A Mg2+-dependent mechanism regulates proximal T cell receptor signaling by modulating ITK activity through a low-affinity Mg2+ binding pocket in the catalytic domain. Dietary Mg2+ deprivation in mice impairs T cell activation and T cell–mediated immunity against influenza.
Abstract
Mg2+ is required at micromolar concentrations as a cofactor for ATP, enzymatic reactions, and other biological processes. We show that decreased extracellular Mg2+ reduced intracellular Mg2+ levels and impaired the Ca2+ flux, activation marker up-regulation, and proliferation after T cell receptor (TCR) stimulation. Reduced Mg2+ specifically impairs TCR signal transduction by IL-2–inducible T cell kinase (ITK) due to a requirement for a regulatory Mg2+ in the catalytic pocket of ITK. We also show that altered catalytic efficiency by millimolar changes in free basal Mg2+ is an unrecognized but conserved feature of other serine/threonine and tyrosine kinases, suggesting a Mg2+ regulatory paradigm of kinase function. Finally, a reduced serum Mg2+ concentration in mice causes an impaired CD8+ T cell response to influenza A virus infection, reduces T cell activation, and exacerbates morbidity. Thus, Mg2+ directly regulates the active site of specific kinases during T cell responses, and maintaining a high serum Mg2+ concentration is important for antiviral immunity in otherwise healthy animals.
Introduction
Mg2+ is required at micromolar concentrations as an essential cofactor for many fundamental biological processes, especially enzymatic reactions involving kinases, nucleic acids, and ATP (Weston, 2008; de Baaij et al., 2015). The typical American diet is insufficient in Mg2+, leading the US Dietary Guidelines Advisory Committee to classify Mg2+ as a shortfall nutrient of public health concern (Costello et al., 2016). Moreover, genetic variation, illnesses, and drugs can cause persistent reductions in serum Mg2+ (Tong and Rude, 2005; Meyer et al., 2010; Cundy and Mackay, 2011). Approximately 95% of intracellular Mg2+ is tightly bound with micromolar affinity as a counterion and cofactor to nucleic acids and nucleotides such as ATP, proteins, and other macromolecules. This “bound” Mg2+ is typically stable at a setpoint concentration between 10 and 20 mM depending on the cell type (de Baaij et al., 2015). By contrast, a small percentage (usually ∼5% or 0.5–1 mM) is basal “free” Mg2+ that is unbound and chemically active and whose concentration, [Mg2+]i, can vary in the low-millimolar range. The free basal Mg2+ dynamically exchanges with free external Mg2+, [Mg2+]e, to establish an electrochemical equilibrium and is not buffered by the bound Mg2+ (MacDermott, 1990; Chaigne-Delalande et al., 2013). Patients with X-linked immunodeficiency with Mg2+ defect, Epstein–Barr virus, and neoplasia (XMEN) disease due to a loss of expression of Mg2+ transporter 1 (MAGT1) have impaired Mg2+ homeostasis, reduced [Mg2+]i, abnormal N-linked glycosylation, and defective immune responses (Zhou and Clapham, 2009; Cherepanova et al., 2014; Shrimal et al., 2015). How changes in [Mg2+]i alter T cell receptor signaling and immune cell function is unknown.
Results
Mg2+ deprivation impairs T cell activation
To investigate T cell responses, we reduced the free basal [Mg2+]i by varying the [Mg2+]e (Chaigne-Delalande et al., 2013). In lymphocytes, we have determined that total Mg2+, [Mg2+]T, = 17 mM based on an assumed resting isotonic lymphocyte cell volume of 200 femtoliters (Deutsch and Lee, 1988; Li et al., 2011; Chaigne-Delalande et al., 2013). To mimic the decreased [Mg2+]i levels observed in XMEN patient T cells without other biochemical effects due to MagT1 gene deficiency (Chaigne-Delalande et al., 2013), we equilibrated T lymphocytes from healthy donors in different levels of [Mg2+]e to achieve different free basal [Mg2+]i using either Mg2+-depleted medium (dRPMI), [Mg2+]e <0.10 mM; reduced Mg2+ medium (rRPMI), [Mg2+]e ≈ 0.13 ± 0.01 mM; or complete medium (cRPMI), [Mg2+]e ≈ 0.59 ± 0.04 mM. To accurately quantitate [Mg2+]i, we adapted a null point titration assay developed by Flatman and Lew (1980) (Grynkiewicz et al., 1985; Ng et al., 1991). We initially used flow cytometry to measure the response of an internal probe in permeabilized cells exposed to various concentrations of [Mg2+]e (Fig. 1 A) and the Grynkiewicz equation (Fig. 1 B; Grynkiewicz et al., 1985). We determined that cycling T cell blasts have a [Mg2+]i = 0.54 ± 0.18 mM in cRPMI, 0.44 ± 0.19 mM in rRPMI, and 0.39 ± 0.1 mM in dRPMI. We confirmed these values using a standard cuvette-based fluorometric assay that allows more rapid equilibration (Fig. 1, B and C). We found this assay had excellent reproducibility between experiments and gave values of [Mg2+]i in cRPMI = 0.77 mM ± 0.23 mM, rRPMI = 0.48 mM ± 0.07 mM, and dRPMI 0.31 mM ± 0.16 mM (Fig. 1 D). Because [Mg2+]T in resting human lymphocytes is 17 mM (Chaigne-Delalande et al., 2013), these values for free basal [Mg2+]i are between 1.7% and 4.5% of [Mg2+]T. Therefore, [Mg2+]i can vary in the millimolar range in T lymphocytes from healthy donors depending on [Mg2+]e.
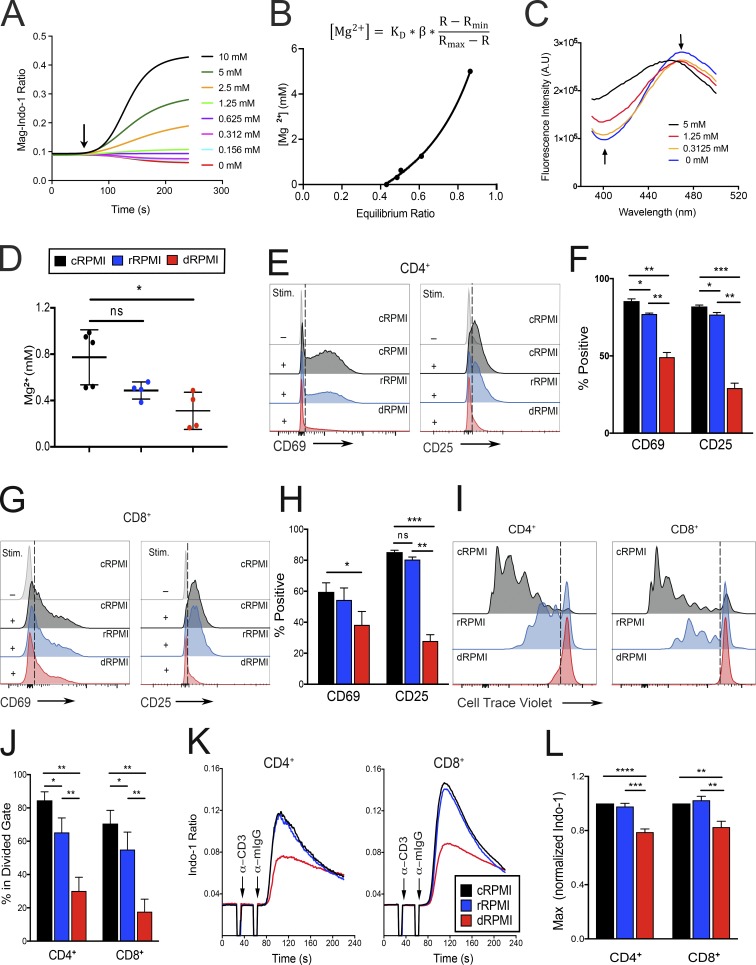
Figure 1.
Defective TCR activation and reduction of basal [Mg2+] in T cells deprived of extracellular Mg2+. (A) Representative curve-fitting plot of the flow cytometric Mg2+ calibration assay. The y axis shows the ratio of bound to unbound (BUV737/BUV380) Mag-Indo-1 dye. Arrow indicates addition of digitonin. Concentration of extracellular MgSO4 for each calibration curve is shown on the right. (B) Grynkiewicz equation and curve-fitting model used for calculation of [Mg2+]i concentration. KD, dissociation constant of Mag-Indo-1; β, scaling factor associated with system; R, measured ratio at any given concentration; Rmax, ratio at 5 mM Mg2+; Rmin, ratio at 0 mM Mg2+. (C) Plots of fluorescence intensity in A.U. versus wavelength of emission for the fluorometer-based version of the Mg2+ calibration assay. Arrows point to the emission wavelengths of the bound and unbound form of the Mag-Indo-1 dye and the direction the peaks shift with increasing Mg2+ concentration. (D) Quantification of internal Mg2+ concentrations in each respective media condition. (E) Flow cytometric analysis of CD69 and CD25 expression on CD4+ cells 18 h and 36 h, respectively, after either no stimulation (−) or the addition of 1 µg/ml anti-CD3/CD28 and 1 µg/ml anti-Ig secondary antibody (+) in cRPMI, rRPMI, and dRPMI as indicated. Dashed line indicates the threshold used for positive staining. (F) Quantification of the percentage of CD69+ and CD25+ cells shown in E. (G and H) Analysis of CD8+ cells as in E and F, respectively. (I and J) Flow cytometric analysis of Cell Trace violet fluorescence of labeled CD4+ and CD8+ cells 5 d after anti-CD3/CD28 stimulation in the indicated media; dashed line indicates the threshold used to determine at least one division quantified as percentage in the divided gate (J). (K) Flow cytometric analysis of Ca2+ flux (plotted as ratio of bound to unbound Indo-1 dye) versus time (s) in CD4+ and CD8+ T cell blasts cultured for 96 h in cRPMI, rRPMI, or dRPMI. Cells were stimulated with 1 µg/ml anti-CD3 and 1 µg/ml anti-Ig secondary antibody as indicated. (L) Quantification of the flux observed in K, represented as a fold change of the maximum ratio, based on the maximum obtained in cRPMI. Legend in D indicates experiments done in cRPMI (black), rRPMI (blue), and dRPMI (red). Panels A–C depict a representative replicate of at least three independent experiments. Error bars represent SEM of three (E–I), four (K and L), or five (D) independent experiments. P values were calculated with a paired t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant).
