Abstract
There remains a need to differentiate between women with a benign or a malignant adnexal mass prior to surgery. As part of an ongoing evaluation of vaginal fluid compounds as potential tumor biomarkers we evaluated whether vaginal lysophosphatidic acid (LPA) predicted the subsequent diagnosis of a malignant adnexal mass. In this prospective pilot study vaginal fluid was obtained from 100 post-menopausal women referred for evaluation of a suspicious adnexal mass and tested for LPA by ELISA. Clinical data and serum CA125 results were obtained only after completion of all laboratory testing. Twenty eight of the women were subsequently diagnosed with an ovarian malignancy, four had a borderline tumor and 68 had a benign diagnosis. Among women with a malignant ovarian mass, 11 (39.3%) had an endometrioid adenocarcinoma +/− Clear cell tumor components, 6 (21.4%) had a high grade serous carcinoma, 3 (10.7%) had a mucinous tumor, 2 each (7.1%) had a malignant mixed mesodermal or a granulosa tumor and 1 each (3.6%) had a Clear cell tumor, a mixed cell tumor, leimyosarcoma or metastatic adrenal tumor. Compared to the median vaginal LPA level in women with benign lesions (1.5 μM), LPA was significantly elevated only in women with endometrioid ovarian cancer (7.9 μM) (p = 0.0137). Of the 6 endometrioid tumors in which values for both plasma CA125 and vaginal LPA were available 5 were positive only for LPA while one was only CA125 positive. Detection of LPA in vaginal secretions may be of value for the noninvasive diagnosis of endometrioid ovarian malignancies in post-menopausal women.
Keywords: Adnexal mass, CA125, Endometrioid ovarian cancer, Lysophosphatidic acid
Introduction
Determination of whether a suspicious adnexal mass is an ovarian cancer is currently made only following invasive surgery and pathological analysis of excised tissue. Since only a minority of these women will in fact have a malignancy [1], the discovery of a biomarker to noninvasively determine whether or not an adnexal mass is malignant could greatly reduce the incidence of unnecessary surgery.
Currently, serum CA125 is the most commonly used biomarker to detect ovarian cancer. However, about 25% of women with stage 1 ovarian cancer will have a normal CA125 and multiple benign conditions may cause CA125 elevations [2]. To date, CA125 has been shown to be most useful in predicting the response to treatment and recurrence rather than detection of early stage malignancy [3].
Recent findings indicate that some ovarian cancers originate as premalignant intraepithelial lesions in the distal fallopian tube and that transformed cells subsequently migrate to the ovary [4,5]. Women who have undergone a tubal ligation have a decreased risk of developing ovarian cancer, especially endometrioid and serous types [6]. Thus, in women with ovarian cancer compounds associated with development of this malignancy may be present in the upper genital tract, migrate to the lower genital tract and accumulate in the vagina.
A scarcity of studies have evaluated vaginal fluid as a potential source of cancer-associated biomarkers. An investigation in 1997 determined that CA125 as well as carcinoembryonic antigen (CEA) and squamous cell carcinoma (SCC) antigen were detectable in vaginal fluid and that the levels of all three compounds were highest in 20 women with benign gynecological diseases as compared to 10 with squamous cell carcinoma, 5 with endometrial cancer, 3 with vulva carcinoma, 8 healthy pre-menopausal and 7 healthy post-menopausal women [7]. A later study reported that the level of CA125 in the vagina and cervix was highest in women with endometrial hyperplasia and endometrial cancer [8]. A comparative evaluation of three tumors biomarkers – CA125, CEA and CA19-9 – in vaginal wash samples from 30 women in each group with either advanced primary ovarian cancer or benign ovarian cysts revealed highly elevated levels of all 3 compounds in the malignant group [9]. In the most recent study women with precancerous endometrial intraepithelial neoplasia or endometrial adenocarcinoma had higher and similar vaginal concentrations of CA125 than did women with non-endometrioma ovarian cysts, endometrial hyperplasia or dysfunctional uterine bleeding [10].
