Abstract
Background
Sex hormone intake in blood donors may affect the quality of red blood cell (RBC) products via modulation of RBC function and predisposition to haemolysis during cold storage. The aims of this study were to evaluate the association between female sex hormone intake and RBC storage outcomes, and to examine possible mechanisms by which sex hormones interact with RBCs.
Materials and methods
Sex hormone intake by race/ethnicity and menopausal status, and association analyses between hormone intake and donor scores of storage, osmotic or oxidative haemolysis, were evaluated in 6,636 female donors who participated in the National Heart, Lung and Blood Institute’s RBC-Omics study. A calcium fluorophore, Fluo-3AM, was used to define RBC calcium influx in response to exogenous sex hormones or transient receptor potential cation (TRPC) channel drugs.
Results
Sex hormone intake was more prevalent in premenopausal women from all racial groups (18–31%) than in postmenopausal women (4–8%). Hormone intake was significantly (p<0.0001) associated with reduced storage haemolysis in all females, reduced osmotic haemolysis in postmenopausal donors (23.1±10.2% vs 26.8±12.0% in controls, p<0.001), and enhanced susceptibility to oxidative haemolysis in premenopausal women. In vitro, supraphysiological levels of progesterone (10 μmol/L), but not 17β-oestradiol or testosterone, inhibited calcium influx into RBC and was associated with lower spontaneous haemolysis after 30 days of cold storage (0.95±0.18% vs 1.85±0.35% in controls, p<0.0001) or in response to a TRPC6 activator.
Conclusions
Sex hormone intake in female donors is associated with changes in RBC predisposition to haemolysis. Menstrual status and the type of hormone preparation may contribute to differences in haemolytic responses of female RBCs to osmotic and oxidative stress. Progesterone modulates calcium influx into RBC via a mechanism that may involve interactions with membrane TRPC6 channels.
Keywords: sex hormones, haemolysis, blood donors, progesterone, TRPC6
Introduction
Sex hormone intake in blood donors occurs in three demographic groups: premenopausal women who consume contraceptive drugs, menopausal hormone therapies in older women, and androgen therapies in men. Among these groups, premenopausal women on birth control medications represent the majority of blood donors who receive exogenous sex hormones. In the USA, it is estimated that among women aged 15–44 years contraception is used by 61.7%, out of whom 25.9% use oral contraceptives (National Survey of Family Growth, 2011–2013)1 that may have combined progestins and oestrogens or progestins alone2. In older women, hormone replacement therapy consists of oestrogens alone or in combination with progestins3. In recent years, the incidence of testosterone replacement therapy among men has increased remarkably4, with a 4-fold increased use in young men (aged 18–45 years) since 20035. It is estimated that nearly 4% of men use androgen replacement therapy, with side effects that may increase the risk of cardiovascular disease and polycythaemia4. Understanding the impact of sex hormone therapies in blood donors on the susceptibility of their red blood cell (RBC) concentrates produced for transfusion is important because units that rapidly haemolyse following transfusion may be associated with adverse outcomes in the recipients.
Recent large population-based studies of haemolysis performed by our group6 and others have demonstrated a sex dichotomy in RBC predisposition to haemolysis during routine cold storage in the blood bank and in haemolytic diseases including sickle cell anaemia7–11. Male sex was associated with enhanced susceptibility to spontaneous storage haemolysis and to stress haemolysis in response to osmotic shock or oxidative stress9,10. Similarly, male patients with sickle cell disease had increased peripheral blood biomarkers of haemolysis (e.g., reticulocyte count, total bilirubin, lactate dehydrogenase) as compared with female patients with sickle cell disease12. Historically, the sex differences in haemolysis were attributed to the protective effects that female sex hormones may exert on the cardiovascular system13. RBC from premenopausal women exhibited enhanced deformability, lower aggregation and viscosity, and higher resistance to experimental mechanical stress13–15. These phenomena were ascribed to menstruation and the consequent reduction in haematocrit and iron levels, along with increased levels of reticulocytes and sex hormones in the circulation. More recently, we made the additional observation that testosterone enhances RBC susceptibility to storage and stress-induced haemolysis, and that inhibition of androgen signalling via castration in mice improved RBC recovery after transfusion of stored murine RBCs10.
Mature erythrocytes have been used as a cellular model for studying the non-genomic effects of various hormones because they lack DNA-containing cell organelles (e.g., nucleoli, mitochondria). Non-genomic hormone actions are typically rapid, as they do not involve classical hormone-receptor pathways, such as gene transcription and protein synthesis. Instead, the actions are mediated via non-specific membrane receptors or via direct interactions with the membrane, whose fluidity can be modified16. Experiments using radiolabelled oestradiol or progesterone suggested that ovarian hormones bind to the RBC membrane at low affinity in a non-saturable manner17. Of note, RBC stored for 42 days in the presence of progesterone exhibited lower levels of spontaneous storage haemolysis and osmotic haemolysis, and better retention of adenosine triphosphate18.
RBC membranes express transient receptor potential cation (TRPC)-6, a cation channel whose activation has been associated with enhanced calcium influx that triggered pre-haemolytic events also known as erythrocyte programmed-cell death or eryptosis19. Several studies have demonstrated the ability of progesterone or its synthetic analogues (e.g., norgestimate) to inhibit diacylglycerol-induced calcium influx into non-erythroid mammalian cells by direct interactions with TRPC channels20,21 including TRPC3, TRPC5, and TRPC6. These interactions may be specific to progesterone, as oestrogens had less impact on TRPC-mediated calcium influx into the cells22. Based on these observations, we hypothesised that progesterone modulates calcium influx and subsequently RBC rheological properties via interactions with membrane TRPC channels.
The purpose of this study was to define the impact of female sex hormone intake by blood donors on RBC storage stability in a cohort of 6,636 pre- and postmenopausal female donors from the National Heart, Lung and Blood Institute (NHLBI) Recipient Epidemiology Donor Evaluation Study (REDS)-III Red Blood Cell-Omics (RBC-Omics) study. Another goal was to define the effect of sex hormones on spontaneous or TRPC-induced calcium influx into RBCs. We report age-specific associations between female sex hormone intake and RBC predisposition to spontaneous or stress-induced haemolysis and demonstrate the inhibitory properties of progesterone on calcium influx and haemolysis in stored RBC.
