Short abstract
Objective
To measure the inductive effect of kartogenin on matrix metalloproteinase-2 levels during the differentiation of human bone marrow mesenchymal stem cells (hMSCs) into chondrocytes in vitro.
Methods
In vitro cultured bone marrow hMSCs were grown to the logarithmic phase and then divided into three groups: control group (0 µM kartogenin), 1 µM kartogenin group and 10 µM kartogenin group. After 72 h of culture, cell proliferation and differentiation were observed microscopically. Matrix metalloproteinase-2 (MMP-2) in the cell supernatant and type II collagen levels in the cells were detected by enzyme linked immunosorbent assay and immunofluorescence staining, respectively.
Results
Kartogenin induced the proliferation and differentiation of hMSCs. With the increase of kartogenin concentration, the level of type II collagen was increased, while the level of MMP-2 decreased.
Conclusion
These findings indicate that kartogenin can induce hMSCs to differentiate into chondrocytes, and with the increase of kartogenin concentration, degeneration of the cartilage extracellular matrix may be inhibited.
Keywords: Stem cells, kartogenin, matrix metalloproteinase-2, cartilage, collagen type II
Introduction
Articular cartilage damage of the knee accounts for as high as 63% of knee injuries.1 Chronic cartilage damage can cause secondary subchondral bone degeneration and osteophyte hyperplasia, which develops into osteoarthritis (OA).2 In people aged over 60, the symptomatic knee OA incidence rate is approximately 37%.2 Advanced stage OA is characterized by severe pain, swelling, deformity and dysfunction. Due to the poor regenerative capability of cartilage and the high probability of progression to knee arthritis, articular chondral injury in weight-bearing joints is an intractable challenge for sports medicine physicians and scientists. The current methods to treat chondral injury include oral medication of cartilage nutrition drugs, intra-articular injections such as steroids and viscosupplementation, physical therapy and surgery.2 Arthroscopy-assisted surgery is widely used for the treatment of cartilage injury, and includes debridement and microfracture, osteochondral autografting, and autogenous or homogenous chondrocyte implantation.3 However, the efficiency is far from satisfactory for severe cartilage defects even though some clinical or experimental success has been reported for full thickness cartilage defects.4,5
As a last resort, artificial joint replacement surgery for elderly patients with severe OA is highly effective, but the limited life of the prosthetic joint hinders its application in young people.6The development of tissue engineering, which focuses on cell biology (various cell sources, including chondrocytes, fibroblasts and stem cells) and biomaterial science, has been deemed an encouraging area of research aimed at finding the ultimate therapy for cartilage defects.7 Multipotent mesenchymal stem cells (MSCs) are of great interest because of their potential to enhance tissue engineering.7 Molecules promoting selective differentiation of MSCs into chondrocytes have been shown to be effective in accelerating the repair of damaged cartilage.7 A previous report demonstrated that the small molecule kartogenin (KGN) promotes chondrocyte differentiation by interfering with the transcription process of proteins that compose the extracellular matrix (ECM) of cartilage, such as collagen type II, aggrecan and tissue inhibitors of metalloproteinase.7 The ECM is essential for the maintenance of normal cartilage and a disrupted balance between ECM synthesis and degradation leading to pathological cartilage destruction is a major feature of OA.7 KGN has demonstrated a chondroprotective effect in OA animal models and is a possible therapeutic target for the treatment of OA.8
Matrix metalloproteinase-2 (MMP-2) is a member of the gelatinase subfamily of the MMP family.9MMP-2 is involved in a wide range of biological processes, including cancer cell metastasis,10 angiogenesis,11 inflammation12 and tissue repair.13 MMPs are also involved in the development of OA by facilitating ECM breakdown.14 Increased gene expression of MMP-2 was detected in patients with OA.15 MMPs are considered to be important molecular markers in the pathological process of cartilage injury and degeneration.14–16
A previous study demonstrated that KGN did not alter MMP-3, MMP-13 or aggrecanase expression in primary chondrocytes, nor did it show any inhibition of aggrecanase or MMP-13 in vitro.7 The authors concluded that their findings suggest that no accelerated apoptosis occurred in primary chondrocytes.7 However, the effect of KGN on MMP-2 expression during the committed differentiation process of MSCs remains unclear. This current study aimed to determine whether kartogenin exhibited chondroprotective effects by altering the levels of MMP-2.
