Short abstract
Objectives
To investigate the expression levels of aromatase cytochrome P450 enzyme (P450AROM) and related molecules—estrogen receptor-beta (ER-β), Ki-67, and p53—in prolactinoma tumor tissue from pre- and post-menopausal women, and to determine the associations of tumor invasiveness with expression levels of these genes.
Methods
This study recruited 90 patients with prolactinoma who underwent adenoidectomy between 2012 and 2017. Information was collected regarding clinical characteristics, hormones, laboratory tests, and magnetic resonance imaging-assessed tumor invasiveness. Expression levels of P450AROM, ER-β, Ki-67, and p53 were examined by immunohistochemistry in prolactinoma tissues.
Results
Increased P450AROM expression was found in invasive prolactinoma tissues in post-menopausal women, compared with its expression in non-invasive prolactinoma tissues. ER-β level was significantly higher in patients resistant to treatment with bromocriptine, a dopamine agonist. However, there were no differences in rate of resistance to treatment (8.2% vs. 3.4%) or expression levels of P450AROM, Ki-67, p53, and ER-β between pre- and post-menopausal patients.
Conclusions
Our results demonstrated that increased P450AROM expression in prolactinoma of post-menopausal women was positively associated with invasiveness. Moreover, ER-β level was higher in both pre- and post-menopausal patients who were resistant to dopamine agonist treatment.
Keywords: Aromatase, prolactinoma, invasion, estrogen receptor beta, dopamine agonists, CYP19A1 protein, bromocriptine, tumor suppressor protein p53, post-menopause, adenoidectomy
Introduction
Prolactinoma, which comprises 32% to 66% of all pituitary adenomas, presents with symptoms of amenorrhea, loss of libido, galactorrhea, and infertility in women.1,2 Serum prolactin level is measured and magnetic resonance imaging (MRI) is performed to assess tumor presence and evaluate invasiveness. Treatment of prolactinoma aims to preserve or restore anterior pituitary function; medical therapy using dopamine agonists is the preferred treatment.1,2 Cabergoline and bromocriptine are commonly used dopamine agonists: 85% to 90% of patients using cabergoline and 75% using bromocriptine can achieve normalization of serum prolactin level.1,2
Several mechanisms are suspected to contribute to pituitary prolactinoma expansion and invasion,3–5 including actions mediated by nuclear proteins Ki-67 and p53; these are often used as markers of prolactinoma invasiveness and other biological behavior.6 Estrogen receptor activity also contributes to the disease; although little is known regarding its expression during or prior to the onset of prolactinoma, a previous study suggested that the estrogen receptor contributed to prolactinoma occurrence and development.7
Women have higher serum prolactin levels than men, and pituitary size increases up to 136% throughout pregnancy;8 this is accompanied by increased prolactinoma secretion in pregnant women. Prolactinomas are commonly diagnosed in women between 20 and 50 years of age, most of whom are at the pre-menopausal stage;6,9 thus, it is rarely diagnosed in the post-menopausal stage. Similar to prolactinoma in men, prolactinoma in post-menopausal women exhibits large and invasive macroadenomas at the time of diagnosis, which secrete high levels of prolactins.9 This particular type of prolactinoma may be underdiagnosed and no specific factors are known to be associated with its development and aggressive behavior.
Aromatase cytochrome P450 enzyme (P450AROM), which aromatizes testosterone to estrogen, is present in normal pituitary tissues, and is expressed at higher levels in prolactinoma tissue.1 In men, invasive prolactinoma tumors have higher levels of P450AROM, which suggests that it could be involved in tumor development and subsequent behavior.3,4 It is not yet known whether P450AROM plays a critical role in prolactinoma invasiveness in post-menopausal women.
To investigate the roles of genes involved in prolactinomas in pre-menopausal women, this study measured the expression levels of P450AROM, estrogen receptor-beta (ER-β), Ki-67, and p53 in prolactinoma tissues from pre- and post-menopausal women. In addition, this study explored the associations of these molecules with prolactinoma invasiveness and resistance to treatment.
Materials and methods
General characteristics of patients in different menstrual status
This study recruited pre-menopausal and post-menopausal consecutive female patients (menopause was defined as age >50 years and follicle stimulating hormone [FSH] levels >30 IU/l and E2 levels <50 pmol/l, as in a previous study5) with prolactinoma during 2012 and 2017 who were admitted and received treatment in the First Affiliated Hospital of Xinjiang Medical University, a large medical center located in Xinjiang, China. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. Participation was voluntary and written informed consent was obtained from all participants.