Materials and methods
The Red Blood Cell-Omics blood donor cohort
We performed secondary analyses of the RBC-Omics’ donor haemolysis and demographic databases (13,403 male and female donors), which provided data regarding hormone intake in female donors, menstrual status, age, race/ethnicity, percent storage haemolysis, percent osmotic haemolysis determined by a modified pink test9, percent oxidative haemolysis induced by 2,2′-azobis-2-methyl-propanimidamide, dihydrochloride (AAPH), plasma ferritin (ng/mL), and RBC haematological parameters including RBC count, haematocrit (HCT), haemoglobin (Hb), mean corpuscular volume, and RBC distribution width (RDW). Hormone intake (e.g., oral contraceptives, estrogen or testosterone) and menstrual status in 6,636 female donors was determined by a self-reported questionnaire at the time of donor recruitment. Detailed information regarding RBC-Omics’ study design, methodology, goals and outcomes can be reviewed elsewhere7–9,23,24.
Red blood cell storage in the presence of exogenously added female sex hormones
Whole blood (about 20 mL) from seven healthy volunteers was collected into heparinised tubes, centrifuged three times (1,500 g, 10 min, 18 °C) with phosphate-buffered saline (PBS), and packed RBCs were mixed with additive solution-5 (AS-5) and PBS in a 2:1:0.5 ratio. Each RBC suspension was divided into three identical aliquots, which were treated with dimethyl sulfoxide (DMSO, vehicle control, final concentration 0.1%), progesterone or 17β-oestradiol at a final concentration of 10 μmol/L. All drugs were from Sigma-Aldrich (St. Louis, MO, USA). All samples were incubated for 3 h at 37 °C and then stored at 1–6 °C for 24 h or 30 days. Percent haemolysis was determined according to the following equation after 24 h and 30 days:
Sample HCT was determined by collecting a portion of each blood sample into a capillary tube and spinning in a micro-HCT centrifuge (LW Scientific, Lawrenceville, GA, USA). Hbsupernatant refers to the amount of free haemoglobin obtained after centrifugation (1,500 g, 10 min, 18 °C) measured in the supernatant. Hbtotal refers to the total amount of sample haemoglobin before centrifugation. Haemoglobin concentrations (micromolar) were determined by Drabkin’s method25.
Kinetic calcium influx into red blood cells
Washed, packed RBCs, prepared as described above, were suspended in PBS calcium (132.5 mg/L as calcium chloride dihydrate) magnesium (100 mg/L magnesium chloride hexahydrate) buffer (Sigma-Aldrich), which was fortified with glucose (5 mmol/L) and labelled with a mixture of Fluo-3AM calcium indicator and Pluronic F-127 (ThermoFisher Scientific, Waltham, MA, USA). After incubation (at 37 °C) for 45 min on a tube rotator, Fluo-3AM-labelled RBCs were washed three times and suspended in PBS calcium magnesium glucose to a final concentration of approximately 6×106 cells/mL. Each Fluo-3AM-labelled RBC suspension was divided into identical aliquots, which were treated with the indicated concentrations of progesterone, 17β-oestradiol, testosterone, SKF-96365 (an inhibitor of multiple TRPC channels: TRPC3, TRPC6, and TRPC7), Pyr3 (a selective TRPC3 inhibitor) or Hyp9 (a selective TRPC6 activator). All drugs were purchased from Sigma-Aldrich. After adding the drugs, replicates of 200 mL aliquots from each sample were transferred into dark, 96-well microplates (Nunc F96, ThermoFisher Scientific). Fluo-3AM fluorescence was determined at 1 min intervals on a plate reader (Synergy Neo, BioTek, Winooski, VT, USA). In some experiments involving TRPC6 activation by Hyp9, baseline Fluo-3AM fluorescence was determined by incubating Fluo-3AM-labelled RBCs for 10–15 min in the presence of selected drugs, after which Hyp9 was injected into selected wells at a final concentration of 25 μmol/L.
Red blood cell incubation with transient receptor potential cation channel drugs or progesterone
Washed packed RBCs prepared as described above were diluted 1:25 with PBS calcium magnesium glucose buffer. Each suspension was divided into 1 mL aliquots, which were treated with DMSO (0.2%, vehicle control), SKF-96365 (25 μmol/L), Pyr3 (25 μmol/L) or progesterone (10 or 20 μmol/L) in the presence or absence of Hyp9 (25 μmol/L). The samples were then incubated at 37 °C for selected periods, after which percent haemolysis was determined by Drabkin’s method.
Statistical analysis
Data pre-processing for female donor menstrual status
Self-reported menopausal status for all female donors in the RBC-Omics study was used to separate female donors into premenopausal and postmenopausal groups. Self-reported menstrual status was missing for 583 female donors. If the following two criteria were met, these women were defined as postmenopausal: (i) age ≥50 years, and (ii) donor reported that her periods had stopped for other reasons. In total, 87 female donors with missing menstrual status were excluded from analysis. The histograms for premenopausal and postmenopausal female donors were generated in R statistical software for Windows, version 3.5.026.
Race difference in hormone supplement use
Counts and percentages of hormone supplement use in female donors in the RBC-Omics study were calculated in each self-reported race and compared in all females, and separately in premenopausal and postmenopausal females.
Comparison of three haemolysis measurements based on hormone supplement use in the Red Blood Cell-Omics study
We compared each of the three haemolysis measurements (spontaneous storage haemolysis, osmotic haemolysis and oxidative haemolysis) between female donors who used a hormone supplement and those who did not. The comparison was done within all female donors, and separately for premenopausal and postmenopausal donors. Student’s t-test was used to determine the statistical significance within each donor group. All dot and box plots were generated by using the ggplot2 packages27 (version 2.1.1) in R statistical software for Windows, version 3.5.026.
In-vitro haemolysis studies in red blood cells treated with sex hormones or transient receptor potential cation channel modulators
Statistical significance tests determined the differences between two (unpaired t tests) or more (one-way or two-way analysis of variance and Dunnett’s multiple comparisons test where applicable) groups. Probabilities less than 0.05 were considered statistically significant. Statistical analyses were performed using commercial software (GraphPad Prism version 8, GraphPad Software, San Diego, CA, USA).
