Short abstract
Objective
This meta-analysis was performed to evaluate the effect of spinal anesthesia (SA) using bupivacaine combined with dexmedetomidine (DEX) in cesarean section, analyze the adverse drug reactions induced by this mixture, and provide a reference for rational drug use.
Methods
Randomized controlled trials were obtained from the PubMed, Cochrane Library, and Embase databases. The primary outcome measure was the time to the highest sensory block level (min), and the secondary outcome measure was adverse effects.
Results
The time to the highest sensory block level was significantly shorter in the bupivacaine-DEX group than in the control group (standardized mean difference, −0.23; 95% confidence interval, −0.43 to −0.03). The incidence of shivering during the process of anesthesia, especially at a dose of 5 µg DEX, was significantly lower in the bupivacaine-DEX group than in the control group (odds ratio, 0.26; 95% confidence interval, 0.14–0.49). No significant differences were observed in the symptoms of hypotension, bradycardia, nausea/vomiting, or pruritus.
Conclusion
Compared with the use of bupivacaine alone for SA in cesarean section, adding dexmedetomidine during SA can significantly shorten the onset time and decrease the rate of shivering during anesthesia.
Keywords: Dexmedetomidine, bupivacaine, spinal anesthesia, cesarean section, shivering, meta-analysis
Introduction
In recent decades, marked changes have taken place in parturition.1 The overall rate of cesarean section (C-section) is significantly increasing worldwide.2–4 Anesthesia during C-section can eliminate pain and shows few adverse effects in the mother and infant. The ideal anesthetic technique is characterized by a short time to establishment of successful anesthesia, minimal hemodynamic changes, and few adverse effects.5 Choosing the appropriate anesthetic and proper anesthesia method can effectively reduce injury to the mother and newborn. Spinal anesthesia (SA)6 is the preferred method during most surgical operations, especially C-section. Because of the dense and predictable block associated with SA, this technique exhibits a quicker onset and fewer complications compared with other anesthetic protocols. However, the adverse effects of neuraxial analgesia, such as maternal hypotension, shivering, vomiting or nausea, and a faint feeling, cannot be underestimated.7 Adjuvant drugs are required to enhance the safety of anesthesia. The optimal anesthetic and adjuvant drugs should not only maintain the appropriate anesthetic effect during the puerperal period but also have limited transportation from the mother’s body to the fetus.
Dexmedetomidine (DEX) is a highly selective agonist of α2-adrenergic receptors.8 Increasingly more anesthetists are choosing DEX as a safe and effective local adjuvant drug in SA.9–12 Studies have shown that DEX can potentially prolong the blockade duration and enhance the anesthetic action.9,13–15 However, it is necessary to analyze the effectiveness of DEX in C-section because of the special characteristics of this surgery. Although several meta-analyses of randomized controlled trials (RCTs) have indicated that DEX is a potential anesthetic adjuvant that can facilitate better anesthetic effects in local anesthesia,12,16 little research has been focused on the use of DEX in C-section. Whether the addition of DEX is beneficial remains unknown because of the lack of hard evidence. Based on previously published studies, the present meta-analysis was performed to determine the effect of bupivacaine combined with DEX for SA in C-section, analyze the adverse drug reactions induced by this combination of drugs, and provide a reference for rational drug use.
Materials and methods
Search strategy
Relevant studies published in the PubMed (1996–2017), Cochrane Library (1989–2017), and Embase (1980–2017) databases were searched in October 2017 for RCTs involving bupivacaine alone and bupivacaine-DEX in C-section using SA. The following search terms were used: bupivacaine, dexmedetomidine, DEX, and cesarean section. No language restrictions were imposed. Manual searches were also conducted by reviewing the references of all publications. The included studies were published in peer-reviewed journals as full articles; none were published in gray literature or conference proceedings.
Inclusion and exclusion criteria
The retrieved articles were selected according to the following preset inclusion and exclusion criteria.
Inclusion criteria
Population: patients who had undergone C-section under SA
Intervention: hyperbaric bupivacaine
Comparison: bupivacaine alone or bupivacaine-DEX
Outcome: the effect and safety of bupivacaine alone or bupivacaine-DEX used in SA
Study design: RCT
Language: published in English or Chinese
Exclusion criteria
Not written in English or Chinese
Duplicated data
Abstract, comment, review/editorial review, guideline, meeting, or case report
Published without sufficient data or the relevant raw data could not be abstracted
The title, abstract, and full text of each RCT were scanned. The retrieved studies were independently selected by two investigators. Disagreements were resolved by a discussion with a third independent investigator.
Quality assessment and data extraction
Two reviewers independently evaluated the risk of bias of the included RCTs according to the RCT bias risk assessment tools of the Cochrane Handbook Version 5.1.
Characteristics including study information, study design, sample information, surgical information, and sample size calculation were extracted from each study. The anesthesia outcomes were also obtained directly from the recruited articles. The time to achievement of the highest sensory block level (min) and the duration of the sensory block, which might be the main difference between groups, was abstracted as the primary outcome measure. The secondary outcome was the incidence of adverse effects, including hypotension, bradycardia, nausea or vomiting, shivering, and pruritus. Two researchers independently performed the data extraction and settled any disagreements by discussion with a third researcher.
Meta-analysis
The primary and secondary outcomes were analyzed in this study. As a continuous variable, the time to the highest sensory block level was analyzed by combining the standardized mean difference (SMD) and 95% confidence interval (CI). An SMD of >1 and p value of <0.05 suggested that more time was required for anesthesia in the case group than in the control group. For dichotomous outcomes (adverse reactions to anesthesia), the odds ratio (OR) and 95% CI were used as effect indicators. A primary outcome with an OR of >1 and p value of <0.05 indicated that the anesthesia effect was better than that in the control group.