Materials and methods
Human mesenchymal stem cell culture
Human bone marrow mesenchymal stem cells (hMSCs) were purchased from Cyagen Biosciences (Guangzhou) (Guangzhou, China) and stored in liquid nitrogen until required. After retrieving the hMSCs from liquid nitrogen storage, the cells were warmed at 37°C for 3 min in a water bath. The cells were then transferred to a sterile tube and centrifuged at 1000 g for 5 min at 4°C in a MicroCL 17 microcentrifuge (Thermo Fisher Scientific, Rockford, IL, USA). The cell pellet was suspended in Dulbecco’s Modified Eagle’s Medium (DMEM; Cyagen Biosciences [Guangzhou]) and 5 × 106 cells were transferred into a T25 cell culture flask. The cells were cultured in DMEM with 1 g/l glucose, 10% heat inactivated fetal bovine serum, 50 mg/ml gentamicin, 1.5 mg/ml fungizone, 1 ng/ml fibroblast growth factor-2 and 0.1 mM L-ascorbic acid 2-phosphate (MSC culture medium) at 37°C in an atmosphere of 5% CO2. The cell culture reagents were all purchased from Gibco BRL, Life Technologies (Gaithersburg, MD, USA). Cells were passaged using 0.25% trypsin (Gibco BRL, Life Technologies) when approximately 90% confluent. Drug induction began during the logarithmic growth phase.
The study was approved by the Institutional Review Board of Qilu Hospital of Shandong University, Jinan, Shandong Province, China.
Drug induction
Equal numbers of hMSCs were seeded into nine T25 cell culture flasks at a density of 2 × 105 cells/cm2. The flasks were labelled with the sample numbers as shown in Table 1. After allowing the cells to adhere to the cell culture plastic for 24 h, KGN (Medicine School of Shandong University, Jinan, Shandong Province, China) diluted in dimethyl sulphoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA) was added at the concentrations shown in Table 1. The control cells received DMSO alone. Then the cells were incubated for a further 72 h under standard conditions. According to a previous study, the proliferation of chondrocytes was still increased when the KGN concentration was 100 µM, so this current study used concentrations of 1 µM and 10 µM.7
Table 1.
Experimental groups used to measure the effects of kartogenin on human mesenchymal stem cells in cell culture.
| Concentration of kartogenin | Sample numbers | |
|---|---|---|
| Control group | 0 µM | A1, A2, A3 |
| Experimental group | 1 µM | B1, B2, B3 |
| 10 µM | C1, C2, C3 |
Experimental outcome measures
The morphological changes of the hMSCs were examined under an inverted microscope (Eclipse E600; Nikon, Tokyo, Japan) after 72 h of drug treatment. A 3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, a tetrazole (MTT) assay was used to measure the proliferation of hMSCs after KGN treatment for 72 h (Sigma-Aldrich).
An enzyme-linked immunosorbent assay (ELISA) was used to determine the concentration of MMP-2 in the cell culture supernatant according to the manufacturer’s instructions (human total MMP-2 ELISA Kit; Boster Biological Technology, Pleasanton, CA, USA) using an ELISA microplate reader (Beijing Perlong New Technology, Beijing, China). The minimum detectable concentration of MMP-2 was 10 pg/ml. Intra- and interassay coefficients of variation for the ELISA were < 10% and < 10%, respectively.