Results
Female donor demographics and sex hormone intake
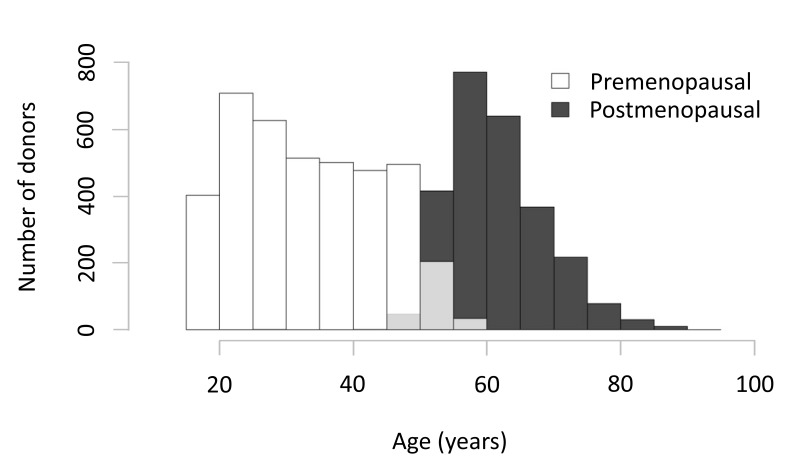
Among the 6,636 female donors who participated in RBC-Omics, 2,583 study participants were defined as “postmenopausal” and 3,966 as “premenopausal”. Menstrual status was self-reported by the donors at the time of recruitment (RBC-Omics donor questionnaire, Online Supplementary Content, Figure S1). We excluded 87 donors from the analyses because their menstrual status could not be verified for various reasons including a blank response on the questionnaire, use of non-steroid contraceptive devices, and possible perimenopausal status as indicated by self-reported appearance of spotting. The distribution of menstrual status by donor age is illustrated in Figure 1.
Figure 1.
Distribution of donor menstrual status by age of the donors in the RBC-Omics study.
Bar graphs represent the numbers of pre- and postmenopausal female donors at each decade. Menstrual status was self-reported by female donors at the time of recruitment into the RBC-Omics study, as detailed in the “Materials and methods” section. Of the 6,636 female donors, 3,966 were defined as premenopausal and 2,583 as postmenopausal. Partial grey bars represent overlap between pre- and postmenopausal women at specific age groups.
Hormone intake was more prevalent among younger premenopausal women in all racial groups (18–31%) as compared with older postmenopausal females (4–8%). Evaluation of hormone intake by donor race/ethnicity (Table I) revealed that consumption of female sex hormones was higher (≥20%) in White, Asian and Other (mixed race, Native American or Hawaiian American) females and lower (15%) in African American females. The lowest rate of hormone intake (9%) was observed in high-intensity donors, defined as nine or more successful blood donations in the prior 24 months without a low haemoglobin deferral. The majority of donors in this category were older White women8.
Table I.
Distribution of sex hormone intake stratified by self-reported menstrual status and race/ethnicity in 6,636 female donors from the National Heart, Lung and Blood Institute’s RBC-Omics study.
| Hormone intake | African American | Asian | White | High-intensity | Hispanic | Other | |
|---|---|---|---|---|---|---|---|
| All female donors | N | 733 | 586 | 2,777 | 581 | 504 | 245 |
| Y | 127 | 148 | 687 | 56 | 105 | 87 | |
| % Y | 15 | 20 | 20 | 9 | 17 | 26 | |
| Premenopause | N | 483 | 481 | 1,298 | 97 | 426 | 177 |
| Y | 117 | 141 | 545 | 22 | 100 | 79 | |
| % Y | 20 | 23 | 30 | 18 | 19 | 31 | |
| Postmenopause | N | 237 | 101 | 1,441 | 473 | 74 | 67 |
| Y | 9 | 7 | 132 | 32 | 4 | 6 | |
| % Y | 4 | 6 | 8 | 6 | 5 | 8 |
Y: yes; N: no.
Hormone intake is associated with changes in red blood cell predisposition to storage or stress haemolysis
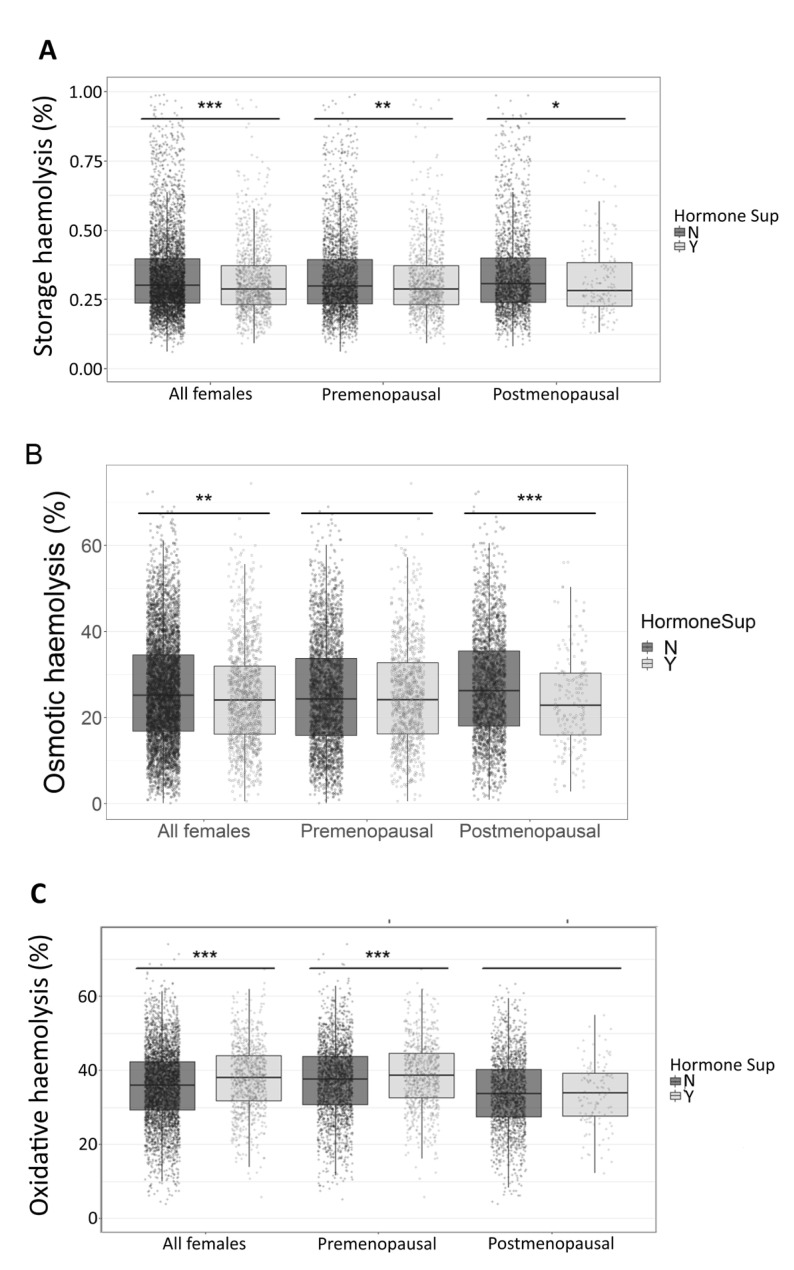
We evaluated the impact of sex hormone intake on haemolysis in stored (39–42 days) leucocyte-reduced RBCs in the entire female cohort and in subsets of pre- and postmenopausal donors. Hormone intake was associated with lower spontaneous storage haemolysis in the entire female population (0.32±0.16% vs 0.35±0.29%, hormone intake vs controls; p<0.0001), and in each menstrual status subgroup (Figure 2A). Hormone intake was also associated with reduced osmotic haemolysis in the entire female cohort (24.9±11.8% vs 26.0±12.3%, hormone intake vs controls; p=0.007) (Figure 2B). However, this effect was ascribed to postmenopausal women (23.1±10.2% vs 