Suppression of endothelial ERK1/1 pathways results in multi-organ failure, similar to what is observed in patients treated with anti-VEGF therapies.
Abstract
The mechanism of maintaining vascular endothelial identity and integrity is largely unknown. In this issue of JEM, Ricard et al. (https://doi.org/10.1084/jem.20182151) discover essential roles of ERK1/2 in maintaining endothelial homeostasis and its deletion-related serious defects in multiple tissues and organs.
Together with perivascular cells, vascular endothelial cells (ECs) may constitute the largest cell population in the body and are broadly distributed in almost all tissues and organs. Arterial, venous, and capillary ECs organize into endothelium with distinct features. For example, endothelium in endocrine organs contains fenestrae and exhibits sinusoidal structure in hematopoietic tissues (Roberts and Palade, 1995). Tissue-context endothelium organization, survival, EC identity, and integrity are tightly regulated by a balance between positive and negative endothelial factors. Overproduction of angiogenic factors such as vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs) often initiates an angiogenic response. Particularly, pathological processes such as tumor growth and age-related macular degeneration often accompany an angiogenic phenotype (Folkman, 1971), and an excessive amount of VEGF is largely responsible for neovascularization. In addition to angiogenesis, VEGF potently induces vascular permeability (Senger et al., 1983). Heterozygous mice lacking only one allele of the Vegf gene exhibit haploinsufficiency and embryonic lethality (Carmeliet et al., 1996; Ferrara et al., 1996). While the impact of these factors on neovascularization is relatively well studied, little is known about their physiological functions in the quiescent endothelium. Notably, VEGFs and FGFs are also ubiquitously expressed in most adult tissues and organs without augmenting angiogenesis. Systemic treatment of adult animals with anti-VEGF agents results in significant regression and loss of integrity of microvasculatures in multiple tissues and organs, indicating the crucial survival effect of VEGF (Yang et al., 2013). Activation of VEGFR2 and FGFRs by their respective ligands markedly induces ERK phosphorylation. Genetic deletion of mouse Erk2, but not Erk1, results in embryonic lethality with multifarious developmental defects (Hatano et al., 2003). The quiescent nature of endothelium is thought to be maintained by many endogenous negative factors such as thrombospondin-1, angiostatin, and endostatin (Cao, 2008), but the mechanisms of these negative regulators are still not known despite their early discovery.

Insights from Yihai Cao.
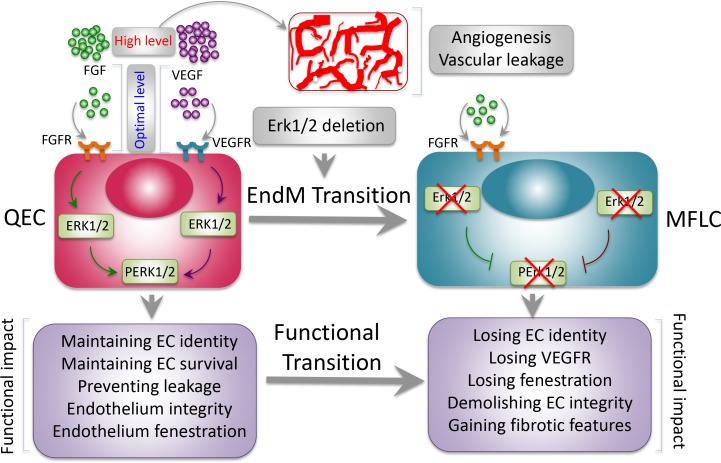
Functional consequences of Erk1−/− Erk2iEC−/− mice. Under a physiological condition, FGFs and VEGF are expressed at an optimal level to maintain endothelium homeostasis through activation of ERK1/2. Maintenance of EC identity, survival, and integrity by ERK1/2 are essential for sustaining vascular functions. Overexpression of these and other angiogenic factors in pathological tissues such as tumors often triggers angiogenesis and vascular permeability. Deletion of global Erk1 and endothelial Erk2 leads to EndMT, resulting in fibrosis in multiple organs. ECs lose their identity and gain fibrotic features. Erk1−/− Erk2iEC−/− mice experience sudden death and manifest functional failures of multiple organs, including heart, liver, kidney, and lung. MFLC, myofibroblast-like cell; QEC, quiescent EC.