Cochran’s Q test and the I2 test17 were used to determine the heterogeneity among the included articles. A p value of <0.05 in Cochran’s Q test or an I2 value17 of >50% indicated significant heterogeneity, and the data were analyzed using a random-effects model. Otherwise, a fixed-effects model was chosen. All statistical analyses were performed using STATA 12.0 software (StataCorp, College Station, TX, USA).
Sensitivity analysis
To evaluate the sensitivity, each study was removed one by one during the meta-analysis. The combined effect values were then compared. Reversal of a combined result after removing a study indicated an unstable analysis result. The sensitivity analysis was also performed using STATA 12.0 software (StataCorp).
Ethics
Ethics approval was unnecessary for this meta-analysis.
Results
Literature research
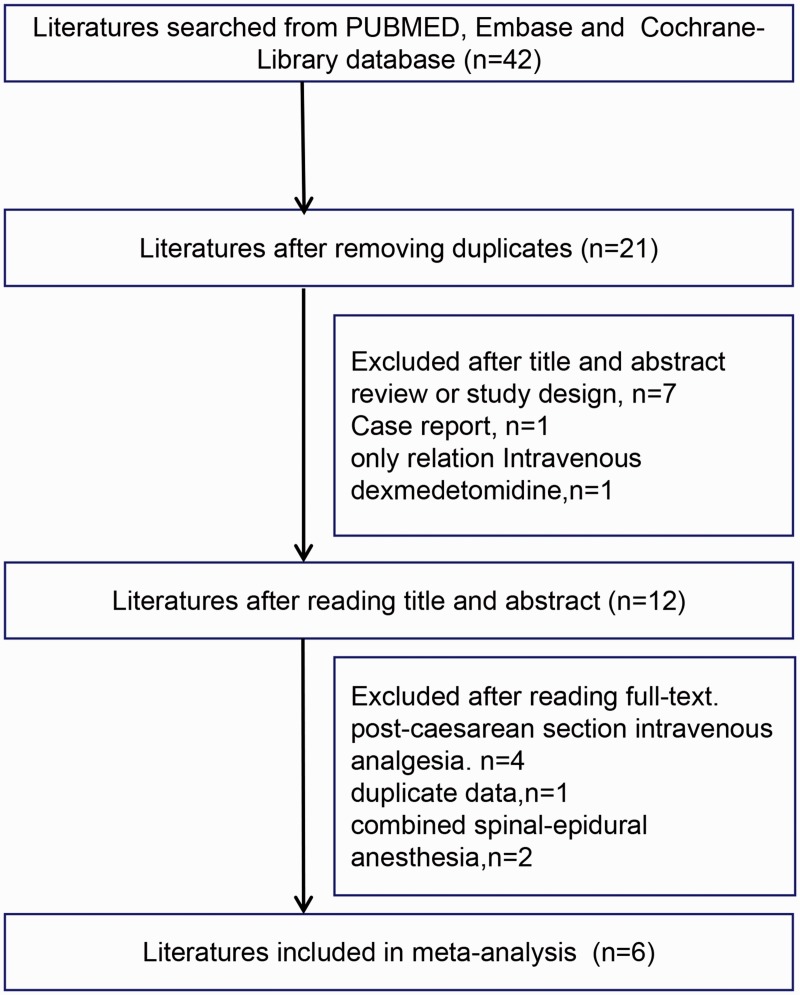
As shown in Figure 1, articles were identified using index words from the PubMed, Embase, and Cochrane Library databases. Twenty-one duplicate articles were then eliminated. According to the inclusion and exclusion criteria, seven reviews, one case report, and one study involving only intravenous DEX were eliminated after reading the titles and abstracts. For studies of post C-section intravenous analgesia, one article with duplicate data, and two studies using combined spinal-epidural anesthesia were excluded after reading the full text. Finally, six studies14,18–22 were included in this meta-analysis.
Figure 1.
Study selection process for the meta-analysis
Characteristics of the included studies
All six studies were well-designed RCTs, and the type of anesthesia was SA. The general data of each study, such as the sample inclusion/exclusion criteria, anesthetic medications, and number of patients in each group, are shown in Table 1. In total, 494 C-sections and 3 DEX doses were included.
Table 1.