26.8±12.0%, hormone intake vs controls; p<0.0001), whereas no significant changes were observed in premenopausal women (25.4±12.4% vs 25.4±12.2%, hormone intake vs controls; p=0.99). Conversely, hormone intake was associated with enhanced susceptibility to AAPH-induced oxidative haemolysis (37.9±9.3% vs 35.8±9.9%, hormone intake vs controls; p<0.0001) (Figure 2C) in the entire female cohort and in premenopausal women (38.7±9.2% vs 37.3±10.0%, hormone intake vs controls; p=0.0003), but not postmenopausal females (33.1±8.5% vs 33.9±9.6%, hormone intake vs controls; p=0.338).
Figure 2.
Effect of female sex hormone intake on red blood cell predisposition to storage or stress-induced haemolysis.
Leucocyte-reduced red blood cell concentrates from 6,549 pre- and postmenopausal female donors enrolled in the RBC-Omics study were stored for 39–42 days (at 1–6 °C) and then evaluated for spontaneous storage, osmotic or oxidative haemolysis. Each panel compares the levels of haemolysis between women who responded yes (Y) or no (N) to hormone supplements (Hormone Sup).
Comparisons were made between all female donors or by menstrual status. (A) Percent storage haemolysis. (B) Percent osmotic haemolysis. (C) Percent AAPH-induced oxidative haemolysis. Box plots demonstrate the median and interquartile range (IQR) of the three haemolysis measurements in all female, premenopausal and postmenopausal donors, separated by hormone supplement use. *p<0.05, **p<0.01, ***p<0.001 by the Student’s t-test.
AAPH: 2,2’-azobis-2-methyl-propanimidamide, dihydrochloride.
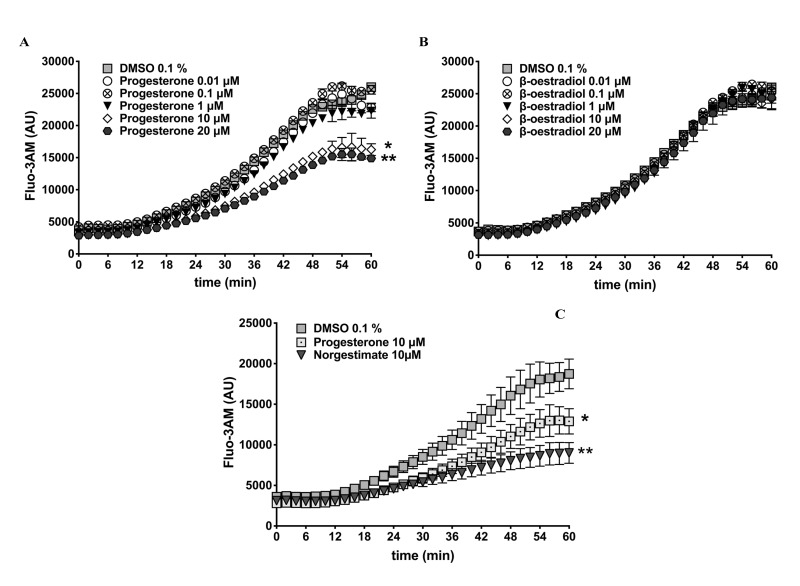
Exogenous progesterone, but not oestradiol, decreases calcium influx into human red blood cells
We examined the interactions between female sex hormones and calcium influx into RBCs by incubating Fluo-3AM-labelled RBCs (37 °C, 60 min, mild agitation) in the presence or absence of progesterone or 17β-oestradiol at concentrations from 0.01 to 20 μmol/L (Figure 3). Under the tested conditions, about a 5-fold increase in Fluo-3AM fluorescence was observed at 60 min in DMSO-treated RBCs (vehicle control). Low dose progesterone treatments (10 or 100 nmol/L) did not have significantly different effects than the control treatment, whereas significant (p<0.0001) inhibition of Fluo-3AM fluorescence was observed with higher doses of progesterone (10 or 20 μmol/L) (Figure 3A). Conversely, treating RBCs with 17β-oestradiol at all selected doses had no impact on Fluo-3AM fluorescence, beyond that of the DMSO control (Figure 3B).
Figure 3.
Effect of female sex hormones on red blood cell calcium influx.
Human red blood cells (RBC) from healthy volunteers (n=3) were labelled with a fluorescent calcium probe (Fluo-3AM) and treated with selected concentrations of progesterone, 17β-oestradiol or norgestimate (a progesterone contraceptive analogue) or dimethyl sulfoxide (DMSO 0.1%, vehicle control). Kinetic curves represent the rates of RBC calcium influx during incubation for 60 min (37 °C, mild agitation). (A) Calcium influx in the presence of progesterone. Asterisks represent significant differences (p<0.0001) between results for DMSO and 10 μmol/L (*) or 20 μmol/L (**) progesterone. (B). Calcium influx in the presence of 17β-oestradiol. (C) Comparison of RBC calcium influx kinetics between cells treated with progesterone or norgestimate (each at 10 μmol/L). Asterisks represent significant differences between DMSO and progesterone (*p=0.033) or norgestimate (**p=0.003). AU: arbitrary units.
