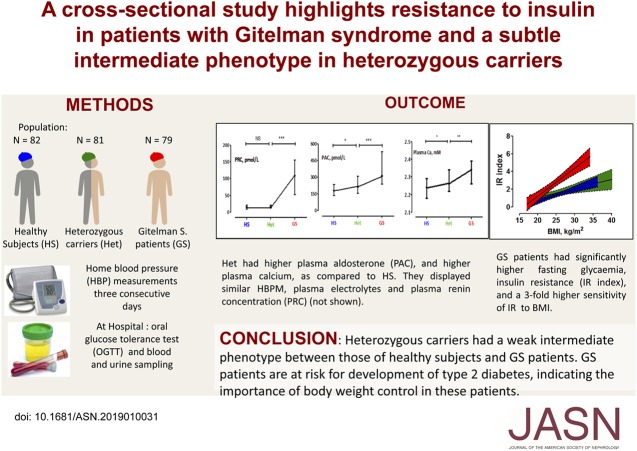
Significance Statement
About 1% of the population is heterozygous for loss-of-function variants in SLC12A3, which encodes the thiazide-sensitive sodium-chloride cotransporter. Biallelic SLC12A3 mutations are responsible for Gitelman syndrome, a salt-losing tubulopathy. In a cross-sectional study of 81 heterozygous carriers, 82 healthy noncarriers, and 79 patients with Gitelman syndrome of similar age, body mass index, and sex ratio, the authors assessed findings from home BP monitoring, oral glucose tolerance testing, and assays of plasma and urine electrolyte and hormone levels. They found evidence for a subtle intermediate phenotype in heterozygous carriers and demonstrated a resistance to insulin in the patients with Gitelman syndrome. These findings indicate that counseling of such patients is warranted to reduce their risk of type 2 diabetes and to reassure them about the health of their heterozygous relatives.
Keywords: Gitelman-s syndrome, heterozygous, carriers, blood pressure
Visual Abstract
Abstract
Background
Gitelman syndrome is a salt-losing tubulopathy caused by mutations in the SLC12A3 gene, which encodes the thiazide-sensitive sodium-chloride cotransporter. Previous studies suggested an intermediate phenotype for heterozygous carriers.
Methods
To evaluate the phenotype of heterozygous carriers of pathogenic SLC12A3 mutations, we performed a cross-sectional study of patients with Gitelman syndrome, heterozygous carriers, and healthy noncarriers. Participants measured their BP at home for three consecutive days before hospital admission for blood and urine sampling and an oral glucose tolerance test.
Results
We enrolled 242 participants, aged 18–75 years, including 81 heterozygous carriers, 82 healthy noncarriers, and 79 patients with Gitelman syndrome. The three groups had similar age, sex ratio, and body mass index. Compared with healthy noncarriers, heterozygous carriers showed significantly higher serum calcium concentration (P=0.01) and a trend for higher plasma aldosterone (P=0.06), but measures of home BP, plasma and urine electrolytes, renin, parathyroid hormone, vitamin D, and response to oral glucose tolerance testing were similar. Patients with Gitelman syndrome had lower systolic BP and higher heart rate than noncarriers and heterozygote carriers; they also had significantly higher fasting serum glucose concentration, higher levels of markers of insulin resistance, and a three-fold higher sensitivity to overweight. According to oral glucose tolerance testing, approximately 14% of patients with Gitelman syndrome were prediabetic, compared with 5% of heterozygous carriers and 4% of healthy noncarriers.
Conclusions
Heterozygous carriers had a weak intermediate phenotype, between that of healthy noncarriers and patients with Gitelman syndrome. Moreover, the latter are at risk for development of type 2 diabetes, indicating the heightened importance of body weight control in these patients.
Gitelman syndrome (GS; Online Mendelian Inheritance in Man no. 263800) is a rare, salt-losing tubulopathy characterized by hypokalemic metabolic alkalosis, hypomagnesaemia, hypocalciuria, and normal or low BP.1 It may be associated with impaired glucose tolerance.2 Loss-of-function variants in SLC12A3, which encodes the thiazide-sensitive sodium-chloride cotransporter, are responsible for most cases.
The reported prevalence of GS is one in 40,000.3,4 On the basis of this prevalence, the heterozygous carrier frequency is estimated at 1% of the population. However, the measured frequency in a Chinese population was 3%.5 Simon et al.6 suggested that heterozygous carriers of variants in SLC12A3 might present with modestly reduced BP and reduced sodium reabsorption, leading to a shift in the renal pressure natriuresis relationship.
Accordingly, low office BP values were reported in 49 patients recruited in the Framingham Health study who were Het for loss-of-function variants of the SLC12A3, SLC12A1, and KCNJ1 genes involved in GS and Bartter syndromes, suggesting that these heterozygous variations may protect from development of hypertension (HTN).7 Nandakumar et al.8 replicated the study in a population of 7444 European ancestry individuals from the Atherosclerosis Risk in Communities study. They detected 65 different variants in the same three genes in 121 individuals, among them 92 heterozygous for SLC12A3 variants. A significant decrease of BP was present only in a subset of 29 patients harboring ten variants common to the Framingham study, including five variants in SLC12A3. These studies analyzed a small number of individuals from very large unselected cohorts, and participants expressed SLC12A3 variants that may differently affect the function of the sodium-chloride cotransporter. Family studies that allow for investigation of larger populations of heterozygous relatives of patients with GS with known loss-of-function variants have provided inconsistent results with regards to the phenotype of heterozygous carriers. Cruz et al.9 studied 199 members of a large Amish kindred, including 26 patients with GS who were homozygous for a deletion in SLC12A3, 113 heterozygous carriers of the deletion, and 60 noncarriers. They reported that heterozygous children, but not adults, had significantly lower office BP levels than wild-type relatives. This result was not confirmed in a cohort of 29 heterozygous Chinese children compared with 471 noncarrier children. Finally, other studies have observed intermediate calcium excretion and fasting glucose in heterozygous carriers,3,5,9 or higher urinary pH suggestive of metabolic alkalosis.10
The aim of this study was to evaluate the effect of pathogenic SLC12A3 heterozygous variants by analysis of a large group of heterozygous carriers related to patients with GS compared with healthy noncarrier controls. We measured home BP (HBP) and evaluated renal homeostasis of electrolytes and calcium and bone remodeling. Glucose metabolism was investigated in response to an oral glucose tolerance test (OGTT).
Methods
Cross-Sectional Study Design
All participants provided written informed consent for participation in the study. The protocol was approved by the “Comité de Protection des Personnes,” Paris-Île de France III, France. All investigations were performed in accordance with the principles of the Declaration of Helsinki. This study was registered at Clinicaltrials.gov (identifier NCT02035046).
