The human intestinal ecosystem is characterized by a complex interplay between different microorganisms and the host. The high variation within the human population further complicates the quest toward an adequate understanding of this complex system that is so relevant to human health and well-being.
KEYWORDS: animal model, gut-on-a-chip, in vitro model, intestinal microbiota, minimal microbiota
SUMMARY
The human intestinal ecosystem is characterized by a complex interplay between different microorganisms and the host. The high variation within the human population further complicates the quest toward an adequate understanding of this complex system that is so relevant to human health and well-being. To study host-microbe interactions, defined synthetic bacterial communities have been introduced in gnotobiotic animals or in sophisticated in vitro cell models. This review reinforces that our limited understanding has often hampered the appropriate design of defined communities that represent the human gut microbiota. On top of this, some communities have been applied to in vivo models that differ appreciably from the human host. In this review, the advantages and disadvantages of using defined microbial communities are outlined, and suggestions for future improvement of host-microbe interaction models are provided. With respect to the host, technological advances, such as the development of a gut-on-a-chip system and intestinal organoids, may contribute to more-accurate in vitro models of the human host. With respect to the microbiota, due to the increasing availability of representative cultured isolates and their genomic sequences, our understanding and controllability of the human gut “core microbiota” are likely to increase. Taken together, these advancements could further unravel the molecular mechanisms underlying the human gut microbiota superorganism. Such a gain of insight would provide a solid basis for the improvement of pre-, pro-, and synbiotics as well as the development of new therapeutic microbes.
INTRODUCTION
Given its involvement in metabolic, nutritional, physiological, and immunological processes, the human intestinal microbiome can be regarded as an essential organ of the human body (1). Further strengthening its clinical relevance, the intestinal microbiome has been linked to numerous disease conditions, including metabolic and immune disorders, cancer, and neurodegenerative diseases (2). However, apart from a remarkable increase in the amount of genome sequence data of the human gut microbiota, progress in functional insight has been hampered by its complexity: the existence of more than 1,000 prevalent species (3), combined with the high interpersonal variation within the human population in terms of genetics, environment, and habits, results in a complex entity termed the human microbiome superorganism (4). The number of known host-microbe interactions has grown rapidly over the past decades, yet many aspects still remain obscure.
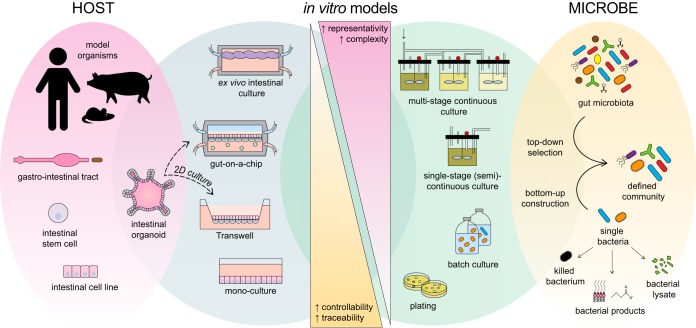
To solve this complexity, there is a need for a reductionist approach in which both host and microbiome are simplified to the extent that experimental variables can be tightly controlled and deliberately manipulated. Regarding the microbiota, synthetic or defined communities have been proposed as useful models to study microbial ecology (5). In recent years, the number of cultivable gastrointestinal microbial species has rapidly expanded (3) by the use of sophisticated or brute-force culturomics approaches (6, 7). These strategies have allowed for the design of defined communities that are representative of the normal human intestinal microbiota. With respect to the human host, laboratory animals, notably mice, have proven valuable models for developing human medicine. The colonization of germfree (GF) animals with defined bacterial communities, resulting in gnotobiotic animals, has already been applied for decades. During the 1960s and 1970s, it was recognized that the intestines of GF animals display aberrant histological, anatomical, and physiological characteristics compared to conventional laboratory animals (8). The development of the Schaedler cocktail for colonization of the murine gut (9) marked one of the first attempts to normalize GF mice. An altered version has been widely adopted as a standardized gut microbiota by animal breeders and biomedical researchers ever since. Over time, various other defined communities have been designed to generate gnotobiotic animals for purposes beyond standardization; they have proven to be a valuable in vivo tool to study microbial ecology (e.g., microbial invasion, microbe-microbe interactions, and metabolism) and host-microbe interactions. However, mice and other animal models have various limitations that hamper their use as models for the human microbiome, as was recently reviewed (10, 11). Interesting alternatives concern the development of sophisticated in vitro models, such as organ-on-a-chip systems and organoids.
This review summarizes existing models of host-microbe interactions in which defined communities, as models of the (human) gut microbiota, were applied. We aim to present all in vivo studies that used defined microbial communities representing the intestinal microbiota of healthy individuals and in which host parameters were considered. The designs of these model communities, as well as the selection of their host, are compared and critically evaluated. The potential uses of defined communities in in vitro (cellular) models, as a surrogate host, are outlined as well. We conclude by discussing the increased value, opportunities, and possible obstacles when applying defined communities in to-be-developed in vitro host-microbe interaction models.
DEFINED COMMUNITIES MIMICKING THE NORMAL INTESTINAL MICROBIOTA IN VIVO
A number of recent studies addressed host-microbe interactions in vivo by using defined communities representative of the healthy human gut microbiota (Tables 1 to 3). These include various mouse studies with more- or less-defined intestinal microbiota, which are summarized below. Studies in which animals were antibiotic treated before bacterial colonization are excluded from our analysis, as their reproducibility and gnotobiology cannot be ensured (12). The following section first discusses the specifically named defined communities applied in rodents (Table 1) (n = 31), followed by non-specifically-named communities in rodents (Table 2) (n = 16). Finally, the defined communities administered to nonrodent models are discussed (Table 3) (n = 6).
TABLE 1.
Studies using specifically named defined communities to study host-microbe interactions in vivo (n = 31)a
| Consortium (no. of speciesb) | Division of phylae | Strain source(s) | Host species (strain) | Part of the gut studiedf | No. of animals/group | Chow | Sex | Age (collection time[s]c) | Study outcome(s)d | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Schaedler flora (5 species) 2 Lactobacillus spp., anaerobic Streptococcus sp. (group N), Bacteroides strain, Enterococcus sp., coliform strain |
|
Mouse | Mouse (NR) |