We next tested the responsiveness of T cells and found that the CD69 activation marker was significantly decreased in both CD4+ and CD8+ cells 18 h after TCR stimulation in dRPMI and rRPMI compared with cRPMI (Fig. 1, E–H). Similarly, CD25 was significantly reduced 36 h after activation in rRPMI and dRPMI (Fig. 1, E–H). The cell viability using these media ranged from 80–92% (Fig. S1, A and B). Importantly, the proliferation of both CD4+ and CD8+ cells was dramatically reduced when cells were activated and expanded in lower Mg2+ concentrations for five days (Fig. 1, I and J), with a decrease titrating from cRPMI to rRPMI to dRPMI. At this time point, we observed a significantly reduced cell number in dRPMI medium (Fig. S1, C–F), which could be due to the inability of T cells to proliferate in dRPMI. Thus, even an approximately twofold reduction in [Mg2+]i in the millimolar range was detrimental to T lymphocyte activation and proliferation.
We subsequently investigated the impact of chronic Mg2+ deprivation on TCR signal transduction by culturing T cell blasts in cRPMI, rRPMI, or dRPMI for 96 h and then measuring Ca2+ upon TCR engagement. Our assay buffer contains both Ca2+ and Mg2+ at 1.2 mM each to allow acute fluxes of those ions. Ca2+ flux was clearly blunted after TCR stimulation in both CD4+ and CD8+ T cells cultured in dRPMI, but not in rRPMI, as compared with cRPMI (Fig. 1, K and L). Extended incubation in dRPMI also adversely affects cell viability in rapidly proliferating cells (Fig. S1, G and H). Thus, Mg2+ deprivation impaired T cell activation, and short-term Mg2+ supplementation at the time of stimulation was not sufficient to rescue TCR signaling in cells grown in Mg2+-deprived medium.
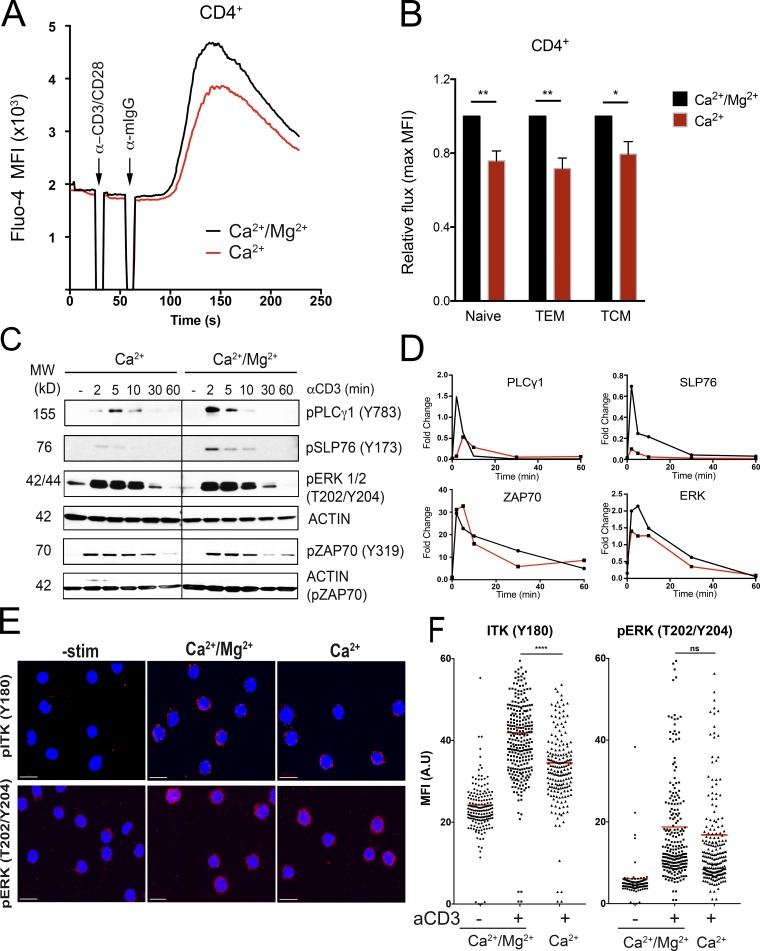
We next examined the effect of acute Mg2+ deprivation during TCR engagement, which would prevent any transient increase in [Mg2+]i (Li et al., 2011). To measure immediate TCR responses, we assayed Ca2+ fluxes in healthy donor CD4+ and CD8+ T cells in presence or absence of [Mg2+]e. We found that assay buffer with Ca2+ only (Ca2+) showed a decreased amplitude of the Ca2+ flux in both CD4+ and CD8+ cells compared with buffer with both Ca2+ and Mg2+ (Ca2+/Mg2+; Fig. 2 A and Fig. S2, A and B). Furthermore, gating on naive (CD45RA+, CCR7+), central memory (CD45RA−, CCR7+), and effector memory (CD45RA−, CCR7−) CD4+ cells revealed a significant reduction in Ca2+ fluxes in all three subsets when using assay buffer with no Mg2+ (Fig. 2 B). We next performed Western blots to assess the effects on TCR-triggered phosphorylation events with or without Mg2+ during TCR engagement. In ex vivo–isolated CD4+ cells stimulated without Mg2+, there was decreased phosphorylation of PLCγ1 (Y783) and SLP76 (Y173) compared with cells stimulated in the presence of both Ca2+ and Mg2+ (Fig. 2, C and D). TCR activation of the tyrosine kinase IL-2–inducible T cell kinase (ITK) is responsible for both PLCγ1 (Y783) and SLP76 (Y173) phosphorylation (Readinger et al., 2009; Sela et al., 2011). By contrast, ZAP70 and ERK phosphorylation, which does not depend on ITK, was not significantly affected (Fig. 2, C and D). Finally, we evaluated autophosphorylation of ITK on the tyrosine 180 (Y180) site as another measure of ITK activity and found that it was also significantly reduced 3 min after stimulation in the absence of extracellular Mg2+ (Fig. 2, E and F), while ERK phosphorylation was only slightly affected (Fig. 2, E and F). This is the step where signal propagation is inhibited in T cells from XMEN patients (Chaigne-Delalande et al., 2013) and is consistent with defects in ITK activity (Fig. S3 A).
Figure 2.
Acute Mg2+ deprivation impairs TCR signaling. (A) Flow cytometric analysis of Ca2+ flux in ex vivo–isolated PBMCs loaded with Fluo-4 and gated on CD4+ cells. 1 µg/ml anti-CD3/CD28 and 1 µg/ml anti-Ig secondary antibody was added at the indicated time. Stimulation was performed in buffer containing only 1.2 mM CaCl2 (Ca2+) or 1.2 mM CaCl2 and 1.2 mM MgSO4 (Ca2+/Mg2+). (B) Quantification of the peak of the Ca2+ flux in different CD4+ cells subsets (naive, CD45RA+CCR7+; central memory T cells [TCM], CD45RA−CCR7+; and effector memory T cells (TEM), CD45RA−CCR7−). (C) Representative Western blot analysis of phosphorylated proteins as indicated using lysates from ex vivo–isolated CD4+ cells stimulated with 10 µg/ml anti-CD3 and 10 µg/ml anti-Ig secondary antibody at the indicated times in buffer containing only 1.2 mM CaCl2 (Ca2+) or 1.2 mM CaCl2 and 1.2 mM MgSO4 (Ca2+/Mg2+). β-Actin is shown as a loading control. MW, molecular weight. (D) Quantification of the signal shown in C from one representative experiment. (E) Representative immunofluorescence images of CD4+ cells activated with soluble anti-CD3 and anti-IgG secondary (10 µg/ml each) antibody in different buffers as in C for 3 min and stained with the indicated antibodies. Scale bars represent 10 µM. (F) Quantification of the mean fluorescence intensity (MFI) in A.U. using >100 cells/condition for the images in E. Data are the mean of six independent experiments (A and B) or depict a representative replicate of three independent experiments (C–F). Error bars represent SEM. P values were calculated with a paired t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant).
