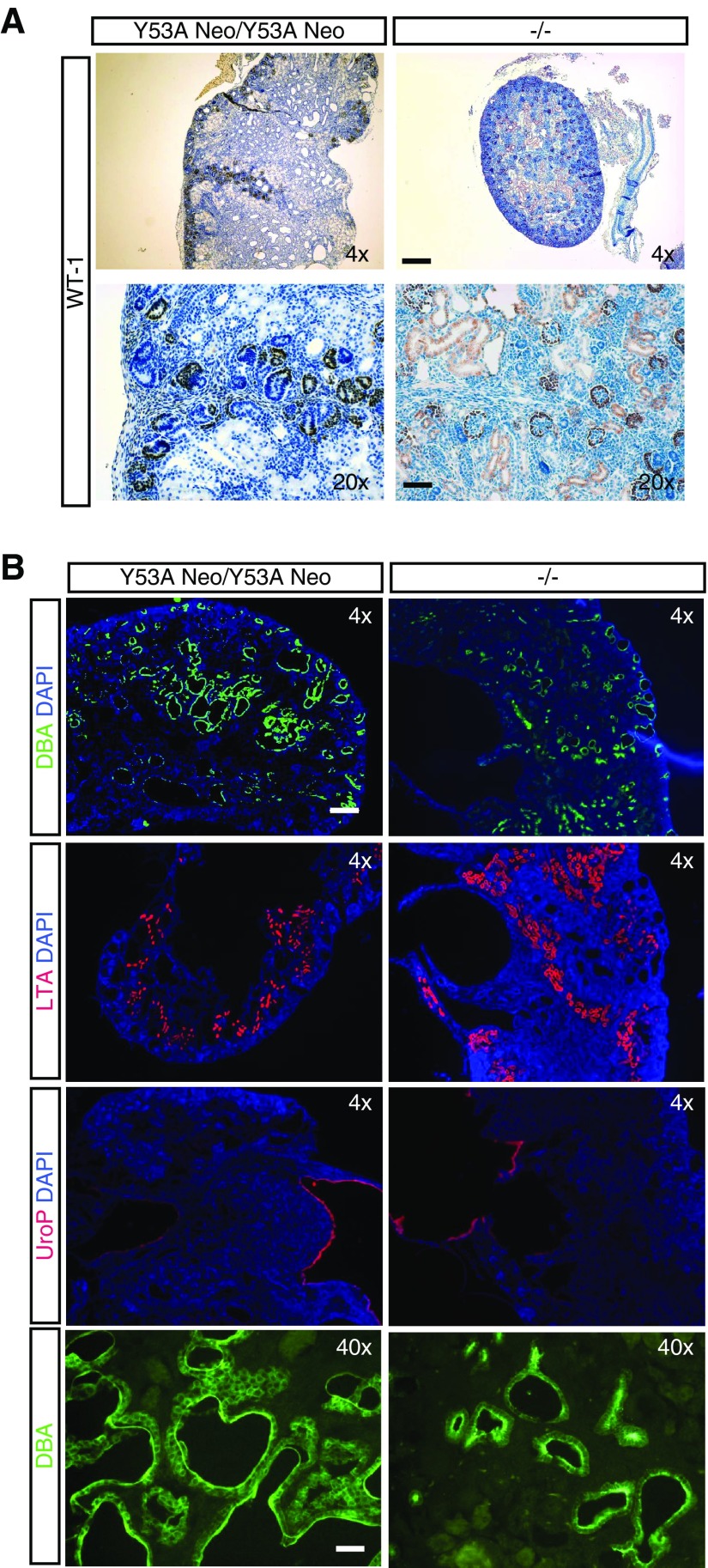
Examination of GU systems of knockin animals at birth revealed a series of lower urinary tract abnormalities, among which bilateral megaureter was by far the most common. Duplex and blind ureters were also observed, albeit to a much lower incidence. Histologic examination of kidneys revealed that they consisted of two or more supernumerary kidneys fused together, with nephrogenic zones not restricted to the periphery (Figure 4B), that stained positive for the marker Wilms’ Tumor 1 (Figure 5A), large cystic cavities, and hydroureteronephrosis (Figure 4, A and B). These defects were fully penetrant in homozygous mice, with a small proportion (approximately 10%) of heterozygous mice presenting unilateral megaureter with concomitant kidney defects (Figure 4, D and E). Moreover, all of these abnormalities were indistinguishable from those found in Spry1 knockout mice, indicating that tyrosine 53 is essential for the functions of Spry1 in GU development (Figure 4, A and B). In contrast, female Spry1Y53ANeo/Y53ANeo mice presented a series of gross internal genitalia abnormalities at birth including ovaries ectopically placed ventral to the kidneys, with uterine horns attached to ureters and in most cases ending blindly instead of fusing to each other (Figure 4C). Knockin males presented testis set next to kidneys, vasa deferentia were fused to ureters and consequently seminal vesicles were absent (Figure 4C). Again, all of these malformations were also found in Spry1 knockout mice (Figure 4A and9). Knockin mice generated from the two independent ES clones presented similar phenotypes. Most of the analyses were performed with the clone 4AD4. Close