Short abstract
Objective
Femoral artery puncture (FAP) is an effective method for interventional percutaneous vascular procedures. However, FAP leads to complications including hematomas and pseudoaneurysms. This study was performed to determine whether pituitrin infusion or vascular closure devices (VCDs) increase the risk of complications after FAP.
Methods
This single-center retrospective study included 3641 patients who underwent FAP. The patients were divided into two groups: a groin complication group (including hematomas and pseudoaneurysms) and a no-groin complication group.
Results
In the multivariate analysis, perioperative pituitrin infusion and the use of VCDs were strongly associated with inguinal hematomas and pseudoaneurysms. The complication rate was obviously higher in patients who underwent bronchial artery embolization (BAE). Because high dosages of pituitrin and VCDs were used in patients undergoing BAE, postoperative hematoma development occurred significantly earlier in these patients. Hematomas occurred within 14 days of the operation in all patients who underwent BAE.
Conclusion
Perioperative pituitrin infusion and the use of VCDs are associated with an increased risk of complications after FAP, including hematomas and pseudoaneurysms. Notably, patients who underwent BAE, who are subject to higher pituitrin and VCD use, showed a higher complication rate. The incidence of complications was highest within 2 weeks postoperatively.
Keywords: Pituitrin, femoral artery puncture, inguinal hematoma, vascular closure devices (VCDs), bronchial artery embolization (BAE), pseudoaneurysm
Introduction
Femoral artery puncture (FAP) through the modified Seldinger technique is a relatively safe and effective method for interventional percutaneous vascular procedures, such as thoracic aortic surgery, bronchial artery embolization (BAE), and coronary intervention.1–3 However, FAP is associated with local vascular complications including bleeding, arteriovenous fistula formation, venous thrombosis, hematomas, and pseudoaneurysms.4 The vascular complication rate from transfemoral arterial access ranges from 0.6% to 18.0%.4–6 Hematomas and pseudoaneurysms are the most common complications.7 Three types of risk factors for hematomas and pseudoaneurysms have been identified: patient-, technique-, and pharmacotherapy-related risk factors.4
Pituitrin is a vasoconstrictor and hemostat that consists of oxytocin (OT) and vasopressin (VP). OT and VP share a similar molecular structure and are both released by the posterior pituitary.8,9 OT is an effective agent for prevention of primary postpartum hemorrhage.10 VP regulates contraction of smooth muscle cells through VP receptor subtype V1a.11 Hence, VP is a key drug in the cure of hemoptysis caused by pulmonary diseases such as tuberculosis and bronchiectasis.12,13 In patients with hemoptysis who are irresponsive to VP or pituitrin, BAE is a substitutive treatment.14 Notably, BAE requires FAP.2 Pituitrin is commonly used in the perioperative period of FAP.15
We found that patients who received pituitrin and underwent FAP developed hematomas or pseudoaneurysms at the access sites. We thus hypothesized that pituitrin increases the incidence of FAP complications, including hematomas and pseudoaneurysms. In the present study, we investigated the role of pituitrin in the development of FAP complications.
Patients and methods
Patients
In this single-center retrospective study, we collected data from hospital records in Sir Run Run Shaw Hospital from January 2012 to December 2016. In total, 3641 patients with available outcome data who underwent FAP with the standardized Seldinger technique were enrolled in this study. Patients who underwent interventional operations through other vessels, such as the femoral vein or internal jugular vein, were excluded from this study (n = 301). All operators were surgeons from the same group of the Invasive Technology Department and were equally experienced in interventions involving both radial and femoral access.
This study was performed in accordance with the guidelines of the Ethics Committee of Sir Run Run Shaw Hospital.
Methods
A groin hematoma was defined as blood loss at the access site resulting in blood transfusion, a drop in the hemoglobin level of 3 g/L per day, and development of a ≥5-cm hematoma at the incision site.16 Groin hematomas were differentiated from pseudoaneurysms based on the blood signals on B-scan ultrasonography: blood flow signals were absent in hematomas but present in pseudoaneurysms.