ITK catalytic activity is regulated by millimolar changes in [Mg2+]
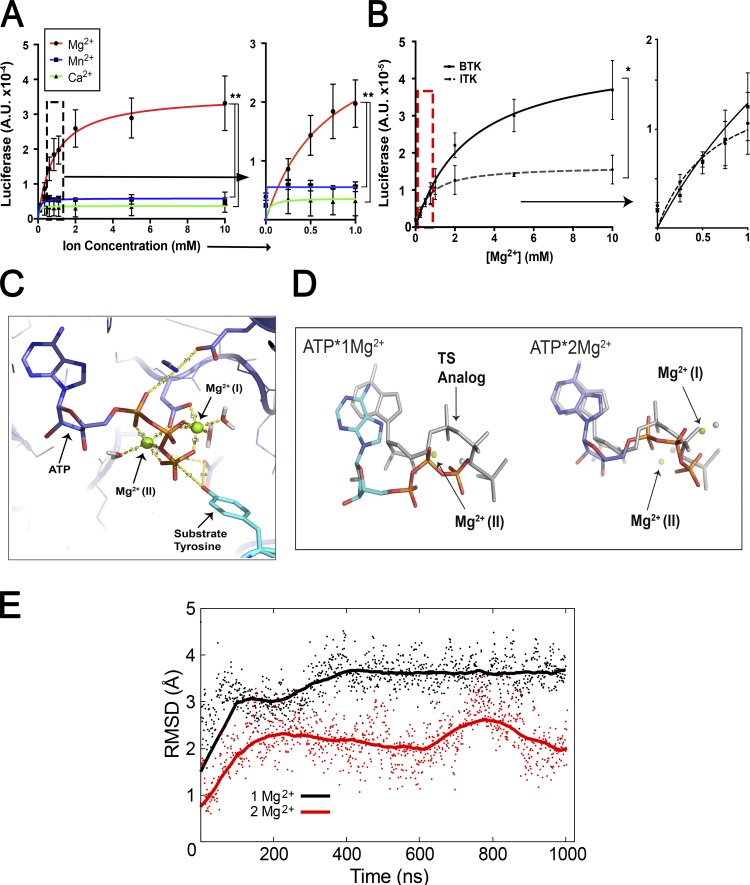
Like all kinases, ITK requires Mg2+ as a cofactor at ∼30 μM to form the ATP:Mg2+ chelate for activity (Bao et al., 2011). However, we hypothesized that there is additional regulation by Mg2+ at millimolar concentrations, possibly involving a second, low-affinity binding site. To test this, we measured the effect of varying [Mg2+] on the catalytic activity of purified ITK (Fig. S3 B). Keeping ATP:Mg2+ and substrate concentrations constant, we titrated [Mg2+] from 0.1 to 10 mM and observed increased ITK activity following Michaelis–Menten kinetics (Fig. 3 A). The Michaelis constant (Km) for [Mg2+] was 0.71 mM, predicting that ITK catalytic activity would change markedly at the different [Mg2+]i, as was observed when using Mg2+-deprived or replete conditions (Figs. 1 and 2). The dramatically increased velocity caused by Mg2+ in the low-millimolar range indicates a second function for Mg2+ in catalysis besides its role as a cofactor for ATP:Mg2+. By comparison, Ca2+, a related alkaline metal cation, and Mn2+, a transition metal often substituted for Mg2+, had no effect on ITK activity. Since BCR signaling closely parallels TCR signaling and there are a number of other tyrosine kinases in both pathways (Fig. S3 C), we tested the effect of [Mg2+] on the activity of Bruton’s tyrosine kinase (BTK), the Tec family counterpart of ITK found predominantly in B cells (Kurosaki, 1999; Joseph et al., 2013), lymphocyte-specific protein tyrosine kinase (Lck), and ZAP70. All three kinases exhibited an increase in activity, but the effect on initial velocity was less pronounced than for ITK (Fig. S3, D and E; and Table 1). Strikingly, BTK was more active when Mn2+ was substituted for Mg2+, indicating that although ITK and BTK are homologous enzymes, their active sites are regulated differently by divalent cations. To verify these results and directly compare ITK and BTK, we purified active FLAG-tagged kinases using a mammalian expression system. These protein preparations also confirmed a dependence on millimolar levels of Mg2+ and showed that ITK was less active than BTK, consistent with previous observations (Fig. 3 B; Joseph et al., 2013).
Figure 3.
Mg2+ can regulate ITK kinase activity at physiological concentrations via two Mg2+ binding pockets in the catalytic domain. (A) Kinase activity assay for ITK as a function of increasing MgCl2 (red), MnCl2 (blue), and CaCl2 (green) concentrations. The y axes represent luciferase activity (A.U.). (B) Comparison of ITK and BTK kinase activity as a function of [Mg2+] using purified FLAG-tagged proteins. Graph to the right shows an expanded axis containing the region boxed by the dashed lines representing the approximate [Mg2+]i measured in lymphocytes (A and B). (C) Spatial model of the ITK catalytic pocket complexed with ATP. Mg2+ ions are depicted in green. (D) MD simulations of ITK with ATP and one Mg2+ ion (left) and two Mg2+ ions (right) predict that presence of the second Mg ion affects both the orientation and the dynamics of the phosphates. Specifically, the phosphates and Mg2+ ion more closely resemble the transition-state mimic conformation (shown in gray) when two Mg2+ are bound. Average positions of the ATP and Mg2+ ions over the entire trajectory are shown for each 1-µs simulation. Structures were aligned on the protein backbone. The gray ATP and Mg2+ reference structures shown are the conformation of the ATP transition-state mimic in CDK2, i.e., ADP/2Mg2+/MgF3− from the Protein Data Bank (3QHW). (E) RMSD plot of the position of the γ-phosphate in each simulation relative to the position in the transition state. Points are shown every 1 ns; solid lines are 250-ns rolling average values. Data are the mean of at least three independent experiments or simulations (A–E) ± SEM (A and B). Error bars represent SEM. P values were calculated with a paired t test (*, P < 0.05; **, P < 0.01).
Table 1. Calculated Km values for kinases tested in the in vitro activity assay with ion titrations.
| Km [Mg2+] mM | Km [Mn2+] mM | Km [Ca2+] mM | |
|---|---|---|---|
| ITK | 0.71 ± 0.16 | 0.074 ± 0.04 | 0.036 ± 0.13 |
| BTK | 2.61 ± 0.51 | 0.333 ± 0.13 | 0.032 ± 0.1 |
| Lck | 2.80 ± 0.26 | ||
| ZAP70 | 2.032 ± 0.54 | ||
| ERK1/2 | 0.374 ± 0.2 | ||
| DYRK | 0.184 ± 0.05 | ||
| PKCθ | 0.260 ± 0.07 | ||
| PKD2 | 0.265 ± 0.06 |
Calculated Km values for kinase assays with Mg2+, Mn2+, and Ca2+ for ITK and BTK. Calculated Km values for kinase assays with Mg2+ for LCK, ZAP70, ERK, DYRK, PKCθ, and PKD2.
ITK utilizes a second low-affinity Mg2+ to configure its catalytic pocket
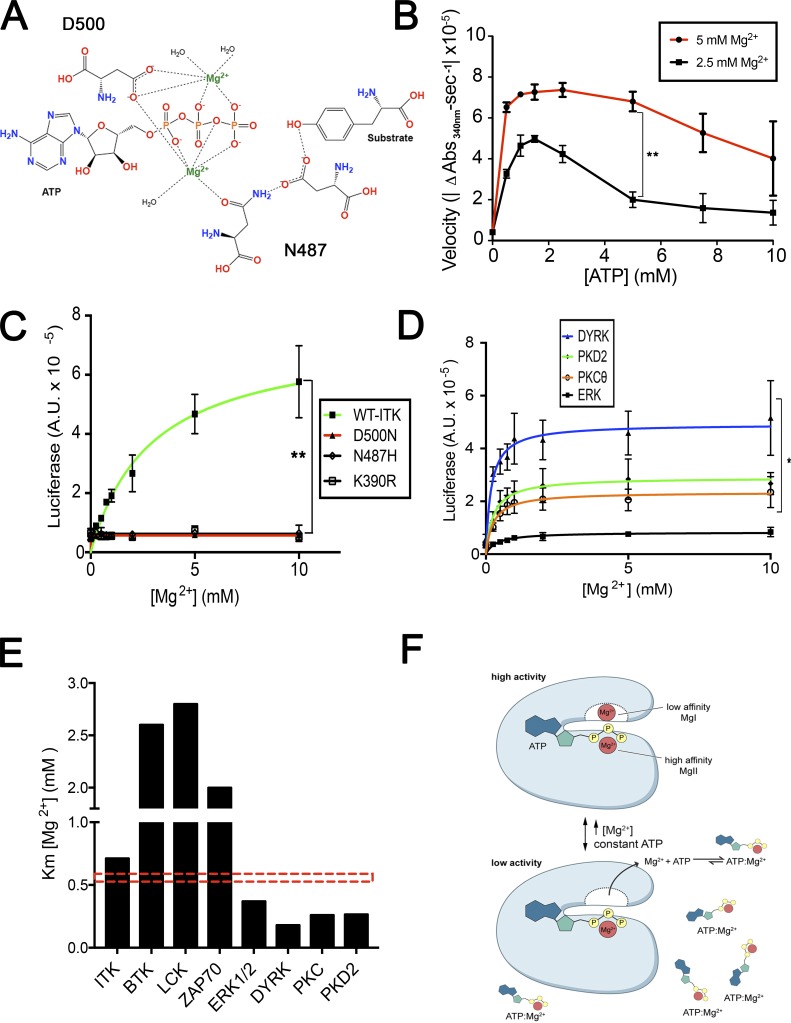
The variation of ITK enzyme activity with [Mg2+] in the range of 0–2 mM is particularly interesting, because [Mg2+]i in different types of mammalian cells has been reported to be 0.1–1 mM (Fig. 3 A, right panel; rectangle in Fig. S3, D and E; Buri and McGuigan, 1990; MacDermott, 1990). Thus, ITK could be a “sensor” or rheostat of [Mg2+]i that controls the physiological response to antigen receptor engagement in T cells with varying [Mg2+] (Li et al., 2011). To understand how millimolar changes in [Mg2+]i influence ITK activity, we examined the possibility of a second low-affinity Mg2+ binding site in its catalytic pocket, which has been reported for cyclin-dependent kinase 2 (CDK2; Sun and Budde, 1997; Shaffer and Adams, 1999; Bao et al., 2011). The available ITK structures do not contain Mg2+ ions or ADP/ATP and are typically complexed with chemical inhibitors, so we threaded ITK using I-TASSER (Yang and Zhang, 2015) onto the CDK2 structure containing two Mg2+ ions and ADP in the catalytic pocket (Protein Data Bank accession number 3QHW; Bao et al., 2011). We then performed molecular dynamics (MD) modeling with AMBER 16 to evaluate the stabilities and structural effects of these ions on the threaded ITK structure (Case et al., 2017). Like CDK2, ITK can bind two Mg2+ ions with appropriate bipyrimidal tetrahedron hexa-coordinate chelation geometry in two distinct hypothetical sites in the catalytic pocket (Fig. 3 C; Bao et al., 2011). By reference to previous convention, the Mg2+ ion in the apparent low-affinity, “second” site was designated MgI, and the Mg2+ ion in the high-affinity site was designated MgII (Bao et al., 2011; Jacobsen et al., 2012). This model achieved a C score of 1.2, a template modeling score of 0.88 ± 0.07, and a root-mean-square deviation (RMSD) of 3.5 ± 2.4 Å, indicating that it represents a plausible structure that the primary ITK polypeptide chain can adopt (Yang and Zhang, 2015). Several features become apparent when comparing the structures with either one or two Mg2+ ions. The 𝛾 phosphate of ATP becomes aligned better in the two-Mg2+ ion structure with the optimal position for catalysis (shown in gray, with the one Mg2+ ion structure on the left; Fig. 3 D). Also, there is a rearrangement of helices and loops lining the active site are now aligned better with the well-characterized “closed” active site structure of CDK2 (Bao et al., 2011). For example, the two-Mg2+ ion structure reveals that the glycine-rich loop “clamps” down the roof of the active site, thereby stabilizing the position of the substrate for efficient phosphoryl transfer. We also found that the two-Mg2+ conformation has a lower RMSD relative to the transition state conformation, which would contribute to enhanced catalytic activity (Fig. 3 E). In this model, MgII was coordinated sixfold by the oxygens of N487 and D500, the β and gamma phosphate as well as the linking oxygen between them in ATP, and one water (Figs. 3 C and 