Abstract
Background
Despite fulfilling all requirements for blood donation, a large proportion of regular blood donors are iron deficient. Red blood cells (RBC) from iron-deficient donors may be particularly susceptible to damage induced by standard refrigerated storage. Herein, we present a study protocol for testing whether correcting iron deficiency in donors with iron-deficient erythropoiesis will improve the quality of their refrigerator-stored RBC.
Materials and methods
This is a randomised, controlled, double-blind clinical trial. Sixty healthy regular donors who meet donation standards, while exhibiting iron-deficient erythropoiesis by laboratory testing criteria, will donate a single standard RBC unit that will be leucoreduced and stored in a refrigerator under standard conditions for 40–42 days. A 51Cr-radiolabelled 24-hour RBC recovery study will be performed and then these donors will be randomised to receive, in a double-blinded fashion, either intravenous saline, as a control, or low-molecular weight iron dextran (1 g), to provide total iron repletion. Four to six months later, they will donate a second RBC unit, which will be similarly stored, and autologous 51Cr-labelled 24-hour post-transfusion RBC recovery will again be determined.
Results
The primary endpoint will be the change in 24-hour post-transfusion recovery from the first to the second donation. The primary outcome will be the group mean difference in the primary endpoints between the group receiving intravenous saline and the group receiving intravenous iron dextran. Secondary outcomes will be quality of life, fatigue, and emotional health, assessed by surveys.
Conclusion
This study will provide definitive evidence as to whether donor iron deficiency affects the quality of the blood supply and will assess the severity of symptoms affecting iron-deficient blood donors.
Keywords: post-transfusion recovery, iron deficiency, blood donation, red blood cells
Introduction
Iron deficiency is common among regular blood donors. In the United States, 69% of the donors who provided the ~15.7 million units of red blood cells (RBC) that were collected in 2011 were repeat donors1. In Canada, approximately 90% of RBC units collected for transfusion are provided by repeat donors2. Although iron deficiency is surprisingly prevalent in first-time donors3,4, its prevalence is even higher in the particularly altruistic frequent donors, especially among women of childbearing age5,6. In the Recipient Epidemiology Donor Evaluation Study (REDS)-II Donor Iron Status Evaluation (RISE) study7, up to 49% and 66% of male and female frequent donors, respectively, had either iron depletion (i.e., absent iron stores) or iron-deficient erythropoiesis. Similar frequencies of iron deficiency were also reported in Canadian2, Austrian8, Danish9, and Dutch10 populations.
RBC from individuals with iron-deficiency anaemia have decreased levels of endogenous anti-oxidants11,12, have evidence of oxidative damage13,14, and are more sensitive to oxidative stress11,14 and low pH12; the latter, in particular, decreases progressively during RBC storage15. In a recent study by the REDS-III group, RBC collected from frequent donors with low ferritin were found to have altered susceptibility to in vitro haemolysis16. Furthermore, refrigerated storage induces oxidative stress in donor RBC and inhibits their oxidative stress defence mechanisms13,17–22. Oxidative damage per se also impairs RBC deformability23 and impaired deformability was seen in humans14, rats14, and rabbits with iron-deficiency anaemia24 and in stored RBC from healthy human donors25. Rigid RBC are less able to pass through previously negotiable microcirculatory beds and are more prone to undergo extravascular haemolysis in the spleen26. Indeed, circulatory RBC lifespan is decreased in humans with iron-deficiency anemia12,27–29 and in relevant animal models24,30. In humans, decreased circulatory lifespan is most likely due to extravascular haemolysis in the spleen27–29 and is corrected by iron repletion12,28.
Remarkably, in several older studies12,27,31, RBC obtained from donors with iron-deficiency anaemia were transfused into healthy recipients, without prior refrigerated storage. In each study, the transfused iron-deficient RBC had a decreased circulatory lifespan/recovery, most likely due to splenic clearance. Indeed, when RBC obtained from healthy donors were transfused into recipients with iron-deficiency anaemia, the transfused RBC had a normal lifespan, suggesting that the iron deficiency-induced defect was intrinsic to the RBC and not due to enhanced clearance mechanisms31,32.