Dimethyl sulfoxide has toxic effects on RBCs and may induce mild haemolysis even at a low concentration of 0.6%28. To rule out the possibility that the increase in RBC calcium influx was caused by DMSO cytotoxicity, validation experiments were performed in the absence of DMSO by using water-soluble progesterone (Sigma-Aldrich, product number P7556). As shown in Online Supplementary Content, Figure S2A, control samples treated with double-distilled water (0.1%, progesterone solvent) exhibited an increase in Fluo-3AM fluorescence during incubation (37 °C, 60 min, mild agitation) that was equivalent to that of DMSO-treated RBCs (Online Supplementary Content, Figure S2B), whereas the inhibitory action of progesterone against calcium influx was reproduced in the water-soluble formula.
To further validate our observations, we examined the ability of norgestimate (10 μmol/L), a progesterone analogue used in contraceptive drugs, to inhibit calcium influx into RBCs. Similar to progesterone (10 μmol/L), norgestimate treatment significantly (p=0.003) prevented the increase in RBC Fluo-3AM fluorescence observed with the DMSO control (Figure 3C). These observations suggest that progesterone and its analogues are capable of inhibiting calcium influx into RBCs.
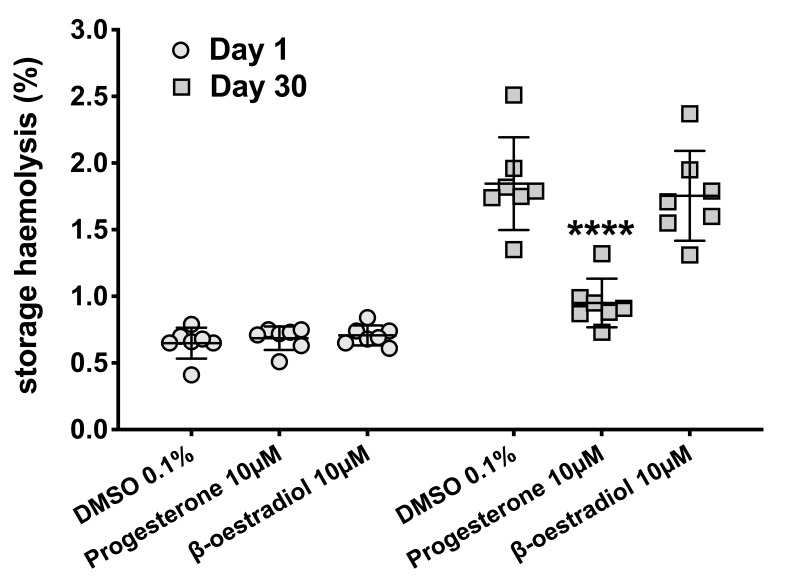
Progesterone may protect against spontaneous haemolysis
We evaluated the interactions between female sex hormones and RBC predisposition to haemolysis by storing RBC aliquots (at 1–6 °C, for 30 days) in the presence of progesterone, 17β-oestradiol or DMSO (vehicle control). We chose the concentration of 10 μmol/L for both hormones, as this dose of progesterone was associated with reduced calcium influx into Fluo-3AM-labelled RBCs (Figure 3A), and because our previous study had demonstrated that nanomolar concentrations of sex hormones had little impact on RBC storage stability including spontaneous haemolysis10. As expected, storage for 30 days was associated with significant increases in spontaneous haemolysis in all tested groups (Figure 4), however, the haemolysis in progesterone-treated RBCs (0.95±0.18%) was significantly (p<0.0001) lower than that observed in RBC treated with DMSO alone (1.85±0.35 %) or in 17β-oestradiol-treated RBCs (1.75±0.34%).
Figure 4.
Red blood cell storage in the presence of female sex hormones.
Human red blood cells (RBCs) from healthy volunteers (n=7) were treated with dimethyl sulfoxide (DMSO 0.1%, vehicle control), progesterone (10 μmol/L) or 17β-oestradiol (10 μmol/L) and stored at 1–6 °C for 30 days as described in the “Material and methods” section. Percent storage haemolysis was determined on days 1 and 30. ****p<0.0001 by one-way analysis of variance at day 30.
TRPC6 drugs modulate calcium influx and haemolysis in human red blood cells
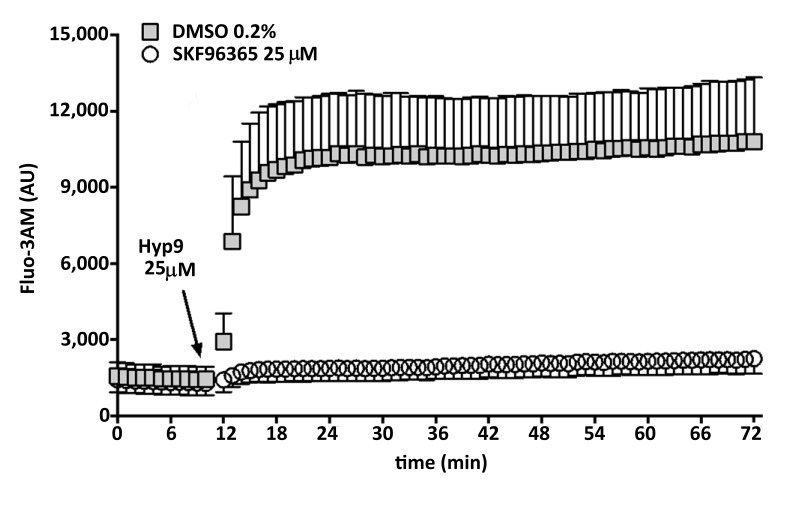
To test the hypothesis that progesterone inhibits calcium influx into RBCs via interactions with membrane TRPC6 channels, we first examined the responsiveness of human RBCs to selected TRPC channel drugs including Hyp9 (a selective TRPC6 activator) and SKF-96563 (a selective TRPC3/6/7 inhibitor). Fluo-3AM-labelled RBCs were incubated (for 12 min) in the presence of SKF-96563 (25 μmol/L) or DMSO (0.2%, vehicle control) prior to stimulation with Hyp9 (25 μmol/L). As illustrated in Figure 5, Hyp9 induced a rapid increase in Fluo-3AM-fluorescence in DMSO-treated RBCs. Conversely, no changes in Fluo-3AM fluorescence were observed in SKF-96563-treated RBCs exposed to Hyp9.