examination of newborn kidneys from both knockin and knockout mice indicated that the cystic cavities stained positive for the collecting duct marker DBA but not the proximal tubule marker LTA (Figure 5B), as previously described.9 As expected, hydroureters and swollen renal pelvises stained positive for the urothelium marker uroplakin (UroP in Figure 5B).
Figure 4.
Spry1Y53ANeo/Y53ANeo phenocopy Spry1−/− mice in terms of GU development. (A) Gross anatomy of newborn GU from the indicated genotypes (top panel). Hematoxylin and eosin–stained sections of newborn kidneys of the indicated genotypes (middle panel). (B) Higher magnification of sections from the indicated genotypes showing ectopic nephrogenic zones and cystic cavities. Scale bar, 100 µm. (C) Detail of some GU abnormalities found in Spry1Y53ANeo/Y53ANeo mice including duplex ureters (left), blind uterine horns (middle), and vasa deferentia fused to ureters (right). (D) Unilateral CAKUT present in approximately 10% of Spry1Y53ANeo/+ mice. (E) Quantification of GU defects found in newborn mice of the indicated genotypes. Original magnification, ×2 in A; ×10 in B. Ad, adrenal gland; K, kidney; Ov, ovary; T, testis; Ur, ureter; Ut, uterus; Vd, vas deferens.
Figure 5.
Characterization of kidneys from newborn Spry1 knockin and knockout mice. (A) Wilms’ Tumor (WT-1) staining of the ectopic nephrogenic zones. Note WT-1–positive developing and mature nephrons, but not cystic cavities. (B) DBA, LTA, and uroplakin (Up) staining of newborn kidneys from the indicated genotypes. Cysts are DBA-positive, revealing their collecting duct origin. Scale bars, 40 µm for 20×, 200 µm for 4×, and 20 µm for 40× pictures. DAPI, 4′,6-diamidino-2-phenylindole.