We used a mouse model to test whether iron deficiency is associated with decreased RBC recovery33. Three donor cohorts were prepared: iron-replete mice, mice with iron-deficient erythropoiesis, and mice with iron deficiency anaemia. Similar to prior studies34, refrigerator-stored, transfused RBC from iron-replete donors had a normal 24-hour post-transfusion recovery (mean 77.1%). In addition, as expected from other publications12,27,31, the 24-hour post-transfusion recovery was poor using RBC from donors with severe iron-deficiency anaemia (mean 46.7%; p<0.001). In contrast, results using donors with iron-deficient erythropoiesis were subnormal (mean 66.5%; p<0.05) and would be less than the minimum mandated by the United States Food & Drug Administration (FDA). Taken together, these data support our hypothesis that, after refrigerated storage, RBC from donors with iron-deficient erythropoiesis without anaemia are suboptimal.
Materials and methods
Study design
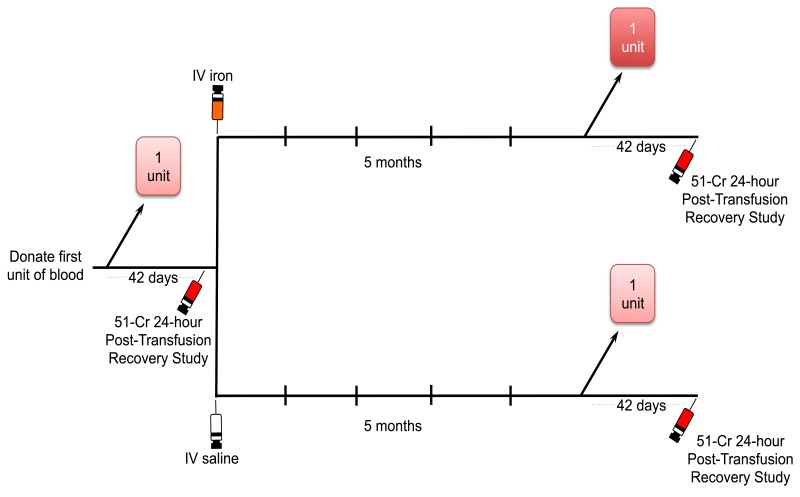
This is a randomised, controlled, double-blind clinical trial (Figure 1). Sixty healthy regular donors who meet donation standards, while exhibiting iron-deficient erythropoiesis by laboratory test criteria, will donate a single standard RBC unit that will be leucoreduced and stored in a refrigerator under standard conditions in AS-3 for up to 42 days. After 40–42 days of storage, a 51Cr-radiolabelled 24-hour RBC recovery study will be performed. Thus, a small aliquot of the donated RBC will be radiolabelled and injected into the volunteers according to a standardised protocol35. RBC recovery will be calculated from samples obtained at 5 min, 7.5 min, 10 min, 12.5 min, 15 min, 30 min, 1 hour and 24 hours after autologous infusion. In a prospective, randomised, double-blind manner, these donors will then receive either intravenous saline or low-molecular weight iron dextran (INFeD; 1 g) from 1 day to 4 weeks (target, 1 day) after the first post-transfusion recovery study. Five months later, they will donate a second RBC unit, similarly stored for 40–42 days (target same as first recovery study), and autologous 51Cr-labelled 24-hour post-transfusion RBC recoveries will again be determined. The primary endpoint will be the change in 24-hour post-transfusion recovery from the first to the second donation. The primary outcome will be the group mean difference in the primary endpoints between the group receiving intravenous saline and the group receiving intravenous iron dextran.
Figure 1.
Study schema.
Sixty frequent blood donors with iron-deficient erythropoiesis will donate one unit of red blood cells (RBC) and a 51Cr-labelled post-transfusion RBC recovery study will be performed after 40–42 days of refrigerated storage. Each volunteer will then be randomised to receive placebo (intravenous saline) or iron repletion (1 g intravenous low molecular weight iron-dextran) within 4 weeks of completing the post-transfusion RBC recovery study. Five months ± 4 weeks later they will donate another RBC unit. Then 40–42 days after the second blood donation, another 51Cr-labelled post-transfusion RBC recovery study will be performed and the result will be compared to that of the first 51Cr-labelled post-transfusion RBC recovery study.
