Short abstract
Objective
Neurobiology studies are increasingly focused on the dorsal root ganglion (DRG), which plays an important role in neuropathic pain. Existing DRG neuron primary culture methods have considerable limitations, including challenging cell isolation and poor cell yield, which cause difficulty in signaling pathway studies. The present study aimed to establish an integrated primary culture method for DRG neurons.
Methods
DRGs were obtained from fetal rats by microdissection, and then dissociated with trypsin. The dissociated neurons were treated with 5-fluorouracil to promote growth of neurons from the isolated cells. Then, reverse transcription polymerase chain reaction and immunofluorescence assays were used to identify and purify DRG neurons.
Results
Isolated DRGs were successfully dissociated and showed robust growth as individual DRG neurons in neurobasal medium. Both mRNA and protein assays confirmed that DRG neurons expressed neurofilament-200 and neuron-specific enolase.
Conclusions
Highly purified, stable DRG neurons could be easily harvested and grown for extended periods by using this integrated cell isolation and purification method, which may help to elucidate the mechanisms underlying neuropathic pain.
Keywords: Dorsal root ganglion, cell isolation, cell purification, microdissection, neurobiology, neurofilament proteins, neuralgia, neurons
Introduction
In recent years, neurobiology studies have increasingly focused on the dorsal root ganglion (DRG),1,2 which contains a diverse subpopulation of primary sensory neurons that are involved in the sensation of back pain and the transmission of non-noxious somatic sensations. The DRG connects the internal nervous system environment with spinal cord and is responsible for the production and transmission of many kinds of neuropeptides.3,4 Many nociceptors are located on the outer membrane of DRG neurons, thus enabling these cells to exhibit sensitivity to diverse stimuli. Moreover, inflammatory factors are known to increase DRG sensitivity and decrease pain threshold.5,6 Although primary culture of DRG neurons is important for characterization of the processes in which these cells are involved, existing methods exhibit a variety of limitations, including challenging cell isolation and poor cell yield; these limitations cause difficulty in signaling pathway studies.7,8 Many researchers have used cytarabine as an inhibitor of non-neuronal cell division during purification of DRG neurons.9,10 Here, we utilized an alternative mitotic inhibitor: 5-fluorouracil (5-FU), a pyrimidine analogue that can be converted into 5-fluoro-2′-deoxyuridine-5′-monophosphate. This metabolite inhibits thymidylate synthase, thereby suppressing DNA synthesis.11 Based on the methods used in previous studies, we established an integrated approach for primary culture of DRG neurons, which can be used to isolate neurons with sufficient longevity and purity to meet the needs of molecular cell biology analyses of neuropathic pain and other somesthetic phenomena.
Materials and methods
Primary culture
The Institutional Animal Care and Use Committee of Soochow University approved this study. E18 Sprague-Dawley embryonic rats were anesthetized by induction of hypothermia in an ice bag, then disinfected with 75% ethanol. Rats were decapitated and fixed in the prone position. The spinal cord was removed by severing the spinal canal with micro-scissors; the exposed DRG (i.e., a white ball between the two spinous processes) was then removed and cleaned under a microscope. Each DRG was then incubated with agitation in 10 mL of 0.25% trypsin (Invitrogen, Carlsbad, CA, USA) in a 15-mL centrifuge tube for 30 minutes at 37°C in an atmosphere of 5% CO2. DRG cells were mechanically dissociated by tapping the tube during digestion. The cell suspension was filtered through a 40-µm cell strainer and centrifuged at 300 × g for 5 minutes at 25°C. The supernatant was then discarded and the cell pellet was washed with Dulbecco’s Modified Eagle’s Medium (Gibco, Carlsbad, CA, USA) containing 10% fetal calf serum (Gibco). The cells were again centrifuged at 300 × g for 5 minutes at room temperature and the supernatant was discarded. The cells were suspended in basal medium (neurobasal medium [Gibco], 2% B27 supplement [Gibco], 2 mM l-glutamine [Gibco], 0.25% glucose [Sigma-Aldrich, St. Louis, MO, USA], and 100 ng/mL NGF [Sino Biological, Beijing, China]) and seeded on six-well plates at a density of 1 × 105 cells per well. Forty-eight hours later, the medium was replaced with basal medium supplemented with 20 mM 5-FU (Sigma-Aldrich); thereafter, the medium was changed every 3 days. All study procedures were conducted in accordance with the guidelines for the use of experimental animals, established by the United States National Institutes of Health. All possible efforts were made to minimize the number of animals used in the experiment and to alleviate the pain and distress experienced by the animals. Moreover, all surgical procedures were performed in an aseptic manner and were approved by the Research Animal Resources and Care Committee of Soochow University.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