|
20 | NR | NR | 4 wk (3 wk–4 mo) | Colonization pattern; cecal size | 9 |
| ASF (8 species) ASF356: Clostridium species ASF360: Lactobacillus intestinalis or Lactobacillus acidophilus ASF361: Lactobacillus murinus or Lactobacillus salivarius ASF457: Mucispirillum schaedleri ASF492: Eubacterium plexicaudatum ASF500: Pseudoflavonifractor sp. ASF502: Clostridium sp. ASF519: Parabacteroides distasonis |
|
Mouse | Mouse (HA/ICR) |

|
30 | NR | Both | Adult (14–56 days) | Death after C. botulinum infection; fecal C. botulinum toxin excretion; colonization pattern of C. botulinum | 36 |
| Mouse | Rat (F344) |
|
1–5 | Sterile food (Charles River) ad libitum | M | NR (2 wk) | Hepatic genotoxicity of mononitrotoluene isomers; metabolic activation of 2NT by intestinal bacteria; cecal bacterial content | 169 | ||
| Mouse | Mouse (scid C.B-17) |

|
4–6 | Autoclaved pelleted diet ad libitum | NR | NR (8–12 wk postreconstitution CD4+ T cells) | After Helicobacter hepaticus infection, rectal prolapse; clinically severe disease; grossly thickened colon, cecum, and rectum on necropsy; colonic inflammation score; colonic epithelial cell proliferation; histopathology | 16 | ||
| Mouse | Rat (HLA-B27 on 33-3/F344) |

|
7–11 | NR | At least M | 2 mo (1 mo) | Gross gut score, levels of MPO and IL-1B in cecal tissue; histological inflammatory score of cecum and antrum | 15 | ||
| Mouse | Mouse (C3H/HeN) |
|
4–8 | Irradiated diet (Harlan Teklad) | NR | 6–8 wk (9–14 wk) | After colonization with Helicobacter bilis or Brachyspira hyodysenteriae, cecal pathological gross and histological scores; serum IgG1 + IgG2a Ab response | 170 | ||
| Mouse | Mouse (C3H/HeN) |

|
7–10 | Irradiated diet (Harlan Teklad) | NR | 6–8 wk (10 wk) | Fecal bacterial contents (after H. bilis infection); cecal pathological scores; cecal histological changes; serum immunoglobulin | 171 | ||
| Mouse | Mouse (SW) |

|
2–5 | NR | NR | 6–9 wk (NR) | Presence of Th17 cells and Foxp3+ regulatory cells in LP of small intestine | 172 | ||
| Mouse | Mouse (C57BL/6) |

|
NR | NR | NR | NR | Total intestinal IgA and intestinal IgA, anti-CBir1; proliferation of splenic CBir1 TgT cells after CBir1 gavage | 173 | ||
| Mouse | Mouse(B6.Rag−/−) |

|
NR | NR | F | 8–10 wk (10 days) | Homeostatic and spontaneous proliferation of TCR TgT cells in LP | 174 | ||
| Mouse | Mouse (C57BL/6) |

|
5–8 | Autoclaved chow | NR | 8 wk (at least 3 dpi) | After infection, S. Typhimurium levels in mesenteric lymph nodes, spleen, cecum, and feces; cecal pathology score; cecal microbiota density; bacterial content and microbiota complexity in feces | 97 | ||
| ASF (8 and 9 species) 8 species: ASF 9 species: ASF + Escherichia coli HA108 or HA107 |
9 species:
|
Mouse | Mouse(C57BL/6) |