IV: intravenous.
The secondary outcome measures are listed in Table I. These secondary outcomes include serum markers of iron status, quality of life surveys, and mental wellbeing questionnaires. These measures will be administered at each donation and post-transfusion recovery study (i.e., a total of four times throughout the study). Markers of iron status will be examined to determine the efficacy of treatment. The quality of life surveys and mental wellbeing questionnaires were chosen based on prior associations with iron deficiency or on their use in prior studies in blood donors36–44.
Table I.
Secondary outcome measures of the donor iron deficiency study.
| Serum ferritin |
| Haemoglobin |
| Zinc protoporphyrin |
| Soluble transferrin receptor |
| Hepcidin |
| Transferrin saturation |
| SF-36 Physical functioning score |
| SF-36 Role functioning/physical score |
| SF-36 Role functioning/emotional score |
| SF-36 Energy/fatigue score |
| SF-36 Emotional well-being score |
| SF-36 Social functioning score |
| SF-36 Pain score |
| SF-36 General health score |
| SF-36 Health change score |
| Beck Depression Inventory (BDI) II score |
| Beck Anxiety Inventory (BAI) score |
| Global Fatigue Index (GFI) score |
| Restless Legs Syndrome (RLS) Rating Scale |
SF-36: Short Form 36 Health Survey
Screening
The New York Blood Center study staff will send a recruitment letter/e-mail to potential subjects, 18–75 years old, who are frequent blood donors. For the purposes of this study frequent blood donors are defined as men who have donated the equivalent of at least two, and women who have donated the equivalent of at least one, RBC units in the preceding year. Volunteers responding to the recruitment request will be screened for eligibility to participation in the study by telephone or e-mail and then invited for a screening visit to confirm eligibility and provide informed consent (see Table II for inclusion/exclusion criteria). Those subjects who meet the criteria of ferritin <15 ng/mL and zinc protoporphyrin >60 μmol/mol haem, but do not qualify because of anaemia may be re-screened between 2 weeks to 3 months later.
Table II.
Inclusion/exclusion criteria.
| Inclusion criteria |
|---|
| 18–75 years old |
| Healthy (by self-report) |
| Body weight >110 lbs (~50 kg) |
| Female haematocrit >38% |
| Male haematocrit >39% |
| Frequent blood donor (male ≥2 and female ≥1 RBC unit donations in past year) |
| Ferritin <15 ng/mL |
| Zinc protoporphyrin >60 μmol/mol haem |
|
|
| Exclusion criteria |
|
|
| Ineligible for donation based on the New York Blood Center autologous donor questionnaire |
| C-reactive protein >10 mg/L |
| Sickle cell trait (by self-report) |
| Systolic blood pressure >180 or <90 mm Hg, diastolic blood pressure >100 or <50 mm Hg |
| Heart rate <50 or >100 bpm |
| Temperature >99.5 °F (37.5 °C) prior to donation (attempts will be made to reschedule donation, if possible) |
| Temperature >100.4 °F (38 °C) or subjective feeling of illness prior to 51Cr-labelled 24-hour RBC recovery study |
| Positive results on standard blood donor infectious disease testing |
| Pregnancy |
| Taking, or planning to take, iron supplements and not willing to stop for duration of study |
| History of severe asthma requiring hospitalisation, allergic eczema (atopic dermatitis) or other atopic allergy associated with anaphylaxis |
RBC: red blood cells
Post-transfusion recovery studies
A urine pregnancy test will be performed on all female participants <55 years old on the day of infusion. A positive pregnancy test will result in exclusion from the study. Two intravenous lines will be placed in contralateral arms. A 30 mL aliquot of the autologous blood unit donated 6 weeks previously will be removed into a syringe by a licensed radiopharmacist using a sterile technique. The radiolabelling will be performed using 20 mCi of sodium chromate (51Cr) based on the methods of Moroff et al.35 and the recommendations from the International Committee for Standardization in Haematology45. Although there are potential measurement errors associated with the 24-hour post-transfusion recovery study46, it is the current FDA gold-standard test. Furthermore, other assessments of RBC clearance involving transfusion of a full unit of RBC followed by the measurement of markers of haemolysis (e.g., serum iron, indirect bilirubin) were not considered because they would partially iron replete subjects randomised to the placebo group. The 51Cr-labelled RBC will be washed with saline and then infused intravenously (over 1 min through one intravenous line) into the volunteer. In order to calculate counts per minute per millilitre of RBC, a blood sample (10 mL) will be taken from the contralateral arm at time zero (T0) and 5, 7.5, 10, 12.5, 15, and 30 min, and 1 and 24 hours after the infusion.