Patients were excluded if they had non-functional adenoma, growth hormone-secreting adenoma, adrenocorticotropic hormone (ACTH)-secreting adenoma, pituitary carcinoma, or multi-hormonal prolactinoma (MEN1). For comparison, age-matched healthy women were recruited as controls for each respective group. Clinical data were collected, including symptoms of headache, visual disturbance, galactorrhea, infertility, oligomenorrhea, amenorrhea, remission status, and resistance to prolactin-lowering effects of dopamine agonists. Patients who had normal prolactin levels without symptoms were judged to be in remission; patients who had higher levels of prolactin with symptoms, and whose tumor size could not be reduced by 50% under maximum tolerable doses of dopamine agonists, were judged to be resistant to treatment, as in a previous study.10
Laboratory tests
All basal hormones were assessed in the central laboratory of our hospital. Thyroid-stimulating hormone (TSH), triiodothyronine (T3), and tetraiodothyronine (T4) levels were assessed using the Cobas 8000 analyzer (Roche, Mannheim, Germany). Prolactin, estradiol, estriol, progesterone, FSH, and luteinizing hormone (LH) were assessed using the microparticle enzyme immunoassay technique with a Dx 1800 analyzer (Beckman Coulter, Brea, CA, USA).
Magnetic resonance imaging
The presence and size of a prolactinoma, as well as its invasiveness, were examined by pituitary MRI to detect microprolactinoma (<1 cm) or macroprolactinoma (≥1 cm). Prolactinoma tissue that had invaded into the cavernous and/or sphenoidal sinuses on MRI was considered indicative of invasion.10
Surgical intervention
Surgical treatment of prolactinoma generally aims to remove the tumor in the pituitary gland using the transsphenoidal approach. Tumor invasiveness was confirmed intraoperatively.11,12 All surgeries were performed in the Neurology Department of our hospital by neurosurgeons with extensive experience in pituitary tumor removal, particularly transsphenoidal pituitary surgery. Surgeries were performed in patients with resistance to dopamine agonists and those who could not tolerate long-term medication therapy. Because of the nature of this retrospective analysis, we could not confirm the inclusion criteria used for each surgical intervention.
Tissue processing and histological study
Archived prolactinoma tissues were obtained from patients who underwent adenoidectomy with prolactinoma that was histologically confirmed by the Pathology Department. Immunohistochemical studies were performed using the streptavidin–biotin complex method.13 Pituitary tissues were collected, fixed in 10% neutral formalin, then cut at 4-µm thickness. Briefly, after deparaffinization, slides were incubated with a primary antibody—anti-P450AROM (PA1-21398, Thermo Fisher Scientific, Waltham, MA, USA), anti-ER-β1 (ab133467, Abcam, Cambridge, UK), anti-Ki-67 (ZM-0167, ZSGB-BIO, Beijing, China), or anti-p53 (ZM-0408, ZSGB-BIO)—for 1.5 hours at room temperature, at a dilution of 1:50 in phosphate-buffered saline. After incubation with secondary antibody—horseradish peroxidase-conjugated goat anti-rabbit IgG (K5007, Dako, Glostrup, Denmark, at a dilution of 1:100)—for 1 hour, the slices were evaluated via microscopy. P450AROM and ER-β were assessed according to H score, as in a prior study.14 Briefly, the reaction staining intensity was evaluated by two independent pathologists who were blinded to the clinical information and graded as follows: 0 = negative, 1+ = weak, 2+ = moderate, and 3+ = strong. The H score was then calculated as the sum of H1, H2, and H3: H1 (percentage of cells graded as “1+” ×1); H2 (percentage of cells graded as “2+” × 2), and H3 (percentage of cells graded as “3+” × 3).3,14 Ki-67 and p53 expression data were assessed as percentages of positive immunostained nuclei among all nuclei by the Pathology Department of our hospital.
Statistical analysis
Statistical analysis was performed using SPSS software (version 22.0, IBM Corp., Armonk, NY, USA). Categorical or non-parametric variables were analyzed using descriptive statistics (median [interquartile range] and percentage), as the data did not exhibit a normal distribution. Medians were compared using the non-parametric Mann–Whitney U and Kruskal–Wallis tests. The chi-squared test or Fisher’s exact test was used to compare frequencies. Spearman’s correlation test was applied to detect relationships between continuous variables. P < 0.05 was considered to be statistically significant.