Aberrant UB Development Generates Supernumerary Kidneys in Spry1 Knockin Mice
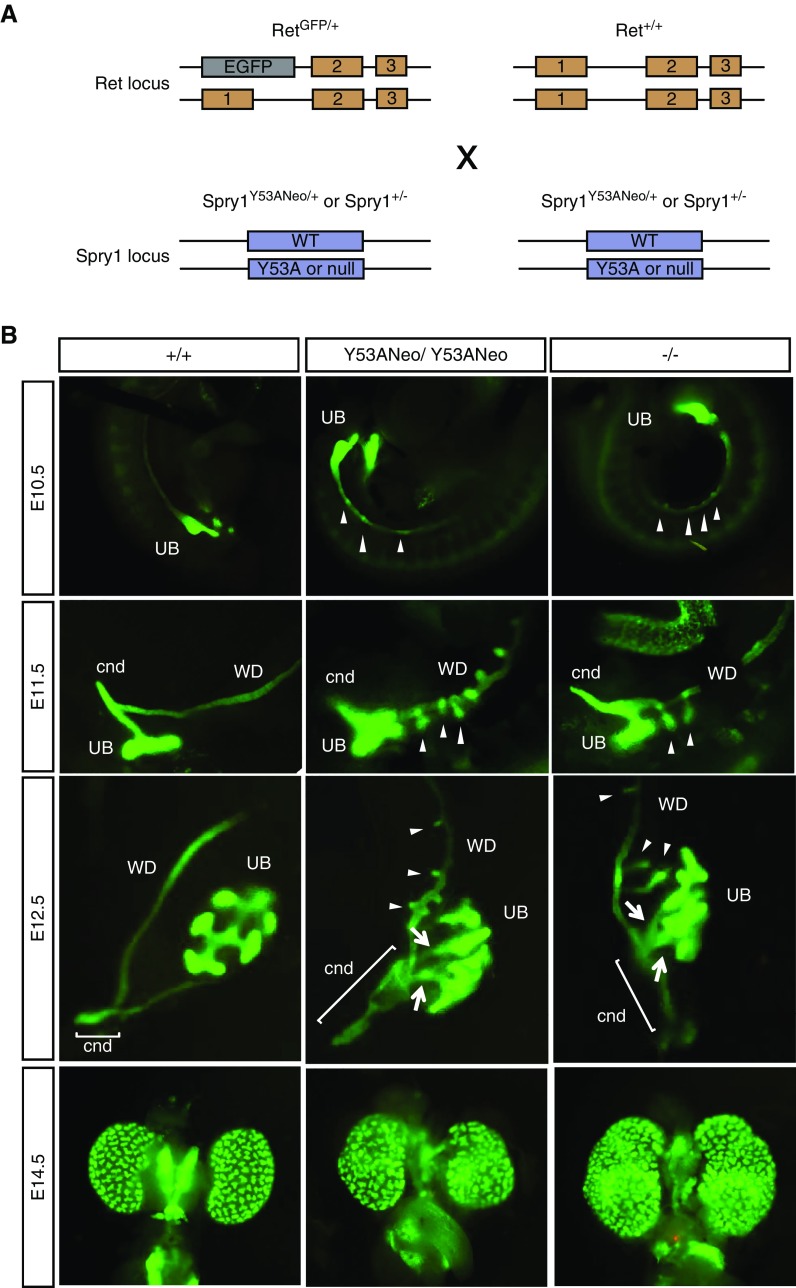
It has previously been shown that these GU defects are readily rescued by genetically reducing the activation of the GDNF-Ret pathway. Thus, kidney defects found in Spry1 are caused by the emergence of supernumerary, ectopic UBs giving rise to multiplex kidneys due to hypersensitivity of the WD to GDNF signaling. To further investigate the developmental origin of GU abnormalities of Spry1Y53A knockin mice, we crossed Spry1Y53ANeo/+ to mice expressing EGFP from the Ret locus,19 thus allowing easy tracking of the WD and the UB (Figure 6A). The UB formed normally in both knockin and knockout mice at E10.5, however several protuberances of the WD cranial to the UB were observed in mutant but not wild-type animals (Figure 6B). At E11.5, a T-shaped UB had emerged from the WD in wild-type mice. At this age, the UB of either knockin or knockout mice also had two well formed branches, but the stalk was much shorter than in wild-type embryos, and numerous ectopic UBs grew cranially. By E12.5 a well developed ureter, completely separated from the WD, and a short cnd were present in wild-type mice. In contrast, metanephric kidneys from both Spy1Y53ANeo/Y53ANeo and Spry1−/− embryos remained in close proximity to the WD; had many shorter, supernumerary UB; and a much longer cnd (Figure 6B).