Low molecular weight iron dextran or placebo infusion
Recent studies support the convenience, safety, and efficacy of a single infusion of 1 g of low molecular weight iron dextran as therapy for iron deficiency in adults47, along with the efficacy of 1 g of iron to counteract iron deficiency in blood donors48. To this end, one peripheral intravenous line will be inserted. A research pharmacist will provide the placebo (intravenous [IV] saline) or treatment (IV low molecular weight iron dextran; INFeD) and test dose (25 mg INFeD, 12.5 mL of a 2 mg/mL solution of iron dextran diluted in normal saline) in tinted infusion bags and tubing specifically designed to maintain blinding in clinical research studies (Medipak, Winchester, VA, USA). In addition, the pharmacy will provide methylprednisolone 125 mg IV to be given both before and after the infusion, acetaminophen (paracetamol) 650 mg PO and diphenhydramine 25 mg PO to be administered before the infusion. After the intravenous test dose (25 mg of INFeD, infused over 20 min), patients will be observed for side effects for 40 min (1 hour from the start of infusion); if no adverse effects are observed, then the entire dose diluted in 500 mL normal saline (i.e., 2 mg/mL of INFeD) will be infused over a period of 2–6 hours as tolerated (target, 2 hours). Adverse events will be identified by observation, direct inquiry, and physical examination of each volunteer. Vital signs will be measured before, during (after 15 min and then hourly), and after each infusion. Resuscitation equipment and personnel trained in detecting and treating anaphylactic-type reactions will be readily available during drug administration.
Laboratory measures
Laboratory measures of iron status and inflammation will be determined from blood samples obtained at the screening visit and then at each of the two study blood donations and two post-transfusion recovery studies (i.e., once before randomisation and once following randomisation). Zinc protoporphyrin, serum iron, total iron binding capacity, ferritin, C-reactive protein, soluble transferrin receptor, and complete blood counts, including reticulocytes, will be measured using clinically validated instruments. Hepcidin will be measured using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions (Intrinsic LifeSciences, La Jolla, CA, USA).
Surveys
Surveys assessing health status and quality of life will be administered in printed format at each of the two study blood donations and two post-transfusion recovery studies (i.e., twice before randomisation and twice following randomisation). The Short Form 36 Health Survey (SF-36), a 36-item, patient-reported survey, assesses overall health status and quality of life within the preceding 4 weeks49. The Multidimensional Assessment of Fatigue (MAF) 16-item scale is used to measure fatigue according to four dimensions: degree/severity, distress that it causes, timing of fatigue, and its impact on activities of daily living within the preceding week50. The Beck Depression Inventory-II (BDI-II) 21-item, self-report, multiple choice inventory is used to assess for depression experienced within the preceding 2 weeks51. The Beck Anxiety Inventory (BAI) 21-question, multiple-choice, self-report inventory, which is used to measure how the subject has been feeling in the preceding month, focuses primarily on somatic symptoms associated with anxiety52.
Randomisation
The study subjects will be randomised using a computerised system with equal allocation (1:1) to iron repletion or placebo. Randomisation will be stratified by gender; randomly permuted block sizes of 4, 6, or 8 will be used. The moment of randomisation will be recorded and will occur only after successful completion of the first post-transfusion RBC recovery study.
Blinding
The study will be double-blind. Thus, the randomised group will only be known to the Columbia University Irving Medical Center Research Pharmacy. A research pharmacist will provide the placebo (IV saline) or treatment (IV INFeD), and the test dose of iron/placebo, in tinted infusion bags with tubing specifically designed to maintain blinding in clinical research studies (Medipak). A research nurse unaffiliated with the study team will be responsible for the test infusion and total dose iron infusion. With this design, volunteers and study investigators will be blinded to whether volunteers receive the active intervention or placebo. Subjects in both groups will receive similar discharge instructions as if they had received low molecular weight iron-dextran. Scheduling and logistic communications with volunteers will be made by the study coordinator, who will also be blinded to the treatment group.