Results
Recruitment procedure and clinical characteristics
A flow chart of the recruitment procedure is presented in Figure 1. Between 2012 and 2017, 286 women who were admitted and received treatment in our Hospital were screened for inclusion in this study. Based on the pathological tumor results, we excluded patients with non-functional adenoma (n = 128), growth hormone-secreting adenoma (n = 42), ACTH-secreting adenoma (n = 15), multi-hormonal prolactinoma (n = 6), pituitary carcinoma (n = 4), and MEN1 (n = 1). Overall, we recruited 61 pre-menopausal and 29 post-menopausal women who had been diagnosed with prolactinoma. For comparison, we also recruited 50 pre-menopausal and 30 post-menopausal healthy women as controls for the respective patient groups.
Figure 1.
Flow of participant recruitment. ACTH: adrenocorticotropic hormone; MEN1: multiple endocrine neoplasia 1.
General clinical characteristics are presented in Table 1. Compared with healthy women who had the same menstrual status, both pre- and post-menopausal patients with prolactinoma exhibited higher rates of symptoms, including headache, visual disturbance, and hypogonadism. Pre-menopausal patients had increased rates of infertility and oligomenorrhea, compared with healthy women who had the same menstruation status (both P < 0.05). Post-menopausal patients had an increased rate of visual disturbances, compared with that in pre-menopausal patients (P = 0.001). There was no significant difference in galactorrhea between pre- and post-menopausal patients, although pre-menopausal patients tended to have an increased incidence.
Table 1.
General clinical characteristics of patients with prolactinoma and healthy controls.
|
Pre-menopause (n = 111) |
Post-menopause (n = 59) |
||||||
|---|---|---|---|---|---|---|---|
| Prolactinoma (n = 61) | Control (n = 50) | P | Prolactinoma (n = 29) | Control (n = 30) | P | P* | |
| Age at diagnosis (years) | 34.8 ± 8.2 | 35.7 ± 8.4 | 0.574 | 56.9 ± 5.7 | 57.9 ± 7.6 | 0.610 | <0.001 |
| Headache, n (%) | 36 (59.0) | 6 (12.0) | <0.001 | 17 (58.6) | 9 (30.0) | 0.027 | 0.972 |
| Visual disturbance, n (%) | 20 (32.8) | 2 (4.0) | <0.001 | 20 (69.0) | 7 (23.3) | <0.001 | 0.001 |
| Galactorrhea, n (%) | 6 (9.8) | 0 | 0.023 | 1 (3.4) | 0 | 0.305 | 0.290 |
| Infertility, n (%) | 10 (16.4) | 2 (4.0) | 0.036 | – | – | – | – |
| Oligomenorrhea, n (%) | 16 (26.2) | 5 (10.0) | 0.030 | – | – | – | – |
| Amenorrhea, n (%) | 17 (27.9) | 9 (18.0) | 0.222 | – | – | – | – |
| Hypogonadism, n (%) | 50 (82.0) | 1 (2.0) | 0.001 | 17 (58.6) | 2 (6.7) | 0.001 | 0.018 |
| MRI results: invasive, n (%) | 16 (26.2) | – | – | 20 (69.0) | – | – | 0.001 |
| Cavernous sinus | 5 (31.2) | – | – | 8 (40.0) | – | – | 0.014 |
| Sphenoidal sinus | 4 (25.0) | – | – | 6 (30.0) | – | – | 0.046 |
| Both sinuses | 7 (43.8) | – | – | 6 (30.0) | – | – | 0.245 |
| Macroadenoma, n (%) | 45 (88.9) | – | – | 29 (100) | – | – | 0.002 |
| Size of macroadenoma (φ, mm) | 25.4 ± 8.0 | – | – | 27.1 ± 9.6 | – | – | 0.379 |
| Size of microadenoma (φ, mm) | 8.0 ± 3.0 | – | – | – | – | – | – |
| Bromocriptine, n (%) | 16 (26.2) | – | – | 3 (10.3) | – | – | 0.103 |
Data are expressed as mean ± standard deviation or n (%). P*: comparison between pre-menopausal and post-menopausal patients.