On the 6th day of growth in culture, total RNA was extracted from neurons using the TRIzol reagent (Invitrogen) and quantified by UV spectrophotometry. cDNA was synthesized from total RNA using a cDNA synthesis kit (TakaRa Bio, Kusatsu, Japan), in accordance with the manufacturer’s instructions. This cDNA was used to determine mRNA levels of neuron-specific enolase (NSE) and neurofilament-200 (NF200) by RT-PCR. The PCR amplification program was as follows: initial denaturation at 95°C for 5 minutes; 33 cycles of denaturation at 95°C for 30 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 45 seconds; and a final extension step at 72°C for 5 minutes. RT-PCR products were separated in 1.5% agarose gels; then, ethidium bromide-stained bands were visualized under UV illumination. The synthetic oligonucleotide primer sequences for NSE and NF200 were as follows:
NSE: 5′-ATGTGATCAACGGTGGCTCT-3′ (forward primer)
5′-TCCTTGCCGTACTTGTCCTT-3′ (reverse primer) (predicted length: 157 bp);
NF200: 5′-CTCCCAAAAATTCCCTCCAT-3′ (forward primer)
5′-CTCCTCCCTCTTCTGCCTCT-3′ (reverse primer) (predicted length: 169 bp).
Fluorescence immunocytochemistry analysis
On the 6th day of growth in culture, neurons were transferred to serum-free medium for 12 h, then identified by immunofluorescence staining. Briefly, 12 mm coverslips (Fisher Scientific, Pittsburgh, PA, USA) were coated with poly-L-lysine under sterile conditions. One hundred milliliter aliquots of DRG neuron suspension (approximately 5 × 103–1 × 104 cells) were added to each coverslip in four-well plates; the cells were rinsed in 0.1 g/L phosphate-buffered saline (PBS) and then fixed in 40 g/L paraformaldehyde for 20 minutes at 25°C). Cells were washed in PBS 3–4 times, then blocked with 10 g/L bovine serum albumin (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h. Cells were incubated with mouse anti-NF200 antibody (BM0100, 1:100 dilution; Boster Biological Technology, Pleasanton, CA, USA) overnight at 4°C and washed three times with PBS. Cells were then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (BA1101, 1:500 dilution; Boster Biological Technology) at room temperature for 1 hour and washed three times with PBS, followed by incubation with rabbit anti-NSE antibody (BA0535, 1:100 dilution; Boster Biological Technology) overnight at 4°C. After cells had been washed three times with PBS, they were stained with allophycocyanin-conjugated goat anti-rabbit IgG (BA1090, 1:500 dilution; Boster Biological Technology) at room temperature for 1 h; cells were then imaged under a fluorescent microscope.
Results
Morphological observations of DRG neurons
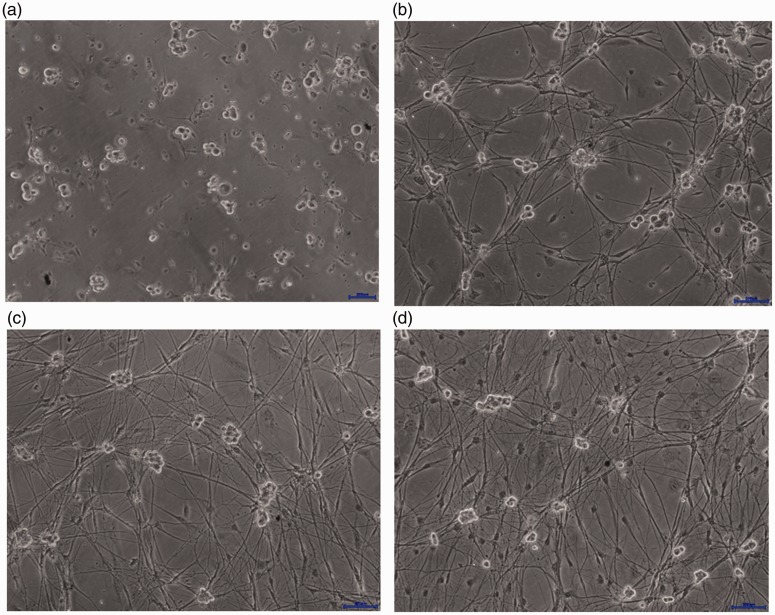
Most DRG neuron cells began to adhere to the plates after 12 hours of culture in basal medium. Approximately 2 × 105 DRG neuron cells were obtained from each rat. All DRG neuron cells in each coverslip were counted under the microscope with a hemocytometer; neurons comprised 85.4% ± 1.75% of the cells on day 6. Schwann cells and other non-neuronal cells increased on the 11th day; during the first 10 days, the ratios remained approximately 10% ± 0.98%. Ganglion cells were significantly larger than other types of cells, with a round or oval shape and a circular halo around the somata (Figure 1a). After 2 days of culture, small synapses appeared around the haloes of the somata and growth cones were observed at the protruding ends. Neurons formed clusters and were surrounded by dendritic protrusions (Figure 1b). During growth in culture, neuronal dendrites became longer and thicker; neurite networks became more dense and neuronal cell clusters gradually grew larger. On the 7th day of culture, mature cells, which exhibited larger volumes and obvious haloes, were considerably interwoven with neurites (Figure 1c). On the 11th day of culture, cells showed gradual degenerative changes, including irregular shape and reduced haloes. Cell debris were present in the somata and protrusions were retracted (Figure 1d).