We used high blood pressure and cardiovascular disease as risk factors for hematoma occurrence. The blood pressure level before and after the operation were defined and classified according to the 2013 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension.17 Cardiovascular diseases included peripheral vascular disease, coronary heart disease, carotid atherosclerosis, intracranial atherosclerosis, and atherosclerosis of both lower extremities. Blood diseases included coagulation disorders, thrombocytopenia, hemophilia, leukemia, thrombocythemia, hypofibrinogenemia, diffuse intravascular coagulation, and others. We collected data on pituitrin use and the methods of hemostasis after the operation. Generally, in patients who underwent BAE, 12 to 24 U of pituitrin was diluted in 50 mL of saline and administered at 0.48 to 2.4 U/hour by continuous infusion until the bleeding stopped. FAP was performed through 5- to 9-French arterial sheaths. The arterial sheaths were removed once the operation ended. A vascular closure device (VCD) or manual compression (MC) was used to stop bleeding. The ProGlide (Abbott Vascular, Santa Clara, CA, USA) and Angio-Seal (Terumo Corp., Tokyo, Japan) were included among the VCDs. Patients with VCDs underwent application of pressure bandaging, while the remaining patients underwent MC for 15 to 20 minutes followed by application of pressure bandaging. Patients who had undergone MC stayed in bed for 24 hours, and the affected limb was mechanically braked for about 12 hours. Patients with VCDs stayed in bed for 4 to 5 hours, and the affected limb was mechanically braked for 4 to 6 hours. Some patients received anticoagulants daily, but they stopped these medications before the operation. Anticoagulants included aspirin, clopidogrel, warfarin, and low-molecular-weight heparin. Written informed consent was obtained from all patients.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (range or interquartile range) and were compared using Student’s t test or the Mann–Whitney–Wilcoxon test. The Shapiro test and Q–Q plots were used to check the normality of the continuous variables. Nominal variables are presented as frequency and percentage and were compared using the chi-squared test or Fisher’s exact test when appropriate. Group proportions were compared by Fisher’s exact test. A two-tailed P value of <0.05 was considered statistically significant.
A multivariate logistic regression model was used to identify the independent predictors of hematoma formation. Variables with a P value of <0.05 in the univariate analysis were included in the multivariable models. The relevance was presented as odds ratios (ORs) with 95% confidence intervals (CIs). Kaplan–Meier survival curves were constructed to investigate the relationship between hematoma formation and BAE. Receiver operating characteristic (ROC) curves were constructed to estimate the optimal method for predicting hematoma occurrence. All statistical analyses were two-tailed, and P < 0.05 was regarded as statistically significant. Analyses were carried out with SPSS 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
Results
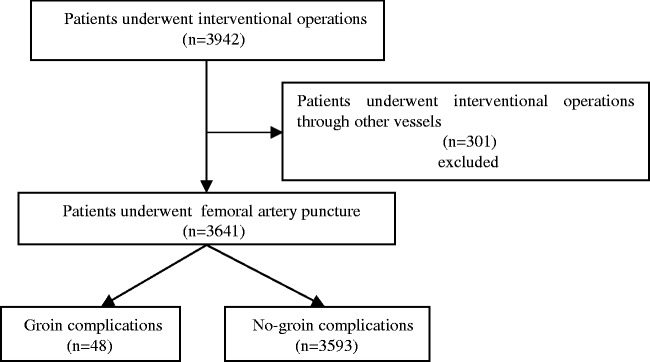
The patient screening process is shown in Figure 1. In total, 3641 patients were screened for enrollment in the study; 301 patients were excluded because interventional operations were performed through other vessels, such as the femoral vein or internal jugular vein, and 325 patients were excluded because of lack of data on the blood pressure level before and after the operation.
Figure 1.
Flow chart showing the selection of patients undergoing femoral artery puncture (FAP) for this study. The total number of patients was 3942, and 301 patients were excluded from this study because they underwent interventional operations through other vessels such as the femoral vein or internal jugular vein. A total of 3641 patients underwent FAP, and they were divided into two groups: a groin complication group (including hematomas or pseudoaneurysms) (n = 48) and a no-groin complication group (n = 3593).
