Short abstract
Objectives
We investigated the role of long non-coding (lnc) RNA-NEF (neighboring enhancer of FoxA2), a characterized oncogene in cancer biology, in postmenopausal osteoporosis and its diagnostic and prognostic value in this disease.
Methods
Expression of lncRNA-NEF in plasma was detected by RNA extraction and real-time quantitative PCR. The diagnostic value of lncRNA-NEF and interleukin (IL)-6 for postmenopausal osteoporosis was evaluated by receiver operating characteristic curve analysis, with postmenopausal osteoporosis patients as true positive cases and healthy volunteers as true negative cases.
Results
We showed that plasma lncRNA-NEF was downregulated and plasma IL-6 was upregulated in postmenopausal osteoporosis patients compared with healthy controls. Altered plasma levels of lncRNA-NEF and IL-6 separated postmenopausal osteoporosis patients from healthy controls, and lncRNA-NEF and IL-6 were inversely and significantly correlated in osteoporosis patients. Patients were divided into high (n = 68) and low lncRNA-NEF (n = 73) groups according to Youden’s index. Patients with high lncRNA-NEF levels required a significantly shorter treatment course and had a lower post-treatment recurrence rate.
Conclusion
We showed that lncRNA-NEF is downregulated in postmenopausal osteoporosis and is related to course of treatment and recurrence. The involvement of lncRNA-NEF in postmenopausal osteoporosis is likely related to IL-6.
Keywords: Postmenopausal osteoporosis, lncRNA-NEF, IL-6, recurrence, treatment, long non-coding RNA
Introduction
Menopause following the reduced ovarian production of estrogen causes persistent and aggressive loss of structural elements and bone mineral, which in turn leads to an increased risk of fracture and loss of bone strength.1 Postmenopausal osteoporosis is a leading cause of fractures of the vertebrae, the proximal femur, and the distal radius.2 Postmenopausal osteoporosis affects more than 50% of women older than 50 years.3,4 Because of the high prevalence rate, postmenopausal osteoporosis is considered a major public health burden in many countries, including China.4 Efforts have been made to improve the diagnosis and treatment of postmenopausal osteoporosis,5,6 but treatment of this disease is still challenged by the high recurrence rate.5
In addition to messenger RNAs that encode proteins, the human genome transcribes a large set of non-coding RNAs that participate in diverse biological processes.7 Long (>200 nt) non-coding (lnc) RNAs are functional RNAs8 and key players in human diseases, including postmenopausal osteoporosis.9–11 LncRNA-NEF (neighboring enhancer of FoxA2) is a tumor suppressor lncRNA with known functionality only in liver cancer.12 Interleukin (IL)-6 is involved not only in inflammatory responses but also in the regulation of bone mineral density (BMD) and osteoclast differentiation and activation;13 however, its clinical value in postmenopausal osteoporosis is unknown. We showed that lncRNA-NEF may participate in postmenopausal osteoporosis and has diagnostic and prognostic value for this disease. In addition, lncRNA-NEF may inhibit IL-6 secretion.
Methods
Patients and specimen collection
A total of 366 patients with postmenopausal osteoporosis were diagnosed and treated in Affiliated Hospital of Hebei University from May 2013 to May 2015. The patients were diagnosed according the diagnostic criterion established by the World Health Organization: a decrease in BMD more than 2.5 standard deviations below that of young, healthy women. Among these patients, 141 were enrolled in this study according to the following inclusion criteria: (1) diagnosed and treated for the first time; (2) completed treatment in Affiliated Hospital of Hebei University; and (3) completed the follow-up study. The exclusion criteria were as follows: (1) patients received treatment before admission; (2) patients had other medical conditions; or (3) patients failed to complete the whole treatment and follow-up studies. Blood was extracted from the enrolled patients on the day of admission to prepare plasma samples through conventional methods. Blood was also extracted from 66 healthy volunteers (control group) who received systemic physical examinations in Affiliated Hospital of Hebei University during the same time and showed normal physiological indexes.
This study passed the review of Ethics Committee of Affiliated Hospital of Hebei University. All participants were informed about the experimental details and provided written informed consent.
