Short abstract
Objective
Patients with interstitial lung disease (ILD) are at increased risk of developing lung cancer. We aimed to investigate the clinical significance of serum immune factors in this progression.
Methods
We retrospectively screened a hospital database from January 2012 to December 2016 for patients with lung cancer and ILD. We measured serum levels of C3, C4, IgA, IgG, IgM, C-reactive protein (CRP), ceruloplasmin (CER), and rheumatoid factor in these patients and in healthy controls.
Results
We analyzed data for 262 patients with lung cancer, 220 with ILD, and 57 healthy controls. CER levels were significantly higher in patients with lung cancer (0.35 ± 0.10 g/L) compared with both ILD patients (0.31 ± 0.25 g/L) and healthy individuals (0.25 ± 0.04 g/L). C3 and C4 levels were both significantly higher in healthy individuals compared with patients with lung cancer (C3: 1.70 ± 0.29 vs 1.04 ± 0.26 g/L, C4: 0.27 ± 0.24 vs 0.24 ± 0.09 g/L) and ILD (C3: 1.70 ± 0.29 vs 0.97 ± 0.25 g/L, C4: 0.27 ± 0.24 vs 0.21 ± 0.09 g/L). Optimal scaling analysis demonstrated that lung cancer was closely associated with CRP, CER, C3, and C4.
Conclusions
Increased levels of CRP and CER and decreased levels of C3 and C4 may identify patients with ILD at high risk of developing lung cancer.
Keywords: Lung cancer, interstitial lung disease, immune factor, risk factor, C-reactive protein, ceruloplasmin, C3, C4
Introduction
Lung cancer is the leading cause of cancer-related death and accounted for an estimated 1.59 million cases worldwide in 2012.1,2 Mortality rates due to lung cancer are still increasing in China, with a large proportion of patients diagnosed at an advanced stage, resulting in delayed treatment.1,3 Similar pathological mechanisms have been shown to underlie the occurrence of lung cancer and interstitial lung diseases (ILDs), including the inflammatory response, abnormal coagulation, dysregulated apoptosis, and the accumulation of myofibroblasts and extracellular matrix.4,5 In addition, patients with ILD have an increased risk and incidence of progression to lung cancer. One study found that >30% of patients with ILD developed lung cancer,6,7 while a multicenter study showed that patients with ILD had a 7–14-fold risk of developing lung cancer compared with the general population.8 Furthermore, the mortality of patients with lung cancer developed from ILD is higher than that of patients diagnosed with lung cancer alone, with shorter survival and poorer quality of life.9 This can adversely affect the diagnosis and treatment of the diseases and result in long-term economic burdens to the patient’s family in low-income countries. It is therefore necessary to understand the difference and association between ILD and lung cancer. However, clinical information regarding the comparison between ILD and lung cancer is currently limited, including in relation to levels of serum immune factors in patients with these respective diseases. A retrospective study found that rheumatoid factor (RF) positivity was closely correlated with ILD,10 and complement has been reported to play a role in the diagnosis or prognosis of lung cancer;11,12 however, current studies of complement in patients with ILD are lacking.
This study therefore examined levels of serum immune factors in patients with ILD and patients with lung cancer, as well as in healthy individuals, and compared the results among these three populations. The results will provide support for the diagnosis and treatment for ILD and for evaluating the risk of progression to lung cancer.
Methods
Ethical statement
This study was approved by the Institutional Review Board of the First Affiliated Hospital (GYFYY-2008-02-23) of Guangzhou Medical University. The use of human serum samples was carried out in accordance with Chinese legislation and with written informed consent from the donors, their legal guardians, or next of kin, where applicable, for use of the serum samples for future unspecified research purposes.
Study subjects
This study was a retrospective analysis of the database generated by the Clinical Laboratory of the First Affiliated Hospital of Guangzhou Medical University, from January 2012 to December 2016. We screened the database for patients clinically diagnosed with lung cancer or ILD (including connective tissue disease-associated ILD, idiopathic interstitial pneumonia, and sarcoidosis) who had also undergone detection of complement component 3 (C3), complement component 4 (C4), immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), ceruloplasmin (CER), C-reactive protein (CRP), and rheumatoid factor (RF). Healthy age- and sex-matched volunteers were recruited from the First Affiliated Hospital of Guangzhou Medical University.