Figure 1.
Morphological changes in dorsal root ganglion neurons. (a) Cells initially exhibited a round or oval shape. (b) Neurons subsequently formed clusters and were surrounded by dendritic protrusions. (c) On the 7th day of culture, cells were mature with obvious haloes, and showed significant interweaving with neurites. The areas of neuron cell clusters were enlarged. (d) On the 11th day of culture, cells showed gradual degenerative changes, including irregular shape. More non-neuronal cells are observed due to the absence of the 5-fluorouracil effect.
Scale bars (blue) = 200 µm.
Fluorescence immunocytochemistry
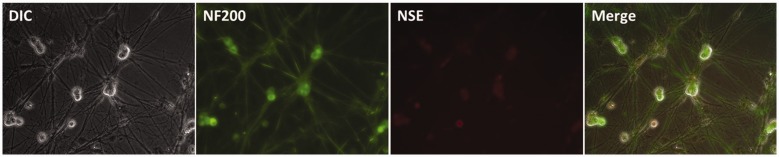
On the 6th day of culture, ganglion cells were used for immunofluorescence staining. NF200 and NSE were used as markers of the neurochemical phenotype. NF200 was expressed in both the somata and neurites of cultured cells, similar to NSE. The two proteins showed clear colocalization in ganglion cells (Figure 2).
Figure 2.
Fluorescence immunocytochemistry showing expression of NSE (red) and NF200 (green) in dorsal root ganglion neurons. Non-neuronal cells were not stained with the fluorescent markers.
Abbreviations: DIC, differential interference contrast; NF200, neurofilament-200; NSE, neuron-specific enolase.
RT-PCR identification of DRG neurons
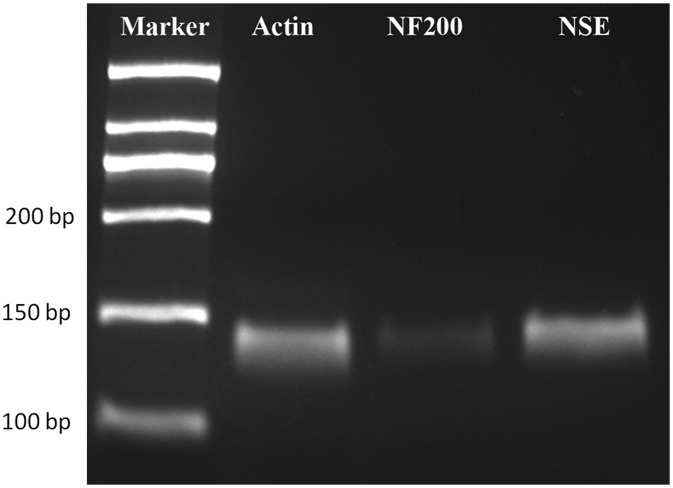
The predicted lengths of the NSE and NF200 PCR products were 157 bp and 169 bp, respectively. Both PCR products showed bands that closely matched these predicted lengths (Figure 3).
Figure 3.
Reverse transcription polymerase chain reaction assay (products separated in agarose gel and stained with ethidium bromide) shows bands that closely match the predicted lengths of the NSE and NF200 amplification products.
Abbreviations: NF200, neurofilament-200; NSE, neuron-specific enolase.