Lysophosphatidic acid (LPA, 1-acyl-2hydroxy-sn-glycero-3-phosphate) is a phospholipid produced by malignantly transformed ovarian epithelial cells. It has been shown to stimulate cell proliferation and invasion, increase expression of factors involved in angiogenesis and levels are elevated in sera and ascites fluid from women with ovarian cancer [[11], [12], [13], [14]]. LPA has also been shown to inhibit autophagy in a prostate tumor cell line [15]. We previously reported that vaginal fluid from women with a malignant adnexal mass inhibited autophagy in peripheral blood mononuclear cells to a greater extent than did vaginal fluid from women with a benign mass [16].
As a component of our ongoing investigation of possible predictive biomarkers of a malignant adnexal mass in vaginal fluid, and based on prior associations between circulating LPA levels and ovarian cancer, we evaluated whether the LPA concentration in vaginal fluid would be elevated in women subsequently diagnosed with a malignant adnexal mass.
Material and methods
Subjects The subjects in this prospective study were 100 women referred to the Division of Gynecologic Oncology at Weill Cornell Medicine for evaluation of a suspicious adnexal mass. All subjects were referred for evaluation due to a pelvic mass of presumed gynecologic origin noted on pelvic sonography or magnetic resonance imaging. Inclusion criteria were post-menopausal status, the ability to collect vaginal samples that could be sent to the laboratory for processing within a 3 h time period and ability to provide written informed consent. Exclusion criteria were women with prior hysterectomy, history of malignancy, presence of vaginal bleeding, signs or symptoms of infection or no plan for undergoing surgical evaluation of their mass at Weill Cornell. Subsequent to vaginal sample collection all women underwent surgical exploration by laparoscopy or laparotomy and surgical resection of the adnexal mass, as described previously [16]. Briefly, the adnexal masses were resected and intraoperative pathology consultation was utilized. When an invasive malignancy was present, further surgical staging procedures including total hysterectomy, bilateral salpingo-oopherectomy, pelvic and paraarortic lymph node sampling and oomentectomy were performed when clinically indicated. The final pathological diagnosis was determined by an attending gynecologic pathologist utilizing techniques and tissue samples standard at our hospital. The study was approved by the Institutional Review Board of Weill Cornell Medicine and all subjects signed informed written consent.
Samples Vaginal fluid was obtained during a speculum-based examination by scraping a cotton swab against the posterior vaginal wall and shaking the contents into a tube containing one ml sterile phosphate-buffered saline. The tube was centrifuged and the supernatant stored in aliquots at −80 °C until assayed. Samples with visible blood contamination were discarded.
Assays Thawed aliquots of vaginal secretions were assayed for LPA by a commercial ELISA kit (Echelon Biosciences, Salt Lake City, Utah). LPA was detected with a monoclonal antibody that did not exhibit reactivity with LPA analogs. Values were converted to μM by reference to a standard curve generated in parallel to each assay. The lower limit of sensitivity was 0.064 μM. Intra-and inter-assay variability was <10%. Peripheral blood serum was assayed for CA125 in the Weill Cornell clinical laboratory by a standard chemiluminescent immunoassay. The laboratory staff was blinded to all clinical data including CA125 results that were obtained by chart review only after completion of the lab analyses.
Statistics Differences in LPA and CA125 levels between women with a benign or malignant mass were analyzed by the non-parametric Mann-Whitney test since values were not normally distributed. A serum CA125 concentration of 35 U/ml is typically used as the cutoff for malignancy [3], and we utilized this value in our study. An LPA concentration >7.25 μM was operationally designated as the cutoff for detection of a malignant lesion, based on the observation that >90% of women with a benign diagnosis had a vaginal LPA level below this value. Differences between positive values for CA125 and LPA for each type of malignancy were assessed by Fisher’s exact test. A two sided p value <0.05 was considered significant. GRAPH PAD INSTAT (Graph Pad Software, San Diego, CA) was utilized for the analysis.
Results
Characteristics of the study population are detailed in Table 1. A malignant adnexal mass was detected in 28 women, four had a borderline tumor while 68 had a benign lesion. Women in the three groups were of a similar mean age, had a comparable mean age at menarche and menopause and were of similar gravidity, parity and body mass index. The majority of women in each group were White. Analysis of these multiple variables negated their possible confounding influence on vaginal LPA levels. There was no difference in the use of hormone replacement therapy (HRT) between groups. The median CA125 level was 52.2 U/ml in women with a malignant mass, 14.6 U/ml in those with a borderline tumor and 11.0 U/ml in those whose mass was benign (p < 0.0001 malignant vs. the other two groups).