Figure 6.
Aberrant UB development in Spry1 Y53A mice is indistinguishable to that of and Spry1 knockout mice. (A) Diagram showing Ret and Spry1 loci and mouse crosses to generate genotypes of interest. (B) EGFP fluorescence of UB and WD development from embryos of the indicated ages and genotypes (all of them bearing one RetEGFP allele to allow visualization of these structures). Note ectopic UBs (arrowheads) and long cnds (brackets) in both Spry1 knockin and knockout mutant mice. WT, wild type.
Defective Ureter Maturation Causes Vesicoureteral Reflux, Megaureter, and Internal Genitalia Defects in Spry1 Knockin Mice
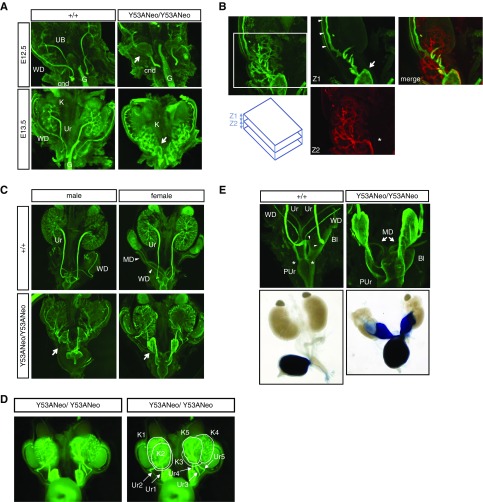
To image GU development at older embryonic ages we performed whole mount cytokeratin staining because Ret expression gets progressively confined to UB tips and caudal WD, including seminal vesicles, but not ureters (data not shown).19 In wild-type E12.5 embryos, as observed with EGFP fluorescence, long, completely separated ureters converged with the WD behind a very short cnd. One day later ureters had elongated caudally and cnds were almost eliminated (Figure 7A, left panels). In contrast, at E12.5 knockin mice presented a flat, triangle-shaped structure (Figure 7A, arrow in top-right panel) that was continuous with in a very long cnd on its caudal side. At E13.5, kidneys from knockin mice were caudally misplaced, in close proximity to this enlarged planar structure (Figure 7A, bottom right panel) to which both multiple UBs and the WD were connected (Figure 7B). At E15.5, numerous ectopic kidneys arising at different points of the WD that attached together to form a single anatomic unit were present in knockin mice (Figure 7D). Each of these supernumerary kidneys had a ureter that, together with the WD, fused to the flat triangle-shaped structure, which was continuous with a greatly enlarged cnd, acquiring the shape of a megaureter (Figure 7C, arrows). At this age, ureter maturation had proceeded completely in wild-type animals, with ureters completely segregated from the WD. Ureters connected to the bladder at their definitive location (Figure 7E, arrowheads), whereas WDs remained attached to the pelvic urethra (Figure 7E, asterisks). Ureter maturation never occurred in mutant mice, thus leaving WD and ureters fused together and connecting to the pelvic urethra (Figure 7E). Such broad, ectopic insertion precluded correct formation of the vesicoureteral junction, giving rise to vesicoureteral reflux as demonstrated by trypan blue injection into the bladder (Figure 7D, bottom panel). This observation explains both the presence of urine-filled megaureters and hydroureteronephrosis found in newborn mutant mice. In contrast, Müllerian ducts failed to fuse to each other at the midline but instead coiled around themselves, giving rise to blind uterine horns in newborn mice (Figure 7D, top panel). Finally, failure of ureters to segregate from WD caused fusion of vasa deferentia to ureters in newborn mutant males.
Figure 7.