Discussion
The DRG arises from neural crest cells and the derivatives of these multipotent stem cells exhibit characteristics of the three classic mesoderm derivatives.12 There is evidence that neural crest cells exist in the gastrointestinal (GI) tract and skin tissue of adult animals; they can differentiate into tyrosine hydroxylase-positive neurons in the GI tissue of 15-day-old rats, while cells from the epidermis can further differentiate into neurons, glial cells, smooth muscle cells, and melanocytes.13,14 In the present study, we successfully isolated DRG neurons and confirmed that they expressed both NF200 and NSE in mRNA and protein forms. Immunocytochemistry demonstrated that most DRG neurons expressed NF200 and NSE in the soma and axon.
Because the DRG typically exhibits low volume, it is difficult to extract under direct vision. In the present study, we used the method described by Banker et al. to prepare ganglia from embryonic rats.15 DRG can be cultured in vitro from rats of all ages; however, DRG from fetal rats is reportedly easy to prepare and provides high-purity cell cultures through simple fracturing of the soft spine and removal of the spinal cord.16 This operation should be gentle, as the embryo is fragile at this stage. Synapses spread during the growth process and are generally observed in mature DRG. Notably, there are fewer fibroblasts and Schwann cells in the DRG of embryonic rats, which contributes to the high purity of isolated neurons. In older rats, the ganglia are often tightly connected to the spinal cord and can be difficult to extract.16 Furthermore, during DRG separation, it is important to expose the spinal cord from the ventral side. In our initial studies, we attempted to extract the spinal cord from the dorsal side, but failed to obtain satisfactory results.
The digestion time and strength of pipetting are also important to consider during the process of DRG preparation. The digestion time should be restricted to 60 minutes. Our findings in this study show that prolonged digestion can cause irreversible damage to DRG neurons, thus reducing the numbers of adherent DRG neuron cells and decreasing their survival time. When cells were poorly separated due to insufficient duration of digestion, we found greater success in dissociating them with a pipette, followed by low-speed centrifugation. Single-cell suspensions were obtained after repeating this step multiple times. Importantly, air bubbles should be avoided when pipetting cell pellets, as cells might be present within the air bubbles and would thus have low contact with liquid during cell culture or digestion. Moreover, bubbles can influence optical density values and may promote the aggregation of microbes.
Here, neurobasal medium supplemented with glucose and B27 was selected to promote the survival and growth of neurons. We also used NGF in this study because it has been reported to effectively promote the survival of fetal rat DRG neurons, promote the growth and extension of neurites, and stimulate the expression of critical proteins.17 We found that the inclusion of 100 ng/mL NGF in the medium could significantly promote the growth of synapses in DRG neurons and prolong the survival of these neurons.
Mature neuronal cells are highly differentiated cells that rarely proliferate in vitro, while non-neuronal cells continue to proliferate during in vitro culture. The early existence of non-neuronal cells, such as glial cells, can feed and support neurons in vitro.16 However, non-neuronal cells compete with neurons for nutrients in the late stages of culture, thus inhibiting the growth and survival of neurons. To obtain DRG neurons with sufficient purity for experimental purposes, non-neuronal cells (e.g., Schwann cells and fibroblasts) must be removed.16 Thus, the purification method is of great importance, as is the selection of appropriate neonatal rats from which to isolate DRGs. The differential adhesion purification method is based on the observation that neurons adhere later than non-neuronal cells.18 In the present study, we attempted to utilize this method: non-neuronal cells were removed by culturing the cell mixture in uncoated plates for 50 minutes. We found that non-neuronal cells cannot be entirely removed simply by differential adhesion, despite minimal incorporation of non-DRG tissue when collecting samples. Although there were few residual non-neuronal cells, they grew rapidly and threatened the growth of neurons. However, the combination of a differential adhesion purification method and use of anti-mitotic chemical drugs (e.g., cytarabine or 5-FU) has been reported to remove non-neuronal cells. Some researchers have suggested that cytarabine might be toxic to DRG neurons and might inhibit their survival and growth; indeed, cytarabine has been confirmed to exhibit neurotoxicity, decreasing neuronal survival and slowing neurite extension.9,10 In the present study, we found that the addition of an optimal concentration of 5-FU—after DRG neurons had been cultured for 48 h—successfully suppressed the growth of non-neural cells by preventing DNA synthesis in those cells, without damaging DRG neurons. The timing of this addition of 5-FU was critical: if it was performed too early (1 day after inoculation), it reduced the neuron yield. In addition, non-neuronal cells proliferated rapidly; thus, late addition of 5-FU (i.e., 6 days after inoculation) could also reduce neuron yield.