The cross-sectional study included three groups with participants ranging from 18 to 75 years old. Men and women were recruited from six tertiary-care French university hospitals in Paris, Lyon, Toulouse, Limoges, and Bordeaux, between December 2013 and August 2016. Participants were distributed into three groups on the basis of genetic status carrying 0, 1, or 2 variants of 4–5 class in the SLC12A3 gene, according to the classification of the American College of Medical Genetics (ACMG)11 (see flow chart in Supplemental Figure 1). One group included patients with GS phenotype according to the Kidney Disease: Improving Global Outcomes guidelines,1 who were homozygous or compound heterozygous for SLC12A3 pathogenic variants (n=79). Patients with GS with a pathogenic variant identified on only a single allele were excluded from the study. The second group were heterozygous carriers (Het) selected mainly from relatives of patients with GS (n=81). The third group were noncarrier healthy subjects (HS) without known history of HTN or of type 2 diabetes or ongoing antihypertensive treatment (n=82). The HS were recruited from relatives of patients with GS and healthy volunteers registered with the clinical investigation center of the Hôpital Européen Georges-Pompidou. Attempts were made to match age, sex, and body mass index (BMI) of Het and HS groups. Exclusion criteria for all participants were history of inflammatory or autoimmune diseases or cancer in the past 3 years, treatment with thiazide diuretics, and pregnancy or breast-feeding. Individuals with variants of uncertain significance were excluded.
Molecular Genetics
Sanger sequencing of coding regions of SLC12A3 was used to identify point variants, and multiplex ligation-dependent probe amplification was used to identify large rearrangements as previously described.12 Four patients with GS were analyzed by next-generation sequencing as recently described.13 SLC12A3 coding regions were sequenced by Sanger in all healthy controls. In all groups the first results were confirmed in an independent second sample. All genetic analyses were centrally performed at the Genetics Department at Hôpital Européen Georges-Pompidou, Paris.
Study Protocol
Patients were asked to measure seated BP and heart rate (HR) at home with a validated electronic device (OMRON M6) three times in the morning and evening on the 3 days preceding admission. Permanent HTN was defined as a mean home systolic BP (SBP) ≥135 mm Hg and/or diastolic BP ≥85 mm Hg.14 Masked HTN was defined as normal office BP (<140 or 90 mm Hg) and high BP at home (≥135 or 85 mm Hg). White-coat HTN was defined as high office BP (≥140 or 90 mm Hg) and normal BP at home (<135 and 85 mm Hg).14
For the OGTT, blood was sampled at approximately 09:00 am. in fasting conditions after 1 hour of rest in a semirecumbent position. Participants then ingested 75 g glucose with 250 ml water over a period of <5 minutes. Plasma glucose and insulin concentrations were measured before and 120 minutes after the oral glucose load. Participants were instructed to collect their 24-hour urine the day before the visit and to follow their usual diets before testing. The biologic samples were stored and managed by the Biologic Resources Center and Tumor Bank Platform (Biobank number: BB-0033–00063).
Laboratory Methods
Biochemical and hormone measurements were performed blind to the group in a centralized laboratory (Department of Physiologic Functional Investigations, Hôpital Européen Georges-Pompidou) with exceptions of lipid and liver panels that were measured in local laboratories.
Plasma renin and aldosterone concentrations were measured by immunoradiometric assay (Renin III, Cisbio Bioassays) and by chemiluminescent immunoassay (LIAISON Aldosterone; DiaSorin), respectively on the Liaison automat. Plasma and urine sodium and potassium concentrations were measured using flame photometry (IL943; Instrumentation Laboratory). The 24-hour urine aldosterone output was measured after hydrolysis at pH 1.
Insulin was measured with paramagnetic chemiluminescent immunoassay with Access Ultrasensitive Insulin reagents, DxI800 system (Beckman Coulter). Insulin sensitivity was assessed by two indices, the index of insulin resistance of the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI). HOMA-IR was calculated as follows: HOMA-IR=fasting plasma insulin (mUI/l)×plasma glucose (mmol/l)/22.5.15 The QUICKI, proposed by Katz et al.,16 was calculated as follows: 1/(log[fasting plasma insulin]+log[fasting plasma glucose]). Impaired glucose tolerance was defined as impaired fasting glucose ranging from 5.6 to 6.9 mmol/l or 2-hour values in the OGTT of 7.8–11.0 mmol/l.17
Calcium and magnesium in plasma and urine were measured using atomic absorption spectrometry (Solar iCE 3000; ThermoScientific). Intact parathormone (PTH) and bone remodeling markers (osteocalcin and collagen 1 C telopeptides) were measured using sandwich chemiluminescent immunoassays (Cobas e411; Roche Diagnostics). Vitamin D was measured using competition chemiluminescent immunoassay (Isys; ImmunoDiagnostic Systems). Calcitriol was measured with a radio-immunoassay (ImmunoDiagnostic Systems). Creatinine was measured in plasma and urine with enzymatic and Jaffe kinetic assays, respectively, using Konelab 20 (IDMS traceable; ThermoScientific). Plasma cystatin C was measured by immunoturbimetric assay (Cobas c502; Roche Diagnostics). eGFR was calculated by the CKD Epidemiology Collaboration formula, on the basis of plasma cystatin C.18
Statistical Analyses
The primary objective of the study was to determine whether Het carriers had different home SBP than HS or patients with GS. The secondary objectives were to determine whether Het carriers differed from HS and patients with GS in terms of hormonal, metabolic, or electrolyte parameters. Assuming a 10 mm Hg difference in home SBP between any of the three groups, an SD of 19 mm Hg, and a two-sided type 1 error rate of 0.017%, a sample size of 77 evaluable participants per group would yield 80% power. To account for up to 10% missing data on the primary end point, we planned to include a total 250 individuals in the study.
We used ANOVA or a Kruskal–Wallis test for continuous variables and chi-squared tests or Fisher tests for categorical variables. Normal distribution of the variables was tested with the Shapiro–Wilks test. Logarithmic or square-root transformation was used where appropriate for non-normally distributed parameters. When the overall tests were significant, we performed 2×2 comparisons with the Tukey test or Dunn post hoc test. The relationship between variables was studied by linear regression. We estimated Pearson and Spearman correlation coefficients for normally and non-normally distributed variables, respectively. The slopes and intercepts of the regression lines were compared between the three groups.
Results are presented as medians (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. Between-group differences are expressed as arithmetic means or ratios of geometric means with their 95% confidence intervals (95% CIs). Except as otherwise stated, we performed two-sided tests and a P value <0.05 was considered statistically significant. The code for data analysis was generated using SAS software and the dunn.test package in “R” software (https://www.R-project.org/).