Diagnosis of lung cancer and ILD
A diagnosis of lung cancer was made based on clinical records, including information on clinical symptoms established by respiratory clinicians, and the results of physical examinations such as chest X-ray and computed tomography scanning.13 Patients who met at least three of the following criteria were clinically diagnosed with ILD:14,15 1) polypnea after physical activity or at rest; 2) cough or Velcro rales; 3) interstitial pulmonary lesions consistent with imaging results (chest X-ray or high-resolution computed tomography), such as honeycombing, ground-glass opacity, lobular interval thickening of peripheral lung; 4) restrictive ventilatory disorder and/or pulmonary exchange dysfunction according to pulmonary function tests (% forced vital capacity, % forced expiratory volume in 1 second, % total lung capacity, % inspiratory capacity, % diffusing capacity of the lungs for carbon monoxide); and 5) pulmonary pathology meeting the 2002 European Society Diagnostic Criteria.
Blood sample testing
Concentrations of serum immune factors including C3, C4, IgA, IgG, IgM, and CER were measured by immunoturbidimetry using an IMMAGE 800 (Beckman Coulter, Brea, CA, USA). CRP and RF concentrations were measured by immunonephelometry using an AU5800 Clinical Chemistry Analyzer (Beckman Coulter). On the basis of the clinical laboratory system used by the First Affiliated Hospital of Guangzhou Medical University, concentrations of CER ≥0.42 g/L, CRP ≥0.6 mg/L, and RF ≥20 IU/mL were considered to be abnormally high.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Numerical data were expressed as percentages, and parametric quantitative data for age and serum C3, C4, CER, CRP, RF, IgA, IgG, and IgM were presented as mean ± standard deviation. Differences among the three groups were analyzed using the non-parametric Kruskal–Wallis test. Optimal scaling was carried out by categorical principal component analysis. Briefly, optimal scaling quantifies categorical data by assigning numerical values to the categories, resulting in an optimal linear regression equation for the transformed variables. When the calculated data are plotted on a two-dimensional graph, the distance between two points represents the strength of the correlation between the two factors (i.e., a shorter distance between two points indicates a higher correlation). A value of P < 0.05 was considered to be statistically significant.
Results
Patients
A total of 539 subjects (271 men, 268 women, age 25–72 years) were included in this study, comprising 262 patients with lung cancer, 220 with ILD, and 57 healthy controls. Detailed patient information is presented in Table 1.
Table 1.
Characteristics of patients with lung cancer, patients with interstitial lung disease, and healthy individuals.
| Characteristic | LC | ILD | Normal |
|---|---|---|---|
| Total (n) | 262 | 220 | 57 |
| Age (y) | 58.63 ± 13.46 | 57.74 ± 11.74 | 56.53 ± 12.62 |
| Sex (male/female) | 154/108 | 89/131 | 28/29 |
| BMI (kg/m2) | 22.08 ± 1.2 | 24.7 ± 2.9 | 24.5 ± 3.2 |
| Cough (%) | 43.9% | 68.1% | 0.0% |
| Hospitalization (%) | 100.0% | 87.4% | 0.0% |
| Combined with rheumatoid arthritis (%) | 32.1% | 42.7% | 0.0% |
LC: lung cancer, ILD: interstitial lung disease, Normal: healthy individuals, BMI: body mass index.