|
3 | NR | NR | NR (119 days) | No. of IgA plasma cells per intestinal villus in duodenum, jejunum, ileum, and colon; IgA-bacterium binding in intestine; anti-E. coli IgA titer | 21 |
| ASF (8 species) | Mouse | Mouse (NMRI, C57BL/6, BALB/c, NIH Swiss, SW, NMRI, MyD88−/− Ticam1−/−, SMARTA, C57BL/6.CD45.1+) |

|
3–10 | NR | NR | NR (up to 28 days) | Cecal bacterial contents; colonic Treg cell response and relative IL-10 expression in spleen, MLN, Peyer’s patches, colonic and small intestinal LP, thoracic duct lymph; IL-17 production; relative abundance of strains; microscopic localization in colon and small intestine | 175 | |
| Mouse | Mouse (Nod1−/− and Nod2−/− on C57BL/6 background) |

|
NR | NR | NR | 6–9 wk (NR) | Cecal bacterial contents; intestinal tissue conductance and Cr-EDTA flux; E-cadherin protein expression and RegIII-gamma mRNA expression in colon; survival, colitis disease severity, histology score, and myeloperoxidase activity after DSS induction; colonic IL-6, IL-10, MCP-1, IFN-c, TNF-α, IL-12p70 levels | 17 | ||
| Mouse | Mouse (C57BL/6) |

|
NR | Autoclaved food | Both | 8–12 wk (8–12 wk) | RegIII-gamma RNA and protein expression in ileum and colon | 176 | ||
| Mouse | Mouse (C57BL/6 and C57BL/6 TSLPR−/−) |

|
3–5 | NR | NR | NR (28 days) | Expression of thymic stromal lymphopoietin mRNA in intestinal epithelial cell or colonic LP (LP); % of CD4+ T cells secreting IL-17A and IFN gamma in the colonic LP and MLN; expansion of colonic Treg cells in colonic LP and MLN; expression of receptor for TSLP by CD4+ and regulatory T cells | 177 | ||
| Mouse | Mouse (NIH Swiss) |

|
4 | NR | NR | 3 days (3 days) | Structure of myenteric plexus, nerve density, average no. of HuC/D-positive myenteric neurons per ganglion, cell body size, and average no. of nNOS-positive neurons per myenteric ganglion in duodenum, jejunum, and ileum; small intestinal motility (frequency and amplitude of muscle contractions) in duodenum, jejunum, and ileum before and after general neural or specific nitrergic blockade | 178 | ||
| Mouse | Mouse (C57BL/6) |

|
5–14 | Autoclaved mouse breeder’s diet (Harlan), unlimited access | Both | 6–12 wk (3 wk) | Colonic histology, inflammatory (MPO) activity, enteropathy (presence of fecal albumin), and cytokine expression; fecal microbiota profiles; colonic gene expression; proportion of T-cell subtypes in colonic LP and other mucosal and systemic immune compartments | 81 | ||
| ASF (8 species) (Oligo-MM12 was also used, but no host parameters were assessed) | Mouse | Mouse (C57BL/6) |

|
3 (ASF), 5–23 (Oligo-MM) | NR | Both | NR | Thicknesses of total colon and colon inner mucus (ASF); mucus turnover time (ASF); alpha diversity in colon and cecum (Oligo-MM) | 26 | |
| ASF (8 and 9 species) 8 species: ASF 9 species: ASF + Oxalobacter formigenes |
9 species:
|
Mouse | Mouse (SW) |

|
4–7 | LM-485 autoclavable rodent diet, free access | M (no gender effect observed) | 3–9 mo (3–9 mo + 6 wk) | Bacterial levels in stomach, cecum, proximal colon, and cecal mucosa; body wt; dietary oxalate intake; cecal and fecal oxalate levels; urine vol; urinary metabolite levels; cecal wet wt; cecal water metabolites | 20 |
| Partial ASF (6 species) ASF356, -361, -492, -502, -519, and -500 (ASF360 and -457 did not colonize) |
|
Mouse | Mouse (NOD.MyD88KO) | None | 9–23 | NR | Both | NR (up to 30 wk) | Incidence of diabetes; histological scores of pancreatic islet destruction | 18 |
| Partial ASF (4 and 5 species) 4 species: ASF360, ASF361, ASF457, ASF519 5 species: 4 species + Butyrivibrio fibrisolvens (type I, ATCC 19171; type II, ATCC 51255) |
4 species: 5 species: 
|
Mouse and bovine | Mouse (BALB/c) |