Table 1.
Characteristics of post-menopausal women evaluated for an adnexal mass.
| Characteristic | Benign | Borderline | Malignant |
|---|---|---|---|
| N = 68 | N = 4 | N = 28 | |
| Mean age in years (SD) | 63.2 (9.4) | 63.0(11.7) | 66.2(10.2) |
| Using HRT | 12 (18.2%) | 0 | 9 (26.5%) |
| Mean age at menarche (SD) | 12.5(1.2) | 13.0(0) | 13.3 (1.9) |
| Mean age at menopause (SD) | 49.1(5.8) | 47.5(3.3) | 50.7(4.1) |
| Median gravida (range) | 2 (0–10) | 3 (0–4) | 2 (0–9) |
| Median parity (range) | 2 (0–9) | 2(0–3) | 2 (0–5) |
| Mean body mass index (SD]b | 26.4(5.8) | 27.1(5.2) | 25.3 (5.0) |
| Per cent White race | 70.7% | 75.0% | 85.2% |
| Median CA125 (range) | 11.0(2.8–75) | 14.6(9.3–117) | 52.2 (5.7–7778)a |
HRT, hormone replacement therapy.
p < 0.0001 vs. benign and borderline.
kg/m2.
Among women with a malignant ovarian mass, 11 (39.3%) had endometrioid ovarian cancer with or without Clear cell tumor components, six (21.4%) had high grade serous cancer, three (10.7%) had a mucinous tumor, two each (7.1%) had a malignant mixed mesodermal or a granulosa tumor and one of four women (3.6%) had a Clear cell tumor, a mixed cell tumor, leimyosarcoma or metastatic adrenal tumor. The majority of women with a benign diagnosis had either a cystadenoma (44.1%) or another type of cyst (33.8%).
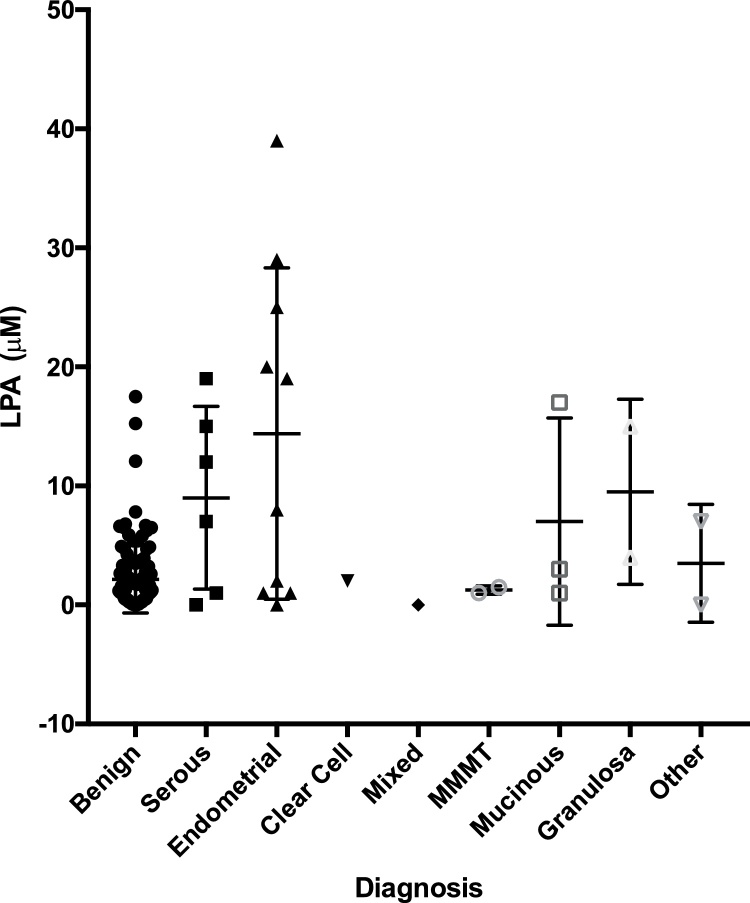
The concentration of LPA in vaginal fluid from women with each of the different ovarian malignancies as well as the individual benign conditions is shown in Table 2. Individual values for women with a malignant or benign mass are presented in Fig. 1. The median vaginal LPA concentration was highest in the six women with serous ovarian cancer (9.5 μM), one woman with a granulosa tumor (9.1 μM) and eleven with an endometrioid malignancy (7.9 μM). However, most likely due to the low number of subjects evaluated only in women with an endometrioid ovarian malignancy was the LPA level significantly elevated (p = 0.0137) compared to the median level in women with benign diagnoses (1.5 μM). None of the women with a malignant mass and 6 women with a benign mass had an undetectable LPA level in their vaginal sample. Only four women with benign conditions – two with a serous cystadenoma and one each with a mucinous cystadenoma and a mesothelial cyst - had a vaginal LPA concentration >7.25 μM. HRT administration had no effect on the vaginal LPA level (data not shown).
