Abstract
Background
The aim of our study was to elucidate the biological targets and pharmacological mechanisms for calycosin (CC) against colorectal cancer (CRC) through an approach of system pharmacology.
Material/Methods
Using a web-based platform, all CRC-causing genes were identified using a database of gene-disease associations (DisGeNET), and all well-known genes of CC identified using the databases of prediction of protein targets of small molecules (Swiss Target Prediction), drug classification, and target prediction (SuperPred). The carefully selected genes of CRC and CC were concurrently constructed by using a database of functional protein association networks (STRING), and use of software for visualizing complex networks (Cytoscape), characterized with production of protein-protein interaction (PPI) network of CC against CRC. The important biological targets of CC against CRC were identified through topological analysis, then the biological processes and molecular pathways of CC against CRC were further revealed for testing these important biotargets by enrichment assays.
Results
We found that the key predictive targets of CC against CRC were estrogen receptor 2 (ESR2), ATP-binding cassette sub-family G member 2 (ABCG2), breast cancer type 1 susceptibility protein (BRCA1), estrogen receptor 1 (ESR1), cytochrome p450 19A1 (CYP19A1), and epidermal growth factor receptor (EGFR). Visual analysis revealed that the biological processes of CC against CRC were positively linked to hormonal metabolism, regulation of genes, transport, cell communication, and signal transduction. Further, the interrelated molecular pathways were chiefly related to endogenous nuclear estrogen receptor alpha network, forkhead box protein A1 (FOXA1) transcription factor network, activating transcription factor 2 (ATF2) transcription factor network, regulation of telomerase, plasma membrane estrogen receptor signaling, estrogen biosynthesis, androgen receptor, FOXA transcription factor networks, estrogen biosynthesis, and phosphorylation of repair proteins.
Conclusions
Use of system pharmacology revealed the biotargets, biological processes, and pharmacological pathways of CC against CRC. Intriguingly, the identifiable predictive biomolecules are likely potential targets for effectively treating CRC.
MeSH Keywords: Colorectal Neoplasms; Defense Mechanisms; Pharmacology, Clinical; Phytoestrogens
Background
Colorectal cancer (CRC) is a highly lethal gastroenteric tumor with high invasiveness [1]. CRC has the third highest morbidity and mortality rates of all cancers worldwide. Diagnostically, the early symptoms of CRC are difficult to detect, and the terminal stage of CRC is hard to treat due to lack of effective biomarkers for clinical screening. If left untreated, advanced CRC will develop to cancer-induced remote metastasis, and the fatality rate increases sharply [2,3]. Pathologically, the exact pathogenesis of CRC is unclear. There are few currently available chemotherapeutic options for advanced CRC, especially in metastatic stage. The clinical effectiveness of surgical resection, chemotherapy, and radiotherapy for CRC is insufficient [4,5]. Therefore, more effective and less cytotoxic bioactive treatments for CRC need to be screened and developed, and there is an urgent need to define the pharmacological mechanisms and biotargets of potential CRC treatments.
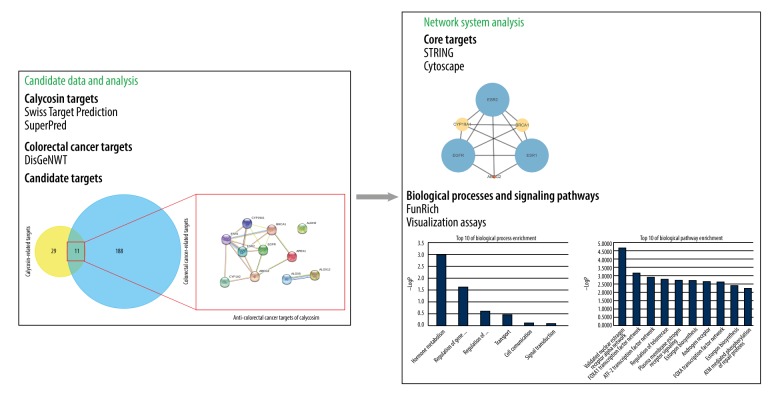
Traditional Chinese medicine uses Astragalus root to enhance immunity, protect cardiovascular function, and treat hypoglycemia. Astragaloside A, formononetin, and calycosin are among the bioactive ingredients extracted from Astragalus root that have promising pharmacological benefits [6,7]. Calycosin (CC), a functional phytoestrogen, is pharmacologically beneficial due to its neuroprotection, cytoprotection, antioxidative, hypolipemic, and hypoglycemic effects [8]. Moreover, CC has potent anti-neoplastic activities, including action against breast cancer and colorectal cancer. In some biological mechanisms, the signaling pathways exerted by CC are related to regulation of WDR7-7-GPR30, HOTAIR/p-Akt, ERβ/miR-17, ERβ/MiR-95, and IGF-1R [9,10]. However, the network molecular mechanisms of CC against CRC are not entirely defined. System pharmacology, also known as network pharmacology, is an emerging methodology to uncover the bioinformatic findings of predictive targets, signaling pathways, and protein-to-protein interaction networks in “drug treating disease” [11]. Interestingly, mounting evidence shows herb-isolated components to treat disorders using the system pharmacological approach [12]. In the present study, we used the system pharmacological approach to screen and reveal the biological targets, bio-processes, and signaling pathways of CC in treating CRC. As a result, the whole schematic diagram was illuminated, as shown in Figure 1.
