Abstract
Reports from both adult and paediatric populations indicate that approximately two-thirds of platelet transfusions are used prophylactically to prevent bleeding, while the remaining one-third are used therapeutically to manage active bleeding. These two indications, prophylactic and therapeutic, serve two very distinct purposes and therefore will have two different functional requirements. In addition, disease aetiology in a given patient may require platelets with different functional characteristics. These characteristics can be derived from the various manufacturing methods used in platelet product production, including collection methods, processing methods, and storage options. The iterative combinations of manufacturing methods can result in a number of unique platelet products with different efficacy and safety profiles, which could potentially be used to benefit patient populations by meeting diverse clinical needs. In particular, cold storage of platelet products causes many biochemical and functional changes, of which the most notable characterised to date include increased haemostatic activity and altered expression of molecules inherent to platelet:leucocyte interactions. The in vivo consequences, both short- and long-term, of these molecular and cellular cold-storage-induced changes have yet to be clearly defined. Elucidation of these mechanisms would potentially reveal unique biologies that could be harnessed to provide more targeted therapies. To this end, in this new era of personalised medicine, perhaps there is an opportunity to provide individual patients with platelet products that are tailored to their clinical condition and the specific indication for transfusion.
Keywords: platelets, cold storage, haemostasis, immune, endothelial
Introduction
Over two million platelet concentrates (PC) were distributed for transfusion in the United States (US) in 20151. Reports from both adult and paediatric populations indicate that approximately two-thirds of platelet transfusions are used prophylactically to prevent bleeding, while the remaining one-third are used therapeutically to manage active bleeding2–4. These two indications, prophylactic and therapeutic, serve two very distinct purposes and therefore will have two different functional requirements. In addition, disease aetiology in a given patient may require platelets with different functional characteristics. Let’s consider Patient 1, who presents with intracranial bleeding from traumatic brain injury, in comparison to Patient 2, who presents with severe serosal bleeding secondary to hypoproliferative thrombocytopenia after a bone marrow transplant. Patients 1 and 2 may need platelets that affect haemostatic, endothelial, and immune function in different ways. PC manufacturing methods include many collection, processing, and storage options. The multiple permutations of manufacturing methods result in a number of unique PC with different efficacy and safety profiles5, which could potentially be used to meet a variety of clinical needs. Moreover, sourcing platelets from donors with specific functional characteristics may also help to provide unique products capable of directly addressing individual patient needs. To this end, in this new era of personalised medicine, perhaps there is an opportunity to provide patients with platelet products that are tailored to their clinical condition and the specific indication for transfusion.
Many methods are used to manufacture PC. Platelets can be collected either from whole blood derivation (platelet-rich-plasma or buffy-coat method) or apheresis via different collection platforms (e.g. Trima Accel; TerumoBCT, Lakewood, CO, USA, and Fenwall Amicus; Fresnius Kabi, Bad Homburg, Germany, both licensed in the US). Collected platelets are then stored in either plasma or in various types of platelet additive solutions (PAS)6–8. There are also additional processing methods for PC, such as pathogen reduction technologies, irradiation, volume reduction, and multiple storage temperatures. Each PC manufactured from these different methods develops a specific biochemical and morphological signature or profile9–13. These biological changes occur over time in PC and are collectively referred to as the platelet storage lesion (PSL). Platelet manufacturing methods and storage time have been demonstrated to affect haemostatic, endothelial, and immune function of PC. For example, platelet storage increases phosphatidylserine (PS) expression on platelets, which has prothrombotic effects and increases microparticle and soluble mediator release, which may affect the immune status of PC recipients14,15. The phenotypic and functional changes resulting from PSL formation could directly impact clinical course and outcome, depending on the pathophysiology of a given patient. Therefore, benefit or harm from PC manufacturing methods can only be determined in the context of individual PC transfusion recipients and their specific disease status.
Maintaining an adequate PC inventory has proven to be a burden with respect to both cost and the effort required16,17. This is due to many factors, including length of time and costs associated with apheresis collection, required pathogen testing, and a limited shelf life. Of note, over 90% of distributed PC were derived from single donor apheresis1, and standard practice dictates that PC be stored for a maximum of 5–7 days at room temperature (RT; 22 °C), which increases the risk of bacterial contamination18. Recent estimates suggest 9% of PC inventories are discarded due to the room-temperature storage constraints having expired1. In the US, at an average cost of $ 524 per apheresis PC1, this resulted in a loss of roughly 89.6 million US dollars for hospital systems in 2015 alone. Furthermore, declining numbers of volunteer donors, the regional nature of the blood banking systems, and the perishable nature of platelet products all combine to create environments with reduced PC availability or “PC shortage”19,20.
Cold storage (4 °C) of platelets provides an attractive alternative to RT storage for many reasons. First, cold storage of PC reduces platelet metabolism, therefore maintaining haemostatic function for longer than RT-stored platelets and potentially making them ideal for patients with active bleeding (therapeutic use)21. Second, cold storage of PC mitigates bacterial contamination, thereby reducing the risk of transfusion-transmitted infections (TTI) and the costs associated with pathogen testing22. Third, cold storage has significant potential to increase platelet storage duration, extending the current shelf life from 5–7 days potentially to 10–15 days23. Increased storage duration of cold-stored PC would theoretically increase the availability at hospitals that currently do not have access to platelets due to the short shelf life of RT-stored platelets. Increasing platelet availability to these non-tertiary care centres may impact the 30,000 preventable deaths per year due to traumatic haemorrhage that predominantly occur before arrival to tertiary care centres24. Increased PC storage duration would also mitigate the PC shortage, as well as reduce the costs associated with maintaining a PC inventory with a 5–7 day shelf life. Collectively, cold storage of PC not only provides a more haemostatically active platelet, but may also allow for a more tailored approach to transfusion medicine in bleeding patients and additional economic benefits for institutions maintaining a PC inventory.
As current data show that cold storage could prove to have quite an impact in improving patient outcomes for actively bleeding patients, in addition to improving the logistics of blood banking practices, we advocate a more physiological and thorough examination of the biological effects of cold-stored platelets. There is a need for both advanced in vitro technologies and robust clinical trials25 to determine whether platelet storage temperature and other manufacturing methods can be used to provide PC that are personalised to a patient’s individual needs based on their pathophysiology. We also contend that differences in both donor variability26 (with respect to platelet function) and manufacturing methods are not necessarily undesirable, and can likely be capitalised upon to generate PC that have increased efficacy in either preventing or treating bleeding, as donor genetics plays a role in platelet adhesion and aggregation on biological surfaces27,28. To promote this, this review will highlight recent advances in platelet biology, and how these new findings may be impacted by cold storage of platelets. In addition, we hope to generate a discussion as to how platelet manufacturing methods, including storage conditions, affect other cells in the vascular space as the dearth of data on this topic is alarming. Lastly, we will discuss how platelet storage temperature may help to personalise transfusion medicine.