After the cultured cells were grown on sterile glass slides overnight at 37°C, collagen type II protein levels were detected using immunofluorescence staining according to the manufacturer’s instructions (immunofluorescence kit for type II collagen; Boster Biological Technology). Expression levels of the collagen type II (COL2A1) gene were detected by real-time polymerase chain reaction (RT–PCR). In brief, total RNA was extracted from 2 × 105 hMSCs using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA (2 mg) was reverse transcribed using a High Capacity cDNA Reverse Transcriptase kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The resulting cDNA was used for real-time PCR using the SYBR® Green Master PCR Mix (Applied Biosystems, Foster City, CA, USA) in triplicates using a TP800 Thermal Cycler Dice™ Real Time System (TaKaRa, Dalian, China). The primer sequences for real-time PCR for COL2A1 were: 5ʹ-AGCACGCAGCAGA TC GAG-3ʹ (sense) and 5ʹ-CTCCTTGTT CCAT CGTCTC-3ʹ (antisense). Primers for the control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were: 5ʹ-TGACTTC AACA GCGACACCCA-3ʹ (sense) and 5ʹ-ACCCT GTTGCTGTAGCCAAA-3ʹ (antisense). All primers were synthesized by GeneChem (Shanghai, China). The cycling programme involved preliminary denaturation at 94°C for 1 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and elongation at 72°C for 45 s, followed by a final elongation step at 72°C for 5 min. The relative type II collagen mRNA levels were compared with those of GAPDH using the 2–ΔΔCT method. The CT value used for these calculations was the mean of the triplicate for each reaction.
Statistical analyses
All statistical analyses were performed using GraphPad Prism software version 8.0 (GraphPad Software, San Diego, CA, USA). All in vitro experiments were undertaken in triplicate for each concentration of KGN. The concentration of MMP-2 is expressed as the mean value and differences between the three groups were compared using Kruskal–Wallis test. A P-value < 0.05 was considered as statistically significant.
Results
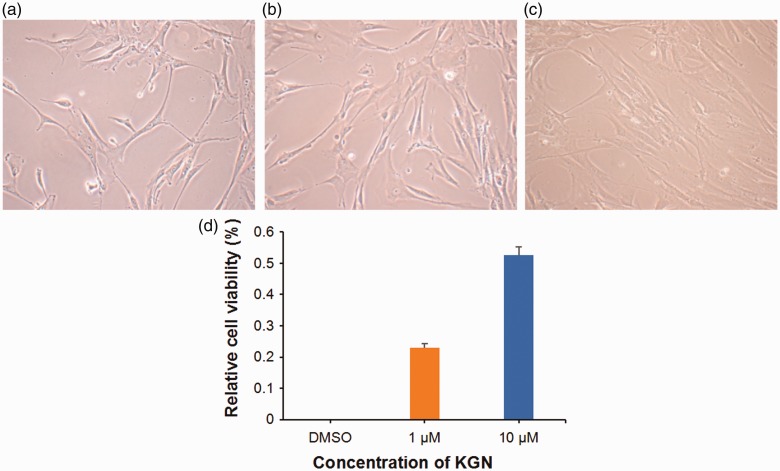
When hMSCs were viewed under the inverted microscope, most of the cells were spindle-shaped or had irregular adherent growth. With increasing KGN concentration, the hMSCs grew more vigorously and fused more, forming fish-like colonies (Figures 1a–c). As the KGN concentration increased, the number of cells gradually increased and the cells were arranged closely. Also, the morphology gradually became slender with the cytoplasm getting dense, the gap between cells becoming closer and some cells overlapped to form cell clusters. In contrast, cells in the control group were stretched, proliferated slowly and were arranged on the bottom of the dish like paving stones. The control-treated cells were triangular, polygonal or fusiform, with obvious protrusions and a strong stereoscopic form. The rate of proliferation of the KGN-treated cells as shown by relative cell viability (%) in the MTT assay was greater than the DMSO-treated control group (Figure 1d), which was in accordance with the morphological findings.
Figure 1.
Morphological changes and cell proliferation of human mesenchymal stem cells (hMSCs) under chondrogenic induction with kartogenin (KGN). Representative light photomicrographs of hMSCs treated for 72 h with vehicle alone (dimethyl sulphoxide [DMSO]) (a), 1 µM KGN (b) and 10 µM KGN (c). Scale bar 30 µm. Proliferation of hMSCs presented as the relative cell viability (%) compared with the control group (d). Data presented as mean ± SD. The colour version of this figure is available at: http://imr.sagepub.com.