Treatment and follow-up
All patients received intramuscular injections of 40 IU of elcatonin, a calcitonin derivative, twice per week. The level of pain (scale of 4 to 0) was assessed and recorded daily by patients: 4, unbearable pain and activities are restricted; 3, tolerable pain with obvious limited activities; 2, obvious pain but activities are not limited; 1, pain is not obvious; 0, no pain. A pain level of 0 was reached in all patients after 5 weeks of treatment. The duration of treatment ranged from 28 days to 3 months, depending on BMD measurement; recovery of BMD to within the normal range was observed after 3 months of treatment in all patients. All patients were followed for 3 years with monthly visits to record disease recurrence.
Cells and cell transfections
Human bone marrow mesenchymal stem cells (hBMSC, SCC034) were purchased from Sigma-Aldrich (St. Louis, MO, USA). A NEF expression vector was constructed using pcDNA3 vector (Sangon, Shanghai, China). Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA) was used to transfect 10 nM NEF expression vector or empty pcDNA3 vector (negative control, NC) into 105 hBMSC. Control (C) cells were not transfected.
ELISA
Levels of IL-6 in plasma and cell culture medium were detected using the Human IL-6 Quantikine ELISA Kit (D6050, R&D Systems, Minneapolis, MN). All procedures were performed as described in the manufacturer’s instructions.
RT-qPCR
The GenElute Total RNA Purification kit (Sigma-Aldrich) was used to extract total RNA from plasma. RNA was reverse transcribed into cDNA using the RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific). All PCR reactions were prepared using Luna Universal One-Step RT-qPCR kit (New England Biolabs, Ipswich, MA, USA). The PCR thermal conditions were 1 minute at 95°C followed by 40 cycles of 18s at 95°C and 40s at 58.5°C. All data normalizations were assessed using the 2−ΔΔCT method. All PCR products were sequenced to confirm that correct products were obtained.
Statistical analysis
Mean values in this paper represent data from three biological replicates. Diagnostic values of plasma levels of lncRNA-NEF and IL-6 for postmenopausal osteoporosis were evaluated by receiver operating characteristic (ROC) curve analysis, with postmenopausal osteoporosis patients as true positive cases and healthy volunteers as true negative cases. Correlation analyses between expression levels of lncRNA-NEF and IL-6 were analyzed using Pearson correlation coefficients. Patients were divided into high (n = 68) and low (n = 73) lncRNA-NEF expression groups according to Youden’s index, an unpaired t-test was used to compare the two groups. A p-value < 0.05 was considered statistically significant.
Results
Patient demographics
A total of 141 women were enrolled in the postmenopausal osteoporosis group; they ranged in age from 46 to 65 years, with a mean of 54.3 ± 4.4 years. The healthy control group (n = 66) comprised women ranging in age from 46 to 64 years, with a mean of 54.8 ± 4.2 years.
Altered expression levels of lncRNA-NEF and IL-6 were observed in postmenopausal osteoporosis patients
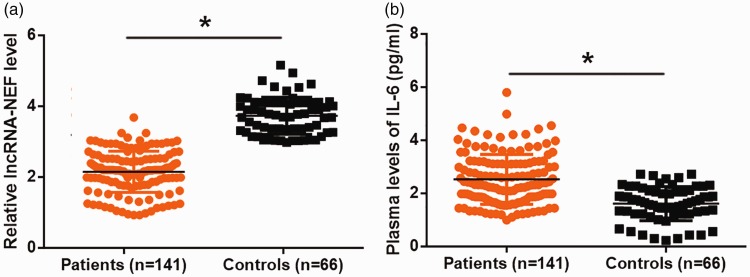
Expression of lncRNA-NEF in plasma was detected. Compared with that of healthy controls, plasma lncRNA-NEF was significantly downregulated in patients with postmenopausal osteoporosis (Figure 1a, p < 0.05). In contrast, ELISA results showed that plasma IL-6 was upregulated in postmenopausal osteoporosis patients compared with healthy controls (Figure 1b, p < 0.05).
Figure 1.