4 A). In the two-Mg2+ structure, MgI formed contacts with a bidentate configuration of the oxygens of the D500 (DFG) carboxylate, the β and γ phosphates, and two water molecules. The distance between Mg2+ and the amino acid chelation points were within 2 Å, consistent with the CDK2 structure (Bao et al., 2011; Jacobsen et al., 2012). The steric constraint, previously observed in the CDK2 structure (Bao et al., 2011), was due to chelation by two oxygens from the D500 carboxylate, which have a 60° interoxygen bond angle rather than an ideal equatorial coordination angle of 90° (Fig. 3 C). This nonoptimal coordination, together with the fact that MgI had two chelation sites occupied by water, suggests that its binding is thermodynamically less favorable, thus accounting for the apparent lower affinity of this site compared with the MgII binding site (Bao et al., 2011). By contrast, MgII, which enters the active site as the ATP:Mg2+ chelate, has three coordinated oxygens in ATP, only one chelation site occupied by a water molecule, and a more ideal geometry, features that collectively contribute to the apparent higher binding affinity of MgII.
Figure 4.
Regulation of ITK activity is dependent on the low-affinity Mg2+ binding site, and mutation of residues that chelate Mg2+ in the catalytic pocket of ITK are highly conserved and required for enzymatic activity. (A) Schematic of the ITK catalytic pocket and the key residues coordinating Mg2+ and ATP. (B) Pyruvate kinase/lactate dehydrogenase (PK/LDH) assay of ITK titrating ATP at constant Mg2+ concentrations (2.5 and 5 mM). (C) Kinase assays of in-house–purified full-length ITK (green) and mutants disrupting Mg2+ chelation. Catalytically dead K390R ITK was purified as a control. The y axis represents luciferase activity (A.U.). (D) Kinase activity assays for DYRK, PKD, ERK, and PKCθ as a function of increasing MgCl2 concentration. (E) Bar graph of the average Km values for Mg2+ across different kinases tested in in vitro kinase assays. Red dashed box indicates an approximation of the basal level of [Mg2+]i. (F) Provisional model of two site Mg2+ regulation for kinases. Data are the mean of three independent experiments ± SEM (B–E). Error bars represent SEM. P values were calculated with a paired t test (*, P < 0.05; **, P < 0.01).
From this analysis, we conjectured that the MgI binding site would be low affinity (KD [dissociation constant of Mg2+ ion] in the millimolar range as opposed to the first site, for which the KD is in the micromolar range) and strongly affect ITK enzyme activity (Bao et al., 2011). To provide evidence for a second Mg2+ affecting catalysis and ensure that the boost in enzyme activity we observed (Fig. 3 A) is not due to the generation of more Mg:ATP−2, we used an approach previously described for CDK2 (Jacobsen et al., 2012), in which one measures enzyme activity while titrating pure, Mg2+-free, ATP. Since ATP is a strong chelator for Mg2+ (KD = 28.6 µM), it will abstract Mg2+ from the second, weak binding site in the enzyme once the [ATP] exceeds that of free Mg2+ in the buffer, resulting in inhibition of kinase activity. We titrated ATP at the indicated concentrations while keeping [Mg2+]e constant at either 2.5 or 5 mM and observed that with increasing ATP, the reaction velocity at low concentrations increased, likely by forming more Mg:ATP−2 complex. However, a maximum was reached, and an inhibitory effect was observed at approximately the same concentration as Mg2+ included in the reaction (Fig. 4 B). The reduction in activity was not due to removal of the Mg2+ from the high-affinity MgII site, since Mg:ATP−2 is in excess under these conditions. These data provide evidence Mg2+ occupies a second binding site in the enzyme, which is likely the MgI binding site predicted by MD modeling, and that this is necessary for optimal ITK catalytic activity.
Both the N487 and D500 residues, predicted by our modeling to be key Mg2+ chelation sites (Fig. 4 A), are highly conserved across tyrosine kinases (TKs; Fig. S4 E; Taylor and Kornev, 2011). The D500 residue in human ITK is part of the canonical DFG loop that regulates kinase activity and contributes two crucial oxygen chelation points in MgI (Taylor and Kornev, 2011). By contrast, N487 is adjacent to MgII and stabilizes the binding of Mg:ATP−2 as the enzyme cofactor. We designed mutants that would selectively disrupt only one of the Mg2+ chelation points for MgI and MgII, namely N487H and D500N, as well as a previously characterized kinase dead mutant, K390R (Heyeck et al., 1997; Fig. S4, A and B). We expressed and isolated FLAG-tagged mutant ITK proteins together with WT-ITK from mammalian cells (Fig. S4, C and D). To estimate the amount of enzyme needed for the kinase assays, we performed a Western blot for both FLAG and the Lck-mediated activating phosphorylation on Y511 (Fig. S4 D). Since Y511 phosphorylation is required for kinase activity but was not equal across the different proteins, we normalized the input for the kinase assays to the amount of phosphorylated ITK in each preparation to ensure equal amounts of activated enzyme were added to each assay. We observed that WT ITK had significant kinase activity and displayed increased activity when Mg2+ was titrated, especially in low-millimolar concentrations, verifying what we observed with the commercially prepared ITK protein (Fig. 3 A). Both mutants, N487H and D500N, predicted to disrupt Mg2+ binding to the cofactor site (MgII) or the regulatory site (MgI) abolished enzyme activity as effectively as the kinase-dead, K390R, mutant (Fig. 4 C). Therefore, both Mg2+ chelation points and the associated MgI and MgII binding sites are required for ITK phosphotransfer activity.
Augmented activity of other kinases by Mg2+
The signaling cascade downstream of the TCR involves several key TKs besides ITK, including Lck and ZAP70, as well as serine/threonine kinases such as protein kinase C theta (PKCθ) that are crucial for Ca2+ flux or subsequent T cell activation events. Conservation of the N487 and D500 residues (Fig. S4 E) suggested these kinases might also have a low-affinity Mg2+ binding site that is important for enzyme activity. As previously mentioned, Lck, ZAP70, and BTK also displayed increased Mg2+-dependent activity. We therefore tested the effects of Mg2+ on representative kinases belonging to different families of the kinome. We found that dual-specificity tyrosine-phosphorylation–regulated kinase (DYRK), ERK, PKCθ, and PKD2 all showed varying degrees of augmented catalytic activity with millimolar increases in [Mg2+] (Fig. 4 D). Using the enzyme kinetic curves, we calculated a Km for each of these kinases, which we found differed markedly (Fig. 4 E and Table 1). The serine-threonine and dual-specificity kinases, DYRK, ERK, PKCθ, and PKD2 all exhibited a Km in the range of 0.2–0.4 mM Mg2+ and would be expected to be fully active in T cells at basal Mg2+, which is 0.72 mM (Fig. 4 E and Table 1). ITK had a Km of 0.71 mM, which would have variable activity reflecting variations in [Mg2+]i observed under physiological conditions (Fig. 4 E and Table 1). By contrast, BTK, Lck, and ZAP70 had Km >2 mM and overall higher catalytic activity, indicating their relative insensitivity to perturbations in [Mg2+]i in the physiological concentration range (Fig. 4 E, Table 1, and Fig.S3, D and E). In conclusion, enzymes essential for lymphocyte activation have a Mg2+ dependence within the range that [Mg2+]i fluctuates, and ITK is the most sensitive to changes near the typical [Mg2+]i setpoint of 0.7 mM. A generalized model of Mg2+-dependent kinase regulation is illustrated in Fig. 4 F.