History of platelet storage temperature
Early platelet transfusion in the US predominantly used freshly isolated platelets within 24 hours of collection. As such, many studies were performed to determine if platelets could be stored for longer periods of time, and if so, if cold storage or RT storage better maintained optimal platelet function. Three studies in the late 1960s and early 1970s reported that prolonged cold storage of PC resulted in platelet products that had shortened circulation times and decreased viability when compared to RT-stored platelets in vivo29–31. Interestingly, one of these studies was a randomised controlled trial (RCT) that indicated that cold-stored platelets resulted in improved aggregation response and reduced bleeding times in both patients with thrombocytopenia and those on aspirin31.
At that time, the maintenance of both cold- and RT-stored platelet inventories to allow for the use of cold-stored platelets for bleeding patients was considered to be a logistical challenge and was not implemented. Therefore, for the past 40–50 years, RT storage of platelets was chosen as the predominant use of platelets for patients with hypoproliferative thrombocytopenia, where longer in vivo circulation time is prioritised as it reduces increased exposure to additional platelet transfusions. Fewer transfusions reduces the risk of infection, transfusion reactions, and alloimmunisation. With the current emphasis on haemostatic resuscitation24,32, and the increased use of plasma and platelets relative to red blood cell (RBC) transfusion for severe bleeding, there has been renewed interest in the use of cold-stored platelets for patients with active bleeding due to their increased haemostatic function21,33.
Platelet storage lesion
During storage, platelets undergo a series of biochemical and morphological changes leading to the formation of the PSL, which reduces haemostatic function and in vivo survival. The PSL in RT-stored platelets has been reviewed in depth elsewhere14,15. Briefly, the hallmark features are metabolic changes resulting in reduced mitochondrial function, release of soluble mediators (such as sCD40L), shedding of microparticles, and increased cell surface receptor expression (such as P-selectin/CD62P). Recent studies have used “omics” technologies to describe platelet-intrinsic metabolic9 and proteomic12,34,35 changes occurring during storage, indicating endocytosis, cytoskeletal rearrangement, and Fc-mediated phagocytosis as the significantly altered pathways during storage. These specific processes are important for platelet interactions with other cells in the vascular space, as well as platelet mobility, thereby highlighting potential mechanisms by which stored platelets may be sensitive to and interact with their surroundings. Further “omics” analyses will provide a better understanding of how stored platelets change over time, and how these changes alter platelet interactions, not only with each other, but also with other cells in the vascular space.
It is disappointing that so few changes to both the storage bag and the storage solution have been made over the past few decades to mitigate the PSL. Platelets have high metabolic activity when stored at RT, and therefore produce large amounts of lactic acid and carbon dioxide while consuming oxygen, which results in a drop in pH in the storage bag, exacerbating the PSL36. As changes in platelet manufacturing methods resulted in more concentrated platelet products over time, storage bags permitting more gas exchange were needed to extend the life of the product. Platelet bags are therefore made with different plasticisers than other blood products (e.g. RBC) to facilitate this gas exchange and maintain a more desirable pH36. Other than this, the storage container itself has not been improved.
With respect to storage solution, platelets have historically been stored in plasma. Storage in plasma causes the formation of microaggregates due to activation of glycoprotein (GP) IIb/IIa and subsequent binding with fibrinogen23,37. To minimise this phenomenon, and also prolong PC shelf life, storage in platelet additive solutions (PAS) has been explored11,23,38–40. PAS also allows more plasma to be collected from the donor than can be used for transfusions or further processing, and potentially reduces antibody-based immune reactions due to either incompatible plasma type or human leucocyte antigen (HLA)-mediated transfusion-related acute lung injury11,41. The in vitro work to date has shown that storage in PAS indeed diminishes platelet activation and aggregation within the PC, and maintains improved aggregation responses in in vitro and in vivo animal studies11,23,39,41. Moreover, in vivo studies have demonstrated that platelets stored in PAS meet US Food and Drug Administration (FDA) standards for recovery and survival up to seven days of storage11,38,40,42, and PAS has long been approved and used for transfusion in Europe11,43. Stolla et al. report reduced recovery for PAS platelets, though the effects on haemostatic function in vivo remain unclear44. However, not all PAS solutions are created equal23,39,42,44, and optimisation of PAS is an ongoing area of study8. A recent study demonstrated that citrate, which is added to most PAS iterations45, actually promoted PSL formation via increased P-selectin and PS expression, as well as increased reactive oxygen species (ROS) production, whereas removal of citrate from PAS diminished these effects46. As with other platelet manufacturing methods, differences in the biological effects caused by platelet storage solutions could potentially be exploited to allow for the development of personalised platelet transfusion therapies in the future.
An additional mechanism which modulates PSL formation is to store platelets in the cold, thereby slowing down metabolic activity. Cold storage of platelets has been shown to retain bioenergetics47, minimise soluble mediator release, and increase expression of activation markers on the platelet surface48, such as P-selectin, GMP-140, and CD40L49–54. Extensive in vitro studies have shown increased haemostatic function of cold-stored platelets31,55–57, demonstrating that platelet storage temperature alters haemostatic efficacy. In addition, storage of pathogen-reduced platelets at 4 °C improved viscoelastic measures of platelet function compared to storage at 22 °C31,55. While supportive of cold storage of platelets, most of these studies focus on intrinsic platelet function as assessed by in vitro methodologies that leave much to be desired in terms of physiological relevance. Data are limited regarding the haemostatic efficacy of cold-stored platelets under physiologically relevant flow conditions, and also little data are available showing improved haemostatic function in vivo in patients with platelets stored for more than 48–72 hours31,58,59.
In vitro haemostatic function of cold-stored platelets
Current clinical standard assessments for bleeding, coagulation, and platelet function are largely restricted to reductionist assays that isolate components of the haemostatic system60,61. Coagulation factors are assessed through clotting times (e.g. prothrombin time, activated partial thromboplastin time) and concentrations (e.g. fibrinogen, factor VIII). Platelet function is typically assessed in isolation of all other haemostatic processes (e.g. thrombin generation, fibrin formation, and lysis) by aggregometry (light transmission, impedance) with single agonists, which is non-physiological. Even assays that are purportedly “global”, such as rotational thromboelastometry (ROTEM) and thromboelastography (TEG), stimulate the sample with high concentrations of single agonists that may mask endogenous platelet inhibition. TEG platelet mapping assays attempt to examine platelet inhibition in response to adenosine diphosphate (ADP) and arachidonic acid (AA) agonists. ROTEM assays attempt to estimate platelet contribution by comparing extrinsic activation with platelets (EXTEM) and extrinsic activation with a platelet inhibitor (FIBTEM). These assays all lack appropriate flow conditions and physiological surfaces, both of which are essential to capture the desired physiological and pathological mechanisms. Recent efforts have been made to develop a point-of-care platelet assay incorporating physiologically relevant flow, the most notable being the PFA-100. While the PFA-100 incorporates flow and closure time, it provides only a binary end point and does not allow for control of shear, and the formation of thrombus cannot be monitored62.