Altered expression levels of lncRNA-NEF and IL-6 were observed in postmenopausal osteoporosis patients. LncRNA-NEF (a) was downregulated, and IL-6 (b) was upregulated in plasma of postmenopausal osteoporosis patients compared with healthy controls (*p < 0.05). lncRNA-NEF, long non-coding RNA neighboring enhancer of FoxA2; IL-6, interleukin-6.
Altered plasma levels of lncRNA-NEF and IL-6 separated postmenopausal osteoporosis patients from healthy controls
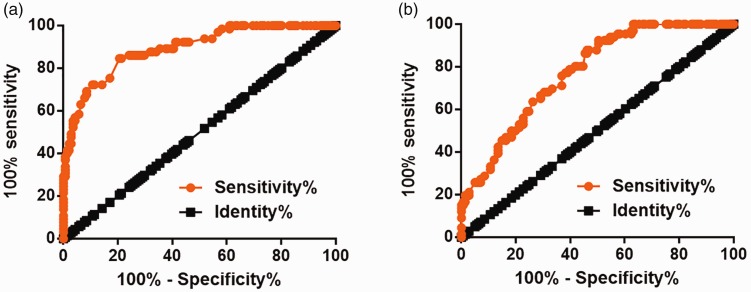
The diagnostic value of plasma levels of lncRNA-NEF and IL-6 for postmenopausal osteoporosis was evaluated by ROC curve analysis, with postmenopausal osteoporosis patients as true positive cases and healthy volunteers as true negative cases. For plasma lncRNA-NEF, the area under the curve was 0.8919 (standard error: 0.02366; 95% confidence interval: 0.8455–0.9382; p < 0.0001) (Figure 2a). For plasma IL-6, area under the curve was 0.7743 (standard error: 0.03209; 95% confidence interval: 0.7114–0.8372; p < 0.0001) (Figure 2b).
Figure 2.
Altered plasma levels of lncRNA-NEF and IL-6 separated postmenopausal osteoporosis patients from healthy controls. ROC curve analysis showed that altered plasma levels of lncRNA-NEF (a) and IL-6 (b) separated postmenopausal osteoporosis patients from healthy controls. lncRNA-NEF, long non-coding RNA neighboring enhancer of FoxA2; IL-6, interleukin-6; ROC, receiver operating characteristic.
LncRNA-NEF and IL-6 were significantly and inversely correlated in postmenopausal osteoporosis patients
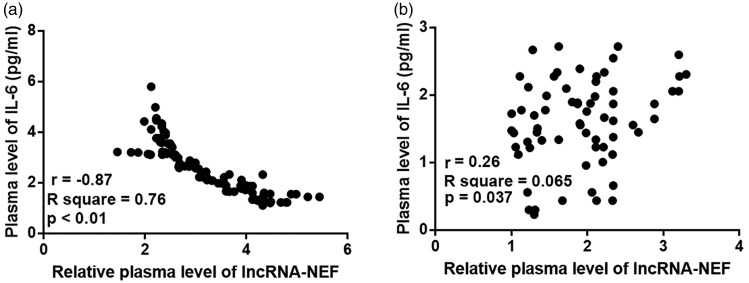
Expression levels of lncRNA-NEF and IL-6 were analyzed using Pearson correlation coefficients. As shown in Figure 3a, a significant and inverse correlation between plasma levels of lncRNA-NEF and IL-6 was observed in patients with postmenopausal osteoporosis (r = −0.87; p < 0.0001). LncRNA-NEF and IL-6 were not correlated in healthy controls (Figure 3b). In addition, NEF was positively correlated whereas IL-6 was inversely correlated with BMD (data not shown).
Figure 3.
Plasma levels of lncRNA-NEF and IL-6 were significantly and inversely correlated in postmenopausal osteoporosis patients but not in healthy controls. Pearson correlation coefficients revealed a significant and inverse correlation between plasma levels of lncRNA-NEF and IL-6 in patients with postmenopausal osteoporosis (a) but not in healthy controls (b). lncRNA-NEF, long non-coding RNA neighboring enhancer of FoxA2; IL-6, interleukin-6.