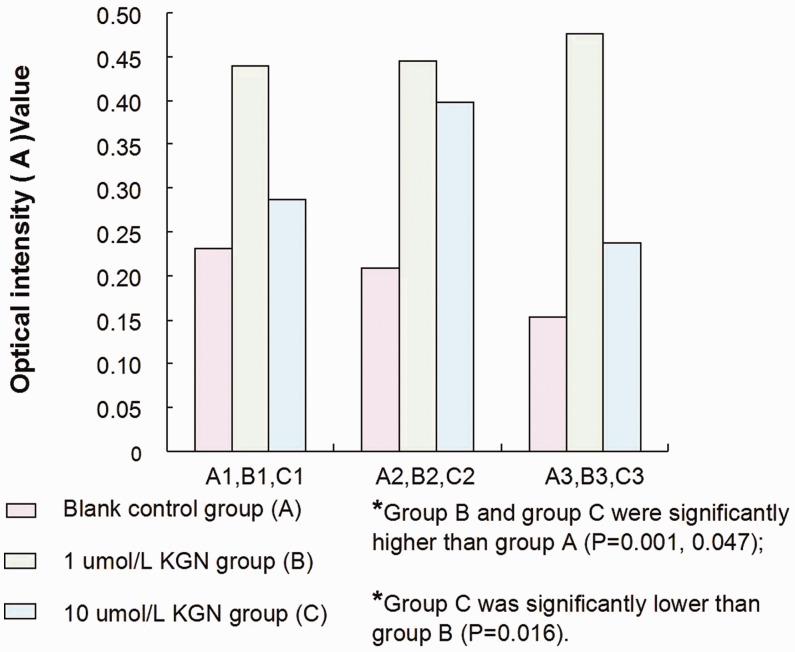
The concentration of MMP-2 in each supernatant sample was detected using an ELISA that measured the optical intensity (A) value (Figure 2). The levels of MMP-2 in hMSC culture supernatant of the 1 µM KGN group (group B) and 10 µM KGN group (group C) were significantly higher than those of the DMSO-treated control group (group A) (P = 0.001, P = 0.047, respectively). The level of MMP-2 was significantly lower in group C than in group B (P = 0.016). The optical density (A) values of MMP-2 in each sample were converted to mass concentration values using a standard curve. The results showed that the concentration of MMP-2 decreased with increased KGN concentration (P = 0.03) (Table 2).
Figure 2.
Matrix metalloproteinase-2 (MMP-2) concentrations in the culture supernatant of the different human mesenchymal stem cell treatment groups as detected by an enzyme-linked immunosorbent assay and presented as optical intensity (A) values. The results showed that the concentration of MMP-2 in the cell culture supernatant increased after induction with 1 µM kartogenin (KGN) compared with the control group (P < 0.05), but the MMP-2 concentration in the culture supernatant decreased significantly when the KGN concentration was increased to 10 µM compared with the 1 µM KGN group (P < 0.05); Kruskal–Wallis test. The colour version of this figure is available at: http://imr.sagepub.com.
Table 2.
Matrix metalloproteinase-2 (MMP-2) concentrations in the culture supernatant of the different treatment groups as detected by an enzyme-linked immunosorbent assay.