The mechanism of clot formation, and thus the composition of the resulting clot, depends entirely on flow62–64. In low shear conditions, a red, gelantinous, fibrinous clot is formed with a distinct reduction of platelets in the gross clot structure. In high shear regimes, white, platelet-rich thrombus forms62,63. This differentiation is due to preferential platelet adhesion to fibrinogen at low shear and von Willebrand Factor (vWF) at high shear63–65. vWF is a multimeric glycoprotein that circulates as a wound-up globule, not unlike a ball of string. At high shear rates, vWF unwinds on collagen due to increased forces, which exposes many additional binding sites, required for capture of unactivated circulating platelets65–67.
Therefore, to accurately mimic in vivo physiology and pathology, clot formation, and certainly platelet function, must be assessed at specifically chosen flow conditions and on specifically chosen surfaces. Furthermore, to obtain a complete picture of platelet function, an in vitro assay must encompass all relevant shear regimes with appropriate surfaces. In vitro microfluidic systems require low volumes of blood and allow functional assessment of a wide variety of blood mechanisms in in vivo flow conditions over biological surfaces, resulting in higher physiological relevancy over traditional haemostasis assays61,68–72, and fulfilling the aforementioned needs that current clinical assays cannot satisfy. They are rapidly fabricated, and the geometry can be easily customised, allowing for the platform to be adjusted to specific disease states or even patient physiologies. Microfluidic assays typically adsorb the surface with a protein (e.g. collagen, tissue factor) prior to blood exposure in order to provide an active surface for thrombus formation that mimics exposure of the extravascular space61,73–76. In addition to the full control over flow conditions and surface offered by microfluidic platforms, the small size offers additional advantages. These assays need small volumes of blood and typically take less than 10 minutes (and in many cases less than 5 minutes) to generate the primary endpoint72. Therefore they lend themselves nicely not only to preclinical in vitro functional assessment, but also to potential point-of-care bedside applications.
Such a tool applied to blood product safety and efficacy would allow ideal storage practices to be studied and developed, products and treatment strategies to be compared, and enable the impact of the products on specific indications, and even individual patients, to be evaluated. Different patient disease status and aetiologies modulate the mechanisms of haemostasis, and thus the morphology of the resulting clot77. This means that real-time observation of physiologically relevant thrombosis can allow for personalised diagnoses, observation of the effects or non-effects of pharmacological agents, and exploration of other avenues of treatment. However, a limitation of microfluidic assays is that they are currently not licensed for clinical use and are, therefore, only available for research purposes, so there is still a long way to go before this tool can be used as a point-of-care assay. As such, understanding the limitations and over-/under-representations of haemostatic function in the currently available assessment platforms is essential for translating in vitro findings to the in vivo setting.
In vivo haemostatic function of cold-stored platelets
RCTs in cold-stored platelets have not been performed since the 1970s29,31,56,78,79. Those original studies indicated that circulation time was reduced due to cold storage, and therefore the practice was abandoned, as we have discussed above. While several of these studies demonstrated improved platelet aggregation in vitro and clotting after transfusion due to cold storage, this effect was only examined for up to 72 hours of storage31,58,59. However, it is important to note that platelet processing methods and storage conditions have very much changed over the past five decades56. Furthermore, the use of platelets for patients with active bleeding is increasing80, and these populations would benefit from a more haemostatically active platelet, even with reduced circulation time54,56. A paediatric RCT found that cold-stored whole blood was more effective in reducing blood loss than reconstituted whole blood containing platelets stored at 22 °C for cardiac surgery patients55, showing potential promise for cold-stored platelets. While only a handful of centres are using cold-stored platelets for active bleeding, its use will likely increase due to the recent FDA approval for the use of cold-stored PCs collected by apheresis in actively bleeding patients37. Due to this renewed interest in cold storage, there are two ongoing clinical trials involving cold-stored platelets. The first is a pilot RCT in cardiac surgery patients where patients received either RT-stored platelets or cold-stored platelets for up to seven days, with the primary end point of post-operative bleeding (NCT02495506). A pilot study using cold-stored platelets with up to 14 days of storage time was added to this study as a third arm, but the results of this pilot study have not yet been published. The second current trial is a transfusion platelet recovery study of apheresis platelets in PAS (NCT02754414).
Non-haemostatic platelet function and cold storage: endothelial and immune cells
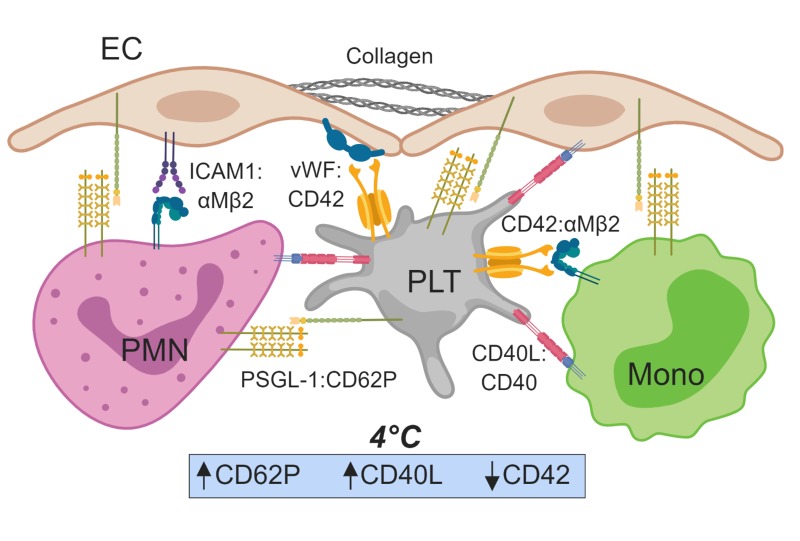
While numerous studies have evaluated the effect of platelet storage temperature on haemostatic potential in vitro, little is known about how storage temperature alters platelet interactions with other cells, such as endothelial cells (EC) and cells of the immune system, as well as interaction with soluble biological mediators, such as extracellular vesicles (Figure 1). Cold storage of platelets results in many changes, and several studies have characterised the molecular and cellular features of the PSL in detail, such as changes in expression of P-selectin/CD62P, CD40L, PS, and levels of degranulation and microparticle release that occur during cold storage15. How these phenotypic changes may perturb downstream endothelial and immune function has yet to be fully elucidated.
Figure 1.
Schematic representation of platelets (PLT) in the vascular space.
Important molecular interactions between PLT, leucocytes, and endothelial cells (EC) as denoted. Cold (4 °C) storage of PLT increases CD62P and CD40L expression, potentially promoting PLT:leucocyte interactions and aggregates, and decreases expression of CD42, potentially diminishing PLT interactions with von Willebrand factor (vWF). PMN: neutrophil; Mono: monocyte; PSGL-1: p-selectin ligand 1; ICAM1: intercellular adhesion molecule 1. Created with ©BioRender - biorender.com.