Characteristics of the included studies
|
Study information |
Study design |
Sample information |
Surgical information |
Sample size calculation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (first author, publication year) | Country | Randomi-zation | Blinding | Sample source | Eligibility criteria | ASA status | Sample size | Sample age, years | Patient position during anesthesia | Needle, anesthesia method | Total volume | Intervention | Comparison | Power | α |
| Nasseri (2017) | Iran | Yes | Double | Healthy parturients undergoing CS | Yes | I or II | 50 | 18–45 | Sitting | 25G spinal Quincke-tip needle, needle-through-needle technique | 3 mL | 3 mL of 12.5 mg 0.5% bupivacaine and normal saline | 3 mL of 12.5 mg 0.5% bupivacaine plus 5 µg of dexmedetomidine | 90% | 0.05 |
| He (2017) | China | Yes | Double | Healthy parturients undergoing CS | Yes | I or II | 90 | 18–40 | Left lateral | 25G Quincke spinal needle, needle-through-needle technique | 3 mL | 2.5 mL of 0.5% hyperbaric bupivacaine and 0.5 mL of normal saline | 2.5 mL of 0.5% hyperbaric bupivacaine and 2.5 and 5 µg of dexmedetomidine | - | - |
| Qi (2016) | China | Yes | Double | Healthy parturients undergoing CS | Yes | I or II | 120 | >18 | Lateral decubitus | 25G pencil point spinal needle, needle-through-needle technique | 2 mL | 2 mL of 0.5% bupivacaine | 2 mL of 0.5% bupivacaine containing 5 µg of dexmedetomidine | 90% | 0.05 |
| Sun (2015) | China | Yes | double | Healthy parturients undergoing CS | Yes | I or II | 90 | 20–35 | Left lateral or sitting | 25G Whitacre needle, needle-through-needle technique | 2 mL | 10 mg of hyperbaric bupivacaine 0.5% plus 1.0 mL of normal saline | 10 mg of hyperbaric bupivacaine 0.5% plus 10 µg of dexmedetomidine in 1.0 mL of normal saline | - | - |
| Bi (2017) | China | Yes | Double | Healthy parturients undergoing CS | Yes | I or II | 60 | 18–40 | Lateral | 25G pencil point needle, needle-through-needle technique | 2 mL | 10 mg of bupivacaine alone | 10 mg of bupivacaine with 3 or 5 µg of dexmedetomidine | - | - |
| Li (2015) | China | Yes | Double | Healthy parturients undergoing CS | Yes | I or II | 84 | 20–35 | Right-wedged supine | 27G or 25G Quincke needle, needle-through-needle technique | 4 mL | 10 mg of bupivacaine alone | 10 mg of bupivacaine plus 10 µg of dexmedetomidine | ||
ASA, American Society of Anesthesiologists; CS, cesarean section
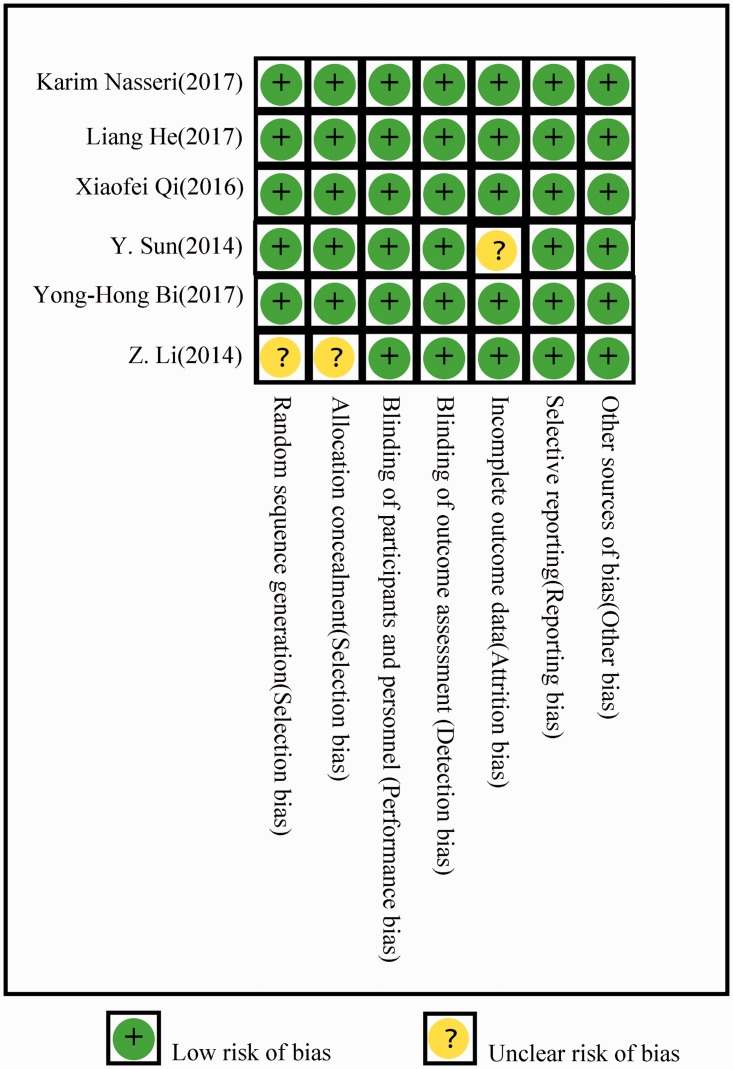
Risk of bias assessment
The quality of the included studies according to the Cochrane Handbook for Systematic Review of Interventions is shown in Figure 2. All six studies showed low bias except for one RCT, in which the randomization was unclear.
Figure 2.