Mg2+ deprivation in vivo affects viral immunity
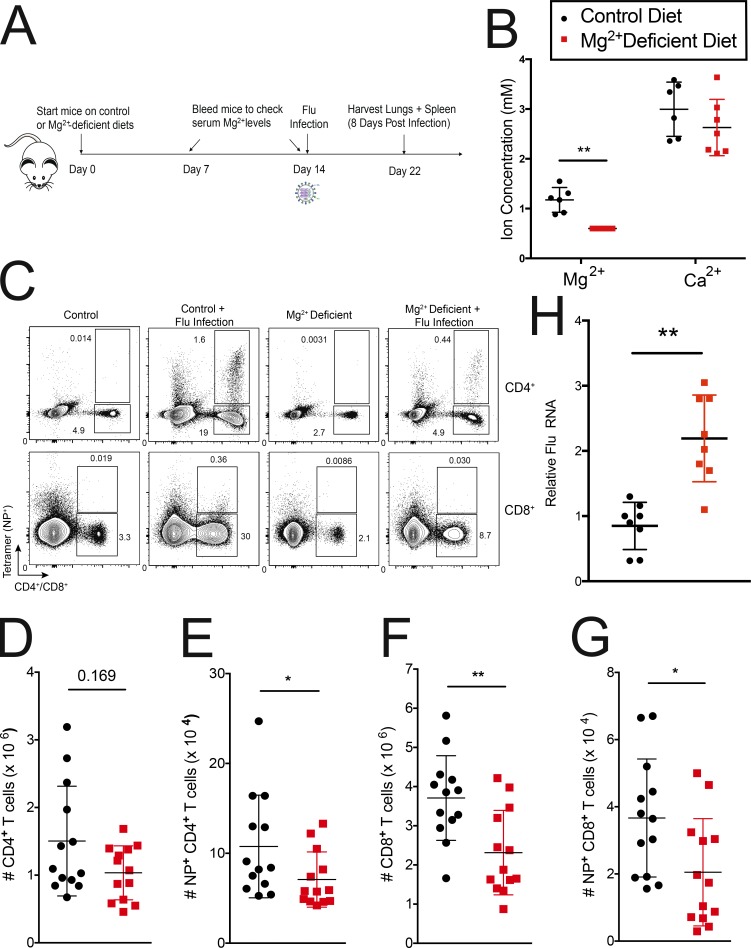
We next tested whether changes in serum Mg2+ levels can affect immune responses in vivo by restricting dietary Mg2+ for ∼2 wk before infecting mice intranasally with influenza A virus; lungs and spleen were harvested 8 d after infection (Fig. 5 A). Serum Mg2+ levels were monitored, and we observed a ∼50% reduction within 1–2 wk. Ca2+ levels were not significantly affected (Fig. 5 B). Consistent with the in vitro defects (Fig. 1, E–L; and Fig. 2), we observed a significant reduction in the numbers of both total CD8+ and tetramer+ CD8+ T cells in the lung of Mg2+-deprived mice 8 d after influenza infection (Fig. 5, C, F, and G). CD4+ cells were also reduced (Fig. 5, C–E), albeit to a lower extent. Splenic T cell numbers were not significant between the control and Mg2+-deprived animals (Fig. S5, D–G). We hypothesize that the difference in cell numbers is because Mg2+ deprivation affects both the expansion and homing/homeostasis of cells at the site of infection, which might give rise to the difference between the spleen and the lungs. Furthermore, low-Mg2+ environments have been demonstrated to induce bronchoconstriction, which could exacerbate the phenotype we see in the Mg2+-deprived animals. Consistent with an impaired immune response, viral titers in the lungs of Mg2+-deprived mice were significantly higher (Fig. 5 H), and these mice had greater morbidity and lost more weight during infection (Fig. S5 H).
Figure 5.
Mg2+ deprivation affects influenza A responses in vivo. (A) Schematic of the experimental design. (B) Mg2+ and Ca2+ serum levels assayed 13 d after mice (Male C57BL/6) were put on a Mg2+-reduced diet. (C) Representative flow cytometric plot of CD4+ and CD8+ cells in lung 8 d after flu infection. (D–G) Quantification of the number of CD4+ (D), CD4+NP tetramer+ (E), CD8+ (F), and CD8+ NP tetramer+ (G) cells in lungs of infected mice. (H) Quantitative RT-PCR quantification of viral titers from lungs of infected mice. Flu RNA is normalized to Hprt1. Data ± SEM shown are from two (H) or three (D–G) independent experiments with four or five mice in each infected group. Error bars indicate SEM. Panel B depicts a representative replicate of three independent experiments. Legend in B indicates the control diet (black) and the Mg2+-reduced diet (red) for B and D–H. Statistical significance was calculated with a Mann–Whitney U test (D–G; *, P < 0.05; **, P < 0.01) or a paired t test (B and H; *, P < 0.05; **, P < 0.01).
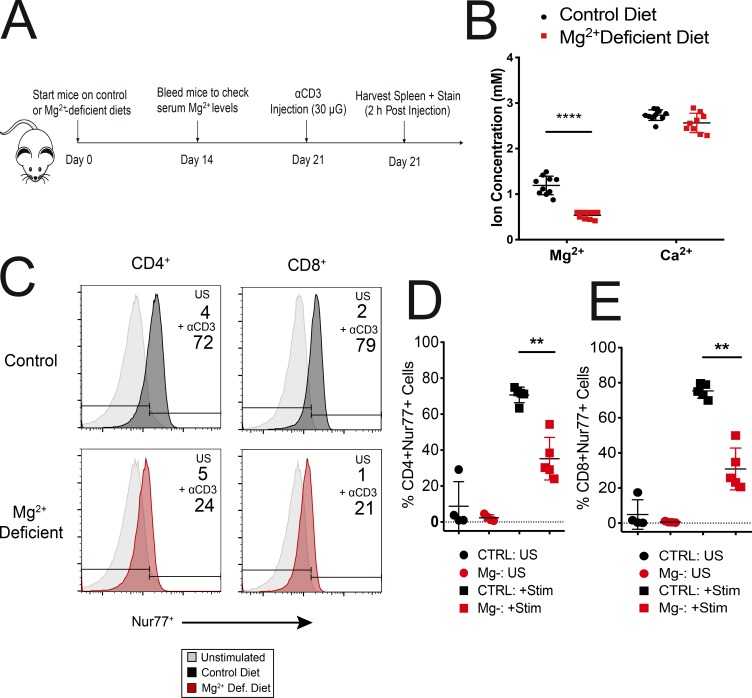
Mg2+ deprivation in vivo affects T cell activation
To exclude the possibility that the impaired flu responses we observed in Mg2+-deprived mice were due to indirect effects on T cell activation or homing, we tested whether chronic Mg2+ deprivation in vivo would recapitulate the T cell activation defects we observed in vitro using Nur77 as a specific T cell activation marker (Ashouri and Weiss, 2017). Mice were deprived of dietary Mg2+ for 2 wk, and αCD3 antibody (30 μg, clone 145-2C11) was intravenously injected. Spleens were harvested 2 h after αCD3 injection (Fig. 6 A). Mg2+ levels were reduced ∼60% within 2 wk, but Ca2+ levels were not significantly affected (Fig. 6 B). Consistent with the in vitro T cell activation defects (Fig. 1, E–L; and Fig. 2), we observed impaired induction of Nur77 in both CD4+ and CD8+ subsets taken ex vivo in Mg2+-deprived samples compared with controls (Fig. 6, C–E). Thus, we conclude that impaired T cell immunity caused by a failure of the Mg2+ regulatory mechanisms we have described may be an important component of poor immune responses to influenza in Mg2+-deprived mice.
Figure 6.
Mg2+ deprivation impairs T cell activation in vivo. (A) Schematic of the experimental design. (B) Mg2+ and Ca2+ serum levels assayed 14 d after mice (male C57BL/6) were put on a Mg2+-reduced diet. (C) Representative flow cytometric plot of Nur77+ cells in CD4+ and CD8+ lymphocyte subsets. (D and E) Quantification of the number of Nur77+CD4+ (D) and Nur77+CD8+ (E) cells in spleens. Unstimulated (US) cells are depicted as circles, while cells stimulated via i.v. α-CD3 injection (Stim) are represented by squares (D and E). Data shown are from three (B and D–E) independent experiments. Error bars represent SEM. P values (****, P < 0.0001) were calculated with a paired t test (B). Panel C depicts a representative replicate of three independent experiments. Plots in D and E represent one or two mice per condition from three independent experiments. Statistical significance was calculated with a Mann–Whitney U test (D and E; *, P < 0.050; **, P < 0.01). CTRL, control.