The patients’ baseline characteristics and clinical outcomes are shown in Table 1. The patients were divided into a groin complication group (including hematomas and pseudoaneurysms) and a no-groin complication group. Among the 3641 patients, sex was not significantly different between the two groups (male: 62.5% vs. 68.9%). Likewise, age was not significantly different between the two groups. There were no significant differences in the proportion of patients with hypertension between the two groups. In addition, similar numbers of patients with cardiovascular diseases other than hypertension were present in the groin complication group and no-groin complication group (43.8% vs. 31.0%). The distribution of blood diseases was also similar between the two groups. Furthermore, differences in blood pressure before and after the operation were not statistically significant. To achieve hemostasis after percutaneous FAP, the patients underwent either use of VCDs or application of MC. We recorded data from 3308 of 3641 patients. Twenty-five patients in the groin complication group were treated with VCDs, which was significantly higher than the number of patients in the no-groin complication group (52.08% vs. 22.46%, P < 0.001). We further compared pituitrin use in the two groups. We found that 37.50% (18 patients) in the groin complication group were treated with pituitrin in the perioperative period, whereas only 3.84% (138 patients) in the no-groin complication group received pituitrin (P < 0.001). The use of anticoagulants in both groups was not significantly different (14.6% vs. 8.7%). Finally, there was no difference in the distribution of arterial sheath sizes between the two groups.
Table 1.
Demographic and clinical characteristics of patients who underwent femoral artery puncture.
| All(n = 3641) | Groin complications(n = 48) | No-groin complications (n = 3593) | P value | Missing | |
|---|---|---|---|---|---|
| Age range, years | 3641 | 0.088 | |||
| ≤39 | 430 | 6 (12.50) | 424 (11.80) | ||
| 40–49 | 675 | 7 (14.58) | 668 (18.59) | ||
| 50–59 | 1061 | 10 (20.83) | 1051 (29.25) | ||
| 60–69 | 953 | 13 (27.08) | 940 (26.16) | ||
| 70–79 | 418 | 7 (14.58) | 411 (11.44) | ||
| ≥80 | 104 | 5 (10.42) | 99 (2.76) | ||
| Male sex | 2506 | 30 (62.50) | 2476 (68.91) | 0.341 | |
| Classification of BP level before operation, mmHg | 0.061 | 325 | |||
| Normal | 1475 | 13 (27.08) | 1462 (40.69) | ||
| High normal | 1125 | 20 (41.67) | 1105 (30.75) | ||
| Grade 1 | 548 | 9 (18.75) | 539 (15.00) | ||
| Grade 2 | 129 | 4 (8.33) | 125 (3.48) | ||
| Grade 3 | 39 | 1 (2.08) | 38 (1.06) | ||
| Classification of BP level after operation, mmHg | 0.320 | 325 | |||
| Normal | 948 | 11 (22.92) | 937 (26.08) | ||
| High normal | 1166 | 18 (37.50) | 1148 (31.95) | ||
| Grade 1 | 832 | 9 (18.75) | 823 (22.91) | ||
| Grade 2 | 281 | 7 (14.58) | 274 (7.63) | ||
| Grade 3 | 89 | 2 (4.17) | 87 (2.42) | ||
| Difference in blood pressure between preoperative and postoperative period, mmHg | 7.09 ± 12.898 | 6.33 ± 14.133 | 0.989 | ||
| Cardiovascular diseases | 21 (43.75) | 1113 (30.98) | 0.058 | ||
| Blood diseases | 1 (2.08) | 74 (2.06) | 1.000 | ||
| Perioperative pituitrin use | 18 (37.50) | 138 (3.84) | <0.001 | ||
| Hemostasis | <0.001 | 333 | |||
| VCDs | 25 (52.08) | 807 (22.46) | |||
| MC | 21 (43.75) | 2455 (68.33) | |||
| Anticoagulant use | 7 (14.60) | 312 (8.70) | 0.19 | ||
| Size of arterial sheaths (French) | 0.147 | 101 | |||
| 5 | 31 (64.58) | 2748 (76.48) | |||
| 6 | 13 (27.08) | 687 (19.12) | |||
| 7 | 1 (2.08) | 27 (0.75) | |||
| 8 | 0 (0.00) | 30 (0.83) | |||
| 9 | 0 (0.00) | 3 (0.08) |
Data are presented as the mean ± standard deviation or n (%). P values were calculated using the Mann–Whitney–Wilcoxon test, chi-squared test, or Fischer’s exact test. BP, blood pressure; VCDs, vascular closure devices, including ProGlide and Angio-Seal; MC, manual compression. Groin complications included hematomas and pseudoaneurysms. Anticoagulants included aspirin, clopidogrel, warfarin, and low-molecular-weight heparin. Blood diseases included coagulation disorders, thrombocytopenia, hemophilia, leukemia, thrombocythemia, hypofibrinogenemia, and diffuse intravascular coagulation.
