Abstract
Takotsubo cardiomyopathy (TCM), also known as broken heart syndrome or stress-induced cardiomyopathy, is a rare condition with an estimated incidence of 0.02% of all hospitalizations in United States and 2% of all acute coronary syndrome presentations. TCM predominately presents as a transient wall motion abnormality of the left ventricular apex due to emotional or physical stress. Cardiac rupture in the setting of TCM is an extremely rare phenomenon with limited published case reports. We present a case of a 75-year-old female who had cardiac rupture secondary to TCM and performed a literature review using Ovid MEDLINE for published cases showing this association. After the literature review, we found 20 cases showing this association, which are listed in a tabular fashion.
1. Introduction
Takotsubo cardiomyopathy (TCM), also known as stress-induced cardiomyopathy, was first described in Japan in 1990 [1]. It mimics acute coronary syndrome and is characterized by transient systolic and diastolic dysfunction of the left ventricle, wall motion abnormalities, and elevated cardiac biomarkers and is frequently preceded by emotional or physical stress [2]. Ballooning of the left ventricular apex with a finding of patent coronary arteries is typically present on left ventriculography and cardiac catheterization, respectively. It is diagnosed in 0.02% of all nationwide hospitalization and predominantly involves elderly females [3]. The inpatient mortality rate in patients with TCM is 4.5% [3]. There has been a gradual increase in the incidence of TCM with the realization that this entity is not as benign as it was thought to be. As per recent TCM registry, on long-term follow-up, rate of major adverse cardiac and cerebrovascular events was 9.9% per patient-year and death was 5.6% per patient-year [4]. While TCM is usually reversible, it may present with rare complications including systemic embolism, life-threatening ventricular arrhythmias, cardiogenic shock, and cardiac rupture leading to cardiac arrest [5]. We present an exceptional rare case of TCM leading to left ventricular wall rupture. To the best of our knowledge, only 20 cases have been reported so far showing this very rare outcome.
2. Case Presentation
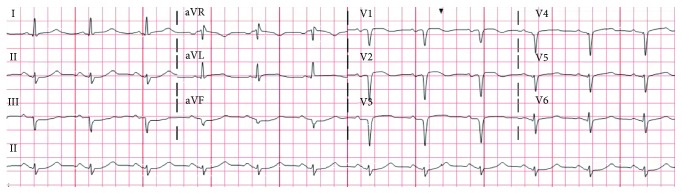
A 75-year-old female with past medical history of coronary artery disease, hypertension, hyperlipidemia, paroxysmal atrial fibrillation, systemic lupus erythematosus, and deep venous thrombosis was transferred from an outside hospital for the management of non-ST segment elevation myocardial infarction (NSTEMI). The patient presented to the local emergency department with substernal chest pain radiating to the left jaw and arm and was found to have an elevated troponin (0.26 ng/ml). She was appropriately given aspirin, heparin, and nitroglycerine and was transferred to our medical centre for further care. On presentation, she was alert and oriented, her blood pressure was 200/100 mmHg, nontachycardic, nontachypneic, and sating at 97-98% on 2 litres per minute of oxygen. Pertinent physical exam findings include bibasilar crackles, no murmurs/rubs, normal heart sounds, and no pedal edema. Pertinent laboratory values include haemoglobin (14.2 gm/dl), platelet count (191 k/μl), white count (14.4 k/μl), INR (1.1), creatinine (0.66 mg/dl), and AST (87 μ/l). Electrocardiogram (ECG) on presentation showed sinus rhythm at 74 beats/min, left axis deviation, Q waves in V1 to V3, 1 mm ST segment elevation of V2-V3 (present in prior ECG), and poor R wave progression (Figure 1). A resting 2D Doppler echocardiogram was performed on admission which showed severely reduced ejection fraction of 30-35%, severe hypokinesis of mid to apical segment with more involvement of the mid anteroseptum, and anterior wall with basal hyperkinesia and basal asymmetric hypertrophy of the septum (Figure 2).
Figure 1.