Table 2.
Association between lysophosphatidic acid concentration in vaginal secretions and differential pathological diagnosis in women with a benign or malignant adnexal mass.
| Diagnosis | No. women | LPA (μM) |
|---|---|---|
| Malignant | ||
| Endometroid +/- Clear cell | 11 | 7.9 (1.9–24.3)a |
| Serous | 6 | 9.5 (1.0–16.1)b |
| Mucinous | 3 | 2.6 (1.2–15.2) |
| MMMT | 2 | 1.1 (0.9–1.4) |
| Granulosa | 2 | 9.1 (4.2–14.1) |
| Other | 2 | 4.4 (1.6–7.3) |
| Clear cell | 1 | 1.6 |
| Mixed | 1 | 0.2 |
| Borderline | ||
| Serous | 2 | |
| Mucinous | 2 | |
| Benign | ||
| Cystadenoma | 30 | 1.2 (0.4–6.6) |
| Other cysts | 23 | 2.7 (1.2–21.8) |
| Teratoma | 6 | 1.1 (0.5–2.3) |
| Fibroma | 6 | 2.0 (0.5–4.4) |
| Endometriosis | 3 | 0.3 (0–7.9) |
| Total benign | 68 | 1.5 (0.5–4.3) |
Values are median (25%–75%) μM; Endometrioid +/− Clear cell, endometrioid tumor with and without clear cell components; MMMT, malignant mixed mesoderm tumor (carcinosarcoma); Other, metastatic adrenal, leiomyosarcoma.
ap = 0.0137 vs. benign; bp = 0.1267 vs. benign; cp = 0.1208 vs. benign.
Fig. 1.
LPA in vaginal fluid from women with a benign or malignant adnexal mass. Vaginal fluid was collected from women being screened for an adnexal mass and tested for LPA by ELISA. The type of malignancy was subsequently determined by a gynecologic pathologist.
MMMT, malignant mixed mesoderm tumor (carcinosarcoma).
Values for serum CA125 were available for 23 of the 28 women with a malignant adnexal mass. The relative ability of vaginal LPA and serum CA125 to detect the various adnexal mass-related malignancies is shown in Table 3. Of six women with endometrioid ovarian cancer five (83.3%) were positive only for vaginal LPA while one (16.6%) was only CA125 positive. None were positive for both LPA and CA125. Of six women with a serous carcinoma five were CA125 positive (three only CA125 positive and two CA125 and LPA positive) and three were LPA positive (one LPA only and two LPA and CA125 positive). The median CA125 level was higher in women with a serous carcinoma (71 U/ml) than in those with endometrioid cancer (20 U/ml). Among the other malignancies only one, a woman with a granulosa tumor, was LPA positive and CA125 negative.
Table 3.