Most information on how blood product transfusion affects EC function comes from studies evaluating RBC transfusion, with little data on how platelet transfusion affects EC function. In 2012, Urner et al. found that of three components of blood products (RBC, fresh frozen plasma [FFP], and RT-stored platelets), RT-stored platelets were the most proinflammatory, as they promoted EC activation and supported neutrophil adherence to EC in vitro81. In addition, when EC were already primed by stimulation with endotoxin (as in the case of sepsis), RT-stored platelets potentiated the release of soluble mediators from EC81. Miyazawa et al. showed platelets and platelet-derived extracellular vesicles (EV) were shown to restore EC junctions in vitro and decrease vascular permeability in a murine tail-snip model of haemorrhage82. These vasculoprotective benefits decrease with the duration of storage, as longer platelet storage times led to increased EC activation in vitro83, and resulted in a loss of vasculoprotective capacity both in vitro and in vivo84. Of note, PS expression on platelets, which increases with storage, was shown to be important for EC phagocytosis and clearance of platelets from circulation, and EC-mediated clearance of endogenous platelets reduced platelet:leucocyte aggregates (PLA) in vivo in a murine model of sepsis85. As certain aspects of the PSL phenotype can clearly modulate platelet:EC interactions, it is important to understand how PC manufacturing and subsequent transfusion can either promote or hinder endothelial function.
Unsurprisingly, how cold storage of platelets modulates EC function is even less well understood. As cold storage induces increased microparticle release as well as increased PS expression, one might hypothesise that cold storage would provide a more pro-inflammatory and pro-thrombotic platelet product. The only study to date to assess the effects of cold-stored platelets on endothelial integrity showed that cold-stored platelets limit endothelial permeability under in vitro static conditions, yet cold-stored platelets were less effective at preventing vascular leak in vivo when compared to platelets stored at room temperature48. These data suggest that perhaps cold-stored platelets are indeed more proinflammatory in vivo. More studies assessing cold-stored platelet:EC interactions are needed to have a thorough understanding of EC function in response to cold-stored platelet transfusion.
Modulation of immune function in response to blood product transfusion is not a new concept, but the mechanisms by which storage temperature may alter immune function have yet to be determined. Platelets themselves are active immune cells, and are also capable of affecting leucocyte function86–89, yet there has been limited discussion on how platelet storage temperature inherently alters platelet immune function, and/or whether platelet transfusion itself (not the plasma fraction of a PC) leads to transfusion-related immunomodulation (TRIM)90. Moreover, there are little functional data on what the phenotypic changes found in the PSL mean with respect to immune responsiveness. For example, PLA are associated with pro-inflammatory and pro-thrombotic disease states91,92, and cold storage increases CD62P and CD40L on platelets, thereby potentially increasing the likelihood of platelet:leucocyte interactions (Figure 1). Interestingly, Wu et al. demonstrated that cold-stored whole blood transfusion in a rodent model of acute traumatic coagulopathy led to increased PLA levels when compared to fresh whole blood transfusion93. While these data highlight the effect of storage temperature on the incidence of PLA in platelet-containing blood products, there is still no concrete answer as to whether cold-stored platelets are more likely to form PLA, and if these cold-stored PLA would be proinflammatory and/or pathogenic.
Refractoriness to platelets is mediated through the alloimmune response specific to both HLA and non-HLA antigens (including human platelet antigens [HPA]). Although platelet refractoriness is not a very common event, it must be remembered that there is an approximately 15% rate of incidence in acute myeloid leucemia (AML) patients after leucoreduced platelet transfusion94. Cold storage is known to modulate HPA (CD42b/GPIα is an HPA) expression57,95, yet whether HLA expression is changed by cold storage has yet to be determined. As these molecules are important mediators of alloimmunisation, it is important to determine whether changes in HLA expression lead to augmented or diminished rates of platelet refractoriness. Of note, cold-stored platelets have elevated PS expression, and recent work by Saris et al. highlighted increased rates of PS-dependent platelet internalisation and subsequent alloimmunisation of stored platelets compared to fresh platelets96. The paucity of data regarding the potential changes in alloimmunisation rates due to different platelet storage temperatures highlights an important area of much needed investigation.
Additional platelet manufacturing methods as alternatives to cold-stored platelets
Additional methods adding to the complexity of manufacturing platelets include pathogen reduction (PR), cryopreservation, lyophilisation, platelet membrane products, as well as nanoparticle mimicry, all of which are either in clinical use or under pre-clinical or clinical development. While PR technologies increase the safety of platelets by reducing risk of infection, the process also increases platelet activation and microparticle release97,98, perhaps making a more proinflammatory platelet product than was originally intended. Moreover, a 2017 review of clinical studies highlighted concerns about the use of PR platelet for controlling bleeding compared to traditional platelet products99, although one retrospective study in massively transfused patients did not indicate any differences in survival outcomes100. Relative to the goal of personalised transfusion medicine, the use of PR platelets is most valuable in immunocompromised patients at high risk of an overwhelming infection with bacterial contamination of a platelet product, and perhaps may not be optimal for patients with massive bleeding. Additional studies are needed to determine if PR treatment does affect outcomes in patients with severe haemorrhage. Moreover, as PR and cold storage are not mutually exclusive (and in fact could be concomitant during the manufacturing process), it is of great importance to study how these methods can alter the PSL when combined.
While cryopreservation of platelets increases product shelf life to two years101, the storage constraints (−80 °C) and thawing protocols may not be conducive to use in all centres102. With respect to in vivo studies, there have been a limited number of trials assessing safety and efficacy of cryopreserved platelets. A report from the Netherlands military forces demonstrated similar efficacy of cryopreserved components as compared to standard components during massive transfusion103; the report focused on all frozen components, thereby confounding the direct effects of cryopreserved platelets alone. In vitro assessment of cryopreserved platelets has highlighted similar phenotypes to those of cold-stored platelets, with increased CD62P and PS expression upon thawing101. While both cryopreserved platelets and cold-stored platelets have increased haemostatic function as assessed by thromboelastometry, cryopreserved platelets have diminished aggregation104. Moreover, cryopreserved platelets were found to be more readily phagocytosed by monocytic cells than cold-stored platelets, and induced pro-inflammatory signalling in the phagocytic cells105. As cryopreservation and cold storage are not synonymous with respect to PSL formation, these differences could be leveraged to target patient specific needs.
Naturally-derived platelet products are an additional class of haemostatic agents. Infusible platelet membranes (IPM) are derived from platelets through a series of laboratory processes. Many of these products, although haemostatically efficacious both in vitro and in vivo in clinical trials, have yet to receive FDA clearance106,107. Thrombosomes, a lyophilised platelet product, were shown to effectively promote haemostasis in preclinical models108, cause no serious adverse effects in a safety trial109, and are currently being assessed in a Phase I clinical trial (NCT03394755). While these naturally-derived platelet products have been assessed for haemostatic efficacy, the long-term effects and their effects on other cells in the vascular space have yet to be clearly delineated and neither has been compared to cold-stored platelets.