According to MRI examinations, tumors were larger and more often invasive in post-menopausal patients than in pre-menopausal patients (both P < 0.01); no microadenomas were found in post-menopausal patients (Table 1). With respect to sites of invasion, there were significant differences in the rates of invasion of the cavernous and sphenoidal sinuses between pre- and post-menopausal patients (both P < 0.05).
Preoperative medical treatment with bromocriptine (2.5–5.0 mg, three times daily) was only administered to patients with non-invasive tumors, and this did not differ between pre and post-menopausal patients (Table 1). Specifically, 12 women with microadenoma and one with macroadenoma among pre-menopausal patients, and three women with macroadenoma among post-menopausal patients, were administered preoperative medical treatment.
Menstrual status influenced endocrine hormone levels in women
High levels of prolactin often cause hypogonadism with decreased levels of FSH, LH, estrogen, and progesterone. Compared with healthy women, pre-menopausal patients who had prolactinoma exhibited reduced levels of estradiol, estriol, and progesterone, as well as an increased level of prolactin. There was no significant difference in prolactin level between pre- and post-menopausal patients; however, we found significant differences in other endocrine hormone levels between the two groups. Notably, estradiol and progesterone levels were significantly higher in pre-menopausal patients, whereas FSH and LH levels were significantly higher in post-menopausal patients (Table 2).
Table 2.
Endocrine hormone levels in patients with prolactinoma and healthy controls.
|
Pre-menopause (n = 111) |
Post-menopause (n = 59) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Prolactinoma (n = 61) | Control (n = 50) | P | Prolactinoma (n = 29) | Control (n = 30) | P | P* | ||
| Prolactin (ng/mL) | 69.8 (29.3–114.3) | 12.0 (7.4–17.3) | <0.001 | 62.2 (33.9–78.2) | 9.1 (5.9–11.6) | <0.001 | 0.537 | |
| Estradiol (pg/mL) | 35.57 (22.9–53.8) | 61.0 (52.94–75.1) | 0.003 | 18.3 (12.6–23.5) | 20.1 (10.8–27.1) | 0.617 | <0.001 | |
| Estriol (pg/mL) | 22.00 (15.0–32.6) | 30.6 (20.4–38.9) | 0.027 | 22.5 (7.5–28.0) | 25.3 (17.2–29.9) | 0.214 | 0.166 | |
| Progesterone (ng/mL) | 0.6 (0.34–0.90) | 0.6 (0.3–1.0) | 0.004 | 0.3 (0.1–0.7) | 0.4 (0.2–0.5) | 0.844 | 0.005 | |
| FSH (IU/L) | 5.5 (3.17–7.32) | 7.3 (3.8–20.1) | 0.022 | 12.3 (7.9–40.0) | 24.0 (9.7–30.4) | 0.159 | 0.003 | |
| LH (IU/L) | 2.5 (1.3–4.3) | 3.5 (1.6–11.7) | 0.011 | 8.0 (4.2–16.5) | 10.9 (7.1–16.7) | 0.820 | <0.001 | |
All values are expressed as 25th–75th percentiles. P*: comparison between pre-menopausal and post-menopausal patients. FSH: follicle-stimulating hormone; LH: luteinizing hormone.