Abnormal levels of CER, CRP, and RF in patients with lung cancer and ILD
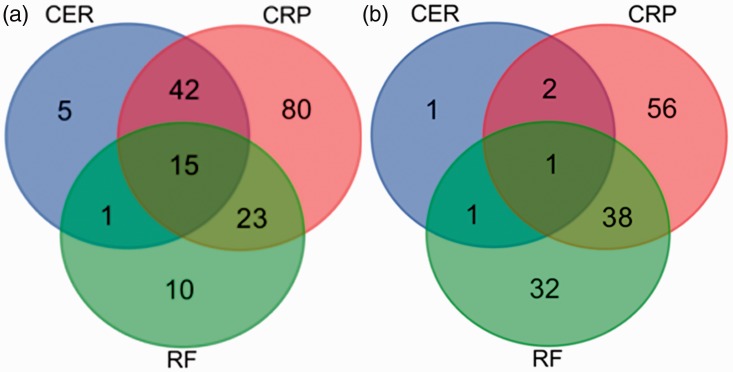
Within the lung cancer and ILD groups, more patients had increased levels of CRP (160/262, [61.1%] and 97/220 [45.5%], respectively) compared with increased levels of either CER or RF. Similarly, the numbers of patients with increased CRP levels alone were highest in both patient groups (80/262 [30.5%] and 56/220 [25.5%], respectively). A significantly higher proportion of patients with lung cancer had increased levels of CER compared with patients with ILD (24.1% vs 2.3%, respectively; (χ2 = 4.16, P = 0.041). In terms of multiple abnormal factors, more patients with lung cancer had increased levels of both CRP and CER (n = 42, 16.03%), while more patients with ILD had elevated levels of both CRP and RF (n = 56, 17.27%), however, these differences were not significant (Figure 1).
Figure 1.
Venn diagrams of abnormally increased levels of CER, CRP, and RF in (a) 262 patients with lung cancer and (b) 220 patients with interstitial lung disease. Levels of CER ≥0.42 g/L, CRP ≥0.6 mg/L and RF ≥20 IU/mL were considered to be abnormally increased. CER: ceruloplasmin, CRP: C-reactive protein, RF: rheumatoid factor.
Comparisons of immune factor among patients with lung cancer or ILD and healthy individuals
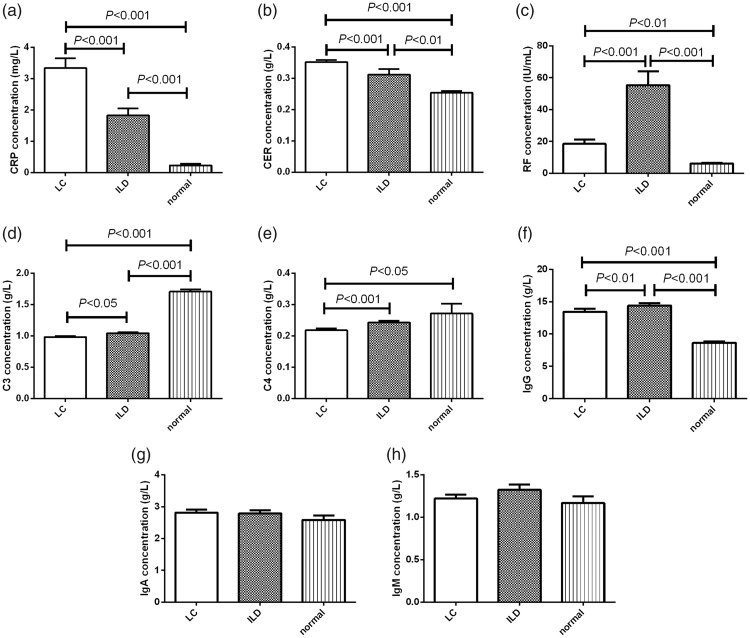
Levels of immune factors are presented in Table 2. CRP and CER levels were significantly higher in patients with lung cancer compared with ILD patients and healthy individuals (Kruskal–Wallis = 72.92 and 74.59, respectively, both P < 0.001) (Figure 2a, 2b). In contrast, RF levels were significantly higher in patients with ILD compared with lung cancer patients and healthy individuals (Kruskal–Wallis = 39.08, P < 0.001) (Figure 2c). Furthermore, C3 levels were significantly higher in healthy individuals compared with ILD and lung cancer patients (Kruskal–Wallis = 137.0, P < 0.001) (Figure 2d), and a similar trend was observed for levels of C4 (Kruskal–Wallis = 74.59, P < 0.001), but there was no significant difference between ILD patients and healthy individuals for C4 (Figure 2e). Additionally, both lung cancer and ILD patients had significantly higher IgG concentrations than healthy individuals (Kruskal–Wallis = 99.33, P < 0.001) (Figure 2f), but there were no differences among the three groups in levels of IgA or IgM (Figure 2g, 2h).