|
4–5 | Autoclaved low-fiber diet (5SRZ, catalog no. 1813680), high-fiber diet (5SVL, catalog no. 1813901), or tributyrin diet (5AVC, catalog no. 1814961) | NR | NR (2.5–5 mo after colorectal cancer induction) | Colorectal tumor multiplicity, tumor size, and tumor grade; levels of LDHA, lactate, butyrate, H3ac, and total H3 in colonic tissue and tumors; luminal SCFA levels; H3ac and expression levels of Fas, p21, and p27 genes in colonic tissue and tumors; apoptosis and cell proliferation levels in colonic tissue and tumors | 19 |
| Partial ASF (4, 5, 7, and 7 species) 4 species: ASF356, ASF360, ASF361, and ASF519 5 species: ASF360, ASF361, ASF457, SB2 (ASF502), and ASF519 7 species: ASF356, ASF360, ASF361, ASF457, ASF500, SB2 (ASF502), and ASF519 7 species: 4 species + E. coli Mt1B1, Streptococcus danieliae ERD01G, Staphylococcus xylosus 33-ERD13C (more with Oligo-MM [see below]) |
4 species: 5 species:  7 species: 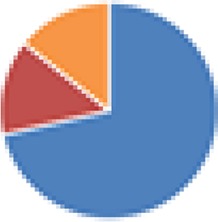 7 species: 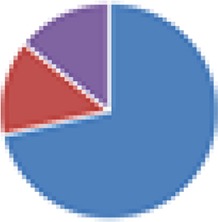
|
Mouse | Mouse (C57BL/6) |

|
4–6 | NR | Both | 0 or 8–12 wk (8–12 wk or 40 days) | Fecal bacterial content; bacterial load of S. Typhimurium in feces, cecum, and MLN; relative cecal wt; functional genomic analysis of bacteria | 22 |
| Oligo-MM (12, 15, and 17 species) 12 species, Oligo-MM: Acutalibacter muris KB18, Flavonifractor plautii YL31, Clostridium clostridioforme YL32, Blautia coccoides YL58, Clostridium innocuum I46, Lactobacillus reuteri I49, Enterococcus faecalis KB1, Bacteroides caecimuris I48, Muribaculum intestinale YL27, Bifidobacterium longum subsp. animalis YL2, Turicimonas muris YL45, Akkermansia muciniphila YL44 15 species: 12 species + 3 facultative anaerobes (E. coli Mt1B1, Streptococcus danieliae ERD01G, Staphylococcus xylosus 33-ERD13C) 17 species: 12 species + 5 ASF members (ASF360, ASF361, ASF457, SB2 [ASF502], ASF519) |
12 species: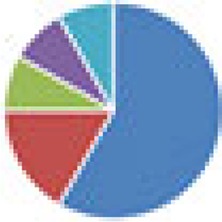 15 species: 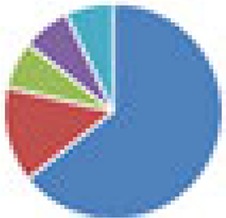 17 species: 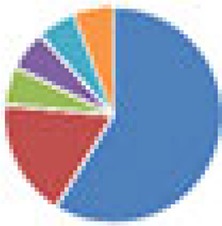
|
Mouse | Mouse (C57BL/6) |

|
4–6 | NR | Both | 0 (8–12 wk) | Fecal bacterial content; bacterial load of S. Typhimurium in feces, cecum, and MLN; relative cecal wt; functional genomic analysis of bacteria | 22 |
| Oligo-MM (12 and 13 species) 12 species: Oligo-MM 13 species: 12 species + Clostridium scindens ATCC 35704 |
12 species: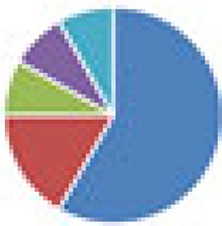 13 species: 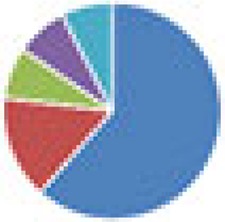
|
Mouse | Mouse (C57BL/6) |

|
5–8 | NR | NR | 0 (6–12 wk) | Fecal and cecal bacterial contents; cecal levels of lipocalin-2; calprotectin expression in cecal tissue; histopathology of cecum; cecal bile acid metabolome | |
| SIHUMI(x) (7 and 8 species): SIHUMI: Anaerostipes caccae DSM 14662 or DSM 14667, Bacteroides thetaiotaomicron DSM 2079, B. longum NCC 2705, Blautia producta DSM 2950, Clostridium ramosum DSM 1402, E. coli K-12 MG1655, Lactobacillus plantarum DSM 20174 SIHUMI(x) Clostridium butyricum DSM 10702 |
7 species: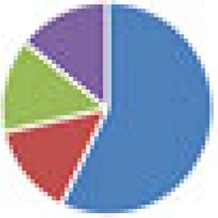 8 species: 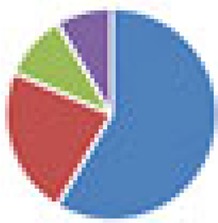
|
Human | Rat (Sprague-Dawley) |