Situations may arise in which breaking the blinding earlier would be in the best interest of the volunteer. In any situation in which a physician or the subject asks to be un-blinded to study treatment, the research pharmacy can be reached on an emergency basis to provide this information. Finally, any un-blinding that occurs will be reported to the Data Safety Monitoring Board (DSMB) and ultimately reported in the resulting publication.
Statistical analysis plan
The primary null hypothesis will be tested in an intent-to-treat analysis using a t-test, or non-parametric equivalent, of the between-group difference in means of the within-subject change in the post-transfusion RBC recovery from the initial study under conditions of iron-deficient erythropoiesis and the subsequent study performed after randomisation to iron repletion or placebo. We will also examine pre-specified demographic variables (gender [male/female], race [white/not white], age [<50/≥50 years]) to see if we can identify one or more variables that may be effect modifiers. These factors will be considered for inclusion in an adjusted model. The specific criteria for inclusion are: (i) difference by treatment group significant at α=0.10 two-sided, and (ii) related to outcome at level α=0.10 two-sided. If any of the pre-specified covariates meet the criteria for inclusion, they will be incorporated in an adjusted model, and that model will become the primary analysis. Otherwise, the simple model will be primary.
The secondary outcome null hypotheses will be tested in an intent-to-treat analysis using mixed-effect models to compare the differences in the iron repletion and placebo group temporal course at the four defined time points on the secondary outcome measures (Table I). We will also examine pre-specified demographic variables, as above, to see if we can identify one or more that may be effect modifiers.
Furthermore, because we expect variable responses to iron repletion in the experimental group, and some crossover in the placebo group, we will also perform a tertiary analysis to explore the effect of iron status on post-transfusion RBC recovery. We will use multiple regression to assess whether RBC zinc protoporphyrin level increases the R2 of a model of post-transfusion RBC recovery predicted by treatment group membership. With 60 subjects, we will have 80% power to detect a partial correlation coefficient increase of 0.33 for the unique contribution of RBC zinc protoporphyrin level53.
Sample size estimate
Based on preliminary data from prior human 51Cr RBC recovery studies, the standard deviation of the measure in our single site is 5.0%. Furthermore, the expected mean difference in post-transfusion RBC recovery between iron-replete mice and mice with iron-deficient erythropoiesis is 10.6%. If the difference were this large in humans, we would require fewer than six subjects (α=0.05, two-sided, power=0.80). However, we expect the difference to be less dramatic in humans than in inbred mice. Thus, we will power the study to detect a clinically relevant difference in post-transfusion recovery of 4%. Under this assumption, the calculated sample size required for each arm is 26 (α=0.05, two-sided, power=0.80). Furthermore, to allow for a dropout rate of up to 15%, we plan to randomise 30 subjects per arm for a total sample size of 60 subjects.
Interim analyses
Interim analysis will be performed twice (after every 20 subjects have completed study participation) in addition to the final analysis. The DSMB will conduct the analyses using a two-sided asymmetric Lan-DeMets α-spending approach with an O’Brien-Fleming two-sided symmetric stopping boundary and overall α=0.05. The DSMB criteria for early stopping will include: (i) the Z-score at an interim analysis lying outside of the group sequential boundaries as calculated (Table III); (ii) major safety violations; and (iii) convincing evidence of futility in the context of adverse events. Interim boundaries together with terminal criteria (Z-scores and associated p-values) calculated using the WinLD version 2 programme (Microsoft, Redmond, WA, USA) are provided in Table III.
Table III.
Lan-DeMets group sequential boundaries calculations.
| Volunteers completed | Lower boundary | Upper boundary | Nominal upper alpha | Cumulative alpha |
|---|---|---|---|---|
| 20 | −3.7103 | 3.7103 | 0.00010 | 0.00021 |
| 40 | −2.5114 | 2.5114 | 0.00601 | 0.01210 |
| 60 | −1.9930 | 1.9930 | 0.02313 | 0.05000 |
Study approval and registration
The study will be conducted according to the Declaration of Helsinki and in accordance with good clinical practice guidelines. The Columbia University Irving Medical Center and New York Blood Center Institutional Review Boards approved the protocol. All research participants will provide written informed consent prior to study participation. The study was registered prior to initial enrolment in ClinicalTrials.gov with the identifier #NCT02889133.