Table 2.
Comparisons of immune factors.
| Immune factor | LC (n = 262) | ILD (n = 220) | Normal (n = 57) | P value |
|---|---|---|---|---|
| CRP (mg/L) | 3.34 ± 5.07 | 1.83 ± 3.14 | 0.23 ± 0.34 | <0.001 |
| CER (g/L) | 0.35 ± 0.10 | 0.31 ± 0.25 | 0.25 ± 0.04 | <0.001 |
| RF (IU/mL) | 18.58 ± 43.47 | 55.46 ± 83.80 | 6.23 ± 2.03 | <0.001 |
| C3 (g/L) | 0.97 ± 0.25 | 1.04 ± 0.26 | 1.70 ± 0.29 | <0.001 |
| C4 (g/L) | 0.21 ± 0.09 | 0.24 ± 0.09g | 0.27 ± 0.24 | <0.001 |
| IgG (g/L) | 13.42 ± 7.56 | 14.38 ± 5.98 | 8.63 ± 1.55 | <0.001 |
| IgA (g/L) | 2.81 ± 1.57 | 2.78 ± 1.53 | 2.58 ± 1.00 | 0.96 |
| IgM (g/L) | 1.22 ± 0.72 | 1.32 ± 0.96 | 1.17 ± 0.59 | 0.77 |
Data expressed as mean ± standard deviation and differences between groups analyzed by Kruskal–Wallis test. P < 0.05 was considered significant. LC: lung cancer, ILD: interstitial lung disease, Normal: healthy individuals, CRP: C-reactive protein, CER: ceruloplasmin, RF: rheumatoid factor.
Figure 2.
Comparisons of (a) CRP, (b) CER, (c) RF, (d) C3, (e) C4, (f) IgG, (g) IgA, and (h) IgM levels among patients with lung cancer, patients with ILD, and healthy individuals. Data expressed as mean ± standard deviation; differences between groups analyzed by Kruskal–Wallis test; P < 0.05 was considered significant. LC: lung cancer (n = 262), ILD: interstitial lung disease (n = 220), normal: healthy individuals (n = 57), CER: ceruloplasmin, CRP: C-reactive protein, RF: rheumatoid factor.
Optimal scaling of immune factors in lung cancer and ILD
Optimal scaling demonstrated that the immune factors RF and IgM were closely associated with the occurrence of ILD, while CRP, CER, C3, and C4 were closely associated with the occurrence of lung cancer (Cronbach’s alpha = 84.7%) (Figure 3). Simultaneous abnormalities in serum levels of CRP, CER, C3, and C4 may thus be associated with the risk of developing lung cancer.
Figure 3.