Electrocardiogram showing sinus rhythm at 74 beats/min, left axis deviation, Q waves in V1 to V3, 1 mm ST segment elevation of V2-V3, and poor R wave progression.
Figure 2.
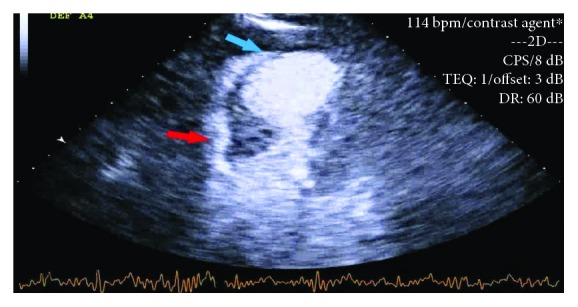
Transthoracic echo showing severely reduced ejection fraction of 30-35%, severe hypokinesis of mid to apical segment with more involvement of the mid anteroseptum, and anterior wall (blue arrow) with basal hyperkinesia and basal asymmetric hypertrophy of the septum (red arrow).
The decision was made to undergo urgent cardiac catheterization as her chest pain continued to worsen while on nitroglycerine drip, and the troponin on arrival to our facility was elevated (6.80 ng/ml). While in the elevator, en route to the cardiac catheterization laboratory, the patient became unresponsive. Resuscitation was immediately started, labs at the time of code blue were significant for acute drop in haemoglobin from 14.2 to 6.2, hypokalaemia of 2.5 mmol/l, and arterial blood gas showed metabolic acidosis (pH of 7.27, pCO2 of 34, pO2 of 28, bicarbonate of 15.2 mmol/l). The catheterization team was unable to establish arterial access hence coronary catheterization was not performed. Unfortunately, despite after aggressive resuscitative efforts for 50 minutes, the patient died.
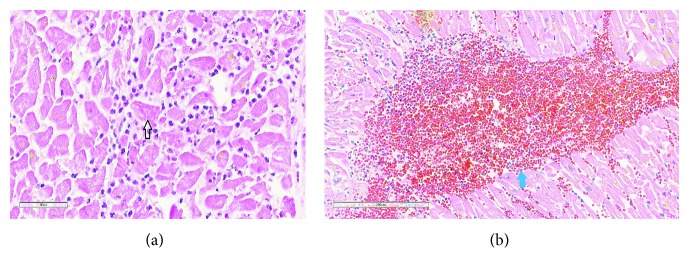
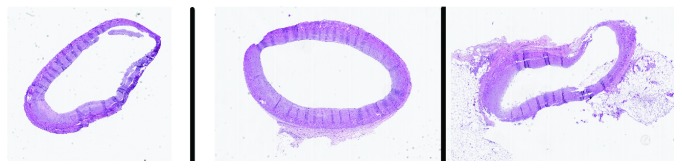
Autopsy was performed and revealed 1000 ml of blood in chest wall cavity. Gross pathology revealed a slit-like rupture of 1 cm × 0.8 cm, transmural and located anteriorly 1.5 cm inferior to the base of the heart. The pericardial surface was smooth and tan red in colour (Figure 3). Microscopically, this area had coagulative necrosis, hypereosinophilic appearance of myocytes with abundant ghost cells (cells without nuclei), and cells with pyknotic nuclei. The neutrophilic infiltrate, haemorrhage, and contraction band necrosis can also be visualised (Figure 4). Sacha et al. reported similar rupture site changes indicative of new acute infarct and transmyocardial necrosis leading to rupture in TCM patient [6]. There was epicardial haemorrhage (3.5 cm in length) adjacent to the left anterior descending artery proximal to the site of rupture. Sections in this area revealed area of infarction with early signs of mottling grossly. Interestingly, this area lacked neutrophils suggesting an area of infarction of less than 10 hours. We speculated that this difference in timeline could be due to the discordance of myocardial contraction seen between the apex and base which is often seen in TCM. Most importantly, her major coronary arteries were patent with minimal atherosclerosis and without evidence of organizing thrombus (Figure 5), supporting the histopathological criteria for the diagnosis of TCM.