|
3–21 | Sterilized standard chow (225 g/kg of body wt protein, 50 g/kg crude fat, 65 g/kg ash, 135 g/kg moisture, 480 g/kg N-free extract), fermentable-fiber-free diet, inulin diet, pectin diet, and high-fat and low-fat diets | Both | 0–3 mo (2–38 wk) | Stability of microbiota in offspring; SCFA concn and pH in cecum, colon, and feces; bacterial counts in cecum, colon, and feces; Midtvedt criteria | 27 |
| SIHUMI(x) (8 and 9 species) 8 species: SIHUMI(x) 9 species: 8 species + A. muciniphila ATCC BAA-835 |
9 species: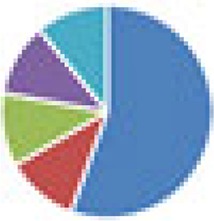
|
Human | Mouse (C3H) |

|
5–10 | NR | NR | 12 wk (5–15 days) | Bacterial cell no. and proportions in cecum and colon; cecal and colonic histopathology scores; expression of proinflammatory cytokines in cecal and colonic mucosa; serum protein levels of proinflammatory cytokines; no. of S. Typhimurium cells in MLN and spleen; size; macrophage infiltration in cecal tissue; localization of A. muciniphila and S. Typhimurium; mucin formation, mucus thickness, mucus composition, and no. of mucin-filled cells | 28 |
| SIHUMI(x) (8 and 9 species) 8 species: SIHUMI(x) 9 species: 8 species + Fusobacterium varium ATCC 8501 |
9 species: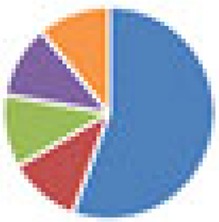
|
Human | Mouse (C3H/HeOuJ) |

|
12 | Irradiated standard chow R03-40 | F | 0 (8 wk) | Body wt; dry mass of cecum and colon; bacterial content in cecum and colon; polyamine concn in cecum and colon; SCFA concn in cecum and colon; histology of cecum and distal colon (thicknesses of crypt, epithelial layer, mucosa, submucosa, muscularis externa); mitosis and apoptosis of cecal and distal colonic tissue | 30 |
| Human | Mouse (Prm/Alf, C3H/He) |

|
12–13 | Sterilized pelleted standard chow R03-40 | F | 0 (56 ± 1 days) | Lengths of small, large, and whole intestines; thicknesses of muscle, crypt, and villi in proximal and small intestine and colon; fecal and cecal microbial contents; cecal concn of SCFAs and polyamines | 31 | ||
| SIHUMI(x) (7 and 8 species) 7 species: SIHUMI(x) without C. ramosum 8 species: SIHUMI(x) |
7 species: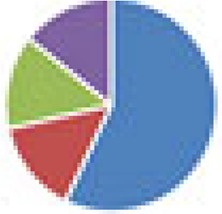 8 species: 
|
Human | Mouse (C3H/HeOuJ) |

|
3–9 | Irradiated low-fat or high-fat diet ad libitum | M | 0 (16 wk) | Body wt; % body fat; adipose tissue wt (epididymal, mesenteric, and subcutaneous); energy intake; food efficiency; digestibility of high-fat diet; digestible energy; cecal and colonic bacterial contents per species; blood glucose; leptin gene expression in epididymal tissue; liver wt; liver triglyceride levels; liver glycogen contents; expression of genes involved in lipid transport, lipid synthesis, cholesterol synthesis, and lipid catabolism; gene expression of proteins involved in small intestinal glucose uptake; SCFA formation in cecum, colon, and portal vein plasma; gene expression of SCFA-related proteins in colonic mucosa; gene expression of lipid transport and storage proteins in ileum; parameters of intestinal permeability and low-grade inflammation | 32 |
| SIHUMI(x) (8 and 9 species) 8 species: SIHUMI(x) 9 species: 8 species + A. muciniphila ATCC BAA-835 |
9 species:
|
Human | Mouse (C57BL/6.129P2-Il10tm1Cgn) |