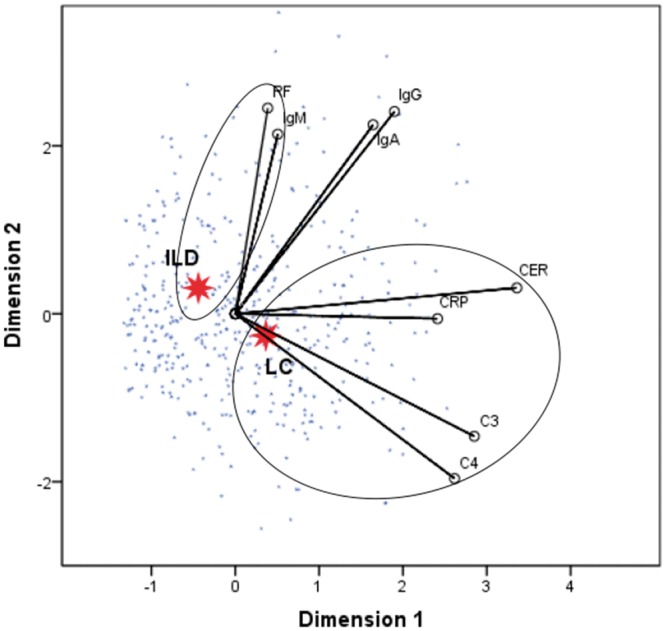
Optimal scaling analysis of immune factors in patients with lung cancer and patients with ILD. Shorter distance between dots indicates a stronger correlation between the disease and the corresponding factor (Cronbach’s alpha = 84.7%). LC: lung cancer (n = 262), ILD: interstitial lung disease (n = 220), CER: ceruloplasmin, CRP: C-reactive protein, RF: rheumatoid factor
Discussion
ILD, first reported in 1975, comprises several disorders with varying clinical presentations. The incidence of ILD continues to increase year on year. ILD is associated not only with a high risk of progression to lung cancer, but is also a significant predictor of a poor prognosis of lung cancer.16,17 Immune factors can reflect the association between lung cancer and ILD.8 We therefore detected levels of the immune factors C3, C4, IgA, IgG, IgM, CER, CRP, and RF in patients with lung cancer or ILD and in healthy controls, and found that lung cancer and ILD patients were most likely to have elevated levels of CRP, as an important indicator of the inflammatory response (61.1% and 45.5% of patients, respectively). Following an inflammatory response, repeated cell injury and repair can increase the risk of abnormal proliferation of epithelial cells in the airway in ILD, ultimately resulting in their transformation into invasive and metastatic cancer cells.18 IgG is also an inflammatory indicator, and our results showed that IgG levels were significantly elevated in both lung cancer and ILD patients compared with healthy individuals. Jiang et al.19 reported that lung cancer-produced IgG is likely to play an important role in cancer growth and metastasis. However, the role of IgG in ILD is still not clear. The increased levels of IgG in ILD patients detected in the current study indicated that IgG may contribute to the progression of ILD to lung cancer, and suggest that CRP and IgG levels should be monitored in patients with ILD.
Levels of complement components were lower in patients with lung cancer or ILD compared with healthy individuals in the present study. C3 and C4 are important components of the complement activation process, and the similar defects in these complement components in both lung cancer and ILD patients may reflect the mechanisms underlying the progress of the diseases. A previous study demonstrated that levels of C3 declined during disease progression in patients with non-small cell lung cancer, suggesting that C3 may have anti-tumor and anti-cancer activities.20,21 Monitoring complement components in patients with ILD may help to assess the risk of disease progression to lung cancer.
Other physiological indicators differ between patients with lung cancer and patients with ILD. For example, plasma CER levels were 4.6-times higher in patients with lung cancer compared with patients with other lung diseases.22 Ninety-five percent of the plasma copper is present in CER.23 As the main oxidase, it is involved in a series of oxidation reactions, including iron and amine metabolism, which mainly occur in the blood.24,25 Activity of the CER oxidase and serum copper concentrations were significantly higher in patients with various types of cancer compared with normal individuals.25 We accordingly found that CER levels were significantly higher in patients with lung cancer compared with ILD patients (P < 0.001). Copper overload in lung cancer patients leads to increased production of CER to participate in a series of oxidation reactions, which can generate abundant reactive oxygen species that can in turn induce cell apoptosis via activation of the calpain and caspase-3 pathway, ultimately leading to tissue damage.26 Damage induced by copper overload is an underlying pathology in many diseases, including cancer and cirrhosis. Oxidative stress has been shown to be significantly increased in patients with advanced lung cancer, while levels of antioxidant molecules were decreased.27 In addition, copper is a basic component in cancer metastasis, and many studies of tumor latency have shown that copper consumption can reduce the proliferation, growth, metastasis, and blood supply to cancer cells.28 Relevant animal studies demonstrated that CER protein and mRNA levels were increased in GPrc5a (an anti-oncogene) knockout compared with normal mice, and CER expression was also increased in lung neoplasms.29 The level of CER may thus reflect the growth status and metastasis of lung cancer, suggesting that monitoring CER levels may aid the diagnosis and clinical observation of lung cancer patients.