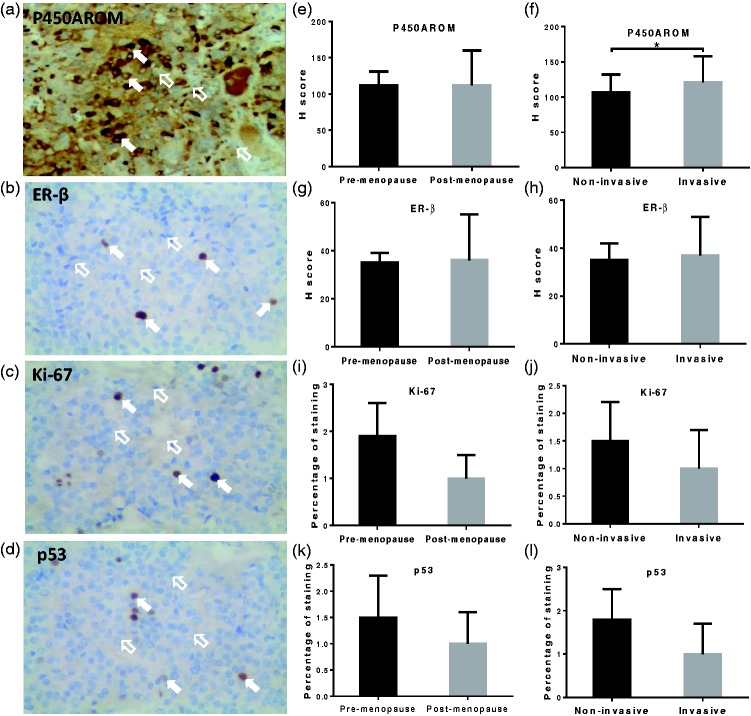
Invasive prolactinoma was associated with increased expression of P450AROM
Immunohistochemistry analysis was performed to examine expression levels of P450AROM, ER-β, Ki-67, and p53 (representative staining: Figure 2a–d). These expression levels were comparable between pre- and post-menopausal patients (Figure 2e, g, i, and k). However, compared with non-invasive prolactinomas, invasive tumors showed increased P450AROM expression in post-menopausal patients (P < 0.05, Figure 2f); moreover, Spearman’s correlation test showed a positive association between increased expression of P450AROM and prolactinoma invasiveness in post-menopausal patients (P = 0.03, r = 0.52). Notably, there were no significant differences in expression levels of Ki-67, ER-β, or p53 between non-invasive and invasive prolactinomas, in either pre-menopausal or post-menopausal patients (Figure 2h, j, and l).
Figure 2.
Expression levels of P450AROM, ER-β, Ki-67, and p53 with respect to menstrual status and tumor invasiveness. Representative immunohistochemical staining for P450AROM (a), ER-β (b), Ki-67 (c), and p53 (d); e, g, i, and k: respective prolactinoma tissue staining scores in pre- and post-menopausal women; f, h, j, l: respective prolactinoma tissue staining scores in non-invasive and invasive prolactinomas of post-menopausal women. In a–d, magnification ×40; open arrows indicate negative immunostaining and solid arrows indicate positive immunostaining. In e–l, error bars indicate standard deviation; *P < 0.05.
Increased expression of ER-β was associated with resistance to dopamine agonist
Resistance to dopamine agonist was defined as a failure to normalize prolactin levels after the administration of > 15 mg of bromocriptine daily for at least 3 months, in accordance with the definition used in a prior study.15,16 We found that eight patients were resistant to dopamine agonist, including five pre-menopausal women and three post-menopausal women. MRI revealed that two of these pre-menopausal patients and three of these post-menopausal patients had macroadenoma.
We compared the expression levels of P450AROM, ER-β, Ki-67, and p53 between patients who were resistant to bromocriptine and those who were not; we found that patients resistant to bromocriptine exhibited increased ER-β expression (P < 0.001). Spearman’s correlation test showed that ER-β expression was associated with resistance in both groups of patients (P = 0.038, r = 0.51). Finally, there were no differences in the expression levels of P450AROM, Ki-67, or p53 between patients who were resistant to bromocriptine and those who were not (Table 3).
Table 3.
Association between prolactinoma resistance to dopamine agonist and expression levels of P450AROM, ER-β, Ki-67, and p53.
|
Menstrual status |
||||||
|---|---|---|---|---|---|---|
| Resistant to bromocriptine |
Pre-menopause (n = 16) |
Post-menopause (n = 3) |
||||
| Yes (n = 5) | No (n = 11) | P | Yes (n = 3) | No (none) | P* | |
| P450AROM (H score) | 145.7 ± 28.4 | 129.9 ± 31.1 | 0.067 | 108.6 ± 30.1 | – | 0.053 |
| ER-β (H score) | 59.8 ± 10.7 | 32.9 ± 11.2 | <0.001 | 35.0 ± 10.7 | – | <0.001 |
| Ki-67 (%) | 1.5 ± 0.6 | 1.5 ± 0.4 | 0.314 | 1.4 ± 0.7 | – | 0.245 |
| p53 (%) | 1.9 ± 0.5 | 2.0 ± 0.6 | 0.187 | 1.7 ± 0.7 | – | 0.147 |
Data are expressed as mean ± standard deviation or n (%). P: resistance versus non-resistance in pre-menopausal patients; P*: resistance versus non-resistance in all participants for whom pre-operative medical histories were available.