Figure 3.
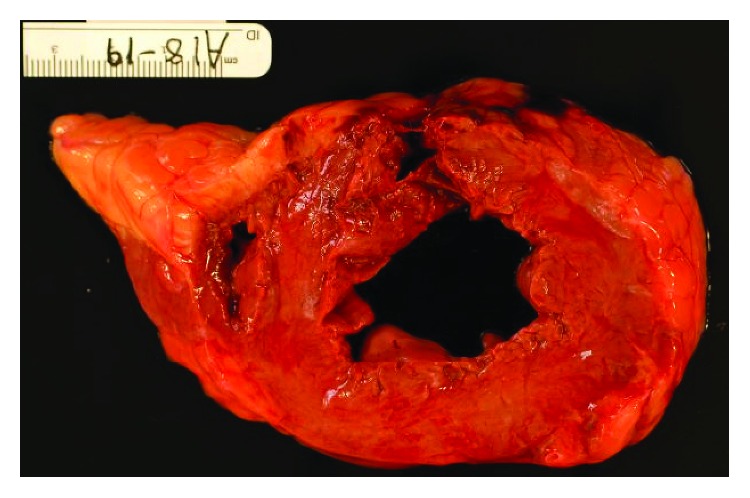
Gross pathology. A cut section of the left ventricle showing an anteriorly located, transmural slit-like rupture (1 cm × 0.8 cm) of the ventricular wall.
Figure 4.
(a) Microscopic pathology. Acute myocardial infarction, H&E, 400x. Note: neutrophilic infiltration of the myocardium, with contraction bands (arrow). (b) Myocardium, H&E, 400x, showing intraventricular haemorrhage (blue arrow).
Figure 5.
Cut sections of coronary arteries showing patent arteries with minimal atherosclerosis.
3. Discussion
TCM is generally classified by an acute and profound but reversible left ventricular dysfunction in the absence of significant coronary artery disease. The revised Mayo criteria are most commonly used currently to establish diagnosis of TCM which includes combination of clinical presentation, ECG, transthoracic echo, and angiographic finding [7]. It is often triggered by an acute emotional or physical stress [4]. TCM is associated with a catecholamine surge and adrenoceptor hyperactivity which can increase the cardiotoxicity leading to increased chances of complications. Increased levels of norepinephrine in the state of stress can cause localized ventricular wall motion abnormalities and ST segment changes [8]. In our case, we believe the rupture of the left ventricular wall was multifactorial and a consequence of increased afterload due to elevated blood pressure, probable increased catecholamine surge, and adrenoceptor hyperactivity in the setting of acute stress.
As per Kumar et al., the characteristics of patients with cardiac rupture in TCM when compared to patients with TCM who did not had cardiac rupture include the following: female gender, older age group, higher systolic and diastolic blood pressure, higher frequency of ST elevations in inferior lead, low ejection fraction (EF), and higher left ventricular peak systolic fraction [9]. Our patient portrayed a number of these characteristics including older age, female gender, higher systolic and diastolic pressures, and severely reduced EF.
As histopathological slides from our patient showed no thrombus and minimal atherosclerosis of coronary arteries, our case falls into the newly diagnosed category of MINOCA (myocardial infarction with nonobstructive coronary arteries). TCM is one of the categories of MINOCA; other categories include coronary spasm, coronary dissection, plaque disruption, spontaneous coronary emboli, myocarditis, and coronary microvasculature dysfunction [10]. Our pathological findings were classical for TCM, and apart from the area of rupture, no other area of infarction was seen. Although coronary spasm can also lead to infarction and subsequent death, hence we cannot exclude it definitively, but when our patient's histological slides are taken in consideration with clinical scenario, TCM is the more likely diagnosis.