Figure 5.
Effect of TRPC6 drugs on red blood cell calcium influx.
Human red blood cells (RBCs) from healthy volunteers (n=3) were labelled with a fluorescent calcium probe (Fluo-3AM) and treated with SKF-96365 (a multiple transient receptor potential cation [TRPC] channel inhibitor that blocks TRPC3/6/7; 25 μmol/L) or dimethyl sulfoxide (DMSO 0.2%, vehicle control). After 12 min incubation (37 °C, mild agitation), Hyp9 (a selective TRPC6 activator; 25 μmol/L) was injected and samples were incubated (same conditions) for an additional 60 min. Kinetic curves represent the rates of RBC calcium influx during the incubation period. AU: arbitrary units.
Progesterone inhibits Hyp9-induced calcium influx and haemolysis in human red blood cells
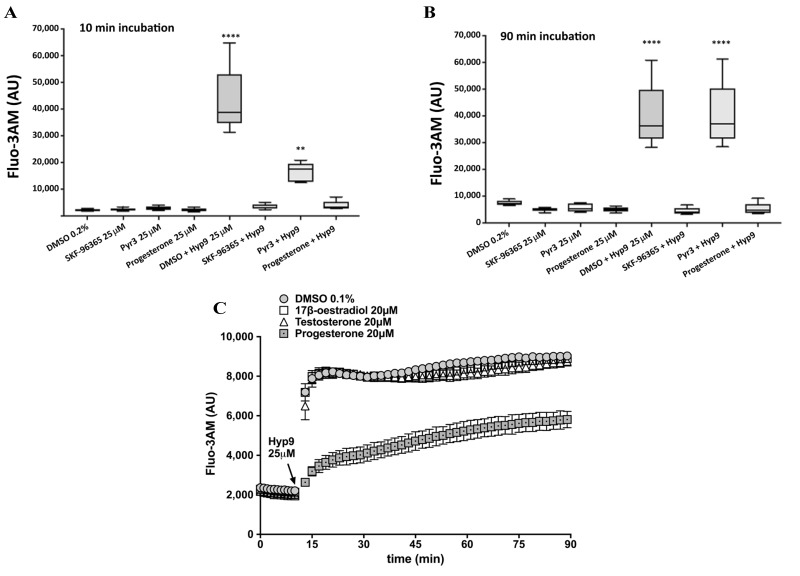
We evaluated the potency of progesterone to inhibit Hyp9-induced calcium influx into RBCs by comparing its action to that of SKF-96365 and Pyr3 (a TRPC3 inhibitor). We monitored RBC Fluo-3AM fluorescence after 10 min (Figure 6A) or 90 min (Figure 6B) of incubation (37 °C, mild agitation). Hyp9 induced a significant (p<0.0001) increase in Fluo-3AM fluorescence compared with all other treatments. Progesterone demonstrated strong inhibition against Hyp9-induced Fluo-3AM fluorescence at 10 and 90 min, which was comparable to that of SKF-96365. Conversely, Pyr3 was less effective in inhibiting Hyp9-induced Fluo-3AM fluorescence at 10 min (Figure 6A), and this inhibitory effect was diminished after 90 min (Figure 6B).
Figure 6.
Effect of TRPC3/6 inhibitors or progesterone on Hyp9-induced red blood cell calcium influx.
(A,B) Red blood cells (RBCs) from five healthy volunteers were labelled with a fluorescent calcium probe (Fluo-3AM) and treated with SKF-96365 (a multiple transient receptor potential cation [TRPC] channel inhibitor that blocks TRPC3/6/7), Pyr3 (a selective TRPC3 inhibitor), progesterone or dimethyl sulfoxide (DMSO 0.2%, vehicle control) in the presence or absence of Hyp9 (a selective TRPC6 activator); all drugs at 25 μmol/L. Fluo-3AM fluorescence was recorded at 10 min (A) and 90 min (B) of incubation (37 °C, mild agitation). Box and whisker plots (median + range). ****p<0.0001 DMSO+Hyp9 compared with all other treatments. **p<0.0001 Pyr3+Hyp9 compared with all other treatments except DMSO+Hyp9 (p=0.99); p values obtained by one-way analysis of variance and Tukey’s correction for multiple comparisons. (C) RBCs from a healthy volunteer were labelled with Fluo-3AM and treated with progesterone, 17β-oestradiol, testosterone (all at 20 μmol/L) or DMSO (0.1%, vehicle control). After 15 min incubation (37 °C, mild agitation), Hyp9 was injected and samples were incubated (same conditions) for an additional 75 min. Kinetic curves represent the rates of RBC calcium influx during the incubation period. Error bars represent the standard error of the mean (n=12 replicates). AU: arbitrary units.
We further verified whether Hyp9 interactions with sex hormones are unique to progesterone by evaluating the potency of 17β-oestradiol or testosterone to inhibit Hyp9-induced calcium influx into RBCs (Figure 6C). Compared to progesterone, 17β-oestradiol or testosterone (all hormones used at a concentration of 20 μmol/L) did not inhibit the spike in RBC Fluo-3AM fluorescence triggered by hyp9 (25 μmol/L), which was similar to that induced by the vehicle control (DMSO 0.1%).
To evaluate associations with haemolysis, human RBCs were incubated (for 2.5 h, at 37 °C) in the presence of progesterone alone (10 or 20 μmol/L) or with Hyp9 (25 μmol/L) (Online Supplementary Content, Figure S3). Hyp9-induced haemolysis at the end of incubation (2.63±0.19%) was significantly (p<0.05) reduced by co-treatment with progesterone in a dose-dependent manner (1.45±0.13% and 1.01±0.09%, at 10 and 20 μmol/L, respectively).