Results
Genetic Analyses of SLC12A3 in the Cohort
The cohort (n=242) comprised 81 healthy Het carriers, 82 HS, and 79 patients with GS. Seventy-four different variants in SLC12A3 were detected (Supplemental Table 1) including missense (57%), frameshift (17%), and splice site variants (15%). Nine pathogenic variants (one large rearrangement, one frameshift, four splice site, one nonsense, and two missense) are newly described. Among patients with GS, nine were homozygous and 70 were compound heterozygous for pathogenic variants. In the Het group, 18% harbored one variant class 4% and 82% had one variant class 5 as classified by the ACMG.
Estimation of Frequency of SLC12A3 Heterozygosity in the General Population
We analyzed the latest release of the gnomAD database v2.1 (http://gnomad.broadinstitute.org), which includes data on 141,448 individuals, to estimate the frequency of SLC12A3 heterozygosity in the general population. Among individuals in the database, 0.72% (1026) harbor heterozygous SLC12A3 loss-of-function variants (nonsense, frameshift, and variants affecting the two first nucleotides of splice sites), and missense variants classified as likely pathogenic or pathogenic after in vitro expression. In addition, 0.42% (593) harbor in-frame or missense variants classified as pathogenic using the ACMG classification (which relies mainly on family segregation criteria). Finally, 3453 individuals (2.44%) have missense and in-frame variants with an allele frequency <0.1%. Thus, we estimate that 1.14% of the population is heterozygous for well established pathogenic mutations in SLC12A3 and 3.6% are heterozygous when nonclassified variants of very low frequency are included.
Comparison of Hemodynamic Parameters, Water and Electrolyte Homeostasis, and Renal Function in the Entire Cohort
The three groups were comparable in age, BMI, and sex ratio (Table 1). None of the 82 HS cohort had HTN history, but HTN was newly discovered in seven HS. Of the 81 Het subjects, 72 were normotensive (eight white-coat HTN), seven had HTN history and were prescribed antihypertensive drugs, and two had newly discovered HTN. Of the 79 patients with GS, 24 were being treated for hypokalemia with potassium-sparing diuretics or aldosterone antagonists. Eleven patients with GS had permanent HTN: six were controlled by antihypertensive drugs (including potassium-sparing diuretics) and five were untreated.
Table 1.
Clinical characteristics of subjects, HTN history and treatment, and home BP measurements (three measurements morning and evening, three consecutive days)
| Characteristics | HS (n=82) | Het (n=81) | GS (n=79) | ANOVA P Value |
|---|---|---|---|---|
| Whole population | ||||
| Age, yr | 40.3 [28.1; 56.1] | 43.2 [25.7; 58.4] | 39 [27.7; 51.1] | 0.85 |
| BMI, kg/m2 | 23.8 [21.5; 25.4] | 23.2 [21.6; 25.7] | 23.8 [20.7; 26.5] | 0.89 |
| Women/men (ratio) | 44/38 (0.53) | 46/35 (0.57) | 42/37 (0.53) | 0.9 |
| Past history of HTN | 0 | 6 | 6 | |
| Newly discovered HTN | 7 | 3 | 5 | |
| White-coat HTN | 3 | 8 | 2 | |
| Antihypertensive drugs | ||||
| Potassium-sparing diuretics | 0 | — | 30 | |
| ACE inhibitors | 0 | 3 | 1 | |
| ARB | 0 | 3 | 1 | |
| β-blockers | 0 | 4 | 4 | |
| Calcium channel blockers | 0 | 2 | — | |
| Home BP | ||||
| SBP, mm Hg | 118 [112; 127]a | 116 [108; 127] | 113 [107; 121] | 0.04 |
| Diastolic BP, mm Hg | 71 [66; 76] | 70 [64; 76] | 70 [66; 74] | 0.82 |
| HR, bpm | 70 [63; 77]b | 69 [64; 77]a | 74 [69; 79] | 0.005 |
| Subpopulations | (n=82) | (n=74) | (n=49) | |
| Antihypertensive drugs | 0 | 0 | 0 | |
| Home BP | ||||
| SBP, mm Hg | 118 [112; 127] | 116 [108; 125] | 113 [107; 124] | 0.13 |
| Diastolic BP, mm Hg | 71 [66; 76] | 69 [64; 76] | 69 [66; 72] | 0.59 |
| HR, bpm | 70 [63; 77]a | 69 [63; 79]a | 75 [69; 79] | 0.009 |
Whole population analyses: all participants were included in the study. Subpopulation: hypertensive or diabetic participants were excluded as were patients with GS treated with potassium-sparing diuretics. Data are presented as median [IQR]. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
For HS or Het versus patients with GS.
P<0.05.
P<0.001.
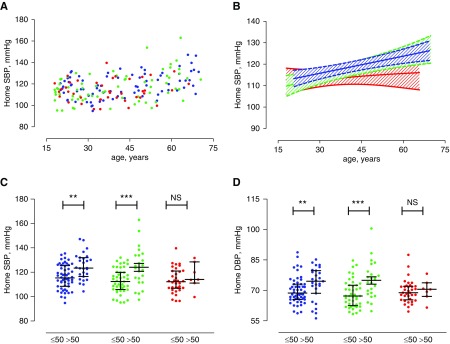
Het carriers had similar home SBP to HS (mean difference −0.3 mm Hg; 95% CI, −2.5 to +3.2; P=0.93). None of the Het carriers had electrolyte or hormonal abnormalities. Patients with GS had significantly lower SBP than HS (P=0.04) but not Het (P=0.11). No between-group difference in diastolic BP was observed (Table 1). Patients with GS had higher HR than HS (P<0.001) and Het (P=0.02) groups (Table 1, whole population). As expected, there was a positive correlation between home SBP and age in the Het group (rho=0.43; P<0.001) and HS (rho=0.38; P<0.001). This increase in home SBP was blunted in patients with GS (rho=0.16; P=0.17). SBP is plotted versus age for participants from the cohort in Figure 1.
Figure 1.
SBP and diastolic BP (DBP) between groups and by age after exclusion of heterozygous carriers with HTN or diabetes history and patients with GS treated with potassium-sparing diuretics. (A) Relationship between home SBP and age in HS (blue), Het (green), and GS (red) groups. (B) Linear regression of relationships with 95% CIs. (C) SBP in participants aged under and over 50 years, with median and IQR. (D) Mean SBP in participants aged under and over 50 years, with median and IQR. Data are means of three consecutive measurements morning and evening on three consecutive days. **P<0.001; ***P<0.001.
