“Mr. Bond, they have a saying in Chicago: ‘Once is happenstance. Twice is coincidence. The third time it’s enemy action’.”
Auric Goldfinger from Ian Fleming’s Goldfinger
Gitelman syndrome is one of two well known autosomal recessive diseases of hypokalemic alkalosis that have received substantial attention in the biomedical literature, both because of their effect on patients and their explanatory power. Bartter syndrome, a constellation of juxtaglomerular hyperplasia and loss of renal salt with secondary hyperaldosteronism, hypokalemic alkalosis, and normal BP, was described in 1962.1 Some years later, Gitelman characterized a similar autosomal recessive syndrome, but noted prominent hypomagnesemia in the setting of normotensive hypokalemic alkalosis.2 For many years, Bartter and Gitelman syndromes were confused with each other, but two groups noted that hypocalciuria may differentiate the two.3,4 This observation suggested that Gitelman syndrome might be a disease of the distal convoluted tubule because the phenotype resembles the effects of thiazide diuretics, which include prominent hypocalciuria. Simon et al.5 later showed that mutations in SLC12A3, which encodes the thiazide-sensitive sodium chloride cotransporter (NCC), cause Gitelman syndrome, confirming it as a disorder of the distal convoluted tubule and conclusively differentiating it from Bartter syndrome. Subsequent work showed that Bartter syndrome results from mutations in any of several genes expressed along the thick ascending limb of the loop of Henle, any of which disrupt activity of the bumetanide-sensitive sodium-potassium-chloride cotransporter.6
The molecular solutions to these hypokalemic alkaloses have highlighted the complex effects of single gene diseases. A recent Kidney Disease Improving Global Outcomes (KDIGO) conference, for example, emphasized that Gitelman syndrome can be phenotypically indistinguishable from “classic Bartter syndrome,” which is caused by mutations in the chloride channel, CLCNKB.7 Because this chloride channel is expressed along both the thick ascending limb and distal convoluted tubule, it appears that, in some patients, mutations cause a Gitelman-like phenotype, whereas in others, a more Bartter-like syndrome prevails.8 Thus, a mutation in the same gene may cause hypokalemic alkalosis with hypocalciuria in some patients (Gitelman-like), and hypokalemic alkalosis without hypocalciuria in others (Bartter-like). This phenotypic variability emphasizes the importance of genetic information for confident diagnosis. In fact, the KDIGO consensus paper recommends that genetic testing be used for patients with hypokalemic alkalosis whenever resources permit,7 while acknowledging that some patients will have only a single mutation detected.
Genetic diagnosis, in addition to clarifying syndromic features, permits investigators to estimate mutation frequencies in populations and define phenotypes better. A paper by Blanchard et al.9 in this issue of JASN does just that. Although prior estimates of Gitelman mutation gene frequency have ranged from 1%10 to 3%,11 Blanchard et al., using a very recent and large database, estimate that 1.14% of the population is heterozygous for well established pathogenic mutations in SLC12A3, and that 3.6% are heterozygous when nonclassified variants of very low frequency (suggestive of being pathogenic) are included.
Given the relative frequency of heterozygosity in the general population, Blanchard et al. tested whether heterozygous individuals exhibit an intermediate phenotype.9 Gitelman syndrome results from dysfunction of salt transport; thus it is not surprising that it has been associated with low BP.12 Several groups have previously tested the hypothesis that individuals carrying a single mutation in SLC12A3 might exhibit a forme fruste of Gitelman syndrome; in this case, the mutations might be maintained in the gene pool because they help protect individuals from hypertension. If this could be confirmed, it would suggest SLC12A3 as an important hypertension-protection gene for populations worldwide. Lifton and colleagues13 tested the hypothesis that heterozygosity for genes leading to either Bartter or Gitelman syndrome leads to lifelong protection from hypertension and cardiovascular disease. Although the results were positive, many assumptions were required for this analysis and the existence of an intermediate phenotype was considered by many to be inconclusive, a suggestion consistent with other small studies.