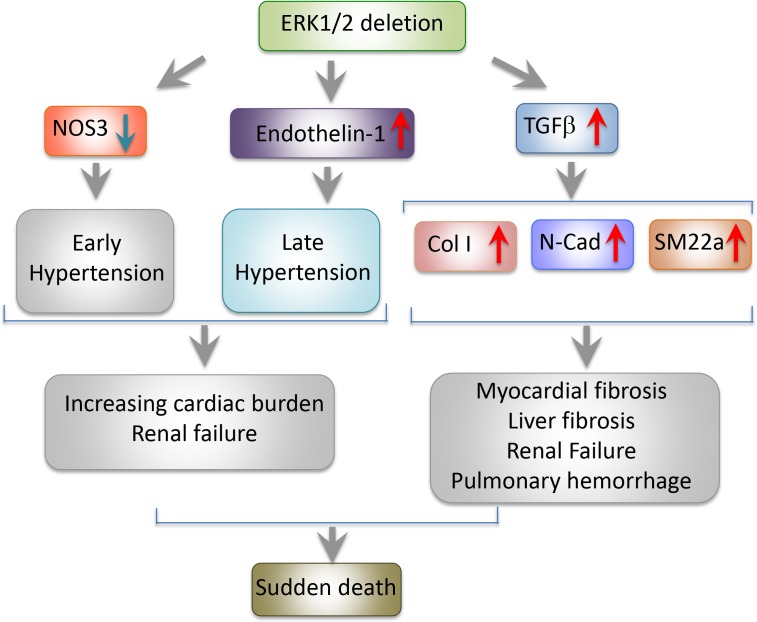
In the current issue of JEM, Ricard et al. take a genetic approach to conditionally delete endothelial Erk2 in a global Erk1−/− background (Erk1−/− Erk2iEC−/−). Surprisingly, inducible Erk2 deletion results in sudden death of all adult mice, owing to functional failures of multiple organs and tissues. The sudden death of mice suggests that vasculatures in crucial organs might be affected. Detailed analysis of the cardiovascular system shows progressive and significant delays of intraventricular conduction, atrial flutter, and atria-ventricular block. These electrocardiographic abnormalities are most likely caused by widespread myocardial fibrosis. Prior to death, Erk1−/− Erk2iEC−/− mice experience a two-phase hypertension period. At the initial phase, shortly followed by tamoxifen administration, a significant decrease of eNOS is likely responsible for inducing hypertension. Elevated levels of endothelin-1, a potent vasoconstrictor, raise blood pressure at a delayed phase. Hypertension plus cardiomyopathy might be largely responsible for inducing mouse sudden death.
Mechanisms of Erk1/2 deletion–triggered signaling pathways. Simultaneous deletion of global Erk1 and endothelial Erk2 down-regulates NOS3, developing an early phase of hypertension. Up-regulation of endothelin-1 is responsible for a delayed development of a late phase of hypertension. TGFβ and its targeted downstream signaling molecules trigger fibrosis in multiple tissues and organs. Erk1−/− Erk2iEC−/− mice experience sudden death and manifest functional failures of multiple organs, including heart, liver, kidney, and lung.
One of the most intriguing findings by Ricard et al. (2019) is the Erk gene deletion–triggered global fibrosis program in multiple organs, including heart, kidney, liver, and lung by the endothelial-to-mesenchymal transition (EndMT). In Erk1−/− Erk2iEC−/− mice, endothelial TGFβ is markedly increased, which is not detectable in wild type of mice. Similarly, TGF-β–targeted genes including Smad2 and collagen I are coordinately up-regulated. To gain mechanistic insights on the causal link between the TGF-β and the broad fibrotic phenotypes, they employ a causal statistical computing framework for “omics” analysis of siERK1- and SiERK2-transfected HUVEC cells. Among the top 600 genes, TGFβ2 was one of the most upstream drivers of the predicted network. TGFβ2-driven EndMT genes, including CDH2, COL25A1, and COL5A1, endothelial fenestration–related plasmalemma vesicle–associated protein, vasoconstrictor END1, and vasodilator NOS3 are all within the core network.
Next, the authors employ the mTmG reporter system, i.e., the Cdh5CreERT2mtmG Erkfl/fl Erk−/− strain to genetically trace ECs. Interestingly, Erk1−/− Erk2iEC−/− ECs in heart, kidney, and liver gain expression of mesenchymal markers, including Sm22a, N-cadherin, and smooth muscle actin. Under a physiological condition, dual deletion of Erk1 and Erk2 genes instructs ECs to become mesenchymal-like cells. In the kidney, thickening of the glomerular basement membrane and tubular proteinosis progressively develop, leading to increase of the albumin/creatine ratio in plasma and urine. Additionally, glomerular and peritubular capillaries often contain blood clots and lack fenestrations. Consistently, VEGFR2, a key receptor for endothelial fenestrations, is markedly decreased in glomerular, perivascular, and pancreatic islet capillaries. The Erk1−/− Erk2iEC−/− mice also show pulmonary hemorrhages and intravascular thrombosis, accompanying irregular and thickened basement membrane.