Low plasma levels of lncRNA-NEF were correlated with a long treatment course and high post-treatment recurrence rate
Patients were divided into high (n = 68) and low (n = 73) lncRNA-NEF groups according to Youden’s index. Compared with that in the high lncRNA-NEF group, treatment course in the low lncRNA-NEF group was significantly longer (Figure 4a, p < 0.05). In addition, the post-treatment recurrence rate was significantly higher in the low lncRNA-NEF level group than in the high lncRNA-NEF level group (Figure 4b, p < 0.05).
Figure 4.
Low plasma levels of lncRNA-NEF were correlated with a longer treatment course and higher post-treatment recurrence rate. Compared with the high lncRNA-NEF level group, treatment course was significantly longer (a) and post-treatment recurrence rate was significantly higher (b) in the low lncRNA-NEF level group (*p < 0.05). lncRNA-NEF, long non-coding RNA neighboring enhancer of FoxA2.
NEF overexpression in hBMSC was associated with inhibited secretion of IL-6 into cell culture medium
At 24 h after transfection and compared with the two control groups (NC and C), NEF was significantly upregulated in hBMSC following transfection of the NEF expression vector (Figure 5a, p < 0.05). In addition, IL-6 levels in the medium of NC and C groups were 5.8 ± 1.2 and 5.9 ± 1.4 pg/mL, respectively, whereas that in the NEF overexpression group was 2.3 ± 0.4 pg/mL, significantly lower than that of NC and C groups (Figure 5b, p < 0.05).
Figure 5.
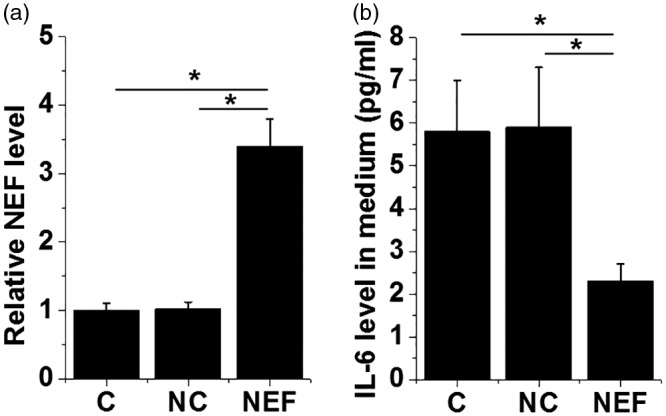
Overexpression of NEF in hBSMC led to the inhibited secretion of IL-6 into cell culture medium. Compared with negative control (NC; empty vector) and control (C; no transfection), NEF was significantly upregulated in hBMSC 24 h after transfection of NEF expression vector (a). In addition, NEF overexpression led to inhibited secretion of IL-6 from hBMSC into cell culture medium (b) (*p < 0.05). NEF, neighboring enhancer of FoxA2; hBMSC, human bone marrow mesenchymal stem cells; IL-6, interleukin-6.
Discussion
LncRNA-NEF is an oncogenic lncRNA that participates in liver cancer.12 However, the roles of this lncRNA in other human diseases have not yet been reported. The key finding of the current study was that lncRNA-NEF was downregulated in postmenopausal osteoporosis, and that this downregulation of lncRNA-NEF has diagnostic and prognostic value in postmenopausal osteoporosis. In addition, lncRNA-NEF may interact with IL-6 to participate in postmenopausal osteoporosis.
Postmenopausal osteoporosis is characterized by an increased susceptibility to fractures and decreased bone mass, which is caused by the reduced production of estrogen.13 Estrogen prevents bone loss by regulating the secretion or release of various cytokines involved in remodeling bone structure.14 It has been reported that levels of IL-6 were significantly upregulated in patients with postmenopausal osteoporosis, and that the expression levels of IL-6 were inversely related to lumbar BMD.15 Therefore, inhibition of IL-6 production may serve as a potential therapeutic target for postmenopausal osteoporosis. Consistently, we observed significantly upregulated plasma levels of IL-6 in postmenopausal osteoporosis patients compared with healthy controls. In effect, upregulation of IL-6 effectively distinguished postmenopausal osteoporosis patients from healthy controls, indicating the potential of IL-6 in the diagnosis of postmenopausal osteoporosis.