The pursuit of an artificial or manufactured platelet-like particle has been underway since the early 1990s. Nanoparticle platelet mimetics overcome multiple current product barriers: ease of manufacture and ability to scale up, longer shelf life, and no risk of alloimmunisation or TTI107,110. The traditional mimetic model has been to join a nanoparticle platform with molecules involved in binding interactions found at the vascular wound site, in order to mimic platelet adhesion, aggregation, or both. While there are a handful of early phase clinical trials looking at albumin particles coated with fibrinogen, more detailed studies are required to understand the safety and efficacy of these platelet-aggregation mimicking products. A recent study demonstrated synthetic platelet nanoparticles (SynthoPlateTM), which mimic both adhesion and aggregation, were capable of a 50% reduction in blood loss and an approximate 75% increase in survival in a porcine traumatic haemorrhage model when compared to saline resuscitation alone111. Currently, there are no data which directly compare nanoparticle platelet mimetics to cold-stored platelets.
Cold storage: personalised transfusion medicine?
The multitude of platelet manufacturing methods, including cold storage, yield a complicated field of platelet products with various phenotypes. Instead of treating this variation as a negative as intuition might suggest, the alternative is to possibly capitalise on these differences and identify the best product for a given application. While the increased haemostatic potential inferred on platelets by cold storage may be optimal in cases of haemorrhage, the additional phenotypes of a cold-stored platelet, such as the potential increase in inflammatory PLA, may not be ideal for patients with immune complications. Clinical data are needed to further examine both the potential benefits and adverse effects of cold storage. In addition, the extended shelf life of cold-stored PC may improve product availability, thereby mitigating current shortages and resulting in an overall improvement in blood banking economics.
Blood donation logistics are an obvious barrier to implementing personalised platelet transfusion therapies, as the rate of donation is declining, and recruitment is difficult given the time required for apheresis procedures. Small donor numbers limit transfusion medicine by not only reducing the sheer number of products available, but also hindering the potential for matching donor platelets to recipient needs. One potential option is to reconsider the use of paid donors with PR technologies112. This strategy would provide a safe product that could increase donation rates and ultimately allow for the recruitment of pedigree donors with specific platelet function characteristics that align with patient needs. This approach is permitted by the FDA in the US and is under consideration by some blood collection centres. If implemented, this has the potential to facilitate the implementation of personalised transfusion medicine with platelet products.
In summary, cold storage of platelets results in many biochemical and functional changes. The most notable of these alterations characterised to date, and probably that with the biggest impact in vivo, include increased haemostatic activity and altered expression of molecules inherent to platelet:leucocyte interactions. While it is important to consider potential deleterious effects on haemostatic, immune, and endothelial function, the variety in platelet products may one day be used to our advantage as practitioners, allowing us to match appropriate function and risk with specific patient populations and individuals.
Footnotes
Authorship contributions
SMS and KAT contributed equally to the preparation of this manuscript.
Conflict of interests disclosure
PCS is a consultant for Secure Transfusion Services and Cerus. The other Authors declare no conflict of interest.
References
- 1.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion. 2017;57(Suppl 2):1588–98. doi: 10.1111/trf.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greeno E, McCullough J, Weisdorf D. Platelet utilization and the transfusion trigger: a prospective analysis. Transfusion. 2007;47:201–5. doi: 10.1111/j.1537-2995.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 3.Cameron B, Rock G, Olberg B, Neurath D. Evaluation of platelet transfusion triggers in a tertiary-care hospital. Transfusion. 2007;47:206–11. doi: 10.1111/j.1537-2995.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 4.Nellis ME, Karam O, Mauer E, et al. Platelet transfusion practices in critically ill children. Crit Care Med. 2018;46:1309–17. doi: 10.1097/CCM.0000000000003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrezenmeier H, Seifried E. Buffy-coat-derived pooled platelet concentrates and apheresis platelet concentrates: which product type should be preferred? Vox Sang. 2010;99:1–15. doi: 10.1111/j.1423-0410.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 6.Acker JP, Marks DC, Sheffield WP. Quality assessment of established and emerging blood components for transfusion. J Blood Transfus. 2016;2016 doi: 10.1155/2016/4860284. 4860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassallo RR, Murphy S. A critical comparison of platelet preparation methods. Curr Opin Hematol. 2006;13:323–30. doi: 10.1097/01.moh.0000239703.40297.a5. [DOI] [PubMed] [Google Scholar]
- 8.van der Meer PF, de Korte D. Platelet additive solutions: a review of the latest developments and their clinical implications. Transfus Med Hemother. 2018;45:98–102. doi: 10.1159/000487513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannsson F, Guethmundsson S, Paglia G, et al. Systems analysis of metabolism in platelet concentrates during storage in platelet additive solution. Biochem J. 2018;475:2225–40. doi: 10.1042/BCJ20170921. [DOI] [PubMed] [Google Scholar]
- 10.Getz TM. Physiology of cold-stored platelets. Transfus Apher Sci. 2019;58:12–15. doi: 10.1016/j.transci.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Getz TM, Montgomery RK, Bynum JA, et al. Storage of platelets at 4 degrees C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56:1320–8. doi: 10.1111/trf.13511. [DOI] [PubMed] [Google Scholar]
- 12.Salunkhe V, De Cuyper IM, Papadopoulos P, et al. A comprehensive proteomics study on platelet concentrates: platelet proteome, storage time and Mirasol pathogen reduction technology. Platelets. 2019;30:368–79. doi: 10.1080/09537104.2018.1447658. [DOI] [PubMed] [Google Scholar]
- 13.Rijkers M, van den Eshof BL, van der Meer PF, et al. Monitoring storage induced changes in the platelet proteome employing label free quantitative mass spectrometry. Sci Rep. 2017;7:11045. doi: 10.1038/s41598-017-11643-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng MSY, Tung JP, Fraser JF. Platelet storage lesions: what more do we know now? Transfus Med Rev. 2018 doi: 10.1016/j.tmrv.2018.04.001. pii: S0887-7963(17)30189-X. [DOI] [PubMed] [Google Scholar]