Discussion
Free basal [Mg2+]i, which is typically 0.5–1 mM depending on the cell type, can undergo long-term and acute changes with circadian rhythms, cell-surface receptor engagement, or other influences (Whang and Ryder, 1990; Li et al., 2011; Feeney et al., 2016; Uetani et al., 2017). The biological importance of these changes, especially for immune function, and how the cell “senses” them has not been established. We now show that either chronic or acute deprivation of Mg2+ decreases the “setpoint” of [Mg2+]i and blocks TCR signal propagation at the level of ITK, which harbors a low-affinity Mg2+ binding site that serves as a “sensor” of acute or chronic changes in [Mg2+]i (Madhusudan et al., 2002; Bao et al., 2011; Jacobsen et al., 2012). Although the role of a second Mg2+ binding site for enzymes has been debated for years, our study of ITK strongly supports the model of “two metal catalysis” for kinases and shows, for the first time, how this could exert a positive regulatory effect on the physiological activity of a cell, specifically in response to an antigenic stimulus (Bao et al., 2011; Jacobsen et al., 2012). We also note that the Km that we measured for the second site for other kinases involved in T cell activation, including ERK and PKC, indicates that severe deprivation may begin to impair their function as well lead to multiple roadblocks to full T cell activation.
Low dietary intake causing reduced serum [Mg2+] is associated with important chronic health problems, including hypertension, diabetes, metabolic syndrome, kidney disease, and cardiovascular disease (Costello and Nielsen, 2017). With modern food processing and agricultural practices, most people in the United States and the developed world have insufficient dietary intake of Mg2+, raising the prospect that Mg2+ supplementation or a Mg2+-rich diet may improve immune function in otherwise healthy people (Whang and Ryder, 1990; Mayer, 1997; World Health Organization, 2009; Rosanoff et al., 2012; de Baaij et al., 2015). We now show that a reduction in dietary Mg2+ can lead to hypomagnesemia and reduced cellular Mg2+. The large amount of bound Mg2+ in lymphocytes (>10 mM) does not “buffer” the external drop. This has the consequence of impairing T cell activation and compromising the immune response in the lung against inhaled influenza virus infection. Taken together, our work demonstrates how lymphocyte responses are modulated at the molecular level by Mg2+ and shows that a Mg2+-sufficient diet, which may not be achieved by currently dietary practices in the United States, is crucial for optimal immune function against a common human pathogen.
Materials and methods
Human biospecimens
Healthy control blood was obtained through the National Institutes of Health Clinical Center’s blood donor program under approved protocols.
Cell culture and Ca2+ flux assays
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy control blood and activated with soluble anti-CD3/CD28 (BioLegend) or CD3/CD28 activation beads (Invitrogen) in cRPMI or dRPMI (USP Biologicals) for 3–4 d as indicated. To expand T cells human recombinant IL-2 (100 U/ml; Roche) was added after 72 h and thereafter, cells were cultured in cRPMI with IL-2. For the ex vivo Ca2+ flux experiments, PBMCs were isolated and rested in cRPMI without IL-2 for 24 h before the loading of the dye. Mg2+ deprivation was performed on cycling T cell blasts for 72 h in cRPMI with IL-2 stripped of Mg2+ (<0.1 mM) and a Ca2+ concentration of 100 mM.
To measure Ca2+ flux, cells were loaded with Fluo-4-AM or Indo-1-AM dye (Invitrogen) at a final concentration of 0.5 µM with PowerLoad (Thermo Fisher) in HBSS with 20 mM Hepes (hereby referred to as assay buffer) for 20 min at room temperature (RT). De-esterification of the probe was performed by washing the cells in 1× PBS for 20 min. In the case of the ex vivo Ca2+ flux, human Fc block (Invitrogen) was added during de-esterification, and cells were labeled with a-CCR7, a-CD45RA, aCD4, and aCD8 (BD Biosciences, eBioscience) antibodies in assay buffer. Cells were diluted in buffered HBSS with 1.2 mM Ca2+ and Mg2+ or 1.2 mM Ca2+ 5 min before stimulation. To stimulate cells, anti-CD3 (HIT3a) at 10 μg/ml (BioLegend) and cross-linked with secondary antibody (F′ab2 fragment goat anti-mouse at 10 μg/ml; Jackson ImmunoResearch) were used. For the ex vivo stimulation, anti-CD28.1 (10 μg/ml; BioLegend) was included with anti-CD3. For all Ca2+ flux experiments, events were acquired for 240 s with a BD LSRFortessa or LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software.
Western blots and immunofluorescence staining for proximal TCR signaling
CD4+ T cells were isolated ex vivo from PBMCs (Stem Cell Technologies). CD4+ cells (12 million) were resuspended (18.5 million cells/ml) in buffered HBSS (650 µl) with 1.2 mM Ca2+ and Mg2+ or 1.2 mM Ca2+ 5 min before stimulation and incubated in a 37°C water bath. 10× radioimmunoprecipitation assay buffer (10 µl) with protease and phosphatase inhibitors (Thermo Fisher) were prepared for each time point (unstimulated and 2, 5, 10, 30, and 60 min) and cells (100 µl) were taken from both buffers for the unstimulated time point, resuspended in radioimmunoprecipitation assay stocks, and then placed on dry ice. Cells were stimulated with anti-CD3 (HIT3a) at 10 μg/ml (BioLegend) and cross-linked with secondary antibody (F’ab2 fragment goat anti-mouse at 10 μg/ml; Jackson ImmunoResearch). Stimulation time points were obtained using the protocol described for the unstimulated time point. All time-point samples were stored at −80°C. Samples were treated with benzonase nuclease (Sigma-Aldrich) for 40 min at 4°C, diluted 1:1 with SDS loading buffer (Quality Biological), and denatured at 95°C for 20 min. Samples were loaded onto 4–20%Tris-Glycine Gels (Thermo Fisher) and run at 150 V for 1.5 h at RT. Proteins were transferred to a nitrocellulose membrane at 25 V for 1.5 h at RT. Membranes were blocked with 5% milk with 0.1% Tween 20 for 1 h at RT. Membranes were cut and incubated with primary antibodies for p-Plcγ1, p-ZAP70, p-ERK, and p-SLP76 (Cell Signaling Technologies) overnight at 4°C. Samples were washed, incubated with secondary HRP-conjugated anti-rabbit antibody (Cell Signaling Technologies), and washed. Blots were incubated in Luminate Forte Substrate (Millipore) and imaged on a Fuji LAS-3000 system (Fujifilm).
For immunofluorescence staining, ex vivo–isolated CD4+ cells were allowed to adhere to poly-L-lysine–coated coverslips for 10 min. Media was aspirated and replaced with HBSS with 1.2 mM Ca2+ or 1.2 mM Ca2+/Mg2+ each, and cells were stimulated as described above. At the indicated time after addition of secondary antibody, cells were fixed with 4% paraformaldehyde for 10 min, and ice-cold methanol was added for ≥1 h at −20°C. Coverslips were washed three times with PBS, blocked for 1 h with PBS/0.5% BSA at RT, and incubated with primary antibody in blocking buffer with 0.05% Tween overnight at 4°C. Cells were washed, secondary antibody (anti-rabbit IgG, Alexa Fluor 594; Thermo Fisher) was added for 1 h at RT and washed once in Hoechst 33342 (Thermo Fisher) containing PBS (1 µg/ml). Coverslips were mounted with ProLong Gold Anti-fade reagent (Invitrogen) and imaged with a Leica SP8 microscope.
In vitro kinase assay
All commercial kinase preps were purchased from SignalChem. Kinase activity was measured using the ADP-Glo kinase activity assay by Promega. A custom synthesized peptide substrate (EQEDEPEGIYGVLF) prepared by the National Institute of Allergy and Infectious Diseases Protein Chemistry Section (Bethesda, MD) was kept at a saturating concentration (0.4 mM). Reaction buffers included 50 mM Tris, pH 7.5, 0.1 mg/ml BSA, 1.92 mM DTT, 200 nM ATP, and 4 nM of the respective kinase. MgCl2 and KCl were added in varying amounts to create the range of free Mg2+ titrations (0–10 mM) used in the assay with a final ionic strength of 160 mM (assuming a KD of 28.6 µM for 1ATP:Mg2+). Reaction mixtures (8 µl total) were prealiquoted in a 384-shallow-well ProxiPlate (PerkinElmer), and kinase was added to initiate catalysis. Following an incubation period of 1 h, the manufacturer’s protocol was followed to detect ATP generation. Chemiluminescent intensity of the final luciferase-based reaction was read by an Omega FluoStar Luminometer (BMG LabTech).
Pyruvate kinase and lactate dehydrogenase coupled enzyme assay
All commercial kinase preps were purchased from SignalChem. Kinase activity as a function of time was measured using a coupled enzymatic assay, which measured the production of ADP via the oxidation of NADH to NAD+ through a pyruvate kinase and lactate dehydrogenase–catalyzed reaction. Reaction buffers (assay buffer) included 100 mM Tris-HCl (pH 7.5), 1.25 mg/ml BSA, 1 mM dithiothreitol, 74 U/ml of pyruvate kinase and lactate dehydrogenase (Sigma-Aldrich), 1 mM phosphoenolpyruvate, 280 µM NADH, and 2.5 mM Mg2+ balanced with KCl for a final ionic strength of 0.15 M. Peptide B was kept at a saturating concentration of 0.4 mM. ATP titrations (0–10 mM) were prealiquoted in a half-volume 96-well plate (Corning). Kinase was added (final concentration, 85 nM) to initiate catalysis, and reaction progress was monitored by absorption at 340 nm at RT (25°C) for 2.5 h using a VersaMax Spectrophotometer (Molecular Devices). Data were processed and reaction velocities were calculated using GraphPad Prism v7 and Microsoft Excel 2016.