This study by Ricard et al. (2019) provides another compelling evidence of the plasticity of ECs that could transdifferentiate into other type of cells. Unlike most other cell types, vascular ECs exist in almost all tissues, and their phenotypic transition would have global impacts on functional alterations in multiple tissues and organs. Indeed, among the examined organs, Erk deletion–induced fibrosis and vascular abnormalities exist in various tissues, validating the broad functional defects. In embryos, EndMT is essential for the development of cardiac valves (Eisenberg and Markwald, 1995). EndMT also occurs under many pathological conditions. For example, EndMT in tumors is an important source of cancer-associated fibroblasts that significantly contribute to cancer invasion and metastasis (Potenta et al., 2008). Excessive EndMT also facilitates cardiovascular diseases, including myocardial infarction, cardiac fibrosis, valve calcification, endocardial elastofibrosis, atherosclerosis, and pulmonary arterial hypertension (Kovacic et al., 2019). The sudden death encountered by Erk deletion mice could be due to multiple organ defects related to fibrosis, thrombosis, hemorrhages, and hypertension. Perhaps the delayed sudden death phenotype might reflect the duration of EndMT to develop severe fibrosis-related pathological changes.
Another interesting notion is that many of these Erk-less phenotypes are reminiscent of clinical adverse effects in cancer patients who receive anti-VEGF treatment. In particular, hypertension, proteinuria, and hemorrhages are common in anti-VEGF–treated cancer patients (Perren et al., 2011). In preclinical animal models, anti-VEGF drugs have been reported to promote cancer metastasis (Ebos et al., 2009). The impact of anti-VEGF treatment on organ fibrosis warrants clinical investigation. It is unclear if anti-VEGF treatment also promotes the EndMT–cancer-associated fibroblast in tumors, which ultimately leads to metastasis. Along this line of thinking, development of small chemical molecules targeting ERKs for drug development should be extremely cautious for clinical use.
In conclusion, this study has provided novel mechanistic insights on transdifferentiation of quiescent ECs into fibroblast-like cells by inhibiting ERK. Fibrosis is a common pathological process that causes functional failures of multiple organs. It would be interesting to study if reduction of endothelial ERK1/2 is also involved in fibrotic diseases such as lung fibrosis, cirrhosis, and kidney fibrosis. If so, modest elevation of ERK expression by drugs would provide a novel approach for therapy. Particularly, chemical-induced toxicity followed by fibrosis in these organs might be involved in suppression of the ERK pathway. Cancer drugs targeting ERK1/2 are under development (Sullivan et al., 2018), and their impacts on causing broad toxicity of various organs as shown in this study warrant careful evaluation.
References
- Cao Y. 2008. Adv. Cancer Res. 100:113–131. 10.1016/S0065-230X(08)00004-3 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., et al. . 1996. Nature. 380:435–439. 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- Ebos J.M., et al. . 2009. Cancer Cell. 15:232–239. 10.1016/j.ccr.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg L.M., and Markwald R.R.. 1995. Circ. Res. 77:1–6. 10.1161/01.RES.77.1.1 [DOI] [PubMed] [Google Scholar]
- Ferrara N., et al. . 1996. Nature. 380:439–442. 10.1038/380439a0 [DOI] [PubMed] [Google Scholar]
- Folkman J. 1971. N. Engl. J. Med. 285:1182–1186. 10.1056/NEJM197111182852108 [DOI] [PubMed] [Google Scholar]
- Hatano N., et al. . 2003. Genes Cells. 8:847–856. 10.1046/j.1365-2443.2003.00680.x [DOI] [PubMed] [Google Scholar]
- Kovacic J.C., et al. . 2019. J. Am. Coll. Cardiol. 73:190–209. 10.1016/j.jacc.2018.09.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren T.J., et al. . 2011. N. Engl. J. Med. 365:2484–2496. 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- Potenta S., et al. . 2008. Br. J. Cancer. 99:1375–1379. 10.1038/sj.bjc.6604662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard N., et al. J. Exp. Med. 2019 doi: 10.1084/jem.20182151. [DOI] [Google Scholar]
- Roberts W.G., and Palade G.E.. 1995. J. Cell Sci. 108:2369–2379. [DOI] [PubMed] [Google Scholar]
- Senger D.R., et al. . 1983. Science. 219:983–985. 10.1126/science.6823562 [DOI] [PubMed] [Google Scholar]
- Sullivan R.J., et al. . 2018. Cancer Discov. 8:184–195. 10.1158/2159-8290.CD-17-1119 [DOI] [PubMed] [Google Scholar]
- Yang Y., et al. . 2013. Proc. Natl. Acad. Sci. USA. 110:12018–12023. 10.1073/pnas.1301331110 [DOI] [PMC free article] [PubMed] [Google Scholar]