Discussion
Consumption of medications or health supplements by blood donors may modulate the quality of blood components and may compromise or enhance transfusion efficacy29. For example, we recently demonstrated that consumption of iron supplements by blood donors was associated with enhanced resistance of the stored packed RBC components to oxidative and osmotic haemolysis8. The present study has identified demographic differences in sex hormone consumption and quantified the associations between female sex hormone intake and RBC predisposition to haemolysis in a large cohort of female donors from NHLBI’s RBC-Omics study. We further demonstrated the uniqueness of progesterone as a modulator of RBC calcium influx and haemolysis in vitro and proposed a mechanism that involves interactions with membrane TRPC6 channels.
We observed age- and race/ethnicity-related differences in the prevalence of female sex hormone consumption among RBC-Omics female donors. Younger age, premenopausal status, and white race/ethnicity were associated with higher rates of sex hormone consumption, whereas lower rates of sex hormone intake were associated with postmenopausal status, frequent blood donations, and African American race/ethnicity. These differences may reflect physiological (age), cultural, and socio-economic differences among the donors. The lowest consumption of sex hormones in all female donors (9%) was observed in high-intensity donors. This can be explained by the demographics of this cohort, which consisted primarily of older (59±11.2 years) postmenopausal white women8.
Sex hormone consumption was associated with lower spontaneous storage haemolysis in all female donors (pre- and postmenopausal). Although those differences were statistically significant (p<0.0001), it may be argued that the overall change in the concentration of free haemoglobin was minor and of little clinical significance. Sex hormone consumption was also associated with stress haemolysis, but this differed by menstrual status. Sex hormone-mediated changes in predisposition to osmotic haemolysis were only observed in postmenopausal women, whose RBCs exhibited enhanced resistance to osmotic stress. On the other hand, sex hormone intake enhanced RBC predisposition to AAPH-induced oxidative haemolysis in premenopausal, but not postmenopausal women. These differences may stem from age-specific differences in erythrocyte rheological properties and antioxidant capacity or from age-related differences in physiological responses to hormone therapy. In support of the latter notion, we recently demonstrated in the entire RBC-Omics cohort that there were remarkable age-related differences in RBC predisposition to oxidative haemolysis; i.e. older age was significantly associated with enhanced resistance to this stressor8. Subsequent metabolomic analysis of a subset of RBC-Omics donors suggested enhanced glutathione antioxidant capacity in RBCs from older donors30. There are also differences in the types of sex hormone therapy in that oral contraceptives are used by premenopausal women, whereas hormone replacement therapy is used in postmenopausal women. Oral contraceptives all have progesterone (with or without oestrogen) whereas hormone replacement therapy contains oestrogen alone or oestrogen plus progesterone.
Our investigations of the mechanisms by which female sex hormones may confer protection against haemolysis revealed the potency of progesterone or its analogue (norgestimate) to inhibit calcium influx into RBCs. Stress or senescence-mediated changes in RBC calcium steady state have been associated with eryptosis, characterised by increased influx of calcium, which may compromise membrane properties via phosphatidylserine exposure in the outer leaflet leading to enhanced recognition and elimination by macrophages31. Our findings support previous studies that demonstrated non-genomic interactions between progesterone and mammalian cells, which lead to inhibition of calcium influx via TRPC channels. It should be noted that in vitro, the concentrations of progesterone required to elicit calcium inhibition or protection against haemolysis are supraphysiological (micromolar) and may not accurately reflect physiological mechanisms. Female sex hormone therapies produce nanomolar levels of progesterone in the patients’ circulation, and our data suggest that in vivo, such concentrations may be sufficient to modulate RBC predisposition to haemolysis.
The hypothesis that progesterone interacts with RBCs via TRPC channels is supported by the potency of progesterone to inhibit Hyp9-induced calcium influx and haemolysis. Although previous studies have proposed that progesterone’s interactions with TRPC channels are non-selective (i.e. similar inhibitory levels of TRPC4, TRPC5 or TRPC6 channels)20,21, our data indicate that in the case of human RBCs, such interactions probably involve TRPC6 channels. This conclusion is based on the observations that: (i) Hyp9 has demonstrated higher selectively for TRPC6 channels over TRPC3 or TRPC7 channels32; (ii) SKF-96365 has demonstrated stronger inhibition of Hyp9-induced calcium influx into RBC as compared with a selective TRPC3 inhibitor, Pyr3; and (iii) progesterone demonstrated a similar inhibitory effect to that of SKF-96365 on Hyp9-induced RBC calcium influx. TRPC6 activation has previously been implicated as a mediator of RBC eryptosis19, which was supported by our observations of Hyp9-induced haemolysis that could be inhibited by progesterone (Online Supplementary Content, Figure S3) or SKF-96365, but not Pyr3 (Online Supplementary Content, Figure S4).
This study has several limitations. First, the analyses were based on donor self-reported sex hormone intake and menstrual status and there was no information in the RBC-Omics questionnaire about the type of administration or the composition of the drugs/contraceptives used for sex hormone therapies. Furthermore, supraphysiological levels of progesterone were required to observe the action of this hormone on RBC calcium influx and haemolysis in vitro and the effects on haemolysis observed in vivo may be mediated via alternative mechanisms that indirectly affect RBC function or rheological properties.
Conclusions
Our study revealed that sex hormone intake by blood donors is capable of modulating RBC predisposition to haemolysis and lead us to propose new mechanistic pathways by which progesterone regulates calcium influx and haemolysis in human RBCs. The implications of our findings can be further validated by characterising RBC TRPC channel biology and its contribution to the regulation of calcium influx and haemolysis under stress conditions including cold storage, by performing studies of haemolysis and assessment of RBC calcium content in blood donors in whom the progesterone and oestrogen content of the sex hormone therapy is well-defined, and by conducting analyses of linked donor-recipient databases to compare outcomes following RBC transfusions from blood donors who received sex hormone therapy or took contraceptives relative to the outcomes following transfusions from matched control donors.
Online supplementary content
Acknowledgements
The Authors thank the REDS-III leadership including Dr. Simone Glynn, Dr. Darrell Triulzi, and Dr. Mark Gladwin for their support, the RBC-Omics research staff at all participating blood centres, the testing laboratories and all blood donors who agreed to participate in this study.