Null point titration
For the flow cytometric method cycling T cells (12 million cells at 2 million cells/ml) were labeled with Mag-Indo-1-AM dye (Invitrogen) at a final concentration of 0.5 µM with 1× PowerLoad (Thermo Fisher) in assay buffer for 20 min at RT. De-esterification of the probe was performed by washing the cells in 1× PBS for 20 min. The cells were resuspended in HBSS without Ca2+ and Mg2+ at 34 million cells/ml (∼350 μl). Graded concentrations of Mg2+ (0, 0.156, 0.312, 0.625, 1.25, 2.5, 5, and 10 mM) were prepared in HBSS (360 µl) without Ca2+ and Mg2+ assuming a total sample volume of 400 µl. Cells (∼1.4 million cells, 40 µl) were added to each Mg2+ sample. Cells were acquired for 30 s, permeabilized (add 1.2 µl of 600 µM digitonin), and reacquired on a BD LSRFortessa Flow cytometer (BD Biosciences) for a total of 240 s. Raw data were prepared with FlowJo software. Sigmoidal curve fitting using a modified Hill equation () was performed using GraphPad Prism v7 with the parameters given in Table S1 to obtain equilibrium ratios for each Mg2+ titration point. With these ratios, curve fitting was performed on the plot of [Mg2+]titration versus equilibrium ratio in GraphPad Prism by means of the Grynkiewicz model () and parameters given in Table S2.
The output parameters of this fit (Table S2), KD × β, Rmin, and Rmax along with the determination of R (average of the baseline values before the addition of digitonin for the lowest five Mg2+ titrations), were inserted into the Grynkiewicz equation () to calculate basal [Mg2+].
For the cuvette method, the same procedure as before was used with the following differences: 30 million cells (2 million cells/ml) were labeled with Mag-Indo-1-AM dye (Invitrogen), and the cells were resuspended in HBSS without Ca2+ and Mg2+ at 20 million cells/ml (∼1,500 µl). 33.3× stock concentrations of Mg2+ (for final concentrations of 0, 0.312, 0.625, 1.25, and 5 mM in cuvette) were prepared in HBSS without Ca2+ and Mg2+ assuming a total sample volume of 120 µl. Cells were added to a UV-compatible quartz microcuvette. Baseline fluorescence emission spectra (390–500 nm) was captured with a fluorometer (Photon Technology International) using an excitation wavelength of 340 nm. Mg2+ (3.6 μl of 33.3× stock) from each titration was added followed by 10 μl of 600 µM digitonin (final volume of 120 µl). Subsequent emission spectra were obtained at 1-min intervals following permeabilization with digitonin until the system achieved equilibrium (superimposition of two emission spectra). The baseline ratio is given by the relative intensities at 416 nm and 476 nm of the emission spectra (). The equilibrium ratio was calculated using the terminal emission spectra set for each Mg2+ titration. With these ratios, curve fitting was performed as previously described.
Molecular modeling
The reference sequence for human ITK was obtained from the UniProt database and was subsequently mapped onto a template crystal structure using the I-TASSER suite. We used a crystal structure of pCDK2 crystallized with two Mg2+ ions and an ATP transition-state analogue (Protein Data Bank accession number 3QHW) as our template structure (Jacobsen et al., 2012). We then manually adjusted our model in PyMol (The PyMol Molecular Graphics System, version 1.7.2.1; Schrödinger) such that the side chain angles of residues chelating the catalytic Mg2+ ions more accurately resembled the geometry of our pCDK2 template. The one-Mg2+ and two-Mg2+ bound structures were then immersed in explicit solvent and neutralizing ions, energy minimized, heated, and simulated for length of 1 μs each in AMBER 16. Average structures in Figs. 3 and 4 were generated by superimposing all protein backbone atoms over the trajectory onto the starting structure coordinates.
Protein purification
C-terminal FLAG ITK was cloned into pCDNA3 (Invitrogen) mammalian expression construct. Mutants were generated by site-directed mutagenesis and In Fusion cloning (Clontech). Constructs were transfected in Expi293 cells (Invitrogen) according to the manufacturer’s protocol. Cells were harvested 72 h after transfection; before harvesting, cells were treated for 10 min with sodium pervanadate (sodium orthovanadate and hydrogen peroxide; NEB, Calbiochem) to induce protein phosphorylation. Cells were lysed in 150 mM NETN (150 mM NaCl, 0.5 mM EDTA, 20 mM Tris-Cl, pH 8.0, and 0.5% NP-40) buffer, and FLAG-tagged proteins were immunoprecipitated with anti-FLAG-M2 magnetic beads (Sigma-Aldrich). Purified protein was eluted with 3xFLAG peptide (250 µg/ml) in elution buffer (50 mM Tris, pH 8.0, 300 mM NaCl, and 10% glycerol) and stored in elution buffer supplemented to 25% glycerol at −20°C.
Influenza infection
Male C57BL/6 (B6) mice were obtained from The Jackson Laboratory (000664). All mice were maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care accredited animal facility at the National Institute of Allergy and Infectious Diseases and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee (LISB 7E). Mg2+-reduced and matching control diets were purchased from Harlan-Teklad. Mouse-adapted influenza A/Puerto Rico/8/1934 H1N1 (influenza PR8) was used for infection (kindly provided by Peter McGuire, National Human Genome Research Institute, Bethesda, MD). Mice (n = 4–5/treatment) were exposed to aerosolized (Glas-Col) 500 PR8 in 10 ml saline. Details of the Mg2+ deprivation and infection are outlined in Fig. 5 A. Expression of viral hemagglutinin in the lungs of infected mice was determined by real-time PCR (Tarasenko et al., 2017).
At the indicated times after infection, spleens and lungs were harvested. Single-cell suspensions were prepared from the specified organs and stained for flow cytometry. The lungs were first perfused with PBS and lymphocytes isolated as previously described (Richard et al., 2015).
For MHC class II tetramer staining, cells were incubated with 1 µg/ml NP (311–325, QVYSLIRPNENPAHK) tetramer in cRPMI medium for 1 h at 37°C. For MHC class I tetramer staining, cells were incubated with 0.5 µg/ml NP (366–374, ASNENMETM) tetramer for 30 min on ice. Tetramers were obtained from the National Institutes of Health tetramer core facility.
In vivo αCD3 stimulation
Male C57BL/6 (B6) mice were obtained from The Jackson Laboratory (000664). All mice were maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care accredited animal facility at the National Institute of Allergy and Infectious Diseases and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee (LISB 7E). Mg2+-reduced and matching control diets were purchased from Harlan-Teklad. Details of the Mg2+ deprivation and ⍺CD3 injection are outlined in Fig. 6 A. Vehicular control (PBS) or 30 µG of ⍺CD3 (2C11 clone) were administered via tail vein injection. Spleens were harvested 2 h after injection. Lymphocytes were fixed in 4% paraformaldehyde and permeabilized overnight in MeOH, and cells were labeled with cells were labeled with aNur77, aCD4, and aCD8 (BD Biosciences, eBioscience) antibodies in assay buffer for 1 h at 4°C. Events were acquired with either a BD LSRFortessa or LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software.
Online supplemental material
Fig. S1 shows cell viability of cells in media containing different concentrations of Mg2+. Fig. S2 shows that acute Mg deprivation impairs TCR signaling in CD8+ cells. Fig. S3 shows that Mg2+ promotes the activity of ITK and other immunologically relevant kinases. Fig. S4 shows purification of ITK mutant proteins. Fig. S5 shows that Mg2+ deprivation in mice decreases body weight but does not affect total lung cellularity and splenic T cells. Table S1 lists the parameters and constraints for the modified Hill equation. Table S2 lists the parameters and constraints for the Grynkiewicz model.
Supplementary Material
Acknowledgments
We wish to thank Dr. Joseph Kao (University of Maryland) for ongoing expert advice and support, Dr. Amy Andreotti (University of Iowa, Iowa City, IA) for sharing ITK constructs, Dr. Debra Yablonsky (Technion - Israel Institute of Technology, Haifa, Israel) for the SLP76 antibody, Drs. David Margulies and Kannan Nattaranjan (National Institute of Allergy and Infectious Diseases) for advice with molecular modeling and protein purification, Dr. Grzegorz Piszczek (National Heart, Lung, and Blood Institute) for help with biophysical measurements, and Ryan Kissinger at the National Institute of Allergy and Infectious Diseases Visual and Medical Arts Department for help with illustrations. We thank the National Institutes of Health Clinical Center Department of Laboratory Medicine for serum ion measurements and Dr. Wei Yang and Ronald Germain for a critical reading of the manuscript.
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, the National Heart, Lung, and Blood Institute Biophysics core, the National Institute of Allergy and Infectious Diseases Structural Biology Section, and Merck and Co.
The authors declare no competing financial interests. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Author contributions: Conceptualization, C. Kanellopoulou, A.B. George, and M.J. Lenardo; Methodology, C. Kanellopoulou, A.B. George, E. Masutani, J.C. Ravell, L. Zheng, and M. Young; Investigation, A.B. George, C. Kanellopoulou, E. Masutani, J.L.C., J.C. Ravell, T.Y., M.G.S., P.D. Jiang, M. Matsuda-Lennikov, J. Reilley, R. Handon, P. Lee, L. Zheng, and M. Young; Writing – Original Draft, C. Kanellopoulou, A.B. George, and M.J. Lenardo; Writing – Review & Editing, C. Kanellopoulou, A.B. George, J.L. Cannons, P.L. Schwartzberg, J.C. Ravell, and M.J. Lenardo; Funding Acquisition, M.J. Lenardo; Resources, J.R. Miller; Supervision, C. Kanellopoulou, N.P. Restifo, P.L. Schwartzberg, M. Young, and M.J. Lenardo.