The development of postmenopausal osteoporosis is accompanied by changes in expression patterns of a large set of lncRNAs.16 Some differentially expressed lncRNAs, such as lncRNA-DANCR, show diagnostic potential for postmenopausal osteoporosis.11 LncRNA-NEF is downregulated in liver cancer.12 We showed that lncRNA-NEF was also downregulated in postmenopausal osteoporosis patients and that reduced plasma levels of lncRNA-NEF separated postmenopausal osteoporosis patients from heathy controls. In addition, we showed that low plasma levels of lncRNA-NEF were correlated with a long treatment course and high post-treatment recurrence rate. Therefore, lncRNA-NEF expression levels not only have diagnostic and prognostic value for postmenopausal osteoporosis but also can guide the treatment of this disease.
Interestingly, we found that plasma levels of lncRNA-NEF and IL-6 were significantly and inversely correlated in osteoporosis patients but not in healthy controls. Therefore, lncRNA-NEF may interact with IL-6 to participate in postmenopausal osteoporosis. However, the molecular mechanism of this interaction between lncRNA-NEF and IL-6 is unknown. Because of the lack of significant correlation between plasma levels of lncRNA-NEF and IL-6 in healthy controls, we speculate that lncRNA-NEF may interact with IL-6 through pathological mediators.
In conclusion, lncRNA-NEF is downregulated in postmenopausal osteoporosis and is related to the length of treatment and rate of recurrence. The involvement of lncRNA-NEF in postmenopausal osteoporosis is likely related to IL-6.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Andreopoulou P, Bockman RS. Management of postmenopausal osteoporosis. Annu Rev Med 2015; 66: 329–342. [DOI] [PubMed] [Google Scholar]
- 2.Eastell R, O'Neill TW, Hofbauer LC, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers 2016; 2: 16069. [DOI] [PubMed] [Google Scholar]
- 3.Xue F, Wagman RB, Yue S, et al. Incidence rate of potential osteonecrosis of the jaw among women with postmenopausal osteoporosis treated with Prolia or bisphosphonates: abstract number 348. Arthritis Rheum, 2015; 67: 492–495. [Google Scholar]
- 4.Si L, Winzenberg TM, Chen M, et al. Screening for osteoporosis in Chinese post-menopausal women: a health economic modelling study. Osteoporos Int 2016; 27: 2259–2269. [DOI] [PubMed] [Google Scholar]
- 5.Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med 2015; 277: 650–661. [DOI] [PubMed] [Google Scholar]
- 6.Maeda SS, Lazaretti-Castro M. An overview on the treatment of postmenopausal osteoporosis. Arq Bras Endocrinol Metabol 2014; 58: 162–171. [DOI] [PubMed] [Google Scholar]
- 7.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet 2006; 15: R17–R29. [DOI] [PubMed] [Google Scholar]
- 8.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10: 155–159. [DOI] [PubMed] [Google Scholar]
- 9.Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett 2013; 339: 159–166. [DOI] [PubMed] [Google Scholar]
- 10.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol 2011; 21: 354–361. [DOI] [PubMed] [Google Scholar]
- 11.Tong X, Gu P, Xu S, et al. Long non-coding RNA-DANCR in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. Biosci Biotechnol Biochem 2015; 79: 732–737. [DOI] [PubMed] [Google Scholar]
- 12.Liang WC, Ren JL, Wong CW, et al. LncRNA-NEF antagonized epithelial to mesenchymal transition and cancer metastasis via cis-regulating FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene 2018; 37: 1445–1456. [DOI] [PubMed] [Google Scholar]
- 13.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11: 1043–1051. [DOI] [PubMed] [Google Scholar]
- 14.Zheng SX, Vrindts Y, Lopez M, et al. Increase in cytokine production (IL-1β, IL-6, TNF-α but not IFN-γ, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas 1997; 26: 63–71. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci 2012; 9: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fei Q, Bai X, Lin J, et al. Identification of aberrantly expressed long non-coding RNAs in postmenopausal osteoporosis. Int J Med Sci 2018; 41: 3537–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.