A thorough literature review of 20 previous cases of TCM complicated with cardiac rupture is listed in Table 1. The characteristics of these patients include 95% females and mean age of 74.9 years. 16 patients presented with chest pain or discomfort (76%); 17 had ST segment elevations (81%); only 9 cases mentioned troponin levels and out of these, 8 showed increased troponin (89%). These findings further support that TCM mimics acute myocardial infarction. Including our case, a total of 17 patients died (81%). The location of rupture was reported in ten cases; six at the apex (60%), two involving the anterior wall (20%), and two involving the posterior wall (20%).
Table 1.
Reported cases with cardiac rupture in takotsubo cardiomyopathy patients (Ovid MEDLINE, 2018).
| Authors | Age (in years) | Gender | Clinical presentation | EKG finding | Troponin (ng/ml) | ECHO findings | Catheterization findings | Outcomes | Autopsy findings |
|---|---|---|---|---|---|---|---|---|---|
| Kumar et al. [9] | 62 | Female | Weakness and lightheadedness | ST elevation in I, II, and V5-V6 | 11.64 | EF 30% and severely reduced LV systolic function in mid and distal segments and preserved at basal segments | Nonstentable 50-75% stenosis at mid LAD artery | Death | Slit-like rupture at the mid portion of the posterior ventricular wall |
| Zalewska-Adamiec et al. [11] | 74 | Female | Chest pain | Sinus rhythm with QS complex and ST segment elevation in V2-V6 | 2.041 | Contractile disturbances in the apex and hyperkinesis of basal segments with EF 56% and cardiac tamponade | No significant stenosis | Surgical repair and good condition on discharge | Not applicable |
| Kudaiberdiev et al. [12] | 63 | Female | Chest pain, lightheadedness, dyspnea | Q waves in III and aVF, T wave inversions in lead II, III, and aVF, and ST-T abnormalities in V5-V6 | 0.0 | LV dilatation EF (35%) moderate MR, hypoakinesia and thinning of LV inferolateral wall with rupture and cross-over blood shunt through two defects into the pericardium | Patent coronary arteries | Surgical repair and good condition on discharge | Not applicable |
| Sung et al. [13] | 73 | Female | Chest pain and dyspnea | ST elevation in V2-V5 | 1.3 | Akinesis of mid to apical left ventricle with EF of 58% | Patent coronary arteries | Death | Not performed |
| Yoshida et al. [5] | 78 | Female | Chest pain and dyspnea | RBBB and ST elevation in V2-V6 with QS pattern | Not mentioned | Apical kinesis with wall thinning and massive pericardial effusion | Patent coronary arteries | In good condition after discharge | Not applicable |
| Indorato et al. [1] | 70 | Female | Chest pain and nausea | Not done | Not done | Not performed | Not performed | Death. Patient died en route to hospital | Hemorrhagic infarction of LV apex. 0.4 cm line of ruptured myocardium from anterior to posterior wall at the apex |
| Shams [14] | 73 | Male | Clinical features of pulmonary edema | Sinus tachycardia with Q waves and ST elevation in inferior leads and depression in anterolateral leads | 2.840 | Left ventriculography: akinesis in the middle and basal-inferior wall and in broad band of mid anterior, mid lateral, and mid septal parts of the left ventricle and hemopericardium. Bedside, limited echo shows cardiac tamponade | Stenosis of all three major arteries. No signs of coronary occlusion | Death | Hemopericardium, perforation of LV free wall at upper posterior part |
| Kurisu and Inoue [2] | 81 | Female | Unconsciousness | ST segment elevation in I, II, III, aVF, and V2-V6 | Not mentioned | Apical akinesia and basal hyperkinesis | Patent coronary arteries | Death | Not performed |
| Sacha et al. [6] | 81 | Female | Chest pain | Diffuse ST elevation in the precordial and limb leads | 1.55 | Balloon-like LV motion abnormalities with akinesis from mid to apical portions and hyperkinesis of base | No coronary artery disease | Death | Hemopericardium with an LV free wall rupture measuring 10 mm in the apical region and no patent coronary arteries. Inside the heart, there was a mural thrombus in the apical area |