As compared with HS, Het had similar water, sodium, and potassium intakes as reflected by daily urinary output and sodium and potassium excretion. HS and Het had similar plasma potassium (P=0.35) and plasma renin (P=0.78) concentrations (Table 2). Plasma aldosterone concentration was higher in Het than in HS, and the difference was just over the limits of statistical significance (P=0.06). Het and HS groups also had similar eGFR, plasma magnesium concentrations (P=0.99), and daily urinary magnesium excretion (P=1.00) (Table 2). Plasma calcium concentration in Het was slightly higher than in HS (mean difference 0.04 mmol/l; 95% CI, 0.01 to 0.07; P=0.01), whereas plasma PTH concentration was slightly but not significantly lower (mean difference −10%; 95% CI, −19% to 0%; P=0.11). There was no difference between Het and HS in terms of daily urinary calcium excretion (P=1.00) or calcium-to-creatinine ratio (P=1.00) (Table 2). The two groups had similar levels of vitamin D, calcitriol, and bone remodeling markers (Supplemental Table 2).
Table 2.
Clinical characteristics and biologic data of participants
| Characteristic | HS (n=82) | Het (n=81) | GS (n=79) | ANOVA P Value |
|---|---|---|---|---|
| Plasma | ||||
| Creatinine, µmol/L | 66 [59; 76]a | 65 [56; 73] | 59 [50; 72] | 0.009 |
| eGFR, ml/min per 1.73 m2 | 92 [81; 110] | 102 [92; 99] | 99 [86; 110] | 0.1 |
| Sodium, mmol/L | 140 [139; 139]a | 140 [139; 142]a | 139 [138; 141] | 0.007 |
| Potassium, mmol/L | 4.0 [3.8; 4.2]b | 3.9 [3.8; 4.1]b | 2.8 [2.5; 3.1] | <0.001 |
| CO2t, mmol/L | 27 [26; 28]b | 27 [26; 29]b | 30 [28; 32] | <0.001 |
| Calcium, mmol/L | 2.24 [2.18; 2.29]b | 2.26 [2.22; 2.34]b,c | 2.34 [2.26; 2.39] | <0.001 |
| Magnesium, mmol/L | 0.82 [0.78; 0.86]b | 0.79 [0.75; 0.83]b | 0.59 [0.51; 0.67] | <0.001 |
| PTH 7–84, pg/ml | 44 [34; 55]b | 39 [31; 46]b | 31 [24; 39] | <0.001 |
| PRC, mUI/L | 15 [9; 21]b | 15 [11; 21]b | 109 [52; 156] | <0.001 |
| PAC, pmol/L | 178 [136; 235]b | 216 [155; 308]b | 311 [238; 530] | <0.001 |
| Urinary excretion | ||||
| Output, L/24 h | 1.6 [1.1; 1.9]a | 1.5 [1.0; 2.2] | 1.7 [1.4; 2.3] | 0.03 |
| Creatinine, mmol/24 h | 11.9 [9.0; 15.0] | 11.3 [9.2; 14.2] | 11.8 [9.9; 15.3] | 0.56 |
| Sodium, mmol/24 h | 147 [99; 172]b | 140 [96; 192]b | 183 [105; 246] | 0.001 |
| Potassium, mmol/24 h | 59 [44; 75]b | 59 [47; 79]b | 108 [92; 154] | <0.001 |
| Calcium, mmol/24 h | 3.8 [2.6; 5.6]b | 3.5 [2.2; 5.2]b | 1.1 [0.7; 2.0] | <0.001 |
| Calcium-to-creatinine ratio, mM/mM | 0.32 [0.23; 0.52]b | 0.33 [0.19; 0.45]b | 0.09 [0.05; 0.18] | <0.001 |
| Magnesium, mmol/24 h | 3.4 [2.5; 4.2]b | 3.3 [2.7; 4.2]b | 4.4 [3.6; 6.1] | <0.001 |
| Aldosterone, nmol/24 h | 56 [33; 87]b | 67 [47; 97]b | 95 [72; 172] | <0.001 |
eGFR calculated with cystatin C-based CKD Epidemiology Collaboration. Data are presented as median [IQR]. Difference between groups was evaluated first with ANOVA. If significant, Dunn test was performed to compare HS versus GS and Het versus GS (a,b) and Tukey test was performed to compare HS and Het (c). CO2t, Total CO2; PRC, plasma renin concentration; PAC, plasma aldosterone concentration.
P<0.05.
P<0.001.
P<0.05.
Patients with GS presented with secondary aldosteronism due to a renal loss of sodium as reflected by a six-fold increase in plasma renin (mean GS/HS ratio, 6.3; 95% CI, 4.9 to 8.1; GS/Het ratio, 5.9; 95% CI, 4.6 to 7.6; P<0.001) and a 50% increase in plasma aldosterone concentration relative to HS (mean GS/HS ratio, 1.8; 95% CI, 1.4 to 2.4; GS/Het ratio, 1.5; 95% CI, 1.1 to 1.9; P<0.001 both). Patients with GS displayed marked hypokalemia (mean difference −1.22 mmol/l; 95% CI, −1.35 to −1.08 and −1.13 mmol/l; 95% CI, −1.2 to −0.99; P<0.001, GS versus HS and Het, respectively) and hypomagnesemia (mean difference −0.21 mmol/l; 95% CI, −0.24 to −0.18; GS versus both groups, P<0.001). GFR estimated using creatinine-based (not shown) or cystatin C-based CKD-EPI did not differ between those with GS and Het or HS groups (Table 2). Finally, patients with GS had higher plasma calcium concentrations and lower daily urinary calcium excretion and fractional urinary calcium excretion than both HS and Het groups (Table 2). This was associated with 28% and 20% lower plasma PTH concentrations as compared with HS and Het, respectively (GS versus both groups, P<0.001), despite similar plasma vitamin D concentrations. Plasma calcitriol and markers of bone remodeling markers were lower in patients with GS than in Het and HS groups (Supplemental Table 2).
Analyses in Subpopulations of Nonhypertensive and Nondiabetic Subjects and Those Not Treated with Potassium-Sparing Diuretics
Because HS were selected for the absence of diabetes, HTN history, and antihypertensive treatments, we applied these criteria to the Het and GS groups and repeated analyses excluding 7 Het and 29 patients with GS. The difference in home SBP observed between patients with GS and their counterparts when the entire cohort was considered was no longer significant in the subpopulation (P=0.13 for GS versus HS and 0.57 for GS versus Het, respectively), but HR was higher in patients with GS than both other groups (mean difference +4.8 bpm; 95% CI, +1.5 to +8.0; P=0.01 for GS versus HS, and +4.3 bpm; 95% CI, +1.0 to +7.6; P=0.03 for GS versus Het) (Table 1, subgroup analysis). The positive correlation between home SBP and age observed in the entire cohort was also observed in the Het subpopulation (rho=0.39; P<0.001). This increase in home SBP was blunted in patients with GS when only those who were nonhypertensive, nondiabetic, and not treated for HTN were considered (rho=0.25; P=0.08). Differences in plasma concentrations of potassium, magnesium, renin, and aldosterone as well as in urinary excretion of sodium, potassium, magnesium, and calcium between these subpopulations were similar to those observed in the whole population (Table 3). The subtle effects of heterozygosity on phenotype are demonstrated by significant differences in plasma aldosterone concentration and plasma calcium and slight differences in aldosterone excretion and plasma PTH concentration in Het compared with HS groups (Supplemental Figure 2).