| Sample number | MMP-2 concentration, pg/ml |
|---|---|
| A1 | 1471 |
| A2 | 1392 |
| A3 | 1192 |
| B1 | 2216 |
| B2 | 2237 |
| B3 | 2348 |
| C1 | 1672 |
| C2 | 2066 |
| C3 | 1493 |
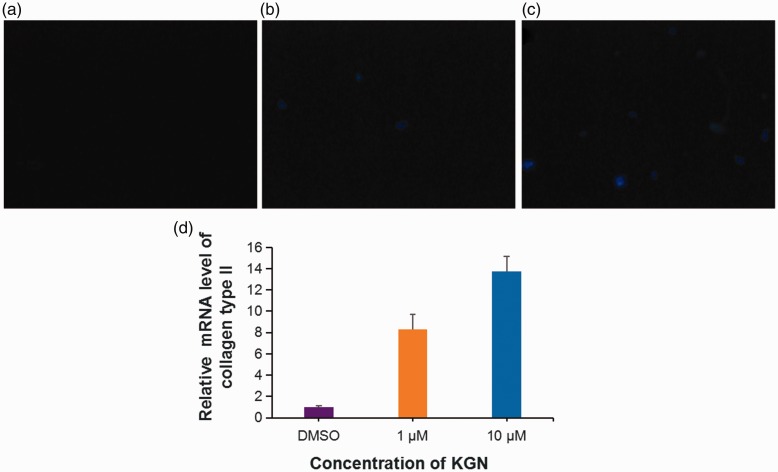
Immunofluorescence staining showed that KGN treatment of hMSCs could significantly increase the levels of type II collagen in a dose-dependent manner (Figures 3a–c). The hMSCs treated with KGN were short fusiform or polygonal in shape, with the cell membrane and cytoplasm showing blue fluorescence, but the nucleus was not clearly visible. Real-time PCR results showed that the levels of type II collagen mRNA were increased in hMSCs treated with 10 µM KGN compared with the control DMSO-treated hMSCs (Figure 3d).
Figure 3.
Changes in the levels of collagen type II protein and mRNA in human mesenchymal stem cells (hMSCs) under chondrogenic induction with kartogenin (KGN). Representative photomicrographs showing immunofluorescence staining of collagen type II protein during KGN-induced chondrogenic differentiation of hMSCs treated for 72 h with vehicle alone (dimethyl sulphoxide [DMSO]) (a), 1 µM KGN (b) and 10 µM KGN (c). Scale bar 30 µm. Relative levels of collagen type II mRNA as determined using polymerase chain reaction (d). Data presented as mean ± SD. The colour version of this figure is available at: http://imr.sagepub.com.
Discussion
Due to the limited potential of articular cartilage to repair itself, even mild articular cartilage damage may lead to degenerative lesions that eventually develop into OA.17 At present, the clinical treatment options for cartilage damage and degeneration cannot obtain ideal long-term results, so the search for effective articular cartilage repair methods has become the focus of sports medicine research. The emergence of tissue engineering technology provides a new pathway for repairing large-area, large-scale cartilage damage. Through the isolation and culture of cells, the scaffold provides temporary space and pre-constructed three-dimensional shape for cell growth, and finally forms regenerated cartilage.18 However, the technique remains limited by several factors such as stress, microgravity, oxygen concentration and cytokines.19–21 According to a previous study,22 there was no significant difference in the effectiveness of three existing methods for the treatment of cartilage injury. This randomized controlled study found excellent or good results in 80% of patients after microfracture, in 82% after autogenous osteochondral transplantation and in 80% after autologous chondrocyte transplantation by tissue engineering.22 In recent years, research on the treatment of cartilage injury and OA has gradually focused on the development of bio-targeted drugs, such as inhibitors of metalloproteinase, antagonists of chondrocyte metabolism and inducers of proliferation and differentiation of chondrocyte cells.22 Selective gene transcription regulates the biochemical processes of chondrocytes at the subcellular level. In vitro and in vivo experiments have shown that KGN promotes chondrocyte differentiation and proliferation.18 With a series of encouraging research results attracting the attention of scholars,23–25 the role of KGN in the process of cartilage repair has been described as a ‘game changer’.26
Metabolic imbalance and the degradation of the cartilage ECM form the pathological basis of OA and articular cartilage degeneration.14 The degradation of the ECM by MMPs is considered to play a key role in the pathogenesis of OA.15The MMP family can be classified into the collagenases (MMP-1, MMP-8 and MMP-13), the stromelysins (MMP-3, MMP-7 and MMP-10) and the gelatinases (MMP-2 and MMP-9).9 The main components of the articular cartilage ECM are collagen type II and proteoglycan.9MMP-1, MMP-7, MMP-8, MMP-10 and MMP-13 degrade collagen type II.15 MMP-3 causes the lysis of proteoglycan, while MMP-2 and MMP-9 degrade the metabolites of collagen type II.15 Research on OA has confirmed that many members of the MMP family are closely related to the pathological changes of chondrocyte degradation and apoptosis.27–29 