Next, we explored the effects of pituitrin by dividing the patients into a BAE group and a control group because only patients who underwent BAE received pituitrin (Table 2). The rate of perioperative pituitrin use was 156 of 208 patients in the BAE group, while none of the 3433 patients in the control group received pituitrin. The rate of hematoma and pseudoaneurysm formation was substantially higher in the BAE group than control group (19/208 vs. 29/3433, P < 0.001). The rate of perioperative pituitrin use was higher in the BAE group than control group (156/208 vs. 0/3433, P < 0.001). A higher proportion of patients was treated with VCDs for hemostasis after FAP in the BAE group than control group (107/208 vs. 863/3433, P < 0.001), indicating delayed hemostasis in the BAE group after FAP.
Table 2.
Comparison of three main factors between BAE group and control group.
| All(n = 3641) | BAE group(n = 208) | Control group(n = 3433) | P value | |
|---|---|---|---|---|
| Perioperative pituitrin use | 156 | 156 | 0 | <0.001 |
| Hemostasis | <0.001 | |||
| VCDs | 970 | 107 (51.44) | 863 (25.14) | |
| MC | 2529 | 96 (46.15) | 2433 (70.87) | |
| Missing | 333 | 5 (2.40) | 328 (9.55) | |
| Hematomas and pseudoaneurysms | 48 | 19 (9.13) | 29 (0.82) | <0.001 |
Data are presented as n (%). P values were calculated using the chi-squared test. BAE, bronchial artery embolization; VCDs, vascular closure devices, including ProGlide and Angio-Seal; MC, manual compression.
Because not all patients who undergo BAE receive pituitrin, we further separated the patients who underwent BAE into two groups: a pituitrin group and a no-pituitrin group as shown in Table 3. The incidence of hematoma formation was much higher in the pituitrin group (11.5% vs. 1.9%, P < 0.05). There were no significant differences in other characteristics. These data indicate that pituitrin increased frequency of hematoma formation after FAP.
Table 3.
Comparison of main factors between pituitrin group and no-pituitrin group among patients who underwent bronchial artery embolization.