- 15.Hegde S, Akbar H, Zheng Y, Cancelas JA. Towards increasing shelf life and haemostatic potency of stored platelet concentrates. Curr Opin Hematol. 2018;25:500–8. doi: 10.1097/MOH.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller AK, Uglik KM, Braine HG, King KE. A comprehensive program to minimize platelet outdating. Transfusion. 2011;51:1469–76. doi: 10.1111/j.1537-2995.2010.03039.x. [DOI] [PubMed] [Google Scholar]
- 17.Riley W, Smalley B, Pulkrabek S, et al. Using lean techniques to define the platelet (PLT) transfusion process and cost-effectiveness to evaluate PLT dose transfusion strategies. Transfusion. 2012;52:1957–67. doi: 10.1111/j.1537-2995.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 18.Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18:195–204. doi: 10.1128/CMR.18.1.195-204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien KL, Mohammed M, Uhl L. Management of a hospital transfusion service during a nationwide blood product shortage. Arch Pathol Lab Med. 2018;142:779–81. doi: 10.5858/arpa.2017-0483-LE. [DOI] [PubMed] [Google Scholar]
- 20.Cap AP. Targeting hemorrhage: alternative storage of platelets for hemostatic transfusion. Blood. 2017;130(Suppl 1):SCI-32. [Google Scholar]
- 21.Cap AP, Spinella PC. Just chill-it’s worth it! Transfusion. 2017;57:2817–20. doi: 10.1111/trf.14399. [DOI] [PubMed] [Google Scholar]
- 22.Ketter PM, Kamucheka R, Arulanandam B, et al. Platelet enhancement of bacterial growth during room temperature storage: mitigation through refrigeration. Transfusion. 2019;59(S2):1479–89. doi: 10.1111/trf.15255. [DOI] [PubMed] [Google Scholar]
- 23.Reddoch-Cardenas KM, Montgomery RK, Lafleur CB, et al. Cold storage of platelets in platelet additive solution: an in vitro comparison of two Food and Drug Administration-approved collection and storage systems. Transfusion. 2018;58:1682–8. doi: 10.1111/trf.14603. [DOI] [PubMed] [Google Scholar]
- 24.Spinella PC, Cap AP. Prehospital hemostatic resuscitation to achieve zero preventable deaths after traumatic injury. Curr Opin Hematol. 2017;24:529–35. doi: 10.1097/MOH.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 25.Krachey E, Viele K, Spinella PC, et al. The design of an adaptive clinical trial to evaluate the efficacy of platelets stored at low temperature in surgical patients. J Trauma Acute Care Surg. 2018;84(Suppl 1):S41–6. doi: 10.1097/TA.0000000000001876. [DOI] [PubMed] [Google Scholar]
- 26.Dzieciatkowska M, D’Alessandro A, Hill RC, Hansen KC. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. J Proteomics. 2015;120:1–6. doi: 10.1016/j.jprot.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neeves KB, Onasoga AA, Hansen RR, et al. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One. 2013;8:e54680. doi: 10.1371/journal.pone.0054680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joutsi-Korhonen L, Smethurst PA, Rankin A, et al. The low-frequency allele of the platelet collagen signaling receptor glycoprotein VI is associated with reduced functional responses and expression. Blood. 2003;101:4372–9. doi: 10.1182/blood-2002-08-2591. [DOI] [PubMed] [Google Scholar]
- 29.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. NEJM. 1969;280:1094–8. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 30.Valeri CR. Hemostatic effectiveness of liquid-preserved and previously frozen human platelets. NEJM. 1974;290:353–8. doi: 10.1056/NEJM197402142900702. [DOI] [PubMed] [Google Scholar]
- 31.Becker GA, Tuccelli M, Kunicki T, et al. Studies of platelet concentrates stored at 22 C and 4 C. Transfusion. 1973;13:61–8. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- 32.Spinella PC, Pidcoke HF, Strandenes G, et al. Whole blood for hemostatic resuscitation of major bleeding. Transfusion. 2016;56(Suppl 2):S190–202. doi: 10.1111/trf.13491. [DOI] [PubMed] [Google Scholar]
- 33.Cap AP, Reddoch-Cardenas KM. Can’t get platelets to your bleeding patients? Just chill... the solution is in your refrigerator! Transfus Clin Biol. 2018;25:217–9. doi: 10.1016/j.tracli.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Jiang T, Fan Y, Zhao S. A proteomic approach reveals the variation in human platelet protein composition after storage at different temperatures. Platelets. 2019;30:403–12. doi: 10.1080/09537104.2018.1453060. [DOI] [PubMed] [Google Scholar]
- 35.Egidi MG, D’Alessandro A, Mandarello G, Zolla L. Troubleshooting in platelet storage temperature and new perspectives through proteomics. Blood Transfus. 2010;8(Suppl 3):s73–81. doi: 10.2450/2010.012S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prowse CV, de Korte D, Hess JR, van der Meer PF Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Commercially available blood storage containers. Vox Sang. 2014;106:1–13. doi: 10.1111/vox.12084. [DOI] [PubMed] [Google Scholar]
- 37.Stubbs JR, Tran SA, Emery RL, et al. Cold platelets for trauma-associated bleeding: regulatory approval, accreditation approval, and practice implementation-just the “tip of the iceberg”. Transfusion. 2017;57:2836–44. doi: 10.1111/trf.14303. [DOI] [PubMed] [Google Scholar]
- 38.Adams G, Swenson S, Rock G. Survival and recovery of human platelets stored for five days in a non- plasma medium. Blood. 1986;67:672–5. [PubMed] [Google Scholar]
- 39.Gulliksson H, AuBuchon JP, Vesterinen M, et al. Storage of platelets in additive solutions: a pilot in vitro study of the effects of potassium and magnesium. Vox Sang. 2002;82:131–6. doi: 10.1046/j.1423-0410.2002.drfgv158.x. [DOI] [PubMed] [Google Scholar]
- 40.Holme S, Heaton WA, Courtright M. Improved in vivo and in vitro viability of platelet concentrates stored for seven days in a platelet additive solution. Brit J Haematol. 1987;66:233–8. doi: 10.1111/j.1365-2141.1987.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 41.Alhumaidan H, Sweeney J. Current status of additive solutions for platelets. J Clin Apher. 2012;27:93–8. doi: 10.1002/jca.21207. [DOI] [PubMed] [Google Scholar]
- 42.Slichter SJ, Corson J, Jones MK, et al. Exploratory studies of extended storage of apheresis platelets in a platelet additive solution (PAS) Blood. 2014;123:271–80. doi: 10.1182/blood-2013-05-501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassallo RR, Adamson JW, Gottschall JL, et al. In vitro and in vivo evaluation of apheresis platelets stored for 5 days in 65% platelet additive solution/35% plasma. Transfusion. 2010;50:2376–85. doi: 10.1111/j.1537-2995.2010.02693.x. [DOI] [PubMed] [Google Scholar]
- 44.Stolla M, Fitzpatrick L, Gettinger I, et al. In vivo viability of extended 4 degrees C-stored autologous apheresis platelets. Transfusion. 2018;58:2407–13. doi: 10.1111/trf.14833. [DOI] [PubMed] [Google Scholar]
- 45.Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 degrees C: development and current experience. Transfus Med Rev. 2006;20:158–64. doi: 10.1016/j.tmrv.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Getz TM, Turgeon A, Wagner SJ. Sodium citrate contributes to the platelet storage lesion. Transfusion. 2019;59:2103–12. doi: 10.1111/trf.15213. [DOI] [PubMed] [Google Scholar]