MMP-2 is the most widely expressed and easy to detect member of the MMP family.12 In recent years, research has confirmed that the levels of MMP-2 in OA cartilage is increased significantly, suggesting that MMP-2 is an important marker of the physiological and metabolic function of chondrocytes that is closely correlated with OA stage.30–32 In a previous study,7 there was no increase in the levels of MMP-3 or MMP-13 in chondrocytes derived from hMSCs induced by KGN. The authors believed that KGN promoted the differentiation of hMSCs into chondrocytes without accelerating the apoptotic process.7 The majority of KGN-related research studies have evaluated the degree of chondrocyte proliferation with small molecular markers such as proteoglycan and its metabolite glycosaminoglycans, lubricin, SOX-9 and collagen II, or the effect of cartilage repair was evaluated with cartilage gross score and pathological tests.33–35 To the best of our knowledge, changes in the levels of MMP-2 have not been reported in KGN-related literature to date. Therefore, this current study selected MMP-2 as the main molecule to study when hMSCs were induced to differentiate into chondrocytes by KGN treatment.
The current study confirmed that KGN could induce chondrocyte differentiation of hMSCs. In accordance with the results of a previous study,7 chondrocyte proliferation and the levels of type II collagen mRNA increased in line with the increase of KGN concentration, while the levels of MMP-2 decreased when the concentration of KGN increased from 1 µM to 10 µM. The current findings demonstrate that chondrocyte differentiation increased proportionally, while the level of MMP-2 decreased, as the concentration of KGN increased, suggesting the presence of chondrocyte activity and the maintenance of the ECM. These findings may serve as further evidence that KGN can promote cartilage repair and meanwhile inhibit the process of chondrocyte apoptosis and ECM degeneration.
In conclusion, KGN was shown to significantly promote the proliferation and differentiation of hMSCs into chondrocytes. With the increase of KGN concentration, the level of type II collagen mRNA was increased, while the level of MMP-2 was decreased. These findings indicate that KGN can induce hMSCs to differentiate into chondrocytes, and with the increase of KGN concentration, destruction of the cartilage ECM may be inhibited.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
Funding for this study was provided in part by the Science and Technology Plan of Shandong Province, China.
References
- 1.Carbone A, Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res 2017; 35: 397–405. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock KJ, Westermann RR, Shamrock AG, et al. Trends in knee articular cartilage treatments: An American Board of Orthopaedic Surgery Database Study . J Knee Surg 2019; 32: 85.–. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019; 393: 1745.–. [DOI] [PubMed] [Google Scholar]
- 5.Capeci CM, Turchiano M, Strauss EJ, et al. Osteochondral allografts: applications in treating articular cartilage defects in the knee. Bull Hosp Jt Dis (2013) 2013; 71: 60–67. [PubMed] [Google Scholar]
- 6.Clouet J, Vinatier C, Merceron C, et al. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov Today 2009; 14: 913–925. [DOI] [PubMed] [Google Scholar]
- 7.Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science 2012; 336: 717–721. [DOI] [PubMed] [Google Scholar]
- 8.Im GI. Application of kartogenin for musculoskeletal regeneration. J Biomed Mater Res A 2018; 106: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 9.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem 1999; 274: 21491–21494. [DOI] [PubMed] [Google Scholar]
- 10.Tien YW, Lee PH, Hu RH, et al. The role of gelatinase in hepatic metastasis of colorectal cancer. Clin Cancer Res 2003; 9: 4891–4896. [PubMed] [Google Scholar]
- 11.Yarani R, Mansouri K, Mohammadi-Motlagh HR, et al. In vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm Biol 2013; 51: 361–368. [DOI] [PubMed] [Google Scholar]