| Jaguszewski et al. [15] | 82 | Female | Chest pain | St segment elevation from V1 to V5 | 14.82 | Abnormal LV contraction with apical ballooning pattern with EF of 55% | Patent coronary arteries | Death | Wide penetrating apical rupture as well as 1500 ml of thrombi and liquid blood in the pericardium |
| Shinozaki et al. [16] | 90 | Female | Chest pain | ST segment elevation in aVL and V1-V4 | Not mentioned | LV apical akinesis and hyperkinesis of base | Intact coronary arteries | Death | Not mentioned |
| Akashi et al. [8] | 70 | Female | Chest discomfort | ST elevation in I, II, III, aVL, aVF, and V2-V6 | Not mentioned | Apical akinesis and basal hyperkinesis with EF of 51% | Normal coronary arteries | Death | Not performed |
| Showkathali et al. [17] | 86 | Female | Chest pain | ST segment elevation in anterolateral and inferior leads | Not mentioned | Shows TCM and no intraventricular gradient | Normal RCA and mild atheromatous LAD artery | Death | Not mentioned |
| Yamada et al. [18] | 71 | Female | Shoulder and back pain | St segment elevation in leads V4-V6 and abnormal Q waves in leads V4-V5 | Not mentioned | Left ventricular apical wall akinesis. Hyperkinesis in the basal wall with mitral valve systolic anterior wall motion | No coronary artery stenosis | Death | Not performed |
| Stöllberger et al. [19] | 71 | Female | Generalized tonic clonic seizure | ST segment elevation in II, II, avF, V5, and V6 | Trop-T positive | Left ventricular apical wall, apical septum, and apical posterior wall akinesia and small pericardial effusion | Normal coronary arteries | Death | 5 mm left ventricular rupture in the apicoposterior region |
| Ohara et al. [20] | 79 | Female | Chest pain | ST segment elevation in 1, aVL, and V1-V5; depression in leads III and avF; and abnormal Q wave in V1-V4 | Not mentioned | Akinesis of the left ventricular apical wall | Patent coronary arteries | Death | Rupture in the anterior portion of the left ventricle, patent coronary arteries, and hemopericardium |
| Mafrici et al. [21] | 87 | Female | Chest pain and dyspnea | ST segment elevation in inferior leads and V2-V6 | Trop-T: 20 | Apical dyskinesis with hyperkinesis of left ventricular basal segment | Patent coronary arteries | Death | Not performed |
| Ishida et al. [22] | 67 | Female | Chest pain | ST segment elevation in I, avL, and V2-V5 | Not mentioned | Apical ballooning, basal hyperkinesis, and left ventricular outflow pressure gradient of 110 mmHg associated with systolic anterior movement of anterior mitral leaflet | Extensive akinesis from the apex to mid portion | Surgical repair to correct the cardiac rupture slit | Not applicable |
| Leva et al. [23] | 65 | Female | Chest pain and dyspnea | ST segment elevation in anterior leads | Not mentioned | Akinesis from mid to apical LV and basal hyperkinesis, EF of 30% | No significant stenosis of epicardial coronary arteries | Death | Not mentioned |
| Iskander et al. [24] | 77 | Female | Unconsciousness, chest pain, and dyspnea | ST segment. Elevation in leads I, aVL, and V2-V6 | Trop-T: 3.60 | EF of 25%. Severe dyskinesis of anterolateral wall of LV, no LVOT obstruction | No coronary artery obstruction with slow flow down the LAD | Death | Fresh clot on epicardial surface, slit-like rupture on anteroapical surface of LV |
| Present case | 75 | Female | Chest pain and dyspnea | Sinus rhythm, no ST segment elevation, poor R wave progression | 6.80 | EF of 30-35%, severe hypokinesis of apical LV, and asymmetric hypertrophy of the basal septum | Not performed | Death | Hemopericardium, patent epicardial coronary arteries, slit-like 1 cm × 0.8 cm rupture of the anterior wall of LV |
Abbreviations: LV: left ventricle; LAD: left anterior descending artery; RCA: right coronary artery; EF: ejection fraction; TCM, takotsubo cardiomyopathy; LVOT: left ventricular outflow tract obstruction; LAD: left anterior descending artery.