| All(n = 208) | Pituitrin group(n = 156) | No-pituitrin group(n = 52) | P value | Missing | |
|---|---|---|---|---|---|
| Hematoma and pseudoaneurysms | 18 (11.54) | 1 (1.92) | <0.05 | ||
| Male sex | 108 (69.23) | 32 (61.54) | 0.394 | ||
| Hemostasis | 0.748 | 5 | |||
| VCDs | 81 (51.92) | 26 (50.00) | |||
| MC | 70 (44.87) | 26 (50.00) | |||
| Blood disease | 1 (0.64) | 2 (3.85) | 0.155 | ||
| Cardiovascular diseases | 54 (34.62) | 17 (32.69) | 0.867 | ||
| Age range, years | 0.925 | ||||
| ≤39 | 12 (7.70) | 3 (5.77) | |||
| 40–49 | 15 (9.62) | 7 (13.46) | |||
| 50–59 | 33 (21.15) | 13 (25.00) | |||
| 60–69 | 54 (34.61) | 18 (36.62) | |||
| 70–79 | 30 (19.23) | 8 (15.38) | |||
| ≥80 | 12 (7.70) | 3 (5.77) | |||
| Classification of BP level before operation, mmHg | 0.178 | 35 | |||
| Normal | 41 (26.28) | 16 (30.77) | |||
| High normal | 43 (27.56) | 9 (17.31) | |||
| Grade 1 | 26 (16.66) | 13 (25.00) | |||
| Grade 2 | 12 (7.69) | 5 (9.61) | |||
| Grade 3 | 8 (5.13) | 0 (0.00) | |||
| Classification of BP level after operation, mmHg | 0.408 | 35 | |||
| Normal | 35 (22.44) | 18 (34.62) | |||
| High normal | 43 (27.56) | 13 (25.00) | |||
| Grade 1 | 37 (23.72) | 10 (19.23) | |||
| Grade 2 | 11 (7.05) | 2 (3.85) | |||
| Grade 3 | 4 (2.56) | 0 (0.00) |
Data are presented as n (%). BP, blood pressure; VCDs, vascular closure devices, including ProGlide and Angio-Seal; MC, manual compression.
Because perioperative pituitrin use and hemostasis were significantly different between these two groups, these two factors were included in the multivariable logistic regression model (Table 4). MC was selected as a dummy variable, also known as a reference variable. This model was regressed on hematoma and pseudoaneurysm occurrence. In the multivariate analysis, pituitrin infusion in the perioperative period was strongly associated with inguinal hematoma formation (OR, 10.46; 95% CI, 5.47–20.02). We also found that if VCDs were used to stop bleeding, the rate of inguinal hematoma increased by 2.5-fold compared with MC (OR, 2.51; 95% CI, 1.36–4.65).
Table 4.
Risk factors for inguinal hematomas and pseudoaneurysms.
| Risk factor | OR (95% CI) |
|---|---|
| Perioperative pituitrin use | 10.46 (5.47–20.02) |
| Hemostasis method | 2.51 (1.36–4.65) |
| VCDs | |
| MC |