Ureter maturation defects leading to vesicoureteral reflux in Spry1 knockin mice. (A) Whole-mount cytokeratin staining of embryonic GU system of the indicated ages and genotypes. Note long cnd and the presence of a flat, triangle-shaped structure connecting WD, UBs, and cnd in mutant mice (arrow). Also note failure of kidneys to migrate away from the point at which WD and UBs converge. (B) Detail of E13.5 mutant GU stained with cytokeratin. Confocal stacks were divided in ventral (Z1) and dorsal (Z2) sections and pseudocolored in green and red for clarity. Note how the WD (arrowheads) is continuous with the triangle-shaped structure (arrow) and the ureter (asterisk). Note also how ectopic UBs integrate into one kidney. (C) Cytokeratin staining of GU systems from E15.5 embryos. The triangle-shaped structure (arrow) is continuous with an enlarged cnd, and together from a megaureter. (D) Stereoscope image of knockin female GU shown in (C) allows clear visualization of supernumerary kidneys (K1–K5) and ureters (Ur1–Ur5). (E) Top panel: Detail of (C) showing complete separation between ureters, which insert into the bladder (arrowheads) and WDs, which join the pelvic urethra at the points marked with asterisks, in wild-type embryos. In knockin embryos, the cnd insert directly into the pelvic urethra. Note the Müllerian ducts (arrows) coiling instead of fusing to each other at the midline. Bottom panel, injection of trypan blue into the bladder of wild-type and mutant dissected GU from newborn mice shows vesicoureteral reflux in the latter. Original magnification, ×4 in A and C; ×10 in B and E. Bl, bladder; G, gut; MD, Müllerian duct; PUr, pelvic urethra; Ur, ureter.
In conclusion, we have demonstrated that mutation of the N-terminal tyrosine of Sprouty1 leads to formation of ectopic UB that ultimately will form supernumerary kidneys, thus phenocopying Spry1 knockout mice. A more detailed analysis of ureter maturation reveals a series of defects that eventually lead to formation of megaureters and vesicoureteral reflux.
Discussion
Phosphorylation of the conserved N-terminal tyrosine is the most abundant post-translational modification detected in Sprouty family members, representing 63%–77% of total post-translational modifications found in high-throughput proteomic experiments (PhosphositePlus23). Accordingly, we have found that tyrosine 53 is the major (but not the only) phosphotyrosine in mouse Sprouty1. Besides tyrosine 53, the same high-throughput experiments have identified two extra phosphotyrosines in Sprouty1, namely tyrosine 89 (identified 50 times versus 247 times for tyrosine 53) and tyrosine 304 (identified seven times). The function of these tyrosines is essentially unexplored and deserves future investigation. Other carboxy-terminal tyrosines found in Sprouty2 such as tyrosines 227, 269, and 28324 have not being identified in their phosphorylated forms in Sprouty1.
We found that knockin mice lacking tyrosine 53 phenocopy the renal malformations found in Spry1−/− mice, namely emergence of ectopic UBs leading to supernumerary kidneys. The role of the conserved N-terminal tyrosine of Sprouty proteins has been previously tested by several independent laboratories, essentially in the context of ERK pathway inhibition—regarded as the primary target of Sprouty proteins. Although some studies showed that mutation of this tyrosine renders Sprouty proteins unable to block the ERK pathway,15–17 others have found that its phosphorylation is dispensable for inhibition of the pathway.18 These discrepancies might be related to differences in the context in which these experiments were conducted; including the cell type, the growth factors used, or the family member(s) analyzed; but also can reflect two distinct modes of action of these proteins. Thus, in experimental paradigms where Sprouty proteins act at the level of Raf-1,25,18 phosphorylation of this tyrosine would be dispensable for their function; whereas in models where Sprouty proteins act upstream of Ras,15,16 this tyrosine would be absolutely necessary for their activity. Our data clearly show that tyrosine 53 of Spry1 is essential for its function at least in the context of GU development, i.e., favoring the latter model. However, we cannot rule out the possibility that mutation of tyrosine 53 in other cellular systems would have no effect on the activity of Spry1.