Risk of bias of the included randomized controlled trials according to the randomized controlled trial bias risk assessment tools of the Cochrane Handbook Version 5.1
Analysis
Primary outcomes
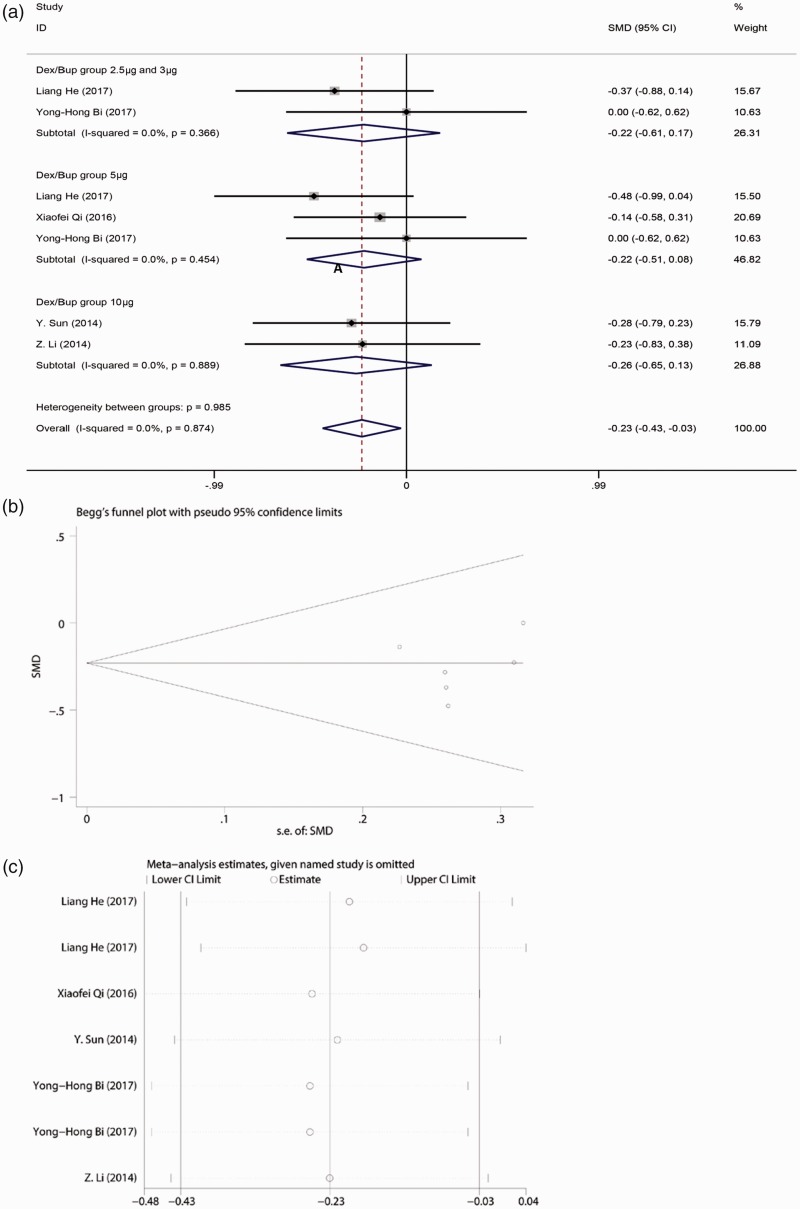
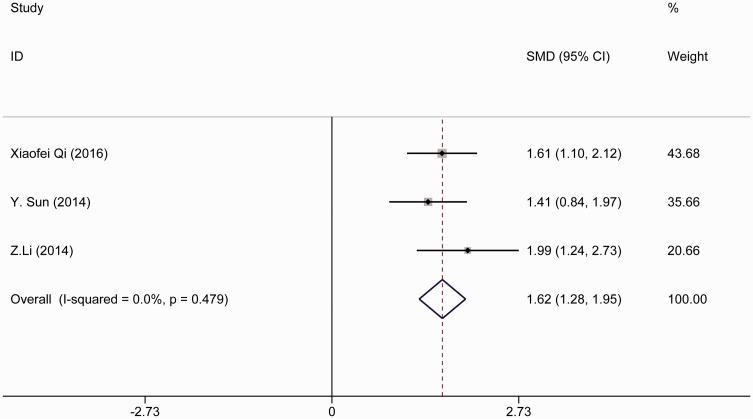
We combined the time to achievement of the highest sensory block level (min). According to the heterogeneity result, a random-effects model was used in our meta-analysis because the heterogeneity was not statistically significant (I2 < 50%). A noticeable difference was present between the case and control groups as shown in Figure 3(a), and a random-effects model was chosen (I2 = 0.0%). The time to achieve the highest sensory block level was significantly shorter in the bupivacaine-DEX group than in the control group (SMD, −0.23; 95% CI, −0.43 to −0.03; p = 0.026). However, no significant differences were found when bupivacaine was combined with different doses of DEX in SA compared with bupivacaine alone: lowest dose of DEX (2.5 or 3 µg): I2 = 0.0%; SMD, −0.220; 95% CI, −0.61 to 0.17; moderate dose of DEX (5 µg): I2 = 0.0%; SMD, −0.22; 95% CI, −0.51 to 0.08; and high dose of DEX (10 µg): I2 = 0.0%; SMD, −0.26; 95% CI, −0.65 to 0.13. No publication bias was found according to the results of Egger’s and Begg’s tests (Figure 3(b)). The significance of the pooled SMD was strongly influenced by omitting any single study in the sensitivity analysis. After eliminating the studies by He et al.19 and Sun et al.,18 the result showed that bupivacaine-DEX could not shorten the time to achievement of the highest sensory block level (Figure 3(c)). Moreover, the duration of the sensory block was analyzed. No significant difference was found between the bupivacaine-DEX group and control group. DEX could not prolong the duration of the sensory block (I2 = 0.0%; SMD, 1.62; 95% CI, 1.28–1.95) (Figure 4).
Figure 3.
(a) Forest plot of time to highest sensory block level analyzed by combining the standardized mean difference (SMD). (b) Publication bias analysis of the combined SMD of time by Begg’s test. (c) Sensitivity analysis of the combined SMD of time
Figure 4.