Discussion
In the present study, we confirmed P450AROM expression in both pre- and post-menopausal patients with prolactinoma. No differences were found between pre- and post-menopausal patients with respect to expression levels of P450AROM or ER-β. However, post-menopausal women with invasive prolactinomas exhibited increased P450AROM expression. Furthermore, we found that increased expression of ER-β in prolactinoma tissue was associated with resistance to treatment with the dopamine agonist, bromocriptine. However, expression levels of P450AROM, Ki-67, and p53 were not associated with resistance to bromocriptine treatment.
Local changes of androgen to estradiol, mediated by P450AROM, play important roles in the regulation of prolactin, proliferation of pituitary prolactin-positive cells, and development of prolactinoma.3,17 In the present study, P450AROM expression was detected in all patients with prolactinoma, and there was no significant difference in its expression level between pre- and post-menopausal women. However, increased P450AROM expression was observed in invasive prolactinoma, compared with that in non-invasive prolactinoma, in post-menopausal patients. A previous study reported a similar expression level of P450AROM in tumor tissues in both men and women; however, P450AROM expression in men was more strongly associated with tumor invasiveness.1 Our results were consistent with the prior finding of an association between P450AROM expression and tumor invasiveness.
Thus far, no conclusive markers have been identified to predict aggressiveness in pituitary tumors.18 Several studies have shown conflicting data with respect to histological markers of aggressiveness, including Ki-67 and p53.19–22 The World Health Organization classification system describes the diagnosis of “atypical” pituitary adenoma, which may be associated with aggressive behavior, based on p53 immunostaining findings.18 A prior report indicated no p53 expression in any non-invasive pituitary adenomas, whereas it demonstrated p53 expression in 15.2% of invasive adenomas and 100% of pituitary carcinomas; moreover, a ≥3% Ki-67 index was able to predict adenoma aggressiveness with 97% specificity and 73% sensitivity.22 However, these conclusions have not been confirmed in other studies.21,23,24 In the present study, we found no significant difference in the expression levels of Ki-67 and p53 in prolactinomas according to aggressiveness. Therefore, a larger clinical and multi-center study is warranted to address the continuing disagreement with respect to prolactinoma markers.
A previous study showed that the biological effect of estrogen on the development of prolactinoma was mediated by estrogen receptors, a nuclear receptor superfamily of steroid hormones.25 Women aged ≥50 years had a lower prevalence of prolactinoma and non-classical clinical symptoms; few had amenorrhea or galactorrhea, which may be the result of reduced ovarian estrogen production.9 Consistent with the results of a previous study,9 we found few cases of prolactinoma in post-menopausal women among all patients in the present investigation. The estrogen level in post-menopausal prolactinoma patients was lower than that of pre-menopausal patients. However, in a comparison with post-menopausal healthy women, post-menopausal prolactinoma patients showed a similar concentration of estrogen in the blood. Estrogen plays important roles in the development of prolactinoma and pathogenesis of dopamine agonist-resistant prolactinomas.26,27 Local estrogen concentration, estrogen receptor expression, and interactions with dopamine receptors in tumor tissue may influence tumor growth and resistance.28 Thus, P450AROM, which produces local estrogen by aromatization of testosterone, may play critical roles in prolactinoma aggressiveness and drug resistance in post-menopausal women.
The roles of estrogen receptors in dopamine agonist resistance and aggressiveness in human prolactinomas have not been fully explored, despite the disparate conclusions of prior studies described above. In addition, a previous study reported no significant differences between estrogen receptor-α and ER-β mRNA levels in dopamine agonist-resistant patients,29 and the most aggressive tumors were those that lacked estrogen receptors.30 However, Bai et al.31 demonstrated increased estrogen receptor-α expression in patients with bromocriptine-resistant prolactinomas, particularly among men. We found an association between increased ER-β expression and bromocriptine resistance, which was consistent with the results of a previous study.3 Both subtypes of estrogen receptors may contribute to prolactinoma resistance to dopamine agonist treatment. Given this hypothesis, prolactinomas that are resistant to dopamine agonist treatment may be managed by using estrogen receptor antagonists; indeed, a previous study showed that an estrogen receptor antagonist, tamoxifen, successfully treated patients with dopamine agonist-resistant prolactinomas.32
Limitations
There were several limitations in this study. First, expression levels of P450AROM, ER-β, Ki-67, and p53 were assessed solely by immunohistochemistry. mRNA and protein levels of these markers could not be quantitatively examined owing to the features of the methodology applied; these markers were limited to immunohistochemistry staining analysis because this study used archived tissues. Second, galectin-3 expression is regarded as a marker of aggressiveness in pituitary adenomas,33–36 but it was not included in this study. Third, this study lacked a positive control tissue, such as prolactin-secreting pituitary cancer. Fourth, the small sample size and retrospective nature of the study limited the generalizability of the findings. Therefore, a large, prospective clinical study with comprehensive marker is needed to confirm these findings.