The prognosis of TCM is usually favourable, and left ventricular function improves in the majority of cases with conservative management. Currently, there is no standardised treatment protocol for the patients with TCM [24]. Although beta blockers have been suggested in studies to prevent the progression and recurrence of TCM [16], but recent TCM registry results show no survival benefit with beta blocker [4]. The same registry states that the use of angiotensin receptor blocker or angiotensin-converting enzyme was associated with improved survival [4]. Further studies are needed to delineate the role of beta blockers in TCM. The ST segment elevation in TCM is usually transient and recovers within few days; persistent ST elevation is a warning sign for continued myocardial injury and portend an impending ventricular free wall rupture [24]. The treatment of cardiac rupture in the setting of TCM is surgical repair [11]. It is unclear to date whether anticoagulation plays any role in TCM, but apical thrombus formation had been reported in patients with TCM [8].
4. Conclusion
Due to devastating complications of TCM, our case highlights the need for close monitoring of patients with TCM for the first few days. Special consideration should be paid to older female patients as they have higher rates of cardiac rupture.
Acknowledgments
We are really thankful to Dr. James L. Fishback (Chancellor's Club Distinguished Teaching Professor (Emeritus), Pathology and Lab Medicine) for his help in interpretation of the pathology slides.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Indorato F., Akashi Y. J., Rossitto C., Raffino C., Bartoloni G. Takotsubo cardiomyopathy associated with rupture of the left ventricular apex: assessment of histopathological features of a fatal case and literature review. Forensic Science, Medicine, and Pathology. 2015;11(4):577–583. doi: 10.1007/s12024-015-9711-7. [DOI] [PubMed] [Google Scholar]
- 2.Kurisu S., Inoue I. Cardiac rupture in tako-tsubo cardiomyopathy with persistent ST-segment elevation. International Journal of Cardiology. 2012;158(1):e5–e6. doi: 10.1016/j.ijcard.2011.10.059. [DOI] [PubMed] [Google Scholar]
- 3.Singh K., Carson K., Usmani Z., Sawhney G., Shah R., Horowitz J. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. International Journal of Cardiology. 2014;174(3):696–701. doi: 10.1016/j.ijcard.2014.04.221. [DOI] [PubMed] [Google Scholar]
- 4.Templin C., Ghadri J. R., Diekmann J., et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. The New England Journal of Medicine. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S., Miwa K., Matsubara T., et al. Stress-induced takotsubo cardiomyopathy complicated with wall rupture and thrombus formation. International Journal of Cardiology. 2012;161(1):e18–e20. doi: 10.1016/j.ijcard.2012.03.152. [DOI] [PubMed] [Google Scholar]
- 6.Sacha J., Maselko J., Wester A., Szudrowicz Z., Pluta W. Left ventricular apical rupture caused by takotsubo cardiomyopathy-comprehensive pathological heart investigation. Circulation Journal. 2007;71(6):982–985. doi: 10.1253/circj.71.982. [DOI] [PubMed] [Google Scholar]
- 7.Scantlebury D. C., Prasad A. Diagnosis of takotsubo cardiomyopathy. Circulation Journal. 2014;78(9):2129–2139. doi: 10.1253/circj.CJ-14-0859. [DOI] [PubMed] [Google Scholar]
- 8.Akashi Y. J., Tejima T., Sakurada H., et al. Left Ventricular Rupture Associated with Takotsubo Cardiomyopathy. Mayo Clinic Proceedings. 2004;79(6):821–824. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S., Kaushik S., Nautiyal A., et al. Cardiac rupture in takotsubo cardiomyopathy: a systematic review. Clinical cardiology. 2011;34(11):672–676. doi: 10.1002/clc.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasupathy S., Tavella R., Beltrame J. F. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation. 2017;135(16):1490–1493. doi: 10.1161/circulationaha.117.027666. [DOI] [PubMed] [Google Scholar]