Forest plot of duration of sensory block level analyzed by combining the standardized mean difference (SMD)
Secondary outcomes
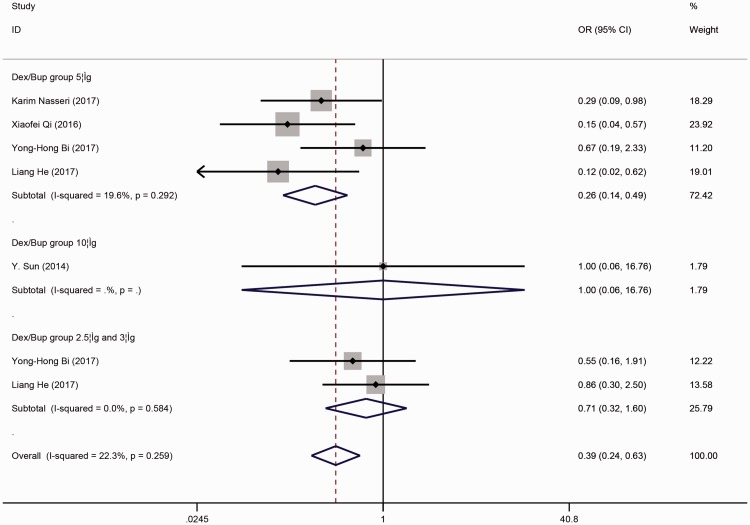
As the secondary outcome, we analyzed the adverse effects of anesthesia between the case and control groups. The pooled results shown in Figure 5 indicate that the incidence of shivering during the process of anesthesia was significantly lower in the case than control group (OR, 0.389; 95% CI, 0.240–0.632; p < 0.001). Figure 5 shows grouping by dose, and bupivacaine combined with 5 µg of DEX in SA was associated with a lower incidence of shivering (OR, 0.259; 95% CI, 0.136–0.491; p < 0.001). No significance differences were found in the other subgroups.
Figure 5.
Forest plot of the combined odds ratio (OR) of the incidence of shivering during the process of anesthesia
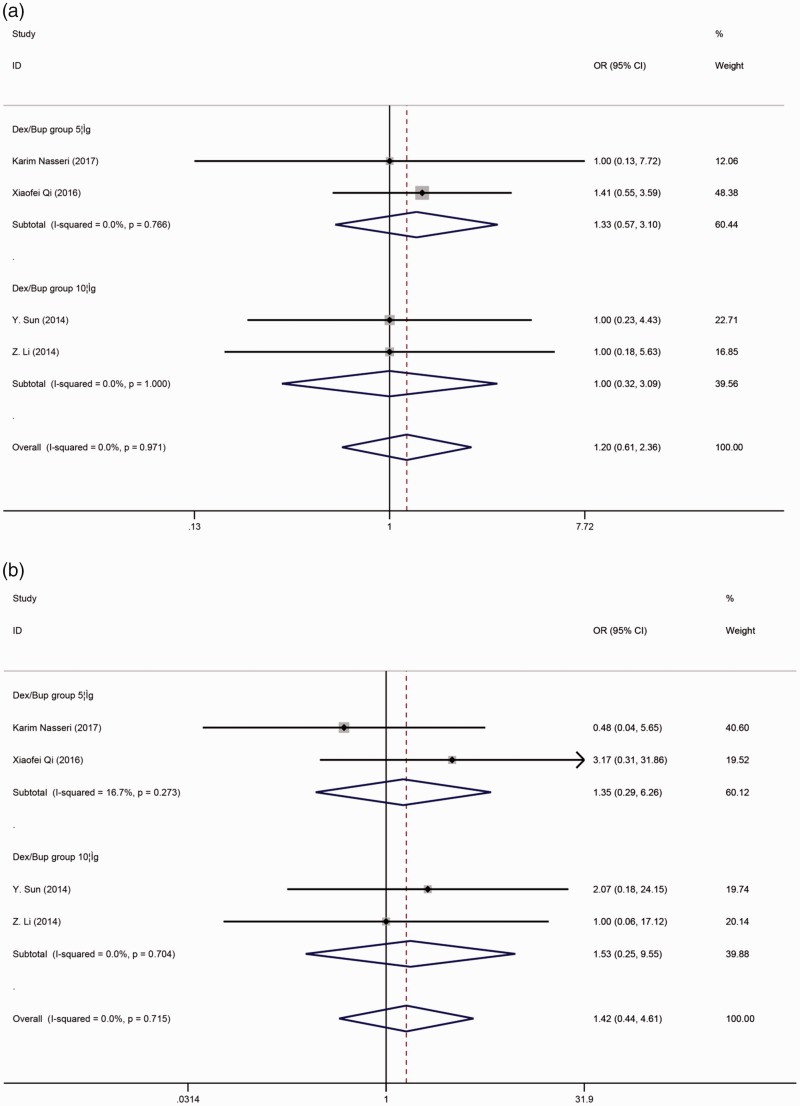
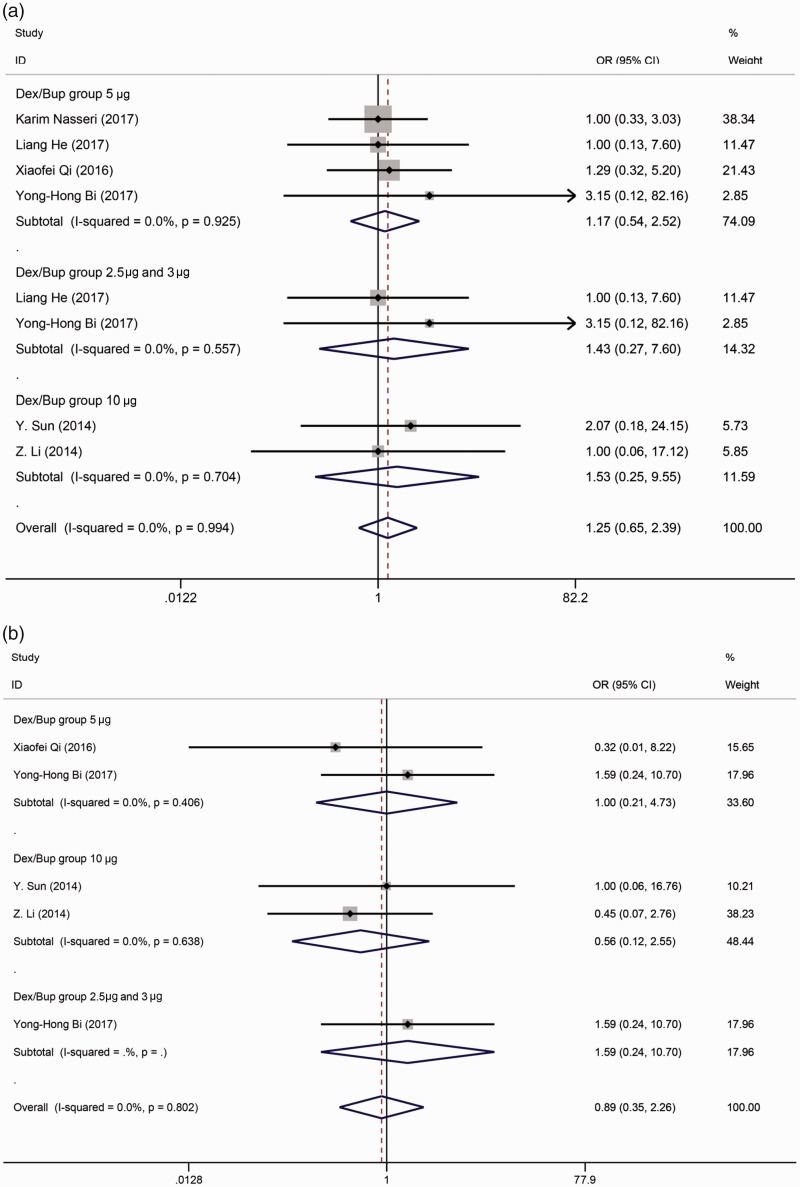
With respect to other adverse effects, no differences were observed in the symptoms of hypotension (OR, 1.197; 95% CI, 0.607–2.358) (Figure 6(a)), bradycardia (OR, 1.423; 95% CI, 0.440–4.607) (Figure 6(b)), nausea/vomiting (OR, 1.246; 95% CI, 0.649–2.391) (Figure 7(a)), or pruritus (OR, 0.894; 95% CI, 0.354–2.260) (Figure 7(b)).
Figure 6.
(a) Forest plot of the combined odds ratio (OR) of hypotension during the process of anesthesia. (b) Forest plot of the combined OR of bradycardia during the process of anesthesia
Figure 7.
(a) Forest plot of the combined odds ratio (OR) of nausea or vomiting. (b) Forest plot of the combined OR of pruritus
No publication bias was found according to the results of Egger’s and Begg’s tests for the pooled incidence rate of adverse effects. The significance of the pooled ORs was not strongly influenced by omitting any single study in the sensitivity analysis. The low sensitivity of the included data in this study indicated good stability of our meta-analysis.
Discussion