- 12.Berry E, Hernandez-Anzaldo S, Ghomashchi F, et al. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J Am Heart Assoc 2015; 4: pii: e001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosselink JV, Hayashi S, Elliott WM, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy G, Knäuper V, Atkinson S, et al. Matrix metalloproteinases in arthritic disease. Arthritis Res 2002; 4: S39–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kevorkian L, Young DA, Darrah C, et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum 2004; 50: 131–141. [DOI] [PubMed] [Google Scholar]
- 16.Kiyotake EA, Beck EC, Detamore MS. Cartilage extracellular matrix as a biomaterial for cartilage regeneration . ; : – . Ann N Y Acad Sci. 2016;1383:139. doi: 10.1111/nyas.13278. [DOI] [PubMed] [Google Scholar]
- 17.Vo N, Niedernhofer LJ, Nasto LA, et al. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res 2013; 31: 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komárek J, Vališ P, Repko M, et al. Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation: long-term results. Acta Chir Orthop Traumatol Cech 2010; 77: 291–295 [Article in Czech, English abstract]. [PubMed] [Google Scholar]
- 19.Martin JA, Klingelhutz AJ, Moussavi-Harami F, et al. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci 2004; 59: 324–337. [DOI] [PubMed] [Google Scholar]
- 20.Place ES, Nair R, Chia HN, et al. Latent TGF-β hydrogels for cartilage tissue engineering. Adv Healthc Mater 2012; 1: 480–484. [DOI] [PubMed] [Google Scholar]
- 21.Falsafi S, Koch RJ. Growth of tissue-engineered human nasoseptal cartilage in simulated microgravity. Arch Otolaryngol Head Neck Surg 2000; 126: 759–765. [DOI] [PubMed] [Google Scholar]
- 22.Lim HC, Bae JH, Song SH, et al. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res 2012; 470: 2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon JY, Lee SH, Na HS, et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep 2018; 8: 13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai G, Liu W, He Y, et al. Recent advances in kartogenin for cartilage regeneration. J Drug Target 2019; 27: 28–32. [DOI] [PubMed] [Google Scholar]
- 25.Spakova T, Plsikova J, Harvanova D, et al. Influence of Kartogenin on Chondrogenic Differentiation of Human Bone Marrow-Derived MSCs in 2D Culture and in Co-Cultivation with OA Osteochondral Explant. Molecules 2018; 23: pii: E181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayek A, Kerstetter-Fogle AE, Sachlos E, et al. Kartogenin: a game-changer in regenerative medicine. Regen Med 2012; 7: 475. [PubMed] [Google Scholar]
- 27.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci 2006; 11: 529–543. [DOI] [PubMed] [Google Scholar]
- 28.Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol 2013; 146: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gargiulo S, Gamba P, Poli G, et al. Metalloproteinases and metalloproteinase inhibitors in age-related diseases. Curr Pharm Des 2014; 20: 2993–3018. [DOI] [PubMed] [Google Scholar]
- 30.Zeng GQ, Chen AB, Li W, et al. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res 2015; 14: 14811–14822. [DOI] [PubMed] [Google Scholar]
- 31.Lipari L, Gerbino A. Expression of gelatinases (MMP-2, MMP-9) in human articular cartilage. Int J Immunopathol Pharmacol 2013; 26: 817–823. [DOI] [PubMed] [Google Scholar]
- 32.Alves AC, Albertini R, dos Santos SA, et al. Effect of low-level laser therapy on metalloproteinase MMP-2 and MMP-9 production and percentage of collagen types I and III in a papain cartilage injury model. Lasers Med Sci 2014; 29: 911–919. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Ma X, Li T, et al. Kartogenin, transforming growth factor-β1 and bone morphogenetic protein-7coordinately enhance lubricin accumulation in bone-derived mesenchymal stem cells. Cell Biol Int 2015; 39: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 34.Kang ML, Ko JY, Kim JE, et al. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 2014; 35: 9984–9994. [DOI] [PubMed] [Google Scholar]
- 35.Jackson MT, Moradi B, Smith MM, et al. Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol 2014; 66: 1525–1536. [DOI] [PubMed] [Google Scholar]