Table 3.
Clinical characteristics and biologic data after exclusion of Het carriers with HTN or diabetes history and patients with GS treated with potassium-sparing diuretics
| Characteristic | HS (n=82) | Het (n=74) | GS (n=79) | ANOVA P Value |
|---|---|---|---|---|
| Plasma | ||||
| Creatinine, µmol/L | 66 [59; 76]a | 65 [58; 72] | 59 [50; 72] | 0.01 |
| eGFR, ml/min per 1.73 m2 | 92 [81; 110] | 103 [89; 114] | 99 [86; 110] | 0.1 |
| Sodium, mmol/L | 140 [139; 141]a | 140 [139; 142]a | 139 [138; 141] | 0.01 |
| Potassium, mmol/L | 4.0 [3.8; 4.2]b | 3.9 [3.7; 4.1]b | 2.8 [2.5; 3.1] | <0.001 |
| CO2t, mmol/L | 27 [26; 28]b | 27 [26; 29]b | 30 [28; 32] | <0.001 |
| Calcium, mmol/L | 2.24 [2.18; 2.29]b | 2.26 [2.21; 2.34]b,c | 2.34 [2.26; 2.39] | <0.001 |
| Magnesium, mmol/L | 0.80 [0.75; 0.83]b | 0.80 [0.78; 0.84]b | 0.59 [0.51; 0.67] | <0.001 |
| PTH 7–84, pg/ml | 44 [34; 55]b | 39 [31; 46]b | 31 [24; 39] | <0.001 |
| PRC, mUI/L | 15 [9; 21]b | 16 [11; 23]b | 109 [53; 154] | <0.001 |
| PAC, pmol/L | 178 [136; 235]b | 213 [154; 305]b | 311 [241; 525] | <0.001 |
| Urinary excretion | ||||
| Output, L/24 h | 1.6 [1.1; 1.9]a | 1.4 [1.0; 2.1]a | 1.7 [1.4; 2.3]a | 0.02 |
| Creatinine, mmol/24 h | 11.9 [9.0; 15.0]a | 11.2 [8.8; 14.2] | 11.8 [9.9; 15.3] | 0.46 |
| Sodium, mmol/24 h | 147 [99; 172]b | 133 [93; 189]c | 183 [105; 246] | <0.001 |
| Potassium, mmol/24 h | 59 [44; 75]b | 58 [47; 79]b | 108 [92; 154] | <0.001 |
| Calcium, mmol/24 h | 3.8 [2.6; 5.6]b | 3.5 [2.2; 5.2]b | 1.1 [0.7; 2.0] | <0.001 |
| Calcium-to-creatinine ratio, mM/mM | 0.32 [0.23; 0.52]b | 0.32 [0.19; 0.47]b | 0.09 [0.05; 0.18] | <0.001 |
| Magnesium, mmol/24 h | 3.4 [2.5; 4.2]b | 3.3 [2.7; 4.3]b | 4.4 [3.6; 6.1] | <0.001 |
| Aldosterone, nmol/24 h | 56 [33; 87]b | 67 [48; 95 ] | 95 [72; 172] | <0.001 |
eGFR calculated with cystatin C-based CKD Epidemiology Collaboration. Data are presented as medians [IQR]. Difference between groups was evaluated first with ANOVA. If significant, Dunn test was performed to compare HS versus GS and Het versus GS (a,b) and Tukey test was performed to compare HS and Het (c). CO2t, Total CO2; PRC, plasma renin concentration; PAC, plasma aldosterone concentration.
P<0.05.
P<0.001.
P<0.05.
Comparison of Glucose Tolerance and Lipid and Liver Panels in the Entire Cohort
In fasting conditions, neither plasma glucose nor insulin concentrations differed between Het and HS (Table 4). Insulin resistance was evaluated using the HOMA-IR and the QUICKI. At baseline, the two indices were similar between Het and HS. Similar increases in plasma glucose and plasma insulin concentrations were observed 2 hours after oral glucose load in both Het and HS (P=1.00). Patients with GS had similar fasting plasma glucose and insulin concentrations to Het and HS groups, but plasma glucose and insulin concentrations after the oral glucose load were much higher in patients with GS (P<0.001; Table 4). A total 14% of patients with GS had prediabetes versus 4% in HS and 5% in Het groups.
Table 4.
Glucose metabolism by group
| Characteristic | HS (n=82) | Het (n=81) | GS (n=77) | ANOVA P Value |
|---|---|---|---|---|
| Antidiabetic drugs | 0 | 6 | 4 | |
| Insulin | 0 | 2 | 0 | |
| Metformin/sulfamides | 0/0 | 5/2 | 2/0 | |
| DPP4 inhibitors | 0 | 2 | 1 | |
| Hypolipemiant treatment | ||||
| Statins/fibrates | 0/0 | 10/9 | 8/6 | |
| Plasma | ||||
| Glucose T0, mM | 4.8 [4.7; 5.2] | 5.0 [4.6; 5.4] | 5.1 [4.8; 5.5] | 0.01 |
| Glucose T120, mM | 5.3 [4.6; 5.9]a | 5.4 [4.7; 6.1]a | 6.2 [5.5; 7.1] | <0.001 |
| Change in glucose (T120–T0) | 0.50 [-0.10; 1.20]b | 0.40 [-0.30; 1.20]b | 1.10 [0.30; 1.80] | <0.001 |
| Insulin T0, mUI/l | 4.4 [3.1; 5.4]a | 4.4 [3.2; 7.3]b | 6.8 [4.3; 10.8] | <0.001 |
| Insulin T120, mUI/l | 15.9 [8.6; 26.2]a | 22.0 [10.4; 35.9]b | 28.6 [17.8; 47.7] | <0.001 |
| Change in insulin (T120–T0) | 11.1 [5.8; 21.7]a | 18.0 [7.4; 32.1] | 21.3 [11.7; 41.0] | <0.001 |
| QUICKI T0 | 0.38 [0.37; 0.40]a | 0.38 [0.35; 0.41]b | 0.35 [0.33; 0.39] | <0.001 |
| IR index T0 | 0.50 [0.38; 0.60]a | 0.51 [0.39; 0.80]a | 0.77 [0.48; 1.22] | <0.001 |
| Cholesterol, mM | 5.09 [4.27; 5.63] | 4.98 [4.37; 5.67] | 5.27 [4.63; 6.01] | 0.1 |
| HDL cholesterol, mM | 1.36 [1.18; 1.58] | 1.48 [1.23; 1.70] | 1.45 [1.13; 1.66] | 0.10 |
| LDL cholesterol, mM | 3.07 [2.56; 3.67] | 3.01 [2.44; 3.72] | 3.34 [2.80; 3.92] | 0.23 |
| Triglycerides, mM | 1.00 [0.71; 1.43] | 1.00 [0.72; 1.33] | 1.10 [0.83; 1.60] | 0.11 |
| ALAT, UI/L | 17 [14; 24]a | 18 [13; 23]a | 23 [17; 39] | <0.001 |
| ASAT, UI/L | 16 [12; 19]a | 17 [13; 22]a | 21 [17; 30] | <0.001 |
| GGT, UI/L | 24 [14; 33] | 20 [14; 28] | 24 [16; 43] | 0.27 |
| ALP, UI/L | 55 [44; 64] | 56 [47; 63] | 51 [42; 61] | 0.10 |
Data are presented as median [IQR]. ANOVA was performed to compare Het or HS versus patients with GS. If significant, Tukey or Dunn test was performed to compare HS and Het and HS or Het versus GS (a,b,c). T0, before glucose load; T120, after glucose load; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkalin phosphaphatase.