This meta-analysis of six RCTs was performed to evaluate the efficacy and safety of bupivacaine combined with DEX in SA among patients undergoing C-section. The main result showed that bupivacaine combined with DEX in SA could improve the characteristics of the anesthetic block by shortening the time to achievement of the highest sensory block level. In addition, we found that the incidence of shivering was lower with bupivacaine-DEX than bupivacaine alone. Bupivacaine and 5 µg of DEX appeared to have the lowest shivering incidence rate among all groups.
DEX, an α2-adrenergic receptor agonist, has been approved by the Food and Drug Administration for short-term sedation and analgesia in the intensive care unit.23,24 Different from other sedative-hypnotic drugs, sedation caused by DEX may lead to natural non-rapid eye movement sleep,25 and patients can be roused and cooperate well during the operation. The use of DEX can also notably reduce the dose of combined anesthetics.26 Studies have shown that DEX plays a role in the regulation of blood pressure,27–29 breathing,30,31 and production of hormones,32 resulting in its extensive application in the clinical setting, especially in anesthesia. Kanazi et al.13 compared the onset and duration of sensory and motor block caused by bupivacaine alone or bupivacaine combined with DEX. The results showed that DEX could significantly enhance the anesthetic effect of local anesthesia. A randomized double-blind study by Fares et al.33 showed that DEX together with bupivacaine can increase the duration of anesthesia and improve hemodynamic stability. Kathuria et al.34 concluded that DEX as an adjuvant to ropivacaine could shorten the sensory and motor block onset time and prolong the duration of anesthesia. Al-Mustafa et al.35 indicated that the onset time of bupivacaine together with DEX was shorter than that in the control group, the duration of anesthesia was longer, and the dose was lower.