- 47.Bynum JA, Meledeo MA, Getz TM, et al. Bioenergetic profiling of platelet mitochondria during storage: 4 degrees C storage extends platelet mitochondrial function and viability. Transfusion. 2016;56(Suppl 1):S76–84. doi: 10.1111/trf.13337. [DOI] [PubMed] [Google Scholar]
- 48.Baimukanova G, Miyazawa B, Potter DR, et al. The effects of 22 degrees C and 4 degrees C storage of platelets on vascular endothelial integrity and function. Transfusion. 2016;56(Suppl 1):S52–64. doi: 10.1111/trf.13455. [DOI] [PubMed] [Google Scholar]
- 49.Connor J, Currie LM, Allan H, Livesey SA. Recovery of in vitro functional activity of platelet concentrates stored at 4 degrees C and treated with second-messenger effectors. Transfusion. 1996;36:691–8. doi: 10.1046/j.1537-2995.1996.36896374372.x. [DOI] [PubMed] [Google Scholar]
- 50.Triulzi DJ, Kickler TS, Braine HG. Detection and significance of alpha granule membrane protein 140 expression on platelets collected by apheresis. Transfusion. 1992;32:529–33. doi: 10.1046/j.1537-2995.1992.32692367196.x. [DOI] [PubMed] [Google Scholar]
- 51.Wood B, Padula MP, Marks DC, Johnson L. Refrigerated storage of platelets initiates changes in platelet surface marker expression and localization of intracellular proteins. Transfusion. 2016;56:2548–59. doi: 10.1111/trf.13723. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery RK, Reddoch KM, Evani SJ, et al. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion. 2013;53:1520–30. doi: 10.1111/j.1537-2995.2012.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rinder HM, Murphy M, Mitchell JG, et al. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31:409–14. doi: 10.1046/j.1537-2995.1991.31591263195.x. [DOI] [PubMed] [Google Scholar]
- 54.Berzuini A, Spreafico M, Prati D. One size doesn’t fit all: should we reconsider the introduction of cold-stored platelets in blood bank inventories? F1000Res. 2017;6:95. doi: 10.12688/f1000research.10363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manno C, Hedberg K, Kim H, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–6. [PubMed] [Google Scholar]
- 56.Apelseth TO, Cap AP, Spinella PC, et al. Cold stored platelets in treatment of bleeding. ISBT Sci Ser. 2017;12:488–95. [Google Scholar]
- 57.Reddoch KM, Pidcoke HF, Montgomery RK, et al. Hemostatic function of apheresis platelets stored at 4 degrees C and 22 degrees C. Shock (Augusta, Ga) 2014;41(Suppl 1):54–61. doi: 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aster RH, Becker GA, Filip DJ. Studies to improve methods of short-term platelet preservation. Transfusion. 1976;16:4–7. doi: 10.1046/j.1537-2995.1976.16176130835.x. [DOI] [PubMed] [Google Scholar]
- 59.Filip DJ, Aster RH. Relative hemostatic effectiveness of human platelets stored at 4 degrees and 22 degrees C. J Lab Clin Med. 1978;91:618–24. [PubMed] [Google Scholar]
- 60.Lassila R. Platelet function tests in bleeding disorders. Semin Thromb Hemost. 2016;42:185–90. doi: 10.1055/s-0036-1571307. [DOI] [PubMed] [Google Scholar]
- 61.Sakurai Y, Hardy ET, Ahn B, et al. A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nat Commun. 2018;9:509. doi: 10.1038/s41467-018-02990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Para A, Bark D, Lin A, Ku D. Rapid platelet accumulation leading to thrombotic occlusion. Ann Biomed Eng. 2011;39:1961–71. doi: 10.1007/s10439-011-0296-3. [DOI] [PubMed] [Google Scholar]
- 63.Cadroy Y, Horbett TA, Hanson SR. Discrimination between platelet-mediated and coagulation-mediated mechanisms in a model of complex thrombus formation in vivo. J Lab Clin Med. 1989;113:436–48. [PubMed] [Google Scholar]
- 64.Ikeda Y, Handa M, Kawano K, et al. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J Clin Invest. 1991;87:1234–40. doi: 10.1172/JCI115124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider SW, Nuschele S, Wixforth A, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 2007;104:7899–903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colace TV, Diamond SL. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol. 2013;33:105–13. doi: 10.1161/ATVBAHA.112.300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casa LDC, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg. 2015;61:1068–80. doi: 10.1016/j.jvs.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 68.Neeves KB, Onasoga AA, Wufsus AR. The use of microfluidics in hemostasis: clinical diagnostics and biomimetic models of vascular injury. Curr Opin Hematol. 2013;20:417–23. doi: 10.1097/MOH.0b013e3283642186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jain A, Graveline A, Waterhouse A, et al. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun. 2016;7:10176. doi: 10.1038/ncomms10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Chen J, López JA. Flow-driven assembly of VWF fibres and webs in in vitro microvessels. Nat Commun. 2015;6:7858. doi: 10.1038/ncomms8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muthard RW, Diamond SL. Blood clots are rapidly assembled hemodynamic sensors: flow arrest triggers intraluminal thrombus contraction. Arterioscler Thromb Vasc Biol. 2012;32:2938–45. doi: 10.1161/ATVBAHA.112.300312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hastings SM, Griffin MT, Ku DN. Hemodynamic studies of platelet thrombosis using microfluidics. Platelets. 2017;28:427–33. doi: 10.1080/09537104.2017.1316483. [DOI] [PubMed] [Google Scholar]
- 73.Zheng Y, Chen J, López JA. Microvascular platforms for the study of platelet-vessel wall interactions. Thromb Res. 2014;133:525–31. doi: 10.1016/j.thromres.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: role of thrombin with and without fibrin. Arterioscler Thromb Vasc Biol. 2012;32:1466–76. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoeman RM, Rana K, Danes N, et al. A microfluidic model of hemostasis sensitive to platelet function and coagulation. Cell Mol Bioeng. 2017;10:3–15. doi: 10.1007/s12195-016-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chappell DC, Varner SE, Nerem RM, et al. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–9. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 77.Tran R, Myers DR, Ciciliano J, et al. Biomechanics of haemostasis and thrombosis in health and disease: from the macro- to molecular scale. J Cell Mol Med. 2013;17:579–96. doi: 10.1111/jcmm.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pietersz RN, Loos JA, Reesink HW. Survival in vivo of platelets stored for 48 hours in the buffycoat at 4 degrees C compared to platelet rich plasma stored at 22 degrees C. Blut. 1987;54:201–6. doi: 10.1007/BF00594194. [DOI] [PubMed] [Google Scholar]
- 79.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. Transfusion. 1976;16:8–12. doi: 10.1046/j.1537-2995.1976.16176130842.x. [DOI] [PubMed] [Google Scholar]
- 80.Whitaker BI, Hinkins S. The 2011 national blood collection and utilization survey report. [Accessed on: 22/02/2019]. Available at: https://www.aabb.org/research/hemovigilance/bloodsurvey/Documents/11-nbcus-report.pdf.