References
- Ashouri J.F., and Weiss A.. 2017. Endogenous Nur77 Is a Specific Indicator of Antigen Receptor Signaling in Human T and B Cells. J. Immunol. 198:657–668. 10.4049/jimmunol.1601301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z.Q., Jacobsen D.M., and Young M.A.. 2011. Briefly bound to activate: transient binding of a second catalytic magnesium activates the structure and dynamics of CDK2 kinase for catalysis. Structure. 19:675–690. 10.1016/j.str.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buri A., and McGuigan J.A.. 1990. Intracellular free magnesium and its regulation, studied in isolated ferret ventricular muscle with ion-selective microelectrodes. Exp. Physiol. 75:751–761. 10.1113/expphysiol.1990.sp003457 [DOI] [PubMed] [Google Scholar]
- Case D.A., Cheatham T.E., Darden T.A., Duke R.E., Giese T.J., Gohlke H., Goetz A.W., Greene D., Homeyer N., Izadi S., et al. 2017. AMBER 2017. University of California, San Francisco, CA. [Google Scholar]
- Chaigne-Delalande B., Li F.Y., O’Connor G.M., Lukacs M.J., Jiang P., Zheng L., Shatzer A., Biancalana M., Pittaluga S., Matthews H.F., et al. 2013. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 341:186–191. 10.1126/science.1240094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanova N.A., Shrimal S., and Gilmore R.. 2014. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J. Cell Biol. 206:525–539. 10.1083/jcb.201404083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello R.B., and Nielsen F.. 2017. Interpreting magnesium status to enhance clinical care: key indicators. Curr. Opin. Clin. Nutr. Metab. Care. 20:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello R., Wallace T.C., and Rosanoff A.. 2016. Magnesium. Adv. Nutr. 7:199–201. 10.3945/an.115.008524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundy T., and Mackay J.. 2011. Proton pump inhibitors and severe hypomagnesaemia. Curr. Opin. Gastroenterol. 27:180–185. 10.1097/MOG.0b013e32833ff5d6 [DOI] [PubMed] [Google Scholar]
- de Baaij J.H.F., Hoenderop J.G.H., and Bindels R.J.. 2015. Magnesium in man: implications for health and disease. Physiol. Rev. 95:1–46. 10.1152/physrev.00012.2014 [DOI] [PubMed] [Google Scholar]
- Deutsch C., and Lee S.C.. 1988. Cell volume regulation in lymphocytes. Ren. Physiol. Biochem. 11:260–276. [DOI] [PubMed] [Google Scholar]
- Feeney K.A., Hansen L.L., Putker M., Olivares-Yañez C., Day J., Eades L.J., Larrondo L.F., Hoyle N.P., O’Neill J.S., and van Ooijen G.. 2016. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 532:375–379. 10.1038/nature17407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P.W., and Lew V.L.. 1980. Magnesium buffering in intact human red blood cells measured using the ionophore A23187. J. Physiol. 305:13–30. 10.1113/jphysiol.1980.sp013346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., and Tsien R.Y.. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450. [PubMed] [Google Scholar]
- Heyeck S.D., Wilcox H.M., Bunnell S.C., and Berg L.J.. 1997. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J. Biol. Chem. 272:25401–25408. 10.1074/jbc.272.40.25401 [DOI] [PubMed] [Google Scholar]
- Jacobsen D.M., Bao Z.Q., O’Brien P., Brooks C.L. III, and Young M.A.. 2012. Price to be paid for two-metal catalysis: magnesium ions that accelerate chemistry unavoidably limit product release from a protein kinase. J. Am. Chem. Soc. 134:15357–15370. 10.1021/ja304419t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R.E., Kleino I., Wales T.E., Xie Q., Fulton D.B., Engen J.R., Berg L.J., and Andreotti A.H.. 2013. Activation loop dynamics determine the different catalytic efficiencies of B cell- and T cell-specific tec kinases. Sci. Signal. 6:ra76 10.1126/scisignal.2004298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555–592. 10.1146/annurev.immunol.17.1.555 [DOI] [PubMed] [Google Scholar]
- Li F.Y., Chaigne-Delalande B., Kanellopoulou C., Davis J.C., Matthews H.F., Douek D.C., Cohen J.I., Uzel G., Su H.C., and Lenardo M.J.. 2011. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 475:471–476. 10.1038/nature10246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott M. 1990. The intracellular concentration of free magnesium in extensor digitorum longus muscles of the rat. Exp. Physiol. 75:763–769. 10.1113/expphysiol.1990.sp003458 [DOI] [PubMed] [Google Scholar]
- Madhusudan P., Akamine P., Xuong N.H., and Taylor S.S.. 2002. Crystal structure of a transition state mimic of the catalytic subunit of cAMP-dependent protein kinase. Nat. Struct. Biol. 9:273–277. 10.1038/nsb780 [DOI] [PubMed] [Google Scholar]
- Mayer A.-M. 1997. Historical changes in the mineral content of fruits and vegetables. Br. Food J. 99:207–211. 10.1108/00070709710181540 [DOI] [Google Scholar]
- Meyer T.E., Verwoert G.C., Hwang S.J., Glazer N.L., Smith A.V., van Rooij F.J., Ehret G.B., Boerwinkle E., Felix J.F., Leak T.S., et al. Meta Analysis of Glucose and Insulin Related Traits Consortium . 2010. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet. 6:e1001045 10.1371/journal.pgen.1001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.L., Davies J.E., and Garrido M.C.. 1991. Intracellular free magnesium in human lymphocytes and the response to lectins. Clin. Sci. (Lond.). 80:539–547. 10.1042/cs0800539 [DOI] [PubMed] [Google Scholar]
- Readinger J.A., Mueller K.L., Venegas A.M., Horai R., and Schwartzberg P.L.. 2009. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 228:93–114. 10.1111/j.1600-065X.2008.00757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A.C., Tan C., Hawley E.T., Gomez-Rodriguez J., Goswami R., Yang X.P., Cruz A.C., Penumetcha P., Hayes E.T., Pelletier M., et al. 2015. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J. Immunol. 194:3567–3582. 10.4049/jimmunol.1401220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanoff A., Weaver C.M., and Rude R.K.. 2012. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr. Rev. 70:153–164. 10.1111/j.1753-4887.2011.00465.x [DOI] [PubMed] [Google Scholar]
- Sela M., Bogin Y., Beach D., Oellerich T., Lehne J., Smith-Garvin J.E., Okumura M., Starosvetsky E., Kosoff R., Libman E., et al. 2011. Sequential phosphorylation of SLP-76 at tyrosine 173 is required for activation of T and mast cells. EMBO J. 30:3160–3172. 10.1038/emboj.2011.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J., and Adams J.A.. 1999. Detection of conformational changes along the kinetic pathway of protein kinase A using a catalytic trapping technique. Biochemistry. 38:12072–12079. 10.1021/bi991109q [DOI] [PubMed] [Google Scholar]
- Shrimal S., Cherepanova N.A., and Gilmore R.. 2015. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin. Cell Dev. Biol. 41:71–78. 10.1016/j.semcdb.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., and Budde R.J.. 1997. Requirement for an additional divalent metal cation to activate protein tyrosine kinases. Biochemistry. 36:2139–2146. 10.1021/bi962291n [DOI] [PubMed] [Google Scholar]
- Tarasenko T.N., Pacheco S.E., Koenig M.K., Gomez-Rodriguez J., Kapnick S.M., Diaz F., Zerfas P.M., Barca E., Sudderth J., DeBerardinis R.J., et al. 2017. Cytochrome c Oxidase Activity Is a Metabolic Checkpoint that Regulates Cell Fate Decisions During T Cell Activation and Differentiation. Cell Metab. 25:1254–1268.e7. 10.1016/j.cmet.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.S., and Kornev A.P.. 2011. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36:65–77. 10.1016/j.tibs.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G.M., and Rude R.K.. 2005. Magnesium deficiency in critical illness. J. Intensive Care Med. 20:3–17. 10.1177/0885066604271539 [DOI] [PubMed] [Google Scholar]
- Uetani N., Hardy S., Gravel S.P., Kiessling S., Pietrobon A., Wong N.N., Chénard V., Cermakian N., St-Pierre J., and Tremblay M.L.. 2017. PRL2 links magnesium flux and sex-dependent circadian metabolic rhythms. JCI Insight. 2:e91722 10.1172/jci.insight.91722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston J. 2008. Biochemistry of magnesium. In The Chemistry of Organomagnesium Compounds. John Wiley & Sons, Ltd, Hoboken, NJ. pp. 315–367. 10.1002/9780470751879.ch8 [DOI] [Google Scholar]
- Whang R., and Ryder K.W.. 1990. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA. 263:3063–3064. 10.1001/jama.1990.03440220087036 [DOI] [PubMed] [Google Scholar]
- World Health Organization 2009. Calcium and Magnesium in Drinking Water. Available at: https://www.who.int/water_sanitation_health/publications/publication_9789241563550/en/ (accessed March 22, 2019).
- Yang J., and Zhang Y.. 2015. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 43(W1):W174-81 10.1093/nar/gkv342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., and Clapham D.E.. 2009. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl. Acad. Sci. USA. 106:15750–15755. 10.1073/pnas.0908332106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.