P<0.001.
P<0.05.
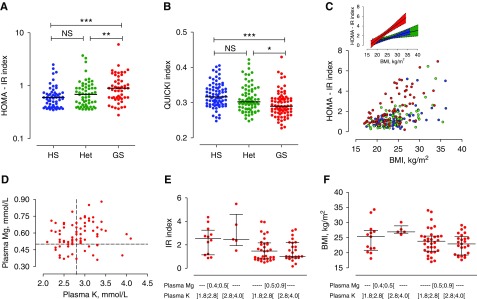
As compared with both Het and HS, patients with GS had a markedly higher HOMA-IR index (P<0.001 and P<0.001) and a lower QUICKI (P<0.001 and P=0.003; Figure 2, A and B, Table 4). Het and HS groups had similar relationships between HOMA-IR and BMI (slope 0.027±0.007 and 0.043±0.008 mUI×L−1/Kg per m2; P=0.14, and intercept −0.05±0.18 and −0.47±0.19 mUI×L−1/Kg per m2; P=0.11, respectively). In contrast, in patients with GS, the slope of the linear regression was steeper and backward shifted (slope of 0.085±0.009 mUI×L−1/Kg per m2; P<0.001 for GS versus HS and P<0.001 for GS versus Het; intercept of −1.11±0.21; P=0.02 for GS versus HS and P<0.001 for GS versus Het; Figure 2C).
Figure 2.
Resistance to insulin in the three groups. (A) IR index, (B) QUICKI, and (C) relationship between IR index and BMI in HS (blue), Het (green), and GS (red) groups. The inset shows semilog linear regression of this relationship and their 95% confidence bands. (D) Relationship between plasma potassium and plasma magnesium in patients with GS. (E) IR index and (F) BMI plotted according to severity of hypomagnesemia and hypokalemia in patients with GS. *P<0.05; **P<0.001; ***P<0.001.
Patients with GS had low potassium and low magnesium concentrations; however, few had both severe hypokalemia (i.e., <2.8 mmo/L) and severe hypomagnesemia (i.e., <0.5 mmol/L) (Figure 2D). Patients with severe hypomagnesemia (<0.5 mmol/l) had higher HOMA-IR index (median 2.29 [IQR, 1.48–2.74] versus 1.34 [IQR, 0.86–2.22]; P=0.02) and slightly higher BMI (median 26.0 [IQR, 21.5–27.0] versus 23.3 [IQR, 20.2–25.5] P=0.2) than those with higher plasma magnesium concentration, irrespective of the presence of severe hypokalemia (Figure 2, E and F). In addition, we found significant correlation between HR and insulin resistance index (r=0.31; P=0.006).
No between-group differences were detected in liver or lipid panels (Table 4). Similar results were obtained when subpopulations of subjects who were nonhypertensive, nondiabetic, and not treated with potassium-sparing diuretics were considered (data not shown).
Discussion
Hemodynamic, Hormonal, and Electrolyte Investigations
Whether heterozygous carriers of pathogenic SLC12A3 variants have phenotypes intermediate between patients with GS and noncarriers has not been conclusively demonstrated. To address this question, we performed detailed phenotyping of 81 heterozygous carriers of pathogenic variants of SLC12A3, 82 noncarrier HS and 79 patients with GS from 68 unrelated families with similar age, BMI, and sex ratio. The strength of our results is supported by the larger sample size of our study as compared with previous studies and the standardized phenotyping methodology used. Moreover, we analyzed BP measurements taken at home rather than office BP measurements to define precisely the hemodynamic status of all participants and to detect white-coat or masked HTN as recommended by the European Society of Hypertension guidelines.19 We standardized all measurements of renal handling of electrolytes, plasma and urine hormones, and glucose metabolism before and after OGTT.
The Het carriers had HBP similar to HBP of HS even when those treated by antihypertensive drugs were excluded. The high frequency of Het carrier status of pathogenic variants of SLC12A3 had previously led to the hypothesis that protection against HTN might be a beneficial evolutionary effect of the presence of an heterozygous functional variant.6 We failed to confirm such a protective effect against HTN in our 81 Het carriers with functional variants. Of our cohort, 17% had HTN, a percentage close to that observed in the French population of around 40 years of age,20 confirming the multifactorial origin of HTN occurring at middle age. Our conclusion that there is no difference in BP between Het and HS groups has limitations. First, the number of the participants was initially set to reach a power of 80% to demonstrate a difference of 10 mm Hg in SBP between Het and HS. This was on the basis of the observation of Ji et al.,7 who found such a difference between 23 carriers of “biochemically proven mutations” in SLC12A3, SLC12A1, and KCNJ1 and 2000 noncarrier participants in the Framingham study. The difference in SBP we found was much smaller and, according to the actual between-group difference and SD in HS and Het, we would have needed about 600 individuals to reach a power of 80% to demonstrate the small between-group differences we observed at a two-sided type 1 error rate =0.05 (Supplemental Table 3). Second, our HS population was selected to have neither history of HTN or diabetes, but mimicked as closely as possible the age, sex ratio, and BMI of the GS and Het groups. Although similar results were obtained after exclusion of Het participants with history of HTN and/or diabetes, the selection of the HS population biased the comparison of BP between Het and HS groups toward the null hypothesis.
We found no obvious difference in homeostasis of sodium or potassium between Het and HS groups. However, plasma aldosterone was slightly increased in the Het group, although plasma concentrations and intakes (reflected by excretion) of sodium and potassium were similar between Het and HS groups. Het carriers have slightly higher plasma calcium concentration and lower plasma PTH concentration compatible with an increase in renal reabsorption of calcium. There were no differences in urinary calcium excretion, calcitriol, or of bone remodeling markers between Het and HS groups. We calculated a posteriori the sample size required to achieve an 80% power to reject the null hypothesis, according to which the difference we observed between the groups is equal to zero, at a significance level of α=0.05 (two-tailed type 1 error). A sample of 150–200 participants per group would achieve acceptable power to be able to declare as significant the difference in plasma concentrations of PTH and aldosterone and in urinary excretion of aldosterone we observed, whereas 500–1000 participants would be required to achieve acceptable power to conclude about the significance of the differences in SBP, plasma magnesium concentration, and urinary calcium-to-creatinine ratio. Altogether, these data are consistent with a subtle intermediate phenotype in Het; a higher powered study will be required to confirm this.