Conclusions
This study will provide definitive evidence as to whether donor iron deficiency affects the quality of the blood supply and whether iron repletion influences the severity of symptoms of iron-deficient blood donors. The proposed research offers a new approach to improve the quality of RBC units obtained from volunteer donors by providing definitive evidence for an unrecognised source of substandard post-transfusion recovery. Current FDA guidelines, which permit up to six blood donations per year with a minimum haemoglobin of 12.5 g/dL, have the consequence that most regular volunteer donors become iron deficient54. Beyond the potential adverse effects of iron deficiency for these committed blood donors, the quality of the RBC units that they altruistically donate has not been rigorously examined. This proposed prospective, randomised, double-blind, placebo-controlled trial will decisively determine whether donor iron-deficient erythropoiesis significantly and substantially decreases 24-hour post-transfusion RBC recovery of refrigerator-stored donor RBC. Because RBC that do not circulate cannot deliver oxygen, measures to improve post-transfusion RBC recovery could produce sustained improvements in patients’ outcomes. Nonetheless, regardless of the study outcome, conducting this carefully-designed trial will yield clinically important information that will help refine guidelines for safe blood donation frequency and blood product approval.
Acknowledgements
This work was supported by the National Institutes of Health, grant number HL133049, and by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1TR001873. The content is solely the responsibility of the Authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authorship contributions
Contributions: ZCB and EAH drafted the first version of the manuscript. All Authors reviewed and edited the manuscript.
Disclosure of Conflicts of Interest
SLS is on the scientific advisory board of Hemanext, Inc. and is a consultant for Tioma, Inc. The other Authors declare that they have no competing financial interests.
References
- 1.Whitaker B. The 2009 National Blood Collection and Utilization Survey Report. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. [Google Scholar]
- 2.Goldman M, Uzicanin S, Scalia V, O’Brien SF. Iron deficiency in Canadian blood donors. Transfusion. 2013;54:775–9. doi: 10.1111/trf.12380. [DOI] [PubMed] [Google Scholar]
- 3.Smith GA, Fisher SA, Dorée C, Roberts DJ. A systematic review of factors associated with the deferral of donors failing to meet low haemoglobin thresholds. Transfus Med. 2013;23:309–20. doi: 10.1111/tme.12046. [DOI] [PubMed] [Google Scholar]
- 4.Bialkowski W, Bryant BJ, Schlumpf KS, et al. The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention. Vox Sang. 2014;108:178–85. doi: 10.1111/vox.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010;99:354–61. doi: 10.1111/j.1423-0410.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 6.Booth AO, Lim K, Capper H, et al. Iron status and dietary iron intake of female blood donors. Transfusion. 2013;54:770–4. doi: 10.1111/trf.12347. [DOI] [PubMed] [Google Scholar]
- 7.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52:702–11. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semmelrock MJ, Raggam RB, Amrein K, et al. Reticulocyte hemoglobin content allows early and reliable detection of functional iron deficiency in blood donors. Clin Chim Acta. 2012;413:678–82. doi: 10.1016/j.cca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Rigas AS, Pedersen OB, Sørensen CJ, et al. No association between iron status and self-reported health-related quality of life in 16,375 Danish blood donors: results from the Danish Blood Donor Study. Transfusion. 2015;55:1752–6. doi: 10.1111/trf.13085. [DOI] [PubMed] [Google Scholar]
- 10.Baart AM, van Noord PAH, Vergouwe Y, et al. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53:1670–7. doi: 10.1111/j.1537-2995.2012.03956.x. [DOI] [PubMed] [Google Scholar]
- 11.Macdougall LG. Red cell metabolism in iron deficiency anemia. III. The relationship between glutathione peroxidase, catalase, serum vitamin E, and susceptibility of iron-deficient red cells to oxidative hemolysis. J Perinatol. 1980;80:775–82. doi: 10.1016/s0022-3476(72)80130-6. [DOI] [PubMed] [Google Scholar]