|
5–6 | Irradiated standard chow (fortified type 1310; Altromin, Lage, Germany) ad libitum | M | 0 or 8 wk (3 wk) | Body wt; histopathology scores in submucosa, LP, surface epithelium, and lumen; colon length; relative mRNA levels of Tnfa, Ifng, and Reg3g; fecal lipocalin-2 concn; fecal and cecal bacterial levels; cecal histology; no. of goblet cells per 100 epithelial cells in cecum and colon; mucus layer thickness in colon; relative Muc2 mRNA levels in distal small intestine, cecum, and colon | 29 |
Abbreviations: M, male; F, female; SW, Swiss Webster; LP, lamina propria; MLN, mesenteric lymph nodes; MPO, myeloperoxidase; NR, not reported; SCFA, short-chain fatty acid; Treg cell, regulatory T cell; IL-1B, interleukin-1B; Ab, antibody; TCR, T-cell receptor; dpi, days postinfection; DSS, dextran sodium sulfate; IFN, interferon; TNF-α, tumor necrosis factor alpha; 2NT, 2-nitrotoluene; H3ac, pan-histone 3 acetylation; LDHA, lactate dehydrogenase A; nNOS, neuronal nitric oxide synthase; TgT cells, transgenic T cells; MCP-1, monocyte chemoattractant protein 1.
Two different strains tested are counted as one species. Strains were not always reported. Pathogenic species, in the case of an infection model, are not included.
The colonization time includes the time from colonization (time zero in the case of transfer of microbiota to offspring) until and including the time of sacrifice or the end of experimental (e.g., dietary) manipulations, in cases where this is clearly stated in the paper. If age is given and animals are colonized at birth, the age is included in the colonization time.
Study outcomes are reported only for animals colonized with the defined community of interest.
 , Firmicutes;
, Firmicutes;
 , Bacteroidetes;
, Bacteroidetes;
 , Actinobacteria;
, Actinobacteria;
 , Proteobacteria;
, Proteobacteria;
 , Verrucomicrobia;
, Verrucomicrobia;
 , other.
, other.
The color codes from left to right in the illustration are as follows:
 , stomach;
, stomach;
 , duodenum;
, duodenum;
 , jejunum;
, jejunum;
 , ileum;
, ileum;
 , cecum;
, cecum;
 , colon;
, colon;
 , rectum;
, rectum;
 , feces.
, feces.
TABLE 2.
Studies using non-specifically-named defined communities in rodents to study host-microbe interactions in vivo (n = 16)a
| Consortium (no. of speciesb) | Division of phylaf | Strain source(s) | Host species (strain) | Part of the gut studiedg | No. of animals/group | Chow | Sex | Age (collection time[s]c) | Study outcome(s)d | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| NA, F-strains, and N-strains (2, 9, 11, 41, and 130 species) 2 species: E. coli C25 + Lactobacillus 9 species: 2 species + Enterococcus + Lactobacillus + Candida + 4 morphologically different strains of Gram-negative anaerobes 11 species: 9 species + 2 strains of Gram-negative anaerobes with fusiform morphology 41 species: 11 species + 30 additional strains of Gram-negative anaerobes 130 species: 50 strains of Gram-negative strict anaerobes (N) + 80 facultative anaerobes (F) |
2 species: Others not specified |
Mouse | Mouse (CD-1) |

|
4–57 | Autoclaved Lobund diet L-356 or pelleted sterile diet from Charles River Mouse Farms | NR | NR (1–60 days) | Cecal number of E. coli C25 bacteria; cecal size; histopathology of stomach, small intestine, cecum, and colon | 33 |
| N- and F-strains (60, 96, and 97 species) 60 species: N-strains + 14 facultative anaerobes + E. coli C25 96 strains: F-strains + E. coli C25, E. coli 40T, or Shigella 97 strains: F-strains + E. coli C25 + Shigella or E. coli 40T |
Not specified | Mouse | Mouse (CD-1) |
|
5–75 | Sterilized Lobund diet L-356, Charles River formula 7RF, Lobund diet L-485, or Purina breeder chow | NR | NR (4 wk) | Cecal size; cecal levels of fatty acids; cecal levels of E. coli; pH of cecal contents | 34 |
| NA (4 species) Lactobacillus species 1 and 2, Bacteroides sp., Streptococcus group N |
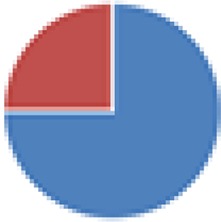
|
Rat? | Rat (Sprague-Dawley) | None | 2 | Autoclaved standard diete supplemented with caffeic acid | NR | NR | Urinary metabolites of caffeic acids | 179 |
| NA (2, 2, 2, 2, 3, 3, 4, 5, 6, 6, 6, 8, 8, 9, 13, 15, and 17 species) 2 species: Actinobacillus s3 + Streptococcus s1 2 species: Bacteroides s8 + Actinobacillus s3 2 species: Eubacterium s10 + Micrococcus s6 2 species: Clostridium C1 + C2 3 species: Bacteroides s8 + Actinobacillus s3 + E. coli s7 3 species: Shigella flexneri + C5 + C6 4 species: C1–C4 4 species: S. flexneri + C3–C5 6 species: C1–C6 6 species: Actinobacillus s3 + Streptococcus s1 + Lactobacillus s4 + Corynebacterium s5 + Micrococcus s6 + Streptococcus s2 6 species: S. flexneri + C5–C9 8 species: 6 species (Actinobacillus, etc.) + Bacteroides s8 + E. coli s7 8 species: S. flexneri + C3–C9 9 species: C1–C9 13 species: C1–C13 15 species: C1–15 17 species: 8 species (Actinobacillus, etc.) + C1–C9 |
2 species (2×):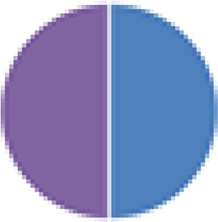 2 species: 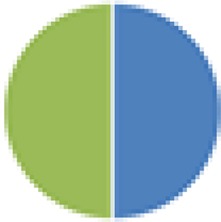  3 species: 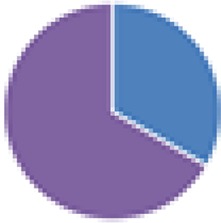 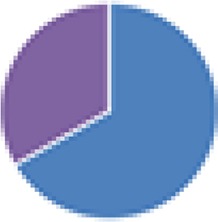 6 species:  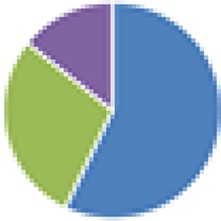 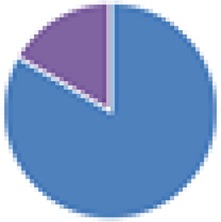 8 species: 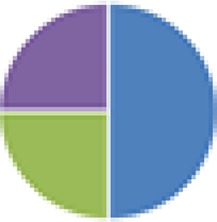 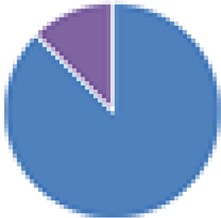 4, 9, 13, and 15 species:  17 species: 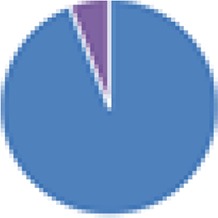
|
Human and mouse | Mouse (CD-1) |