OR, odds ratio; CI, confidence interval; VCDs, vascular closure devices, including ProGlide and Angio-Seal; MC, manual compression.
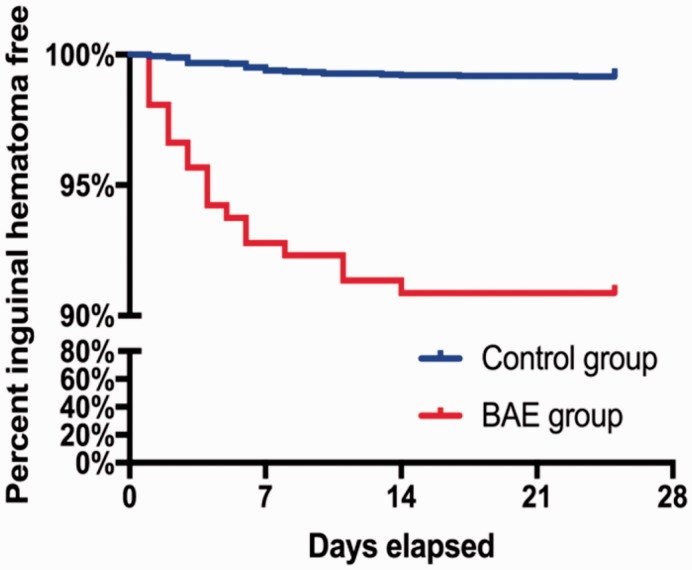
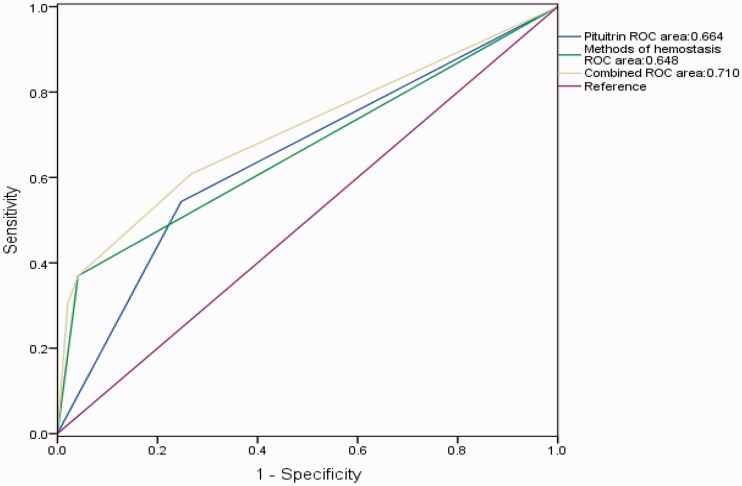
The time of pituitrin cessation was defined as time zero. The numbers of days before and after cessation of pituitrin were recorded when a hematoma or pseudoaneurysm occurred (Figure 2). The number of patients with complications after the cessation of pituitrin was double the number before pituitrin use. We further performed survival analysis and found that hematomas occurred significantly earlier in the BAE group than control group after the operation (P < 0.001) (Figure 3). However, the accumulated incidence of hematoma in the BAE group did not increase after 14 days. The predictive accuracy of pituitrin for hematoma formation was examined by ROC curve analysis. The area under the ROC curve was 0.664 (95% CI, 0.570–0.759; P < 0.001) for pituitrin use and 0.648 (95% CI, 0.563–0.733; P = 0.001) for the method of hemostasis. The combination of these two factors had relatively high accuracy for predicting hematoma formation (95% CI, 0.619–0.800; P < 0.001) with an area under the ROC curve of 0.710 (Figure 4).
Figure 2.
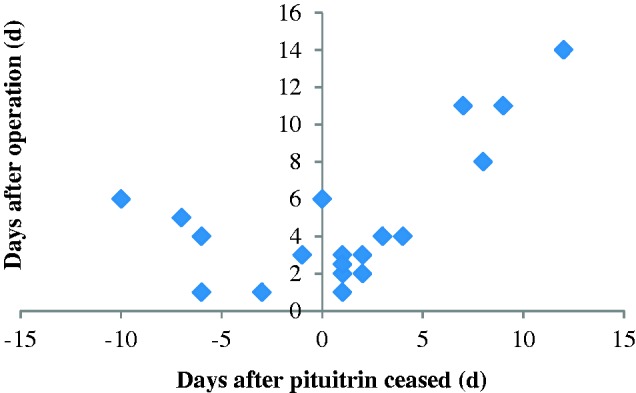
Distribution of patients with hematomas after pituitrin cessation and the operation time in the bronchial artery embolization (BAE) group. The horizontal axis represents the number of days after cessation of pituitrin. The time point of pituitrin cessation was regarded as time 0. If the hematoma occurred after cessation of pituitrin, then it was marked as a positive number, and vice versa. The vertical axis represents the number of days after the operation. The scatterplot in the second quadrant was twice the number in the first quadrant. One patient had a hematoma on the day of pituitrin cessation, which was the sixth day after the operation (0,6). Eleven patients had hematomas within 5 days of ceasing pituitrin.
Figure 3.
Kaplan–Meier survival curves in the bronchial artery embolization (BAE) group and control group. Over time, the number of patients with hematomas increased until the 14th day postoperatively in the BAE group. After that day, there was no significant difference between the two groups.
Figure 4.
Receiver operating characteristic curves for predicting hematomas or pseudoaneurysms.