The work by Blanchard et al. in this issue again tests the hypothesis that SLC12A3 heterozygosity affects BP and electrolyte homeostasis, but uses a larger group of genetically defined individuals who were phenotyped carefully; it finds largely for the null, suggesting that heterozygosity for Gitelman-causing SLC12A3 mutations is not associated with a substantial phenotype.9 The results benefit from several factors that make this data set the most authoritative to date; these include the use of home BP monitoring, the derivation of heterozygous patients as relatives of a population of individuals documented to have Gitelman syndrome, and the careful approach to biochemical analysis. So, then, is this the last word? Are there other approaches to tease out the truth?
Biomedical statistics permit inferences to be drawn about populations from samples, samples that are practical to obtain, but may suffer from small size. In this era of concern regarding rigor and reproducibility in science, some have argued that traditional dividing lines between significance and nonsignificance, such as P<0.05, are not strict enough. Instead, they argue a threshold of P<0.005 should be used to avoid making too many type 1 errors.14 Yet, in biomedicine one also runs the risk of type 2 errors, falsely declaring that a difference does not exist, where in fact it does. The manuscript by Blanchard et al. does not dismiss the possibility of a modest intermediate phenotype resulting from SLC12A3 heterozygosity, but it suggests that any such phenotype is very mild.9
One approach to gain additional insight into this issue is to study animal models of human disease. Unlike some disease classes, where mouse models do not provide highly relevant information regarding human processes, monogenic kidney diseases of electrolyte transport are often faithfully recapitulated by mouse models. This is certainly true of mice lacking, or with mutant, slc12a3: like Gitelman patients, most mice exhibit hypokalemia, hypocalciuria, and hypomagnesemia, findings that may vary with genetic background.15–18 However, two papers describing slc12a3 mutant knockin models concluded that heterozygotes are phenotypically similar to controls, a conclusion mimicking the one reached by Blanchard et al.
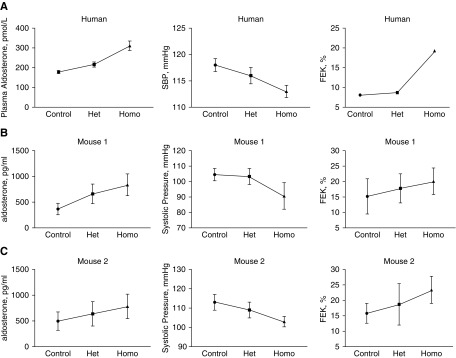
In light of this question, we re-examined the data from the two published animal studies. Yang and colleagues17 introduced a premature stop codon, one commonly observed in Chinese patients with the disease, into the mouse slc12a3 gene. The resulting homozygous mice exhibited a full Gitelman-like phenotype.18 The heterozygous mice, however, were described as being similar to control mice. Yet, plotting the data from that study (Figure 1) suggests that an intermediate phenotype may be present. The values for plasma aldosterone, systolic BP, and fractional potassium excretion (shown) and plasma magnesium concentration (not shown) are, in each case, intermediate between those observed in controls and in the homozygotes.
Figure 1.
Individuals and mice heterozygous for NCC status (Het) exhibit plasma aldosterone, systolic blood pressure, and fractional potassium excretion rates intermediate between controls and homozygotes (Homo). (A) Top panels show data from Blanchard et al. (this issue). (B) Middle panels show data from Yang et al.17 (C) Bottom panels show data from Yang et al.16 Data show means±SEM (except for fractional potassium excretion in humans, for which calculation was impossible). Het, heterozygous; Homo, homozygous.
Although this may indeed reflect coincidence, “enemy action” is suggested by examining a third report, describing mice in which a missense mutation in a key phosphorylation site necessary for NCC activity, was introduced.17 In this case, although slc12a3 mRNA abundance was nearly equivalent in all groups, NCC protein was nearly absent in homozygotes, likely reflecting membrane instability19 and perhaps endoplasmic reticulum–associated degradation.20 Yet, even in the heterozygotes, NCC abundance was significantly lower (about 30% less than controls). Once again, as in the other mouse model, the values for plasma aldosterone, systolic BP, and fractional potassium excretion (Figure 1) were intermediate between those of controls and homozygotes. It seems likely, then, that the mild elevation in plasma aldosterone is a marker of mild extracellular fluid volume depletion, engendered by subtle salt wasting.