- 12.Macdougall LG, Judisch JM, Mistry S. Red cell metabolism in iron deficiency anemia. II. The relationship between red cell survival and alterations in red cell metabolism. J Perinatol. 1970;76:660–75. doi: 10.1016/s0022-3476(70)80283-9. [DOI] [PubMed] [Google Scholar]
- 13.Ogunro PS, Ogungbamigbe TO, Muhibi MA. The influence of storage period on the antioxidants level of red blood cells and the plasma before transfusion. Afr J Med Med Sci. 2010;39:99–104. [PubMed] [Google Scholar]
- 14.Yip R, Mohandas N, Clark MR, et al. Red cell membrane stiffness in iron deficiency. Blood. 1983;62:99–106. [PubMed] [Google Scholar]
- 15.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. PNAS. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanias T, Stone M, Page GP, et al. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion. 2019;59:67–78. doi: 10.1111/trf.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumaswala UJ. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33:517–29. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 18.Dumaswala UJ, Zhuo L, Mahajan S, et al. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280:867–73. doi: 10.1152/ajpcell.2001.280.4.C867. [DOI] [PubMed] [Google Scholar]
- 19.Dumont LJ, Yoshida T, Aubuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009;49:458–64. doi: 10.1111/j.1537-2995.2008.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jozwik M, Jozwik M, Jozwik M, et al. Antioxidant defence of red blood cells and plasma in stored human blood. Clin Chim Acta. 1997;267:129–42. doi: 10.1016/s0009-8981(97)00148-4. [DOI] [PubMed] [Google Scholar]
- 21.Rinalducci S, Marrocco C, Zolla L. Thiol-based regulation of glyceraldehyde-3-phosphate dehydrogenase in blood bank-stored red blood cells: a strategy to counteract oxidative stress. Transfusion. 2015;55:499–506. doi: 10.1111/trf.12855. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida T, Prudent M, D’Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5:1–6. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodvien R, Gillum A, Weintraub LR. Decreased glutathione peroxidase activity secondary to severe iron deficiency: a possible mechanism responsible for the shortened life span of the iron-deficient red cell. Blood. 1974;43:281–9. [PubMed] [Google Scholar]
- 25.Knight JA, Searles DA, Clayton FC. The effect of desferrioxamine on stored erythrocytes: lipid peroxidation, deformability, and morphology. Ann Clin Lab Sci. 1996;26:283–90. [PubMed] [Google Scholar]
- 26.Safeukui I, Buffet PA, Deplaine G, et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood. 2012;120:424–30. doi: 10.1182/blood-2012-01-404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diez-Ewald M, Layrisse M. Mechanisms of hemolysis in iron deficiency anemia. Further studies. Blood. 1968;32:884–94. [PubMed] [Google Scholar]
- 28.Farid Z, Nichols JH, Schulert AR, Bassily S. Chromium-51 red cell half-life in severe iron deficiency anemia. Am J Trop Med Hyg. 1965;14:605–9. doi: 10.4269/ajtmh.1965.14.375. [DOI] [PubMed] [Google Scholar]
- 29.Layrisse M, Linares J, Roche M. Excess hemolysis in subjects with severe iron deficiency anemia associated and nonassociated with hookworm infection. Blood. 1965;25:73–91. [PubMed] [Google Scholar]
- 30.Kempe DS, Lang PA, Duranton C, et al. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006;20:368–70. doi: 10.1096/fj.05-4872fje. [DOI] [PubMed] [Google Scholar]
- 31.Loría A, Sánchez-Medal L, Lisker R, et al. Red cell life span in iron deficiency anaemia. Br J Haematol. 1967;13:294–302. doi: 10.1111/j.1365-2141.1967.tb08743.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown GM, Hayward OC, Powell EO, Witts LJ. The destruction of transfused erythrocytes in anaemia. J Pathol Bacteriol. 1944;56:81–94. [Google Scholar]
- 33.Bandyopadhyay S, Brittenham GM, Francis RO, et al. Iron-deficient erythropoiesis in blood donors and red blood cell recovery after transfusion: initial studies with a mouse model. Blood Transfus. 2017;15:158–64. doi: 10.2450/2017.0349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–53. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moroff G, Sohmer PR, Button LN. Proposed standardization of methods for determining the 24-hour survival of stored red cells. Transfusion. 1984;24:109–14. doi: 10.1046/j.1537-2995.1984.24284173339.x. [DOI] [PubMed] [Google Scholar]