Discussion
Our study showed that the use of perioperative pituitrin and VCDs was highly correlated with complications after FAP, including hematomas or pseudoaneurysms. Controversial evidence exists regarding the use of VCDs or MC. Castillo-Sang et al.6 reported that the use of VCDs in high-risk patients decreased the incidence of groin complications. Another study showed that patients could be physically active earlier in the postoperative period when the Angio-Seal was used.18 In contrast, Chaturvedi19 reported that complications were more severe when VCDs were used. Lee et al.3 also commented on the controversy regarding VCDs versus MC. Both devices are currently used. Our results showed that MC was associated with a lower incidence of groin complications than were VCDs. This finding may be due to the patient getting out of bed earlier after the operation when MC was used. In contrast, the VCD materials occasionally crack, leading to poor adhesion between the anchor polymer and the blood vessel and thus delaying the recovery of patients. We suggest that the anchor polymer should be properly pushed to ensure that the anchor polymer and blood vessel wall are closely adhered to each other during occlusion. At the same time, the patients should not engage in too much activity after the operation, and the increase in activity should be gradual.
Pituitrin is a vasoconstrictor consisting of OT and VP.8,9 OT prevents primary postpartum hemorrhage10 and VP causes contraction of small pulmonary arteries, which facilitates hemostasis.11 VP is also used for refractory hypotension in patients with severe sepsis.20 Hence, pituitrin has been used to treat hemoptysis caused by several pulmonary diseases.12,13 However, few reports have discussed the relationship between pituitrin use and groin complications after FAP. Our results indicate that pituitrin is positively related to groin hematoma or pseudoaneurysm formation, two common groin complications after FAP. These findings are likely caused by the vasoconstriction induced by pituitrin, which makes the vessel fragile and easy to rupture after injury. The femoral artery tends to dilate after the cessation of pituitrin, which renders the puncture site prone to splitting, leading to further complications. Moreover, pituitrin may cause vasculitis, which impairs wound healing in the femoral artery.
In the present study, the complication rate was significantly higher in patients who underwent BAE, who often received perioperative pituitrin and VCDs. The number of patients who developed hematomas after pituitrin cessation was double the number before pituitrin cessation. Interestingly, the onset of hematomas in the BAE group was significantly earlier than that in the control group. However, the accumulated incidence of hematomas in the BAE group did not increase further 14 days after pituitrin cessation. These results suggest that the first 2 weeks after the operation is a critical window during which the incidence of hematoma or pseudoaneurysm should be closely monitored. ROC curve analysis showed that both perioperative pituitrin and VCDs were important predictors of hematoma occurrence. The combination of these two factors had relatively high accuracy in predicting hematomas. Most patients recovered after conservative treatment for hematomas or pseudoaneurysms, and few needed surgery.
Two main limitations of this study should be noted. First, pituitrin use was limited to patients who underwent BAE because it is regarded as a method of perioperative adjuvant hemostasis for hemoptysis. Second, the number of patients who developed hematomas or pseudoaneurysms was relatively small among all patients who underwent BAE. Future studies with larger cohorts are needed to validate these results.
Besides reducing the unnecessary use of pituitrin and VCDs in clinical practice, we should pay attention to the incidence of inguinal hematomas and pseudoaneurysms for patients in whom pituitrin and VCDs are used perioperatively, especially within 2 weeks of the operation. When inguinal hematomas or pseudoaneurysms are suspected, B-scan ultrasonography should be used to confirm these complications in a timely manner.
Conclusions
In the present study, we found that perioperative pituitrin and VCDs were the main risk factors for hematomas or pseudoaneurysms after FAP. As a common interventional operation, BAE had a higher complication rate, and these complications were strongly correlated with frequent use of pituitrin and VCDs in patients undergoing BAE. All hematomas and pseudoaneurysms occurred within 14 days of the operation in patients who underwent BAE, indicating that this is an essential clinical window to monitor and treat hematomas or pseudoaneurysms.
Authors’ contributions
F.W. and H.S. wrote the main manuscript text. F.W., X.W., and H.W. collected the data and prepared Tables 1 to 4. D.X. revised the paper. Y.Z. prepared Figures 1 to 4, and F.Z. was active in planning the study and participated in the writing of the manuscript. All authors reviewed the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by a research grant (Grant No. 81570043) from the National Natural Science Foundation of China and The Natural Science Foundation of Zhejiang Province, China (Grant Nos. LY19H010003 and LQ15H010001).