The current results also revealed that RF levels were significantly higher in ILD compared with lung cancer patients, which was in turn significantly higher than in healthy individuals (P < 0.05). RF is an IgM autoantibody with a high detection rate in patients with rheumatoid arthritis.5 A UK cohort study showed that, among 1460 patients with rheumatoid arthritis, 25% had ILD and a further 25% developed ILD within 3 years following the diagnosis of rheumatoid arthritis.14 We therefore hypothesized that rheumatoid arthritis–ILD–lung cancer may represent a novel disease process.
Finally, optimal scaling showed that ILD was closely associated with RF and IgM while lung cancer was closely associated with CRP, CER, C3, and C4. This was similar to the findings of Aref and Refaat, who indicated that the increases in CRP and CER and decreases in C3 and C4 in lung cancer patients were possibly due to the continuous inflammatory response, decreased complement component function, and increased blood plasma copper in these patients.30 Elevated CRP reflects the inflammatory response, while decreased levels of C3 and C4 reflect defects in complement component functions.18,20 Given that IgM plays a vital role in autoimmune diseases, we speculate that the development of ILD may be closely related to autoimmune diseases.
The major limitation of this study was the lack of follow-up data for the patients, which therefore prevented us from investigating dynamic changes in the immune factors during the transition from ILD to lung cancer. Furthermore, we had no data on exposure to other risk factors for lung cancer (e.g., smoking, toxins, family history) and were therefore unable to account for these factors in this study. Further studies involving long-term observations of immune factors together with detailed information on exposure to lung cancer risk factors are therefore required.
Conclusions
Patients with lung cancer or ILD have lower levels of C3 and C4 and higher levels of CRP compared with healthy individuals. In addition, ILD was associated with elevated levels of RF while lung cancer was associated with increased CER compared with healthy individuals. Clinical monitoring of CER may thus support the diagnosis and monitoring of patients with lung cancer. CRP, CER, C3, and C4 were also closely associated with the development of lung cancer, suggesting that patients with ILD with simultaneously elevated levels of CRP and CER and decreased levels of C3 and C4 should be considered to be at potentially increased risk of progression to lung cancer.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by the National Natural Science Foundation of China (Project No.: 81871736), Bureau of Traditional Chinese Medicine Scientific Research Project of Guangdong (Project No.: 20192048), Science and Technology Innovation Committee Project of Guangzhou (Project No.: 201831802), and Open Project of State Key Laboratory of Respiratory Disease (Project No.: SKLRD-OP-201803, SKLRD-OP-201805 and SKLRD-OP-201809).
References
- 1.He Y, Li D, Song G, et al. Lung cancer burden has increased during the last 40 years in Hebei Province, China. Thoracic Cancer 2016; 7: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh WN, Kong KA, Han Y, et al. Risk factors associated with treatment refusal in lung cancer. Thoracic Cancer 2017; 8: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 4.Archontogeorgis K, Steiropoulos P, Tzouvelekis A, et al. Lung cancer and interstitial lung diseases: a systematic review. Pulmonary Medicine 2012; 2012: 315918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards TJ, Eggebeen A, Gibson K, et al. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum 2009; 60: 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015; 149: 64–69, 70.e1-2. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki K, Satoh H, Kurishima K, et al. Interstitial lung disease in patients with small cell lung cancer. Med Oncol 2010; 27: 763–767. [DOI] [PubMed] [Google Scholar]
- 8.Bouros D, Hatzakis K, Labrakis H, et al. Association of malignancy with diseases causing interstitial pulmonary changes. Chest 2002; 121: 1278–1289. [DOI] [PubMed] [Google Scholar]