- 36.Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118:3222–7. doi: 10.1182/blood-2011-04-346304. [DOI] [PubMed] [Google Scholar]
- 37.Rigas AS, Pedersen OB, Sorensen CJ, et al. No association between iron status and self-reported health-related quality of life in 16,375 Danish blood donors: results from the Danish Blood Donor Study. Transfusion. 2015;55:1752–6. doi: 10.1111/trf.13085. [DOI] [PubMed] [Google Scholar]
- 38.Wouters H, van der Klauw MM, de Witte T, et al. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica. 2019;104:468–76. doi: 10.3324/haematol.2018.195552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page EA, Harrison JF, Jaldow EJ, Kopelman M. Impairment of short-term memory associated with low iron stores in a volunteer multidose plateletpheresis donor. Transfus Med. 2008;18:312–4. doi: 10.1111/j.1365-3148.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- 40.Macher S, Drexler C, Lindenau I, et al. High-dose intravenously administered iron versus orally administered iron in blood donors with iron deficiency: study protocol for a randomised, controlled trial. Trials. 2016;17:527. doi: 10.1186/s13063-016-1648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peuranpaa P, Heliovaara-Peippo S, Fraser I, et al. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet Gynecol Scand. 2014;93:654–60. doi: 10.1111/aogs.12394. [DOI] [PubMed] [Google Scholar]
- 42.Waldvogel S, Pedrazzini B, Vaucher P, et al. Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: a randomized controlled trial. BMC Med. 2012;10:8. doi: 10.1186/1741-7015-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dziembowska I, Kwapisz J, Izdebski P, Zekanowska E. Mild iron deficiency may affect female endurance and behavior. Physiol Behav. 2019;205:44–50. doi: 10.1016/j.physbeh.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Vaucher P, Druais PL, Waldvogel S, Favrat B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184:1247–54. doi: 10.1503/cmaj.110950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berlin NI, Dudley RA, Garby L, et al. Recommended method for radioisotope red-cell survival studies. International Committee for Standardization in Haematology. Br J Haematol. 1980;45:659–66. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 46.Francis RO, Mahajan S, Rapido F, et al. Reexamination of the chromium-51-labeled posttransfusion red blood cell recovery method. Transfusion. 2019;59:2264–75. doi: 10.1111/trf.15310. [DOI] [PubMed] [Google Scholar]
- 47.Wong L, Smith S, Gilstrop M, et al. Safety and efficacy of rapid (1,000 mg in 1 hr) intravenous iron dextran for treatment of maternal iron deficient anemia of pregnancy. Am J Hematol. 2016;91:590–3. doi: 10.1002/ajh.24361. [DOI] [PubMed] [Google Scholar]
- 48.Drexler C, Macher S, Lindenau I, et al. High-dose intravenous versus oral iron in blood donors with iron deficiency: the IronWoMan randomized, controlled clinical trial. Clin Nutr. 2019 doi: 10.1016/j.clnu.2019.03.025. pii: S0261-5614(19)30139-6. [DOI] [PubMed] [Google Scholar]
- 49.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36® Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 50.Piper B, Lindsey A, Dodd M, et al. The development of an instrument to measure the subjective dimension of fatigue. In: Funk S, Tornquist E, Champagne M, Wiese R, editors. Key Aspects of Comfort: Management of Pain, Fatigue, and Nausea. New York: Springer; 1989. pp. 199–207. [Google Scholar]
- 51.Beck AT, Steer RA, Brown GK. Manual for The Beck Depression Inventory Second Edition (BDI-II) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 52.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Harcourt Brace and Company; 1996. [Google Scholar]
- 53.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. [Google Scholar]
- 54.Brittenham GM. Iron deficiency in whole blood donors. Transfusion. 2011;51:458–61. doi: 10.1111/j.1537-2995.2011.03062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]