We also describe ureter maturation defects in our mutant mice, which essentially consisted of failure of the ureters to elongate, to properly separate from the WD, and therefore to migrate to their definitive insertion at the bladder. It has previously been described that excessive activation of the ERK pathway in the developing lung leads to aberrant mitotic spindle orientation and abnormal airway shape.26 We hypothesize that the same mechanism would explain some of the above defects. Thus, in mutant mice, epithelial cells from the UBs would not undergo mitotic divisions oriented parallel to the longitudinal axis of the ureter, but rather place their mitotic spindles in a disorganized fashion, leading to the appearance of the flat, triangle-shaped structure connecting UBs and the WD. In parallel, the cnd fails to degenerate and ends up forming the caudal part of the ectopically inserted megaureters found in Spry1 mutant mice. It has previously been shown that degeneration of the cnd by apoptosis is crucial for proper ureter detachment from the WD during ureter maturation.3,4 On the other hand, increased ERK activity in Ret Y1015F mutants leads to decreased apoptosis of the cnd.19 Finally, these ureter maturation defects are rescued by genetically reducing the activation of the GDNF-Ret signaling pathway.9,11,12 Altogether, these observations strongly suggest that mutation of Sprouty1 leads to an increase of Ret-derived ERK phosphorylation, which in turns inhibits apoptosis of the cnd.
Mechanistically, how deletion of the N-terminal tyrosine blocks Sprouty function is still a matter of debate. It was initially thought that this phosphotyrosine provides a binding site for the Src homology 2 domain of growth factor receptor–bound protein 2, and therefore sequestering of this adaptor by Sprouty proteins upon growth factor stimulation would uncouple receptor tyrosine kinase phosphorylation from ERK pathway activation.16 However, later it was shown that association of growth factor receptor–bound protein 2 to Sprouty was independent of tyrosine phosphorylation.15 Perhaps the most widely accepted protein binding this phosphotyrosine is the ubiquitin ligase cellular-Casitas B-lineage Lymphoma (c-Cbl). According to one model, Spry2 sequesters c-Cbl away from the EGF receptor, thus blocking its proteasomal degradation and potentiating signaling by EGF.27–30 However, such a mechanism would obviously only operate in systems where Spry2 activates instead of inhibits EGF receptor signaling, and would not explain how this tyrosine inhibits signaling by the FGF receptor or Ret. In a more general scenario, the functional consequence of binding of c-Cbl to Spry2 is proteasomal degradation of the latter.15,27,31 Our data are in agreement with these observations because steady-state levels of Spry1 are increased in brain lysates from Spry1Y53A/Y53A mice when compared with their wild-type littermates. However, attenuation of FGF-mediated ERK phosphorylation by Spry2 is not dependent on c-Cbl because Spry2 efficiently inhibits ERK phosphorylation in mouse embryonic fibroblasts from c-Cbl−/− mice.15
In conclusion, the mechanisms by which the N-terminal tyrosine of Sprouty proteins mediate their function require further investigation. We believe that our Spry1Y53A knockin mouse represents an excellent model system to pursue this objective.
Disclosures
None.
Funding
This work was supported by Ministerio de Economía y Competitividad grants BFU2010-47175-P and BFU2017-83646-P (AEI/FEDER, UE) to Encinas. Vaquero was supported by a predoctoral fellowship from Agència de Gestió d’Ajuts Universitaris i de Recerca. Anerillas was supported by a predoctoral fellowship from Universitat de Lleida. Cuesta was supported by a Co-funding of Regional, National, and International Programmes Action grant from the Marie Curie program of the European Union.