Comparison of women with different ovarian malignancies positive for vaginal lysophosphatidic acid and CA125.
| Diagnosis | No. tested | No. (%) positive |
|||
|---|---|---|---|---|---|
| CA125 only | LPA only | Both | Neither | ||
| Endometrioid+/− Clear cell | 6 | 1 (16.6) | 5 (83.3) | 0 | 0 |
| Serous | 6 | 3 (50.0) | 1(16.7) | 2(33.3) | 0 |
| Mucinous | 3 | 1(33.3) | 0 | 1(33.3) | 1(33.3) |
| MMMT | 2 | 2(100) | 0 | 0 | 0 |
| Granulosa | 2 | 0 | 1(50.0) | 0 | 1 (50.0) |
| Other | 2 | 0 | 0 | 1(50.0) | 1 (50.0) |
| Clear cell | 1 | 0 | 0 | 0 | 1(100) |
| Mixed | 1 | 1 (100%) | 0 | 0 | 0 |
Endometrioid +/− Clear cell, endometrioid tumor with and without clear cell components; MMMT, malignant mixed mesoderm tumor (carcinosarcoma); Other, metastatic adrenal, leiomyosarcoma.
Discussion
LPA was detected in vaginal fluid from all women with a malignant adnexal mass as well as in 91% of those whose mass was benign. The vaginal LPA concentration only in women with endometrioid ovarian cancer was significantly higher than the level present in vaginal fluid from women with various benign diagnoses. Furthermore, LPA predicted the detection of an endometrioid ovarian cancer in 83.3% of women with this diagnosis while CA125 was positive in only 16.6% of these women. In contrast, the vaginal LPA assay was inferior to serum CA125 determination for detection of serous ovarian carcinoma. Serum CA125 and vaginal LPA were positive in 83% and 50% of women with this diagnosis, respectively. Among women with a malignant adnexal mass the serum concentration of CA125 was lower in those with an endometrioid ovarian tumor than in those with serous ovarian cancer. This further suggests that CA125 is superior for detection of serous carcinoma than for endmetrioid covarian cancer. The tentative conclusion from these observations is that determination of the vaginal LPA concentration might be a more sensitive measurement than serum CA125 to noninvasively detect the presence of endometrioid ovarian cancer, but not serous carcinoma, in women with a suspicious adnexal mass.
Endometrioid ovarian cancer is classified as a type 1 low grade malignancy. It has been estimated to represent about 10% of ovarian cancers and is often associated with prior endometriosis. Prognosis is usually favorable, especially if confined to the ovary [17,18]. Two studies have reported that vaginal levels of CA125 were higher in women with endometrial adenocarcinoma than in women with benign ovarian cysts, endometrial hyperplasia or uterine bleeding [7,8]. In addition, the incidence of endometriod ovarian cancer is lowered in women who have undergone a tubal ligation [6]. These observations suggest the involvement of genital tract components in development of this malignancy. The finding that vaginal LPA is higher in women with this diagnosis is consistent with this suggestion. Elevated levels of LPA in the vagina might be due to its transduction from the systemic circulation and/or by its local production within the genital tract and subsequent migration and collection in the vagina. The association of LPA with only one type of ovarian malignancy, endometrioid cancer, argues against transduction being a major contributor to vaginal LPA levels.
The source(s) of LPA in vaginal fluid remain to be determined. Platelet activation has been shown to be a source of LPA release into the circulation [19]. In addition, transformed epithelial cells from women with gynecologic malignancies produce LPA [10]. Thus, both a pro—inflammatory immune response that stimulates platelet activation and local production by transformed cells may likely contribute to the elevation in vaginal LPA in women with endometrioid ovarian cancer.
Limitations of the study must be acknowledged. Due to the low number of subjects analyzed the study must be designated as hypothesis testing and is subject to confirmation. In addition, studies on pre-menopausal women with an adnexal mass, as well as pre- and post-menopausal women without an adnexal mass, also remain to be conducted. The use of more sophisticated measurements of vaginal LPA, for example by mass spectrometry-based methods, are needed to provide additional information on fatty acid chain length and saturation. Advantages of the study are that it was prospective, all procedures and diagnoses were from a single institution and samples for CA125 and for LPA were obtained at the same time.
In conclusion, detection of an association between vaginal LPA levels and an ovarian malignancy in this pilot study suggests that analysis of vaginal components as biomarkers for this cancer is biologically plausible and deserves further investigation. The LPA assay may especially be of value for the pre-surgical detection of endometrioid ovarian cancer in post-menopausal women with a suspicious adnexal mass. Further analysis on a larger sample size and among different populations is needed to verify the tentative conclusions and assess the potential clinical utility of vaginal LPA determination.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgement
Supported by a grant from The Macy Foundation.