- 11.Zalewska-Adamiec M., Bachórzewska-Gajewska H., Kozuch M., Marek F., Hirnle T., Dobrzycki S. Cardiac rupture in takotsubo cardiomyopathy treated surgically. Advances in Interventional Cardiology. 2016;3:278–279. doi: 10.5114/aic.2016.61655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudaiberdiev T., Akhmedova I., Imanalieva G., et al. Surgical treatment of left ventricular wall rupture, regarded as a consequence of takotsubo cardiomyopathy. SAGE Open Medical Case Reports. 2017;5 doi: 10.1177/2050313X16689210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung J.-M., Hong S.-J., Chung I.-H., et al. Rupture of right ventricular free wall following ventricular septal rupture in takotsubo cardiomyopathy with right ventricular involvement. Yonsei Medical Journal. 2017;58(1):248–251. doi: 10.3349/ymj.2017.58.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Y-Hassan S. Cardiac rupture in a patient with takotsubo syndrome triggered by acute myocardial infarction: two messages. International Journal of Cardiology. 2014;177(1):162–165. doi: 10.1016/j.ijcard.2014.09.108. [DOI] [PubMed] [Google Scholar]
- 15.Jaguszewski M., Fijalkowski M., Nowak R., et al. Ventricular rupture in takotsubo cardiomyopathy. European Heart Journal. 2012;33(8):p. 1027. doi: 10.1093/eurheartj/ehs054. [DOI] [PubMed] [Google Scholar]
- 16.Shinozaki K., Tamura A., Abe Y., Yano S., Kadota J. Left ventricular free wall rupture in takotsubo cardiomyopathy. International Journal of Cardiology. 2007;115(1):E3–E4. doi: 10.1016/j.ijcard.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 17.Showkathali R., Dworakowski R., MacCarthy P. Catastrophic ruptured takotsubo cardiomyopathy. Journal of Cardiovascular Medicine. 2015;16(9):644–645. doi: 10.2459/JCM.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 18.Yamada R., Watanabe N., Kume T., et al. Left ventricular rupture associated with takotsubo-like left ventricular dysfunction (apical ballooning) Journal of Echocardiography. 2006;4(2):59–62. doi: 10.2303/jecho.4.59. [DOI] [Google Scholar]
- 19.Stöllberger C., Huber J., Enzelsberger B., Finsterer J. Fatal outcome of epileptic seizure‐induced takotsubo syndrome with left ventricular rupture. European Journal of Neurology. 2009;16(6):e116–e117. doi: 10.1111/j.1468-1331.2009.02619.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohara Y., Hiasa Y., Hosokawa S., et al. Left ventricular free wall rupture in transient left ventricular apical ballooning. Circulation Journal. 2005;69(5):621–623. doi: 10.1253/circj.69.621. [DOI] [PubMed] [Google Scholar]
- 21.Mafrici A., Proietti R., Fusco R., De Biase A., Klugmann S. Left ventricular free wall rupture in a Caucasian female with takotsubo syndrome: a case report and a brief literature review. Journal of Cardiovascular Medicine. 2006;7(12):880–883. doi: 10.2459/JCM.0b013e328010410c. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T., Yasu T., Arao K., Kawakami M., Saito M. Bedside diagnosis of cardiac rupture by contrast echocardiography. Circulation. 2005;112(24):e354–e355. doi: 10.1161/circulationaha.105.538348. [DOI] [PubMed] [Google Scholar]
- 23.Ieva R., Correale M., Brunetti N. D., Di Biase M. A “bad” case of tako–tsubo syndrome. Journal of Thrombosis and Thrombolysis. 2009;28(2):248–251. doi: 10.1007/s11239-008-0289-8. [DOI] [PubMed] [Google Scholar]
- 24.Iskander M., Abugroun A., Shehata K., Iskander F., Iskander A. Takotsubo cardiomyopathy-induced cardiac free wall rupture: a case report and review of literature. Cardiology Research. 2018;9(4):244–249. doi: 10.14740/cr728w. [DOI] [PMC free article] [PubMed] [Google Scholar]