Figure 1.
Schematic workflow in this study. The present report aimed to reveal the biotargets, biological processes, and molecular pathways of CC in treating CRC.
Material and Methods
Data collection and assay of CC in treating CRC
The well-known pharmacological genes of CC were obtained from the web-available databases of Swiss Target Prediction and SuperPred, and then the disease-conditioning genes of CRC were collected from the DisGeNET database. Subsequently, identified genes were pooled prior to obtaining the therapeutic targets of CC in treating against CRC [13].
Network target construction and analysis of CC treating CRC
Further, the web-available STRING database was accessed for analysis of pooled therapeutic targets of CC in treating CRC, followed by identification of interrelated network proteins. Then, the system PPI networks with all core targets were created, resulting from the reference data greater than 0.9 using Cytoscape software. In addition, topological indexes following Network Analyzer were screened and determined for identification of core targets. Briefly, clustering assay of system PPIs of CC in treating CRC was performed with the MCODE algorithm, which is a method for automatically finding molecular complexes in large protein interaction networks [14].
Biological processes and molecular pathways by enrichment tests
The web-available functional enrichment analysis tool (Funrich software) was used to find the functional processes and biological pathways of CC in treating CRC. Then, the graphical visualization of these biological functions and signaling pathways was performed in accordance with referencing -LogP value in computational setting [15].
Results
Data collection and identification of biotargets
We isolated and collected 2753 well-established pathogenic genes from the DisGeNET database. According to scores, the optimal 200 genes were identified for further data analysis, including 1 microRNA. We also screened and identified 40 pharmacological targets of CC, and 11 intersection targets of CC in treating CRC were obtained using FunRich software. In further analysis, a PPI network of CC in treating CRC was constructed using these 11 pooled biotargets (Figure 2).
Figure 2.
Network analysis used for construction of a PPI network in CC in treating CRC. As a result, the pharmacological 11 biotargets of CC treating CRC were identified.
Topology parameters of PPI network and core targets
Network Analyzer was used to analyze the topological indexes and PPI network of CC in treating CRC. The median of the calculated degrees of freedom was set as 4, and the maximum degrees of freedom was set as 6. Thus, the core target screening conditions were set as 4 to 6, and 6 core target proteins were identified as ESR2, ABCG2, BRCA1, ESR1, CYP19A1, and EGFR (Figure 3).
Figure 3.

Identification of the most important biotargets of CC treating CRC. The core targets of CC against CRC were ESR2, ABCG2, BRCA1, ESR1, CYP19A1, and EGFR.
Biological processes and molecular pathways of core biotargets
To reveal the detailed mechanisms of CC in treating CRC, enrichment analyses were conducted. Visualization data from FunRich assay showed that the biological processes of explicit core targets were hormone metabolism, regulation of gene expression, epigenetic, regulation of nucleobase, nucleoside, nucleotide, and nucleic acid metabolism, transport, cell communication, and signal transduction (Figure 4). Furthermore, the bioinformatic findings of signaling pathways of explicit core targets in CC in treating CRC were predominantly associated with validated nuclear estrogen receptor alpha network, FOXA1 transcription factor network, ATF2 transcription factor network, regulation of telomerase, plasma membrane estrogen receptor signaling, estrogen biosynthesis, androgen receptor, FOXA transcription factor networks, estrogen biosynthesis, and ATM-mediated phosphorylation of repair proteins (Figure 5).
Figure 4.

Enrichment analysis for revealing biological processes of core biotargets in CC in treating CRC. As a result, top 10 biological processes were identified.
Figure 5.