Acknowledgments
We are grateful to Dr. Sanjay Jain (Washington University, St Louis) for sharing RetEGFP mice, and to Dr. Tung-Tien Sun (New York University) for the uroplakin antibody. We thank Dr. Anna Macià (Institut de Recerca Biomèdica Lleida) for her contribution to the initial development of this manuscript, as well as Ms. Marta Hereu, Ms. Maria Santacana, Ms. Mónica Domingo, and Ms. Maria Carrele for their excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Davis TK, Hoshi M, Jain S: To bud or not to bud: The RET perspective in CAKUT. Pediatr Nephrol 29: 597–608, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al.: Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37: 1082–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Uetani N, Bertozzi K, Chagnon MJ, Hendriks W, Tremblay ML, Bouchard M: Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J Clin Invest 119: 924–935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uetani N, Bouchard M: Plumbing in the embryo: Developmental defects of the urinary tracts. Clin Genet 75: 307–317, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA: Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 92: 253–263, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Guy GR, Jackson RA, Yusoff P, Chow SY: Sprouty proteins: Modified modulators, matchmakers or missing links? J Endocrinol 203: 191–202, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R, et al.: Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci 8: 855–857, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, et al.: Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8: 229–239, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, et al.: Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol 299: 466–477, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Rozen EJ, Schmidt H, Dolcet X, Basson MA, Jain S, Encinas M: Loss of Sprouty1 rescues renal agenesis caused by Ret mutation. J Am Soc Nephrol 20: 255–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D’Agati V, et al.: Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet 6: e1000809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrita MA, Christofori G: Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis 11: 53–62, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Edwin F, Anderson K, Ying C, Patel TB: Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol 76: 679–691, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason JM, Morrison DJ, Bassit B, Dimri M, Band H, Licht JD, et al.: Tyrosine phosphorylation of Sprouty proteins regulates their ability to inhibit growth factor signaling: A dual feedback loop. Mol Biol Cell 15: 2176–2188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Hanafusa H, Torii S, Yasunaga T, Nishida E: Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol 4: 850–858, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Taketomi T, Wakioka T, Kato R, Yoshimura A: Identification of a dominant negative mutant of Sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced ERK activation. J Biol Chem 276: 36804–36808, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki A, Taketomi T, Kato R, Saeki K, Nonami A, Sasaki M, et al.: Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat Cell Biol 5: 427–432, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hoshi M, Batourina E, Mendelsohn C, Jain S: Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development 139: 2405–2415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macià A, Vaquero M, Gou-Fàbregas M, Castelblanco E, Valdivielso JM, Anerillas C, et al.: Sprouty1 induces a senescence-associated secretory phenotype by regulating NFκB activity: Implications for tumorigenesis. Cell Death Differ 21: 333–343, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrester MT, Hess DT, Thompson JW, Hultman R, Moseley MA, Stamler JS, et al.: Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res 52: 393–398, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Impagnatiello MA, Weitzer S, Gannon G, Compagni A, Cotten M, Christofori G: Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J Cell Biol 152: 1087–1098, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E: PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res 43: D512–D520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin C, Zwang Y, Vaisman N, Ron D, Yarden Y: Phosphorylation of carboxyl-terminal tyrosines modulates the specificity of Sprouty-2 inhibition of different signaling pathways. J Biol Chem 280: 9735–9744, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, Lo TL, et al.: Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem 277: 3195–3201, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR: Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science 333: 342–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin C, Litvak V, Medvedovsky H, Zwang Y, Lev S, Yarden Y: Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr Biol 13: 297–307, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Fong CW, Leong HF, Wong ESM, Lim J, Yusoff P, Guy GR: Tyrosine phosphorylation of Sprouty2 enhances its interaction with c-Cbl and is crucial for its function. J Biol Chem 278: 33456–33464, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Wong ESM, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, et al.: Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J 21: 4796–4808, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan JE, Hall AB, Yatsula BA, Bar-Sagi D: The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proc Natl Acad Sci U S A 99: 6041–6046, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall AB, Jura N, DaSilva J, Jang YJ, Gong D, Bar-Sagi D: hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr Biol 13: 308–314, 2003 [DOI] [PubMed] [Google Scholar]