This study had potential limitations. Although the limited concentration of 5-FU could inhibit the growth of non-neural cells without damaging DRG neurons over a period of 10 days, prolonged culture may be needed for further neuropathic studies. In addition, although NF200 and NSE antibodies were suitable for confirmation of the identities of DRG neurons, specific non-neuronal markers should be added to further distinguish neurons from fibroblasts, Schwann cells, and satellite glial cells.
Conclusion
In summary, we have demonstrated a novel method to obtain highly purified rat DRG neurons from neonatal rats by microdissection, trypsin digestion, physical separation via pipetting, and addition of 5-FU to serum-free medium; this approach may provide a foundation for further studies of the mechanisms underlying neuropathic pain.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National High Technology Research and Development Program of China (2015AA020316), the National Natural Science Foundation of China (81301646, 81472132, 81572183, 81601865 and 81672220), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Natural Science Foundation of Jiangsu Province (BK20150299).
References
- 1.Brumovsky PR. Dorsal root ganglion neurons and tyrosine hydroxylase–an intriguing association with implications for sensation and pain. Pain 2016; 157: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunoki T, Takimoto K, Kita K, et al. Differential contribution of Kv4-containing channels to A-type, voltage-gated potassium currents in somatic and visceral dorsal root ganglion neurons. J Neurophysiol 2014; 112: 2492–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarzosa A, Grassme K, Tanaka E, et al. Axolotls with an under- or oversupply of neural crest can regulate the sizes of their dorsal root ganglia to normal levels. Dev Biol 2014; 394: 65–82. [DOI] [PubMed] [Google Scholar]
- 4.Boateng EK, Novejarque A, Pheby T, et al. Heterogeneous responses of dorsal root ganglion neurons in neuropathies induced by peripheral nerve trauma and the antiretroviral drug stavudine. Eur J Pain 2015; 19: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida K, Nomura Y, Kawamori K, et al. Expression profile of vesicular nucleotide transporter (VNUT, SLC17A9) in subpopulations of rat dorsal root ganglion neurons. Neurosci Lett 2014; 579: 75–79. [DOI] [PubMed] [Google Scholar]
- 6.St-Jacques B, Ma W. Peripheral prostaglandin E2 prolongs the sensitization of nociceptive dorsal root ganglion neurons possibly by facilitating the synthesis and anterograde axonal trafficking of EP4 receptors. Exp Neurol 2014; 261: 354–366. [DOI] [PubMed] [Google Scholar]
- 7.Niemi JP, DeFrancesco-Lisowitz A, Cregg JM, et al. Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp Neurol 2016; 275: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong L, Chen Q, Gu X, et al. Expression and identification of olfactory receptors in sciatic nerve and dorsal root ganglia of rats. Neurosci Lett 2015; 600: 171–175. [DOI] [PubMed] [Google Scholar]
- 9.Parson SH, Price JF, Ribchester RR. Cell viability and laminin-induced neurite outgrowth in cultures of embryonic chick neural tube cells: effects of cytosine-B-D-arabinofuranoside. Neurodegeneration 1995; 4: 99–106. [DOI] [PubMed] [Google Scholar]
- 10.Schwieger J, Esser KH, Lenarz T, et al. Establishment of a long-term spiral ganglion neuron culture with reduced glial cell number: effects of AraC on cell composition and neurons. J Neurosci Methods 2016; 268: 106–116. [DOI] [PubMed] [Google Scholar]
- 11.Gmeiner WH, Lema-Tome C, Gibo D, et al. Selective anti-tumor activity of the novel fluoropyrimidine polymer F10 towards G48a orthotopic GBM tumors. J Neurooncol 2014; 116: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res 2008; 333: 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosher JT, Yeager KJ, Kruger GM, et al. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol 2007; 303: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalazonitis A, Kessler JA. Pleiotropic effects of the bone morphogenetic proteins on development of the enteric nervous system. Dev Neurobiol 2012; 72: 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banker GA, Goslin K. Culturing nerve cells. 2nd ed Boston: MIT Press, 1998, p.545. [Google Scholar]
- 16.Maurel P. Preparation of neonatal rat Schwann cells and embryonic dorsal root ganglia neurons for in vitro myelination studies. Methods Mol Biol 2018; 1739: 17–37. [DOI] [PubMed] [Google Scholar]
- 17.Jonas R, Klusch A, Schmelz M, et al. Assessment of TTX-s and TTX-r action potential conduction along neurites of NGF and GDNF cultured porcine DRG somata. PLoS One 2015; 10: e0139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingras M, Gagnon V, Minotti S, et al. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. J Neurosci Methods 2007; 163: 111–118. [DOI] [PubMed] [Google Scholar]