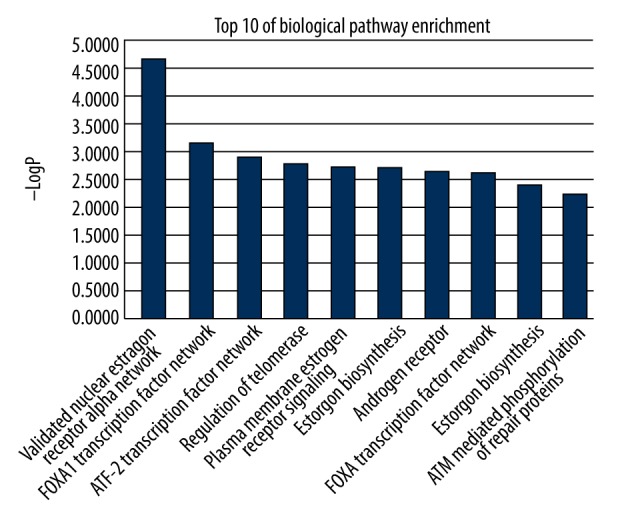
Enrichment assay for disclosing signaling pathways of core biotargets in CC in treating CRC. As a result, the top 10 signaling pathways were identified.
Discussion
By using the bioinformatic assays of system pharmacology, we obtained detailed information on functional processes and signaling pathways of CC in treating CRC. More importantly, the core biotargets of CC in treating CRC were obtained, and the following optimal biotargets were screened: ESR2, ABCG2, BRCA1, ESR1, CYP19A1, and EGFR. Based on our literature review, we reasoned that these important genes/proteins were closely related to the development of CRC. ESR2 (estrogen receptor α) is a nuclear hormone receptor that is functionally linked to modulation of cellular proliferation and differentiation in targeting tissues or cells [16]. Increasing evidence indicates that excessive expression of neoplastic ESR2 is associated with low survival and poor prognosis of colorectal cancer patients [17]. ABCG2 (ATP-binding cassette transporter G2) is a glycosylated transmembrane protein that has a key role in the multidrug resistance of tumor cells [18]. Increasing evidence shows that overexpression of ABCG2 is associated with the progression of stem cell-derived cancer cells, and ABCG2 may be a potential therapeutic target for treating colorectal cancer [19]. BRCA1 is a susceptibility protein that mutates in cancer cells, and it has roles in regulating cell cycle, ubiquitination, and apoptosis [20]. Based on the literature, elevated BRCA2 mutation is related to the risk of developing colorectal cancer [21]. ESR1 (estrogen receptor β) is a nuclear receptor that is highly expressed in malignant tissues, and it promotes cell proliferation and growth of cancer cells in a spatiotemporal manner [22]. In estrogenic signaling, ESR1 is a potential target for treating colorectal cancer [23]. CYP19A1 is a functional enzyme that is involved in gonadal development, sex differentiation, and ontogenesis [24]. CYP19A1 has a functional role in the development of colon and rectal cancer through regulating inflammation-associated pathways [25]. EGFR is a transmembrane tyrosine kinase that regulates cellular proliferation, differentiation, neoplastic growth [26]. Thus, anti-EGFR inhibitor may be a promising treatment of colorectal cancer, including metastatic stage [27]. The literature and our present results indicate that these genes/proteins may be potential pharmacological targets of CC in treating CRC, but this needs to be verified by further research. In addition, these biotargets are consistent with signaling pathways induced by FN, such as endogenous nuclear estrogen receptor alpha network, transcription factor network, estrogen receptor signaling, estrogen biosynthesis, androgen receptor, estrogen biosynthesis, and phosphorylation of repair proteins.
Conclusions
These bioinformatic data sheds light on the clinical and pharmaceutical significance of CC in treating CRC. Intriguingly, the core biotargets, biological functions, and molecular pathways of CC in treating CRC are identified through system pharmacological methods. Further, these optimal biotargets of ESR2, ABCG2, BRCA1, ESR1, CYP19A1, and EGFR may be used for screening and treating colorectal cancer.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81560591, 71764005, 81560134)
Conflict interest
None.