Patients with GS had lower HBP and higher HR than Het and HS groups reflecting the sympathetic adaptive over activity adaptation to both renal salt wasting–induced hypovolemia and peripheral vasodilatation in GS.21 As previously described,22−24 some patients with GS were hypertensive. Because of the high percentage of patients treated with aldosterone antagonists or potassium-sparing diuretics, the exact prevalence of HTN in patients with GS is likely underestimated. Similarly, patients with GS with potassium sparing diuretics/aldosterone antagonists may be the patients with the more severe phenotype and excluding these patients may also biased the comparison of BP between GS and the Het and HS groups. When comparing the two populations of patients with GS with versus without these treatments, patients under diuretics were older, but we found no difference in sex, age at diagnosis, or plasma magnesium levels. Renal function in patients with GS was normal, as previously described.25 Finally, the increase in total plasma calcium concentration observed in patients with GS is likely partly due to extracellular dehydration; unfortunately, we did not measure plasma ionized calcium and plasma albumin concentrations. Thus, the concomitant decrease in serum PTH concentration suggests either higher ionized calcium or less PTH may be required to maintain the same ionized calcium level in patients with GS.
Glucose Metabolism Investigation
Abnormalities in glucose metabolism have been described in patients with GS.2,26,27 Ren et al.2 observed higher responses to oral glucose in 16 patients with GS compared with 12 HS and observed higher plasma glucose and insulin after glucose testing in the GS group with maximal difference 2 hours after administration. In agreement with this, Liu et al.26 reported type 2 diabetes mellitus in 13 of 32 patients with GS,2 and Yuan et al.27 reported higher areas under the curve of the glucose and lower areas under the curve of the insulin after OGTT in 25 patients with GS compared with 20 HS. We measured plasma glucose and insulin twice, after fasting and 2 hours after glucose administration, and calculated the HOMA-IR and QUICKI indices that have been demonstrated to be, respectively, inversely correlated or directly correlated to sensitivity to insulin.16 We found no difference between Het and HS in plasma glucose or insulin concentrations and, consequently, no differences in either the HOMA-IR index or the QUICKI index. Our results are not consistent with those of two other smaller studies reporting slightly but significantly higher fasting plasma glucose concentrations in Het carriers compared with healthy controls; however, OGTTs were not performed in the two previous studies.3,5,28
We confirmed the presence of insulin resistance in our large cohort of patients with GS, as demonstrated by a decrease in QUICKI and increase in HOMA-IR index in fasting conditions and 2 hours after a glucose load. Our results expand on data from a much smaller study that reported a higher plasma glucose and insulin response after an oral glucose test in 16 patients with GS compared with 12 healthy controls.2 We also found that the increase in fasting insulin (a reflection of insulin resistance) by an increase in BMI was three-fold more potentiated in GS as compared with the other groups. Thus, diet control is key to prevention of diabetes in patients with GS. Both hypokalemia and hypomagnesemia, the two main electrolyte abnormalities in GS, are known to impair insulin secretion. Indeed, a large decrease in extracellular potassium concentration is able to inhibit insulin secretion in vitro.29 A low-potassium diet (40 mmol/d) induces a defect in insulin secretion and a decreased sensitivity to insulin in HS.30 Magnesium depletion can lead as well to defects in insulin secretion and decreased sensitivity to insulin. Moreover, inhibition of insulin secretion or sensitivity can worsen hypomagnesemia, because insulin is an important regulator of the renal Mg2+ channel TRMP6.31,32 Patients with GS often have both hypokalemia and hypomagnesemia. The subgroup of patients with GS with severe hypomagnesemia had a higher insulin resistance index than the Het and HS groups, and a tendency toward higher BMI irrespective of the presence of severe hypokalemia, suggesting that magnesium depletion is the predominant contributor to insulin resistance in this population. Finally, we found significant correlation between HR and insulin resistance index, suggesting a contribution of increased sympathetic activity to the pathophysiology of insulin resistance.
Significance of High Prevalence of SLC12A3 Heterozygotes in the General Population
Because of the wide range of frequency of SLC12A3 variants previously reported,4 we analyzed data from the latest release of gnomAD (v2.1) and calculated a worldwide population frequency of 3.6% for expression of SLC12A3 variants (1.14% for well established pathogenic mutations and 2.46% for low-frequency, nonclassified variants). The predicted high prevalence of SLC12A3 heterozygosity led to the hypothesis that it might offer of a beneficial evolutionary effect to the heterozygous carrier, perhaps as protection against HTN.6 Although we failed to confirm a protective effect against HTN in Het carriers with functional variants evaluated here, as stated by Simon et al., “as the morbid clinical consequences of hypertension typically occur well after reproductive age, it seems unlikely that these alleles would increase reproductive fitness.”6 As sodium-chloride cotransporter expression has been reported in placenta,33 loss-of-function variants might directly affect reproductive fitness, a hypothesis that remains untested.
Study Limitations
Our study has limitations. We cannot exclude that there are subtle hemodynamic, electrolyte, or hormonal effects of the Het functional variants that could be manifested in childhood. Moreover, although our study suggests subtle differences between HS and Het, our sample size was not sufficient to confirm this intermediate phenotype or confirm effects on health at the scale of a population.
In conclusion, by evaluating hemodynamic and biologic parameters, we found evidence for an intermediate phenotype of Het carriers of pathogenic variants of SLC12A3 between those of HS and patients with GS. Patients with GS in our cohort had resistance to insulin potentiated by high BMI. To decrease risk of diabetes in patients with GS, both low glucose diet and prevention of weight gain are recommended.
Disclosures
Dr. Azizi reports grants from French Ministry of Health, during the conduct of the study; grants and nonfinancial support from Recor, personal fees from CVRx, grants and personal fees from Novartis, grants and non-financial support from Idorsia, grants from Quantum Genomics, grants from French Ministry of Health, outside the submitted work.
Funding
This work was supported by a grant from the French Ministry of Health and the Assistance Publique des Hôpitaux de Paris (PHRC AOM 12-074) and the European Community (FP7EUNEFRON 201590 and EURenOmics 2012-305608).