|
≥2 | Sterilized commercial diet (Usine d’Alimentation Rationnelle) ad libitum | Both | 2–5 mo (4 wk after last inoculation) | No. of IgA plasmocytes in duodenum | 38 |
| NA (2, 2, 2, 2, 2, 2, 2, 2, and 3 species) 2 and 2 species: Clostridium E or P with E. coli K-12 2 species (×6): Clostridium E + E. coli S, Proteus mirabilis, Klebsiella pneumoniae, Bacteroides (Alistipes) putredinis, Veillonella alcalescens, or Clostridium perfringens 3 species: Clostridium E and P + E. coli K-12 |
2 species (5×):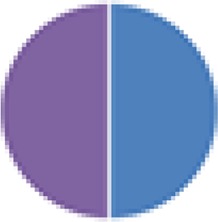 2 species: 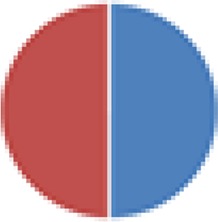 2 species (2×):  3 species: 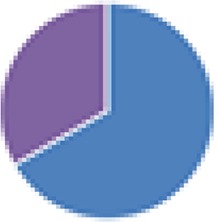
|
Mouse, rat, human | Mouse (C3H) |

|
2–6 | Autoclaved commercial diet | NR | Adult (up to 51 days) | Fecal bacterial counts; (mucosal) histology of stomach, jejunum, ileum, cecum, and colon | 180 |
| UW-GL (9 species) Genera Lactobacillus, Bacillus, Clostridium and Corynebacterium Species not defined |
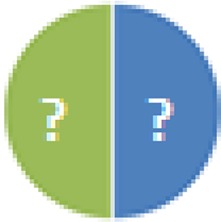 ? = phyla known, but exact composition not defined |
NR | Mouse (BALB/c) |

|
Total of 3 | Sterilized Ralston Purina diet 5010C | Both | 0 (60–90 days) | Cecal levels of bacteria and Candida albicans; histology of tongue and stomach | 37 |
| NA (6 species) Streptococcus (Enterococcus) faecalis, Lactobacillus brevis, Aerobacter aerogenes, Staphylococcus epidermidis, Bacteroides spurius (?), a yeast fungus |
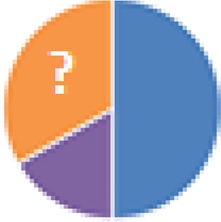 ? = phylum could not be retrieved |
NR | Mouse (BALB/c/ABOMf) | None | 3–6 | Sterilized food (2 different procedures) | NR | 0 (14 wk) | Serum levels of IgG1, IgG2, IgM, and IgA | 108 |
| Partial or complete UW-GL (2, 3, and 9 species) 2 species: Lactobacillus + Clostridium 3 species: 2 species + Bacillus 9 species: UW-GL |
2, 3 species:
|
NR and mouse | Mouse (HA/ICR) |

|
10–48 | NR | Both | Adult (14–56 days) | Death after C. botulinum infection; fecal C. botulinum toxin excretion; colonization pattern of C. botulinum | 36 |
| NA (2 species) B. thetaiotaomicron VPI-5482 + Desulfovibrio piger ATCC 29098 |
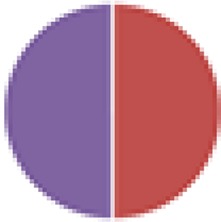
|
Human | Mouse (NMRI/KI) |