References
- 1.Sideris M, Papagrigoriadis S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014;34:2061–68. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. Cancer J Clin. 2017;67:177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberts S, Poston G. OncoSurge: A strategy for long-term survival in metastatic colorectal cancer. Colorectal Dis. 2003;5:20–28. doi: 10.1046/j.1463-1318.5.s3.1.x. [DOI] [PubMed] [Google Scholar]
- 5.Costi R, Leonardi F, Zanoni D, et al. Palliative care and end-stage colorectal cancer management: The surgeon meets the oncologist. World J Gastroenterol. 2014;20:7602–21. doi: 10.3748/wjg.v20.i24.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: A Review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med. 2016;44:1–22. doi: 10.1142/S0192415X16500014. [DOI] [PubMed] [Google Scholar]
- 7.Shahzad M, Shabbir A, Wojcikowski K, et al. The antioxidant effects of Radix astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr Drug Targets. 2016;17:1331–40. doi: 10.2174/1389450116666150907104742. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Ren Q, Zhang X, et al. Neuroprotective mechanisms of calycosin against focal cerebral ischemia and reperfusion injury in rats. Cell Physiol Biochem. 2018;45:537–46. doi: 10.1159/000487031. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zhao X, Li X, Wu Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015;6:3091–97. doi: 10.1039/c5fo00374a. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Li X, Ren Q, et al. Calycosin induces apoptosis in colorectal cancer cells, through modulating the ERβ/MiR-95 and IGF-1R, PI3K/Akt signaling pathways. Gene. 2016;591:123–28. doi: 10.1016/j.gene.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Wu K, Wei P, Liu M, et al. To reveal pharmacological targets and molecular mechanisms of curcumol against interstitial cystitis. J Adv Res. 2019;20:43–50. doi: 10.1016/j.jare.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou R, Wu K, Su M, Li R. Bioinformatic and experimental data decipher the pharmacological targets and mechanisms of plumbagin against hepatocellular carcinoma. Environ Toxicol Pharmacol. 2019;70:103200. doi: 10.1016/j.etap.2019.103200. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Ma X, Song Y, et al. Anti-colorectal cancer targets of resveratrol and biological molecular mechanism: Analyses of network pharmacology, human and experimental data. J Cell Biochem. 2019;120:11265–73. doi: 10.1002/jcb.28404. [DOI] [PubMed] [Google Scholar]
- 14.Su M, Guo C, Liu M, et al. Therapeutic targets of vitamin C on liver injury and associated biological mechanisms: A study of network pharmacology. Int Immunopharmacol. 2019;66:383–87. doi: 10.1016/j.intimp.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Xiao H, Qin X, Wan J, Li R. Pharmacological targets and the biological mechanisms of formononetin for Alzheimer’s sisease: A network analysis. Med Sci Monit. 2019;25:4273–77. doi: 10.12659/MSM.916662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia M, Dahlman-Wright K, Gustafsson JÅ. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29:557–68. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Rudolph A, Toth C, Hoffmeister M, et al. Colorectal cancer risk associated with hormone use varies by expression of estrogen receptor-β. Cancer Res. 2013;73:3306–15. doi: 10.1158/0008-5472.CAN-12-4051. [DOI] [PubMed] [Google Scholar]
- 18.Robey RW, Medina-Pérez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 19.Ma L, Liu T, Jin Y, et al. ABCG2 is required for self-renewal and chemoresistance of CD133-positive human colorectal cancer cells. Tumour Biol. 2016;37:12889–96. doi: 10.1007/s13277-016-5209-5. [DOI] [PubMed] [Google Scholar]
- 20.Esashi F, Christ N, Gannon J, et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 2005;434:598–604. doi: 10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- 21.Sopik V, Phelan C, Cybulski C, Narod SA. BRCA1 and BRCA2 mutations and the risk for colorectal cancer. Clin Genet. 2015;87:411–18. doi: 10.1111/cge.12497. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Leav I, Leung YK, et al. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164:2003–12. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams C, DiLeo A, Niv Y, Gustafsson JÅ. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016;372:48–56. doi: 10.1016/j.canlet.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukami M, Shozu M, Ogata T. Molecular bases and phenotypic determinants of aromatase excess syndrome. Int J Endocrinol. 2012;2012 doi: 10.1155/2012/584807. 584807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slattery ML, Lundgreen A, Herrick JS, et al. Variation in the CYP19A1 gene and risk of colon and rectal cancer. Cancer Causes Control. 2011;2:955–63. doi: 10.1007/s10552-011-9768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20. doi: 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koustas E, Karamouzis MV, Mihailidou C, et al. Co-targeting of EGFR and autophagy signaling is an emerging treatment strategy in metastatic colorectal cancer. Cancer Lett. 2017;396:94–102. doi: 10.1016/j.canlet.2017.03.023. [DOI] [PubMed] [Google Scholar]