Footnotes
Sources of support
This study was supported by the U.S National Institutes of Health grant numbers RO1 HL134653 and RO1 HL098032, and by the National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN2682011-00001I, HHSN2682011-00002I, HHSN2682011-00003I, HHSN2682011-00004I, HHSN2682011-00005I, HHSN2682011-00006I, HHSN2682011-00007I, HHSN2682011-00008I, and HHSN2682011-00009I, which supported the Recipient Epidemiology and Donor Evaluation Study III (REDS-III) RBC-Omics study.
Authorship contributions
TK conceived the study and drafted the manuscript. GPP, FF and YG supervised and performed the analyses from RBC-Omics and created Figures 1 and 2. KH reviewed and edited the manuscript. DS assisted with the RBC calcium influx studies. AEM, SK and MPB developed the RBC-Omics study protocols, participated in data analyses and interpretation, and edited the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15–44: United States, 2011–2013. Natl Health Stat Report. 2015:1–14. [PubMed] [Google Scholar]
- 2.De Leo V, Musacchio MC, Cappelli V, et al. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. 2016;22:634–46. doi: 10.1093/humupd/dmw016. [DOI] [PubMed] [Google Scholar]
- 3.Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy scientific review. JAMA. 2002;288:872–81. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DJ, Dhruva SS, Wright SM, Korenstein D. 2016 update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687–92. doi: 10.1001/jamainternmed.2016.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao PK, Boulet SL, Mehta A, et al. Trends in testosterone replacement therapy use among reproductive-age men in the United States, 2003 to 2013. J Urol. 2017;197:1121–6. doi: 10.1016/j.juro.2016.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanias T, Busch MP. Diversity in a blood bag: application of omics technologies to inform precision transfusion medicine. Blood Transfus. 2019;17:258–62. doi: 10.2450/2019.0056-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion. 2019;59:79–88. doi: 10.1111/trf.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanias T, Stone M, Page GP, et al. Program NREDES-I. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion. 2019;59:67–78. doi: 10.1111/trf.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–41. doi: 10.1182/bloodadvances.2017004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 2016;56:2571–83. doi: 10.1111/trf.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan A, Chen D, Yi QL, et al. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang. 2016;111:8–15. doi: 10.1111/vox.12378. [DOI] [PubMed] [Google Scholar]
- 12.Raslan R, Shah BN, Zhang X, et al. Hemolysis and hemolysis-related complications in females vs. males with sickle cell disease. Am J Hematol. 2018;93:E376–80. doi: 10.1002/ajh.25258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kameneva MV, Watach MJ, Borovetz HS. Rheologic dissimilarities in female and male blood: potential link to development of cardiovascular diseases. Adv Exp Med Biol. 2003;530:689–96. doi: 10.1007/978-1-4615-0075-9_69. [DOI] [PubMed] [Google Scholar]
- 14.Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100:418–21. doi: 10.1111/j.1423-0410.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 15.Kameneva MV, Antaki JF, Yeleswarapu KK, et al. Plasma protective effect on red blood cells exposed to mechanical stress. ASAIO J. 1997;43:M571–5. [PubMed] [Google Scholar]
- 16.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 17.Yoong WC, Tuck SM, Michael AE. Binding of ovarian steroids to erythrocytes in patients with sickle cell disease; effects on cell sickling and osmotic fragility. J Steroid Biochem Mol Biol. 2003;84:71–8. doi: 10.1016/s0960-0760(02)00266-2. [DOI] [PubMed] [Google Scholar]
- 18.DeVenuto F, Wilson SM. Distribution of progesterone and its effect on human blood during storage. Transfusion. 1976;16:107–12. doi: 10.1046/j.1537-2995.1976.16276155103.x. [DOI] [PubMed] [Google Scholar]
- 19.Foller M, Kasinathan RS, Koka S, et al. TRPC6 contributes to the Ca(2+) leak of human erythrocytes. Cell Physiol Biochem. 2008;21:183–92. doi: 10.1159/000113760. [DOI] [PubMed] [Google Scholar]
- 20.Majeed Y, Amer MS, Agarwal AK, et al. Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br J Pharmacol. 2011;162:1509–20. doi: 10.1111/j.1476-5381.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miehe S, Crause P, Schmidt T, et al. Inhibition of diacylglycerol-sensitive TRPC channels by synthetic and natural steroids. PLoS One. 2012;7:e35393. doi: 10.1371/journal.pone.0035393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beech DJ. Integration of transient receptor potential canonical channels with lipids. Acta Physiol (Oxf) 2012;204:227–37. doi: 10.1111/j.1748-1716.2011.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone M, Keating SM, Kanias T, et al. Piloting and implementation of quality assessment and quality control procedures in RBC-Omics: a large multi-center study of red blood cell hemolysis during storage. Transfusion. 2019;59:57–66. doi: 10.1111/trf.15099. [DOI] [PubMed] [Google Scholar]
- 24.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion. 2019;59:46–56. doi: 10.1111/trf.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwart A, van Assendelft OW, Bull BS, et al. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition) J Clin Pathol. 1996;49:271–4. doi: 10.1136/jcp.49.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Accessed on 15/02/2019]. Available at: http://www.R-project.org/ [Google Scholar]
- 27.Wickham H. Ggplot2. 1st ed. New York: Springer-Verlag; 2009. [Google Scholar]
- 28.Yi X, Liu M, Luo Q, et al. Toxic effects of dimethyl sulfoxide on red blood cells, platelets, and vascular endothelial cells in vitro. FEBS Open Bio. 2017;7:485–94. doi: 10.1002/2211-5463.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker CD, Stichtenoth DO, Wichmann MG, et al. Blood donors on medication - an approach to minimize drug burden for recipients of blood products and to limit deferral of donors. Transfus Med Hemother. 2009;36:107–13. doi: 10.1159/000203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Alessandro A, Nemkov T, Reisz J, et al. Increased methylation of deamidated asparagines and aspartates in stored red blood cells from glucose 6-phosphate dehydrogenase-deficient blood donors. Blood. 2018;132:2543. [Google Scholar]
- 31.Bissinger R, Bhuyan AAM, Qadri SM, Lang F. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2019;286:826–54. doi: 10.1111/febs.14606. [DOI] [PubMed] [Google Scholar]
- 32.Leuner K, Heiser JH, Derksen S, et al. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators - identification of a novel pharmacophore. Mol Pharmacol. 2010;77:368–77. doi: 10.1124/mol.109.057513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.