Conclusions
Our study indicated that pre- or post-menopausal status influenced prolactinoma invasiveness. In post-menopausal women with prolactinomas, invasive tumors showed increased P450AROM expression. The expression of ER-β in prolactinoma tissue was significantly associated with dopamine agonist resistance. Our findings suggest that the aromatase-mediated local conversion of testosterone to estrogen contributes to the development and aggressiveness of prolactinoma.
Acknowledgements
The authors wish to thank all participants in the study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Natural Science Foundation of the First Affiliated Hospital of Xinjiang Medical University (2014ZRQN03).
References
- 1.Dos Santos Nunes V, El Dib R, Boguszewski CL, et al. Cabergoline versus bromocriptine in the treatment of hyperprolactinemia: a systematic review of randomized controlled trials and meta-analysis. Pituitary 2011; 14: 259–265. [DOI] [PubMed] [Google Scholar]
- 2.Ji MJ, Kim JH, Lee JH, et al. Best candidates for dopamine agonist withdrawal in patients with prolactinomas. Pituitary 2017; 20: 578–584. [DOI] [PubMed] [Google Scholar]
- 3.Akinci H, Kapucu A, Dar KA, et al. Aromatase cytochrome P450 enzyme expression in prolactinomas and its relationship to tumor behavior. Pituitary 2013; 16: 386–392. [DOI] [PubMed] [Google Scholar]
- 4.Prior JC, Cox TA, Fairholm D, et al. Testosterone-related exacerbation of a prolactin-producing macroadenoma: possible role for estrogen. J Clin Endocrinol Metab 1987; 64: 391–394. [DOI] [PubMed] [Google Scholar]
- 5.Norman C, Rollene NL, Erickson D, et al. Estradiol regulates GH-releasing peptide's interactions with GH-releasing hormone and somatostatin in postmenopausal women. Eur J Endocrinol 2013; 170: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimon I, Hinton DR, Weiss MH, et al. Prolactinomas express human heparin-binding secretory transforming gene (hst) protein product: marker of tumour invasiveness. Clin Endocrinol (Oxf) 1998; 48: 23–29. [DOI] [PubMed] [Google Scholar]
- 7.Delgrange E, Vasiljevic A, Wierinckx A, et al. Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. Eur J Endocrinol 2015; 172: 791–801. [DOI] [PubMed] [Google Scholar]
- 8.Elster AD, Sanders TG, Vines FS, et al. Size and shape of the pituitary gland during pregnancy and post partum: measurement with MR imaging. Radiology 1991; 181: 531–535. [DOI] [PubMed] [Google Scholar]
- 9.Shimon I, Bronstein MD, Shapiro J, et al. Women with prolactinomas presented at the postmenopausal period. Endocrine 2014; 47: 889–894. [DOI] [PubMed] [Google Scholar]
- 10.Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 273–288. [DOI] [PubMed] [Google Scholar]
- 11.Bloomgarden E, Molitch ME. Surgical treatment of prolactinomas: cons. Endocrine 2014; 47: 730–733. [DOI] [PubMed] [Google Scholar]
- 12.Pernicone PJ, Scheithauer BW. Invasive pituitary adenoma and pituitary carcinoma. In: Thapar K, Kovacs K, Scheithauer B, et al. (eds) Diagnosis and Management of Pituitary Tumors New York: Springer, 2001, pp. 369–386.