- 9.Enomoto Y, Inui N, Yoshimura K, et al. Lung cancer development in patients with connective tissue disease–related interstitial lung disease: a retrospective observational study. Medicine 2016; 95: e5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Li H, Wu N, et al. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 2017; 36: 817–823. [DOI] [PubMed] [Google Scholar]
- 11.Ajona D, Razquin C, Elevated levels of the complement activation product C4d in bronchial fluids for the diagnosis of lung cancer. PLoS One 2015; 10: e0119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin K, He S, He L, et al. Complement component 3 is a prognostic factor of non‑small cell lung cancer. Mol Med Rep 2014; 10: 811–817. [DOI] [PubMed] [Google Scholar]
- 13.Wender R, Fontham ETH, Barrera E, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013; 63: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavagna L, Monti S, Grosso V, et al. The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res Int 2013; 2013: 759760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 2002; 19: 794–796. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Lee J, Park YS, et al. Impact of smoking on mortality of patients with non-small cell lung cancer. Thoracic Cancer 2014; 5: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Campos JL, Rodríguez-Becerra E. Incidence of interstitial lung diseases in the south of Spain 1998-2000: the RENIA study. Eur J Epidemiol 2004; 19: 155–161. [DOI] [PubMed] [Google Scholar]
- 18.Bestaev DV, Karateev DE, Nasonov EL. Diagnosis and problems in therapy of interstitial lung disease associated with rheumatoid arthritis. TerArkh 2013; 85: 84–91. [PubMed] [Google Scholar]
- 19.Jiang C, Huang T, Wang Y, et al. Immunoglobulin G expression in lung cancer and its effects on metastasis. PLoS One 2014; 9: e97359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Tomeczko J, Napora P, Dlubek D, et al. Adjuvant spleen ultrafiltrate immunotherapy in unresectable advanced non-small cell lung cancer. Arzneimittel-Forschung 1997; 47: 1411–1416. [PubMed] [Google Scholar]
- 21.Merle NS, Church SE, Fremeauxbacchi V, et al. Complement system part I – molecular mechanisms of activation and regulation. Front Immunol 2015; 6: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn YH, Ji ES, Oh NR, et al. Differential proteomic approach for identification and verification of aberrantly glycosylated proteins in adenocarcinoma lung cancer (ADLC) plasmas by lectin-capturing and targeted mass spectrometry. J Proteomics 2014; 106: 221–229. [DOI] [PubMed] [Google Scholar]
- 23.Chan N, Willis A, Kornhauser N, et al. Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin Cancer Res 2017; 23: 666–676. [DOI] [PubMed] [Google Scholar]
- 24.Cortes L, Roberts BR, Wedd AG, et al. Molecular aspects of a robust assay for ferroxidase function of ceruloplasmin. Inorg Chem 2017; 56: 5275–5284. [DOI] [PubMed] [Google Scholar]
- 25.Zowczak M, Iskra M, Paszkowski J, et al. Oxidase activity of ceruloplasmin and concentrations of copper and zinc in serum of cancer patients. J Trace Elem Med Biol 2001; 15: 193–196. [DOI] [PubMed] [Google Scholar]
- 26.Arnal N, de Alaniz MJ, Marra CA. Cytotoxic effects of copper overload on human-derived lung and liver cells in culture. Biochim Biophys Acta 2012; 1820: 931–939. [DOI] [PubMed] [Google Scholar]
- 27.Esme H, Cemek M, Sezer M, et al. High levels of oxidative stress in patients with advanced lung cancer. Respirology 2008; 13: 112–116. [DOI] [PubMed] [Google Scholar]
- 28.Fouani L, Menezes SV, Paulson M, et al. Metals and metastasis: exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharmacol Res 2017; 115: 275–287. [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Guo W, Hu S, et al. Gprc5a-knockout mouse lung epithelial cells predicts ceruloplasmin, lipocalin 2 and periostin as potential biomarkers at early stages of lung tumorigenesis. Oncotarget 2017; 8: 13532–13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aref H, Refaat S. CRP evaluation in non-small cell lung cancer. Egypt J Chest Dis Tuberc 2014; 63: 717–722. [Google Scholar]