Although DEX works well regardless of whether it is used in general anesthesia or local anesthesia, it is seldom used in C-section because little evidence of safety is available. Some studies have indicated that DEX shows no harm to either the mother or fetus. During general anesthesia in patients undergoing C-section, no difference was found in the maternal arterial blood gas parameters, fetal umbilical blood gas parameters, or neonate Apgar score between the DEX and no-DEX groups.36 In another study, the addition of DEX during local anesthesia in patients undergoing C-section reduced the dose of opioid analgesics with no influence on the neonate Apgar score.37,38 Furthermore, some studies have revealed a faster onset time of DEX used in anesthesia. Kim et al.39 considered that adding DEX to bupivacaine resulted in faster onset when compared with bupivacaine alone among patients of advanced age undergoing transurethral prostatectomy. The same results were found in other studies involving anesthesia for lower limb procedures and transurethral resection of prostate or bladder tumors under SA.13,40 Our study showed identical results in patients undergoing C-section; namely, that bupivacaine combined with DEX can reduce the onset time to achievement of the highest sensory block level.
As a common complication of SA, shivering is observed at a higher rate among patients undergoing C-section and causes discomfort and dissatisfaction in the parturient. The shivering in patients undergoing C-section might be induced by the high metabolic rate in the late third trimester of pregnancy, accelerated blood circulation, and loss of heat accompanied by childbirth. The vascular compensatory contraction in the unblocked area induced by low blood pressure after anesthesia may increase the incidence of shivering. Consistent with our study, Mason and Lerman41 observed anti-shivering effects in the DEX group of their study. The decrease in the shivering rate might be related to the function of DEX,42,43 which can inhibit the heat-regulating center through activation of α2-adrenergic receptors, decrease the temperature threshold of shivering,44 and suppress the afferent temperature information at the spinal level. Moreover, we found that 5 µg of DEX might show more advanced effects than other doses in the present study, although fewer studies were included in the subgroup analysis. No significant difference was found between the 3- and 10-µg doses, which is consistent with the findings by Zhang et al.45 showing that 10 µg of DEX in SA failed to show superiority over 5 µg of DEX in the prevention of perioperative shivering.
The results of our study and the evidence provided by other studies indicate that DEX combined with bupivacaine can be a good choice in SA during C-section. However, the present meta-analysis still has several limitations. First, the literature research was performed using three main databases (PubMed, Embase, and the Cochrane Library), and the language of the articles was restricted to English or Chinese only. Therefore, we cannot assume that we captured all relevant information. Second, because of the small number of analyses and various doses of DEX in the included studies, dose-related conclusions are difficult to make. Additionally, long-term effects, adverse effects, neonatal outcomes, and Apgar scores at least up to 15 minutes after using DEX in SA during C-section were not analyzed because of the lack of relevant research. Finally, because of the limited information acquired from the included articles, we could only analyze the impact on the mother’s body; no effects on the fetus were evaluated. Thus, more studies are needed, and a particular subgroup analysis should be done in the future.
In conclusion, compared with the use of bupivacaine alone for SA in C-section, adding DEX during SA can significantly shorten the onset time and decrease the rate of shivering during anesthesia. DEX is an effective adjuvant drug in C-section.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Banihashem N, Hasannasab B, Esmaeili A. Addition of intrathecal magnesium sulfate to bupivacaine for spinal anesthesia in cesarean section. Anesth Pain Med 2015; 5: e22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavender T, Hofmeyr GJ, Neilson JP, et al. Caesarean section for non-medical reasons at term. Cochrane Database Syst Rev 2012(3):CD004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Hellerstein S, Hou L, et al. Caesarean deliveries in China. BMC Pregnancy Childbirth 2017; 17: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellerstein S, Feldman S, Duan T. China's 50% caesarean delivery rate: is it too high? BJOG 2015; 122: 160–164. [DOI] [PubMed] [Google Scholar]
- 5.Shin YD, Park SH, Kim HT, et al. The effect of anaesthesia technique on caesarean section. Pak J Med Sci 2016; 32: 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aksoy M, Aksoy AN, Dostbil A, et al. Anaesthesia techniques for caesarean operations: retrospective analysis of last decade. Turk J Anaesthesiol Reanim 2014; 42: 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gizzo S, Noventa M, Fagherazzi S, et al. Update on best available options in obstetrics anaesthesia: perinatal outcomes, side effects and maternal satisfaction. Fifteen years systematic literature review. Arch Gynecol Obstet 2014; 290: 21–34. [DOI] [PubMed] [Google Scholar]
- 8.Cormack JR, Orme RM, Costello TG. The role of alpha2-agonists in neurosurgery. J Clin Neurosci 2005; 12: 375–378. [DOI] [PubMed] [Google Scholar]
- 9.Marhofer D, Kettner SC, Marhofer P, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth 2013; 110: 438–442. [DOI] [PubMed] [Google Scholar]
- 10.Keplinger M, Marhofer P, Kettner SC, et al. A pharmacodynamic evaluation of dexmedetomidine as an additive drug to ropivacaine for peripheral nerve blockade: a randomised, triple-blind, controlled study in volunteers. Eur J Anaesthesiol 2015; 32: 790–796. [DOI] [PubMed] [Google Scholar]
- 11.Ugur F, Gulcu N, Boyaci A. Intrathecal infusion therapy with dexmedetomidine-supplemented morphine in cancer pain. Acta Anaesthesiol Scand 2007; 51: 388. [DOI] [PubMed] [Google Scholar]