- 81.Urner M, Herrmann IK, Buddeberg F, et al. Effects of blood products on inflammatory response in endothelial cells in vitro. PLoS One. 2012;7:e33403. doi: 10.1371/journal.pone.0033403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyazawa B, Trivedi A, Togarrati PP, et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J Trauma Acute Care Surg. 2019;86:931–942. doi: 10.1097/TA.0000000000002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sut C, Hamzeh-Cognasse H, Arthaud CA, et al. Platelet concentrate supernatants alter endothelial cell mRNA and protein expression patterns as a function of storage length. Transfusion. 2018;58:2635–44. doi: 10.1111/trf.14973. [DOI] [PubMed] [Google Scholar]
- 84.Baimukanova G, Miyazawa B, Potter DR, et al. Platelets regulate vascular endothelial stability: assessing the storage lesion and donor variability of apheresis platelets. Transfusion. 2016;56(Suppl 1):S65–75. doi: 10.1111/trf.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma R, Xie R, Yu C, et al. Phosphatidylserine-mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis. Sci Rep. 2017;7:4978. doi: 10.1038/s41598-017-04773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapur R, Semple JW. Platelets as immune-sensing cells. Blood Adv. 2016;1:10–14. doi: 10.1182/bloodadvances.2016000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ed Rainger G, Chimen M, Harrison MJ, et al. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets. 2015;26:507–50. doi: 10.3109/09537104.2015.1064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stolla M, Refaai MA, Heal JM, et al. Platelet transfusion - the new immunology of an old therapy. Front Immunol. 2015;6:28. doi: 10.3389/fimmu.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ Res. 2018;122:337–51. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sut C, Tariket S, Aubron C, et al. The non-hemostatic aspects of transfused platelets. Front Med (Lausanne) 2018;5:42. doi: 10.3389/fmed.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cerletti C, Tamburrelli C, Izzi B, et al. Platelet-leukocyte interactions in thrombosis. Thromb Res. 2012;129:263–6. doi: 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 92.Finsterbusch M, Schrottmaier WC, Kral-Pointner JB, et al. Measuring and interpreting platelet-leukocyte aggregates. Platelets. 2018;29:677–85. doi: 10.1080/09537104.2018.1430358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu X, Darlington DN, Montgomery RK, et al. Platelets derived from fresh and cold-stored whole blood participate in clot formation in rats with acute traumatic coagulopathy. BJH. 2017;179:802–10. doi: 10.1111/bjh.14999. [DOI] [PubMed] [Google Scholar]
- 94.Hod E, Schwartz J. Platelet transfusion refractoriness. BJH. 2008;142:348–60. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 95.Sandgren P, Hansson M, Gulliksson H, Shanwell A. Storage of buffy-coat-derived platelets in additive solutions at 4 degrees C and 22 degrees C: flow cytometry analysis of platelet glycoprotein expression. Vox Sang. 2007;93:27–36. doi: 10.1111/j.1423-0410.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 96.Saris A, Peyron I, van der Meer PF, et al. Storage-induced platelet apoptosis is a potential risk factor for alloimmunization Upon Platelet Transfusion. Front Immunol. 2018;9:1251. doi: 10.3389/fimmu.2018.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osman A, Hitzler WE, Provost P. The platelets’ perspective to pathogen reduction technologies. Platelets. 2018;29:140–7. doi: 10.1080/09537104.2017.1293806. [DOI] [PubMed] [Google Scholar]
- 98.Magron A, Laugier J, Provost P, Boilard E. Pathogen reduction technologies: the pros and cons for platelet transfusion. Platelets. 2018;29:2–8. doi: 10.1080/09537104.2017.1306046. [DOI] [PubMed] [Google Scholar]
- 99.Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev. 2017;7:CD009072. doi: 10.1002/14651858.CD009072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nussbaumer W, Amato M, Schennach H, et al. Patient outcomes and amotosalen/UVA-treated platelet utilization in massively transfused patients. Vox Sang. 2017;112:249–56. doi: 10.1111/vox.12489. [DOI] [PubMed] [Google Scholar]
- 101.Kelly K, Dumont LJ. Frozen platelets. Transfus Apher Sci. 2019;58:23–9. doi: 10.1016/j.transci.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 102.Marks DC, Johnson L, Reade MC. A clinical trial of frozen platelets: rationale, protocol and pilot analysis plan. ISBT Sci Ser. 2018;13:331–7. [Google Scholar]
- 103.Noorman F, van Dongen TT, Plat MJ, et al. Transfusion: -80 degrees C frozen blood products are safe and effective in military casualty care. PLoS One. 2016;11:e0168401. doi: 10.1371/journal.pone.0168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson L, Tan S, Wood B, et al. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion. 2016;56:1807–18. doi: 10.1111/trf.13630. [DOI] [PubMed] [Google Scholar]
- 105.Zhao J, Xu B, Chen G, et al. Cryopreserved platelets augment the inflammatory response: role of phosphatidylserine- and P-selectin-mediated platelet phagocytosis in macrophages. Transfusion. 2019;59:1799–808. doi: 10.1111/trf.15183. [DOI] [PubMed] [Google Scholar]
- 106.Hickman DA, Pawlowski CL, Sekhon UDS, et al. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv Mater. 2018;30:201700859. doi: 10.1002/adma.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sen Gupta A. Bio-inspired nanomedicine strategies for artificial blood components. WIREs Nanomed Nanobiotechnol. 2017;9:e1464. doi: 10.1002/wnan.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fitzpatrick GM, Cliff R, Tandon N. Thrombosomes: a platelet-derived hemostatic agent for control of noncompressible hemorrhage. Transfusion. 2013;53(Suppl 1):100S–6S. doi: 10.1111/trf.12043. [DOI] [PubMed] [Google Scholar]
- 109.Barroso J, Osborne B, Teramura G, et al. Safety evaluation of a lyophilized platelet-derived hemostatic product. Transfusion. 2018;58:2969–77. doi: 10.1111/trf.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nandi S, Brown AC. Platelet-mimetic strategies for modulating the wound environment and inflammatory responses. Exp Biol Med (Maywood) 2016;241:1138–48. doi: 10.1177/1535370216647126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hickman DA, Pawlowski CL, Shevitz A, et al. Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Sci Rep. 2018;8:3118. doi: 10.1038/s41598-018-21384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sadler A, Shi L, Bethge S, Muhlbacher A. Incentives for blood donation: a discrete choice experiment to analyze extrinsic motivation. Transfus Med Hemother. 2018;45:116–24. doi: 10.1159/000481142. [DOI] [PMC free article] [PubMed] [Google Scholar]