Supplementary Material
Acknowledgments
We thank the individuals for participating in the study. We also thank the nursing staff, especially the head nurse Mrs. Jeanne Meunier and the pharmacist Dr. Valérie Paquet of the Clinical Investigation Center of HEGP (Paris, France); the nursing staff and Mrs. Deborah Postil of the Clinical Investigation Center of Limoges; and Mrs. Sophie Glippa from the Clinical Research Unit for helping us with regulatory processes.
Drs. Blanchard and Vargas-Poussou conceived the study and drafted the paper. Drs. Vallet, de la Faille, Allard, Haymann, Courand, Houillier, Tack, and Blanchard included and evaluated patients and contributed to data interpretation. Mr. Bergerot and Dr. Becker performed centralized biochemical and hormone measurements. Drs. Hureaux and Vargas-Poussou performed genetic analysis. Mrs. Dinut contributed to data control and acquisition. Mrs. Arnoux performed statistical analysis. Professors Devuyst, Jeunemaitre, and Azizi contributed to protocol development and data interpretation. All the authors reviewed the manuscript and approved the final version.
The funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Enemy Action in the Distal Convoluted Tubule,” on pages 1345–1348.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019010031/-/DCSupplemental.
Supplemental Figure 1. Study flow chart.
Supplemental Figure 2. Evidence for a subtle intermediate phenotype in Het carriers.
Supplemental Table 1. SLC12A3 gene variants and classifications.
Supplemental Table 2. PTH, vitamin D metabolites, and bone remodeling markers.
Supplemental Table 3. A posteriori calculation of participant samples.
References
- 1.Blanchard A, Bockenhauer D, Bolignano D, Calò LA, Cosyns E, Devuyst O, et al.: Gitelman syndrome: Consensus and guidance from a kidney disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 91: 24–33, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Ren H, Qin L, Wang W, Ma J, Zhang W, Shen PY, et al.: Abnormal glucose metabolism and insulin sensitivity in Chinese patients with Gitelman syndrome. Am J Nephrol 37: 152–157, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Fava C, Montagnana M, Rosberg L, Burri P, Almgren P, Jönsson A, et al.: Subjects heterozygous for genetic loss of function of the thiazide-sensitive cotransporter have reduced blood pressure. Hum Mol Genet 17: 413–418, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Knoers NV, Levtchenko EN: Gitelman syndrome. Orphanet J Rare Dis 3: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu YJ, Yang SS, Chu NF, Sytwu HK, Cheng CJ, Lin SH: Heterozygous mutations of the sodium chloride cotransporter in Chinese children: Prevalence and association with blood pressure. Nephrol Dial Transplant 24: 1170–1175, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al.: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, et al.: Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandakumar P, Morrison AC, Grove ML, Boerwinkle E, Chakravarti A: Contributions of rare coding variants in hypotension syndrome genes to population blood pressure variation. Medicine (Baltimore) 97: e11865, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz DN, Simon DB, Nelson-Williams C, Farhi A, Finberg K, Burleson L, et al.: Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension 37: 1458–1464, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Tago N, Kokubo Y, Inamoto N, Naraba H, Tomoike H, Iwai N: A high prevalence of Gitelman’s syndrome mutations in Japanese. Hypertens Res 27: 327–331, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al.: ACMG Laboratory Quality Assurance Committee : Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas-Poussou R, Dahan K, Kahila D, Venisse A, Riveira-Munoz E, Debaix H, et al.: Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol 22: 693–703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashton EJ, Legrand A, Benoit V, Roncelin I, Venisse A, Zennaro MC, et al.: Simultaneous sequencing of 37 genes identified causative mutations in the majority of children with renal tubulopathies. Kidney Int 93: 961–967, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Bobrie G, Frank M, Azizi M, Peyrard S, Boutouyrie P, Chatellier G, et al.: Sequential nephron blockade versus sequential renin-angiotensin system blockade in resistant hypertension: A prospective, randomized, open blinded endpoint study. J Hypertens 30: 1656–1664, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al.: Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Association AD; American Diabetes Association : 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 41[Suppl 1]: S13–S27, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al.: CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al.: [2018 ESC/ESH guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH)]. G Ital Cardiol (Rome) 19: 3–73, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Perrine A, Lecoffre C, Blacher J, Olié V: L’hypertension artérielle en France: Prévalence, traitement et contrôle en 2015 et évolutions depuis 2006. Bull Epidémiol Hebd 10:170–179, 2018 [Google Scholar]
- 21.Calò LA, Davis PA, Rossi GP: Understanding the mechanisms of angiotensin II signaling involved in hypertension and its long-term sequelae: Insights from Bartter’s and Gitelman’s syndromes, human models of endogenous angiotensin II signaling antagonism. J Hypertens 32: 2109–2119, discussion 2119, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Balavoine AS, Bataille P, Vanhille P, Azar R, Noël C, Asseman P, et al.: Phenotype-genotype correlation and follow-up in adult patients with hypokalaemia of renal origin suggesting Gitelman syndrome. Eur J Endocrinol 165: 665–673, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Berry MR, Robinson C, Karet Frankl FE: Unexpected clinical sequelae of Gitelman syndrome: Hypertension in adulthood is common and females have higher potassium requirements. Nephrol Dial Transplant 28: 1533–1542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogihara T, Katsuya T, Ishikawa K, Matsuo A, Rakugi H, Shoji M, et al.: Hypertension in a patient with Gitelman’s syndrome. J Hum Hypertens 18: 677–679, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Walsh SB, Unwin E, Vargas-Poussou R, Houillier P, Unwin R: Does hypokalaemia cause nephropathy? An observational study of renal function in patients with Bartter or Gitelman syndrome. QJM 104: 939–944, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Wang C, Lu J, Zhao X, Lang Y, Shao L: Genotype/phenotype analysis in 67 Chinese patients with Gitelman’s syndrome. Am J Nephrol 44: 159–168, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Yuan T, Jiang L, Chen C, Peng X, Nie M, Li X, et al.: Glucose tolerance and insulin responsiveness in Gitelman syndrome patients. Endocr Connect 6: 243–252, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng MH, Yang SS, Hsu YJ, Fang YW, Wu CJ, Tsai JD, et al.: Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab 97: E1478–E1482, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Howell SL, Taylor KW: Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J 108: 17–24, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe JW, Tobin JD, Rosa RM, Andres R: Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism 29: 498–502, 1980 [DOI] [PubMed] [Google Scholar]
- 31.Nair AV, Hocher B, Verkaart S, van Zeeland F, Pfab T, Slowinski T, et al.: Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci U S A 109: 11324–11329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH: Hypomagnesemia in type 2 diabetes: A vicious circle? Diabetes 65: 3–13, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Chang H, Tashiro K, Hirai M, Ikeda K, Kurokawa K, Fujita T: Identification of a cDNA encoding a thiazide-sensitive sodium-chloride cotransporter from the human and its mRNA expression in various tissues. Biochem Biophys Res Commun 223: 324–328, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.