- 12.Ping Y, Ye Q, Wang W, et al. Dexmedetomidine as an adjuvant to local anesthetics in brachial plexus blocks: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017; 96: e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand 2006; 50: 222–227. [DOI] [PubMed] [Google Scholar]
- 14.Bi YH, Cui XG, Zhang RQ, et al. Low dose of dexmedetomidine as an adjuvant to bupivacaine in cesarean surgery provides better intraoperative somato-visceral sensory block characteristics and postoperative analgesia. Oncotarget 2017; 8: 63587–63595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukry M, Miller JA. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag 2010; 6: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu HH, Wang HT, Jin JJ, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One 2014; 9: e93114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Xu Y, Wang GN. Comparative evaluation of intrathecal bupivacaine alone, bupivacaine-fentanyl, and bupivacaine-dexmedetomidine in caesarean section. Drug Res (Stuttg) 2015; 65: 468–472. [DOI] [PubMed] [Google Scholar]
- 19.He L, Xu JM, Liu SM, et al. Intrathecal dexmedetomidine alleviates shivering during cesarean delivery under spinal anesthesia. Biol Pharm Bull 2017; 40: 169–173. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Tian M, Zhang CY, et al. A randomised controlled trial to evaluate the effectiveness of intrathecal bupivacaine combined with different adjuvants (fentanyl, clonidine and dexmedetomidine) in caesarean section. Drug Res (Stuttg) 2015; 65: 581–586. [DOI] [PubMed] [Google Scholar]
- 21.Qi X, Chen D, Li G, et al. Comparison of intrathecal dexmedetomidine with morphine as adjuvants in cesarean sections. Biol Pharm Bull 2016; 39: 1455–1460. [DOI] [PubMed] [Google Scholar]
- 22.Nasseri K, Ghadami N, Nouri B. Effects of intrathecal dexmedetomidine on shivering after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Drug Des Devel Ther 2017; 11 : 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turunen H, Jakob SM, Ruokonen E, et al. Dexmedetomidine versus standard care sedation with propofol or midazolam in intensive care: an economic evaluation. Crit Care 2015; 19: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res 2011; 5: 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson LE, Lu J, Guo T, et al. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003; 98: 428–436. [DOI] [PubMed] [Google Scholar]
- 26.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol 2011; 28: 3–6. [DOI] [PubMed] [Google Scholar]
- 27.Sezen G, Demiraran Y, Seker IS, et al. Does premedication with dexmedetomidine provide perioperative hemodynamic stability in hypertensive patients? BMC Anesthesiol 2014; 14: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones GM, Murphy CV, Gerlach AT, et al. High-dose dexmedetomidine for sedation in the intensive care unit: an evaluation of clinical efficacy and safety. Ann Pharmacother 2011; 45: 740–747. [DOI] [PubMed] [Google Scholar]
- 29.Gerlach AT, Dasta JF, Steinberg S, et al. A new dosing protocol reduces dexmedetomidine-associated hypotension in critically ill surgical patients. J Crit Care 2009; 24: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci 2014; 10: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebert TJ, Hall JE, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000; 93: 382–394. [DOI] [PubMed] [Google Scholar]
- 32.Venn RM, Bryant A, Hall GM, et al. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth 2001; 86: 650–656. [DOI] [PubMed] [Google Scholar]
- 33.Fares KM, Othman AH, Alieldin NH. Efficacy and safety of dexmedetomidine added to caudal bupivacaine in pediatric major abdominal cancer surgery. Pain Physician 2014; 17: 393–400. [PubMed] [Google Scholar]
- 34.Kathuria S, Gupta S, Dhawan I. Dexmedetomidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block. Saudi J Anaesth 2015; 9: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Mustafa MM, Badran IZ, Abu-Ali HM, et al. Intravenous dexmedetomidine prolongs bupivacaine spinal analgesia. Middle East J Anaesthesiol 2009; 20: 225–231. [PubMed] [Google Scholar]
- 36.Yu M, Han C, Jiang X, et al. Effect and placental transfer of dexmedetomidine during caesarean section under general anaesthesia. Basic Clin Pharmacol Toxicol 2015; 117: 204–208. [DOI] [PubMed] [Google Scholar]
- 37.Nie Y, Liu Y, Luo Q, et al. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol 2014; 31: 197–203. [DOI] [PubMed] [Google Scholar]
- 38.Yousef AA, Salem HA, Moustafa MZ. Effect of mini-dose epidural dexmedetomidine in elective cesarean section using combined spinal-epidural anesthesia: a randomized double-blinded controlled study. J Anesth 2015; 29: 708–714. [DOI] [PubMed] [Google Scholar]
- 39.Kim JE, Kim NY, Lee HS, et al. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull 2013; 36: 959–965. [DOI] [PubMed] [Google Scholar]
- 40.Shukla D, Verma A, Agarwal A, et al. Comparative study of intrathecal dexmedetomidine with intrathecal magnesium sulfate used as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol 2011; 27: 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason KP, Lerman J. Review article: Dexmedetomidine in children: current knowledge and future applications. Anesth Analg 2011; 113: 1129–1142. [DOI] [PubMed] [Google Scholar]
- 42.Bajwa SJ, Gupta S, Kaur J, et al. Reduction in the incidence of shivering with perioperative dexmedetomidine: a randomized prospective study. J Anaesthesiol Clin Pharmacol 2012; 28: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usta B, Gozdemir M, Demircioglu RI, et al. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics (Sao Paulo) 2011; 66: 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talke P, Tayefeh F, Sessler DI, et al. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology 1997; 87: 835–841. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Zhang X, Wang H, et al. Dexmedetomidine as a neuraxial adjuvant for prevention of perioperative shivering: meta-analysis of randomized controlled trials. Plos One 2017; 12: e0183154. [DOI] [PMC free article] [PubMed] [Google Scholar]