References
- 1.Tsiouris A, Elkinany S, Ziganshin BA, et al. Open Seldinger-guided femoral artery cannulation technique for thoracic aortic surgery. Ann Thorac Surg 2016; 101: 2231–2235. [DOI] [PubMed] [Google Scholar]
- 2.Syha R, Benz T, Hetzel J, et al. Bronchial artery embolization in hemoptysis: 10-year survival and recurrence-free survival in benign and malignant etiologies - a retrospective study. Rofo 2016; 188: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 3.Lee MS, Applegate B, Rao SV, et al. Minimizing femoral artery access complications during percutaneous coronary intervention: a comprehensive review. Catheter Cardiovasc Interv 2014; 84: 62–69. [DOI] [PubMed] [Google Scholar]
- 4.Irani F, Kumar S, Colyer WR., Jr. Common femoral artery access techniques: a review. J Cardiovasc Med (Hagerstown) 2009; 10: 517–522. [DOI] [PubMed] [Google Scholar]
- 5.El-Mawardy M, Schwarz B, Landt M, et al. Impact of femoral artery puncture using digital subtraction angiography and road mapping on vascular and bleeding complications after transfemoral transcatheter aortic valve implantation. EuroIntervention 2017; 12: 1667–1673. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Sang M, Tsang AW, Almaroof B, et al. Femoral artery complications after cardiac catheterization: a study of patient profile. Ann Vasc Surg 2010; 24: 328–335. [DOI] [PubMed] [Google Scholar]
- 7.Haude M, Degen H, Schillings C, et al. Femoral artery puncture. EuroIntervention 2010; 5: 751. [DOI] [PubMed] [Google Scholar]
- 8.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 2012; 35: 649–659. [DOI] [PubMed] [Google Scholar]
- 9.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron 2012; 76: 142–159. [DOI] [PubMed] [Google Scholar]
- 10.Adnan N, Boland F, Murphy DJ. Intramuscular oxytocin versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery (LabOR trial): study protocol for a randomised controlled trial. Trials 2017; 18: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamma G, Goswami N, Reichmuth J, et al. Aquaporins, vasopressin, and aging: current perspectives. Endocrinology 2015; 156: 777–788. [DOI] [PubMed] [Google Scholar]
- 12.Aguet F. Hemoptysis and its treatment. Indications in bronchopneumology of a new synthetic posterior pituitary hormone. Schweiz Med Wochenschr 1964; 94: 628–633. [PubMed] [Google Scholar]
- 13.Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care Illustrative case 7: assessment and management of massive haemoptysis. Thorax 2003; 58: 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruchter O, Schneer S, Rusanov V, et al. Bronchial artery embolization for massive hemoptysis: long-term follow-up. Asian Cardiovasc Thorac Ann 2015; 23: 55–60. [DOI] [PubMed] [Google Scholar]
- 15.Noseworthy TW, Anderson BJ. Massive hemoptysis. CMAJ 1986; 135: 1097–1099. [PMC free article] [PubMed] [Google Scholar]
- 16.Cakici M, Yazicioglu L, Baran C, et al. A retrospective analysis of surgical femoral artery closure techniques: conventional versus purse suture technique. Ann Vasc Surg 2017; 44: 103–112. [DOI] [PubMed] [Google Scholar]
- 17.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357. [DOI] [PubMed] [Google Scholar]
- 18.Rittger H, Schmidt M, Breithardt OA, et al. Cardio-respiratory exercise testing early after the use of the Angio-Seal system for arterial puncture site closure after coronary angioplasty. EuroIntervention 2011; 7: 242–247. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi P. Prospective nonrandomized trial of manual compression and Angio-seal and StarClose arterial closure devices in common femoral punctures. Cardiovasc Intervent Radiol 2007; 30: 812. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]