The two reports using genetically altered mice, and the current one studying humans, all have results that might be interpreted statistically to suggest the absence of an intermediate phenotype; when viewed together (a “cross-species quasi-meta-analysis”?), however, they provide strong support for its presence. Thus, it seems likely that plasma aldosterone concentrations are higher in SLC12A3 heterozygotes than in healthy subjects, even though this difference was not “statistically significant” in the paper by Blanchard et al.9 We often think of aldosterone as a pathogenic factor, especially when it coexists with high BP. Yet, it seems that SLC12A3 heterozygotes may also have a slightly lower systolic pressure (mean systolic BP 118 mm Hg in healthy subjects and 116 mm Hg in heterozygous subjects, see table 1 in Blanchard et al.9), which should be protective. This small difference, played out across 7 billion people with a gene frequency of 1.5%, could have mammoth beneficial public health implications.21
This study by Blanchard et al. is the largest and most careful analysis of Gitelman syndrome heterozygotes to date.9 Although it does not itself confirm an intermediate phenotype, in context with animal work which can more easily be controlled, it does suggest that heterozygous individuals are subject to some losing of salt, as indicated by modestly elevated plasma aldosterone concentrations. The long-term consequences of this, however, remain to be determined.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Resistance to Insulin in Patients with Gitelman Syndrome and a Subtle Intermediate Phenotype in Heterozygous Carriers: A Cross-Sectional Study,” on pages 1534–1545.
References
- 1.Bartter FC, Pronove P, Gill JR Jr, MacCardle RC: Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med 33: 811–828, 1962 [DOI] [PubMed] [Google Scholar]
- 2.Gitelman HJ, Graham JB, Welt LG: A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 221–235, 1966 [PubMed] [Google Scholar]
- 3.Sutton RA, Mavichak V, Halabe A, Wilkins GE: Bartter’s syndrome: Evidence suggesting a distal tubular defect in a hypocalciuric variant of the syndrome. Miner Electrolyte Metab 18: 43–51, 1992 [PubMed] [Google Scholar]
- 4.Bettinelli A, Bianchetti MG, Girardin E, Caringella A, Cecconi M, Appiani AC, et al.: Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr 120: 38–43, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al.: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Castrop H, Schießl IM: Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 307: F991–F1002, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Blanchard A, Bockenhauer D, Bolignano D, Calò LA, Cosyns E, Devuyst O, et al.: Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 91: 24–33, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Matsunoshita N, Nozu K, Shono A, Nozu Y, Fu XJ, Morisada N, et al.: Differential diagnosis of Bartter syndrome, Gitelman syndrome, and pseudo-Bartter/Gitelman syndrome based on clinical characteristics. Genet Med 18: 180–188, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Blanchard A, Vallet M, Dubourg L, Hureaux M, Allard J, Haymann J-P, et al. : Resistance to insulin in patients with Gitelman syndrome and a subtile intermediate phenotype in heterozygous carriers: a cross-sectional study. J Am Soc Nephrol 30: 1534–1545, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoers NV, Levtchenko EN: Gitelman syndrome. Orphanet J Rare Dis 3: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu YJ, Yang SS, Chu NF, Sytwu HK, Cheng CJ, Lin SH: Heterozygous mutations of the sodium chloride cotransporter in Chinese children: Prevalence and association with blood pressure. Nephrol Dial Transplant 24: 1170–1175, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Cruz DN, Simon DB, Nelson-Williams C, Farhi A, Finberg K, Burleson L, et al.: Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension 37: 1458–1464, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, et al.: Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis JPA: The proposal to lower P value thresholds to .005. JAMA 319: 1429–1430, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, et al.: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Morris RG, Hoorn EJ, Knepper MA: Hypokalemia in a mouse model of Gitelman’s syndrome. Am J Physiol Renal Physiol 290: F1416–F1420, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Yang SS, Fang YW, Tseng MH, Chu PY, Yu IS, Wu HC, et al.: Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24: 1587–1597, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang SS, Lo YF, Yu IS, Lin SW, Chang TH, Hsu YJ, et al.: Generation and analysis of the thiazide-sensitive Na+ -Cl- cotransporter (Ncc/Slc12a3) Ser707X knockin mouse as a model of Gitelman syndrome. Hum Mutat 31: 1304–1315, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaek LL, Kortenoeven ML, Aroankins TS, Fenton RA: Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289: 13347–13361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunchaparty S, Palcso M, Berkman J, Velázquez H, Desir GV, Bernstein P, et al.: Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman’s syndrome. Am J Physiol 277: F643–F649, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al.: Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362: 590–599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]