- 13.Bratthauer GL. The avidin-biotin complex (ABC) method and other avidin-biotin binding methods. Methods Mol Biol 2010; 588: 257–270. [DOI] [PubMed] [Google Scholar]
- 14.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995; 48: 876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini I, Rasolonjanahary R, Gunz G, et al. Resistance to bromocriptine in prolactinomas. J Clin Endocrinol Metab 1989; 69: 500–509. [DOI] [PubMed] [Google Scholar]
- 16.Wong A, Eloy JA, Couldwell WT, et al. Update on prolactinomas. Part 2: treatment and management strategies. J Clin Neurosci 2015; 22: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 17.Garcia Barrado MJ, Blanco EJ, Carretero Hernandez M, et al. Local transformations of androgens into estradiol by aromatase P450 is involved in the regulation of prolactin and the proliferation of pituitary prolactin-positive cells. PLoS One 2014; 9: e101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLellis RA, Lloyd RV, Heitz PU, et al. (eds). World Health Organization classification of tumours: Tumours of endocrine organs. Lyons: IARC, 2004. [Google Scholar]
- 19.Salehi F, Agur A, Scheithauer BW, et al. Biomarkers of pituitary neoplasms: a review (Part II). Neurosurgery 2010; 67: 1790–1798. [DOI] [PubMed] [Google Scholar]
- 20.Salehi F, Agur A, Scheithauer BW, et al. Ki-67 in pituitary neoplasms: a review–part I. Neurosurgery 2009; 65: 429–437. [DOI] [PubMed] [Google Scholar]
- 21.Mastronardi L, Guiducci A, Spera C, et al. Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol 1999; 52: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thapar K, Scheithauer BW, Kovacs K, et al. p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery 1996; 38: 765–770. [PubMed] [Google Scholar]
- 23.Scheithauer BW, Gaffey TA, Lloyd RV, et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 2006; 59: 341–353. [DOI] [PubMed] [Google Scholar]
- 24.Pizarro CB, Oliveira MC, Coutinho LB, et al. Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz J Med Biol Res 2004; 37: 235–243. [DOI] [PubMed] [Google Scholar]
- 25.Li CZ, Gui SB, Zong XY, et al. The expression of estrogen receptor subtypes in prolactinomas and their relationship to tumor biological behavior. Biomed Environ Sci 2015; 28: 820–822. [DOI] [PubMed] [Google Scholar]
- 26.Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 2006; 65: 265–273. [DOI] [PubMed] [Google Scholar]
- 27.Maurer RA. Estradiol regulates the transcription of the prolactin gene. J Biol Chem 1982; 257: 2133–2136. [PubMed] [Google Scholar]
- 28.Heiman ML, Ben-Jonathan N. Rat anterior pituitary dopaminergic receptors are regulated by estradiol and during lactation. Endocrinology 1982; 111: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 29.Wu ZB, Zheng WM, Su ZP, et al. Expression of D2RmRNA isoforms and ERmRNA isoforms in prolactinomas: correlation with the response to bromocriptine and with tumor biological behavior. J Neurooncol 2010; 99: 25–32. [DOI] [PubMed] [Google Scholar]
- 30.Burdman JA, Pauni M, Heredia Sereno GM, et al. Estrogen receptors in human pituitary tumors. Horm Metab Res 2008; 40: 524–527. [DOI] [PubMed] [Google Scholar]
- 31.Bai J, Gui S, Zhang Y. Suppression of MMQ cells by fulvestrant: possible mechanism of action and potential application for bromocriptine-resistant prolactinomas. J Clin Neurosci 2013; 20: 721–725. [DOI] [PubMed] [Google Scholar]
- 32.Lopez JM, Oestreicher E. Reversal of hypogonadotropic hypogonadism with tamoxifen in a patient with hyperprolactinemia resistant to dopamine agonists. Fertil Steril 2005; 84: 756. [DOI] [PubMed] [Google Scholar]
- 33.Chauvet N, Romano N, Meunier AC, et al. Combining cadherin expression with molecular markers discriminates invasiveness in growth hormone and prolactin pituitary adenomas. J Neuroendocrinol 2016; 28: 12352. [DOI] [PubMed] [Google Scholar]
- 34.Righi A, Morandi L, Leonardi E, et al. Galectin-3 expression in pituitary adenomas as a marker of aggressive behavior. Hum Pathol 2013; 44: 2400–2409. [DOI] [PubMed] [Google Scholar]
- 35.Ruebel KH, Leontovich AA, Jin L, et al. Patterns of gene expression in pituitary carcinomas and adenomas analyzed by high-density oligonucleotide arrays, reverse transcriptase-quantitative PCR, and protein expression. Endocrine 2006; 29: 435–444. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Wang MD, Ma WB, et al. [Expression of galectin-3 in invasive prolactinomas]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2005; 27: 380–381. [PubMed] [Google Scholar]