References
- 1.Radosa M.P., Camara O., Vorwergk J. Preoperative multimodal strategies for risk assessment of adnexal masses. Analysis of 1362 cases in a gynecologic cancer center. Int J Gynecol Cancer. 2011;(21):1956–1962. doi: 10.1097/IGC.0b013e3182187eb0. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs I., Bast R.C., Jr. The CA 125 tumor-associated antigen: a review of the literature. Hum Reprod. 1989;4:1–12. doi: 10.1093/oxfordjournals.humrep.a136832. [DOI] [PubMed] [Google Scholar]
- 3.Bast R.C., Jr, Klug T.L., John St E. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Eng J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 4.Piek J.M., van Diest P.J., Zweemer R.P. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 5.Kurman R.J., Shih I.-M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cibula D., Widschwendter M., Zikan M., Dusek L. Underlying mechanisms of ovarian cancer risk reduction after tubal ligation. Acta Obstet Gynecol. 2011;90:559–563. doi: 10.1111/j.1600-0412.2011.01114.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarandakou A., Phocas I., Botsis D., Rizos D., Trakakis E., Chryssikopoulos A. Vaginal fluid and serum CEA, CA125 and SCC in normal conditions and in benign and malignant diseases of the genital tract. Acta Oncol. 1997;36(7):755–759. doi: 10.3109/02841869709001350. [DOI] [PubMed] [Google Scholar]
- 8.He S.-M., Xing F., Sui H., Wu Y., Wang Y., Wang D. Determination of CA-125 levels in the serum, cervical and vaginal secretions, and endometrium in Chinese women with precancerous disease or endometrial cancer. Med Sci Monit. 2011;17(11):CR618–25. doi: 10.12659/MSM.882046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terzi H., Kale E., Turkay U., Chong G.O., Lee Y.S. New method: Are tumor markers in vaginal-washing fluid significant in the diagnosis of primary ovarian carcinoma? Eur J Gynaecol Oncol. 2015;36(5):560–563. [PubMed] [Google Scholar]
- 10.Calis P., Yuce K., Basaran D., Salman C. Assessment of cervicovaginal cancer antigen 125 levels: A preliminary study for endometrial cancer screening. Gynecol Obstet Invest. 2016;81:518–522. doi: 10.1159/000444321. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y., Shen Z., Wiper D.W., Wu M., Morton R.E., Elson P. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 12.Fang X., Schummer M., Mao M. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;23:57–64. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 13.Sedlakova I., Vavrova J., Tosner J., Hanousek L. Lysophosphatidic acid (LPA) – a perspective marker in ovarian cancer. Tumor Biol. 2011;32:311–316. doi: 10.1007/s13277-010-0123-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y.J., Cao L.Y., Fu Z.Z., Wang Y.J., Wang G.X., Gu T. Clinical significance of plasma lysophosphatidic acid levels in the differential diagnosis of ovarian cancer. J Cancer Res Ther. 2015;11(2):375–380. doi: 10.4103/0973-1482.157335. [DOI] [PubMed] [Google Scholar]
- 15.Chang C.-L., Liao J.-J., Huang W.-P., Lee H. Lysophosphatidic acid inhibits serum deprivation-induced autophagy in human prostate cancer PC-3 cells. Autophagy. 2007;3(3):268–270. doi: 10.4161/auto.3909. [DOI] [PubMed] [Google Scholar]
- 16.Orfanelli T., Doulaveris G., Holcomb K., Jeong J.M., Sisti G., Kanninen T.T. Inhibition of autophagy in peripheral blood mononuclear cells by vaginal fluid from women with a malignant adnexal mass. Int J Cancer. 2015;137:2879–2884. doi: 10.1002/ijc.29665. [DOI] [PubMed] [Google Scholar]
- 17.Gurung A., Hung T., Morin J., Gilks C.B. Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathol. 2013;62:59–70. doi: 10.1111/his.12033. [DOI] [PubMed] [Google Scholar]
- 18.Kurman R.J., Shih I.-M. The dualistic model of ovarian carcinogenesis revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichholitz T., Jalink K., Fahrenfort I., Moolenaar W.H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]