|
4–5 | Autoclaved polysaccharide-rich diet (B&K) ad libitum | M (subset) | Adult or 12 wk (14–28 days) | Bacterial contents in cecum and distal colon; bacterial gene expression; glycan levels in cecum; SCFA production in cecum; serum acetate; liver triglycerides; epididymal fat pad | 109 |
| NA (2, 6, and 10 species) 2 species: Staphylococcus epidermidis + Veillonella parvula 6 and 10 species: anaerobic strains isolated from a conventional male mouse (not specified) |
2 species:
|
Mouse | Mouse (B10.BR) |

|
45–73 | Sterilized ST1 (Institute of Physiology AS CR) | M | 21 days (12 mo) | Occurrence of ankylosing enthesopathy of the ankle; colon histology; bacterial contents in ileum and colon | 181 |
| NA (2 species) B. thetaiotaomicron + Eubacterium rectale |
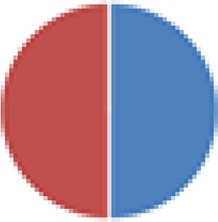
|
Human | Mouse (NMRI/KI) |

|
4–5 | Irradiated standard low-fat, plant polysaccharide-rich diet (diet 2018 from Harland Teklad); high-fat, “high-sugar” Western-type diet (catalog no. 96132; Harlan Teklad); or low-fat, high-sugar diet (catalog no. 03317; Harlan Teklad) | M | 11 wk (14 days) | Bacterial gene expression; cecal colonization levels; fermentation efficiency in cecum; colonic gene expression; protein expression in cecum | 85 |
| NA (3, 8, 9, and 10 species) 3 species: E. coli HS, Bacteroides vulgatus DSM 1447, B. thetaiotaomicron DSM 2079 8 species: 3 species + B. longum NCC2705, Blautia hansenii DSM 20583, C. scindens DSM 5676, Eubacterium ventriosum DSM 3988, Lactobacillus rhamnosus NCC4007 9 species: 8 species + Collinsella aerofaciens DSM 3979 (colonized most mice) 10 species: 9 species + Faecalibacterium prausnitzii DSM 17677 (did not colonize mice) |
3 species: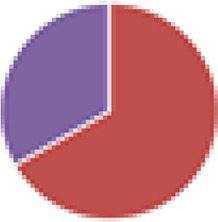 8 species: 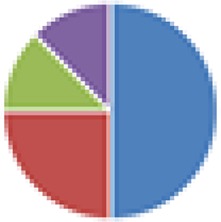 9 species: 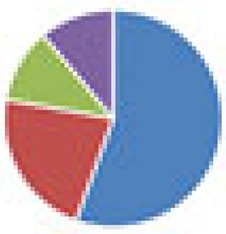 10 species: 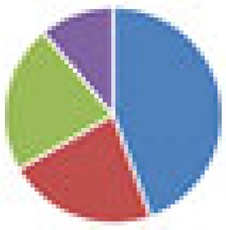
|
Human | Mouse (C3H/HeN) |

|
15 in total | Sterile standard chow diet or switch to high-fat diet ad libitum | Both | 7 wk (70 days after 1st inoculation) | Fecal and cecal bacterial cell counts; body wt; metabolites in urine and plasma | 39 |
| NA (15 and 19 species) 15 species: Bacteroides caccae, Bacteroides ovatus, B. thetaiotaomicron, Bacteroides uniformis, B. vulgatus, Bacteroides strain WH2, C. scindens, Clostridium spiroforme, C. aerofaciens, Dorea longicatena, E. rectale, F. prausnitzii, Parabacteroides distasonis, Ruminococcus obeum, Ruminococcus torques (strain information not accessible) 19 species: 15 species + Bifidobacterium animalis subsp. lactis CNCM I-2494, Lactobacillus delbrueckii subsp. bulgaricus CNCM I-1632 + CNCM I-1519, Lactococcus lactis subsp. cremoris CNCM I-1631, and Streptococcus thermophilus CNCM I-1630 |
15 species: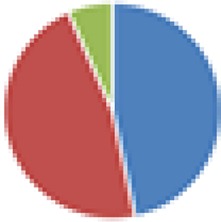 19 species: 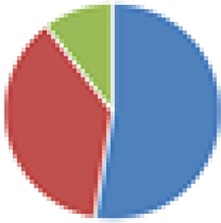
|
Human | Mouse (C57BL/6J) |

|
5 | Autoclaved low-fat, plant polysaccharide-rich diet (B&K rat and mouse autoclavable chow, catalog no. 7378000) | M | 6–8 wk (42 days) | Fecal and cecal bacterial content; bacterial gene expression; urinary metabolites | 40 |
| NA (2 species) B. thetaiotaomicron VPI-5482 (ATCC 29148) + F. prausnitzii A2-165 (DSM 17677) |
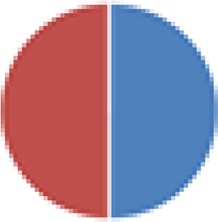
|
Human | Rat (F344) |

|
6–16 | Irradiated polysaccharide-rich diet (R03, SAFE) | M | <3 mo (30 days after inoculation of F. prausnitzii) | Host gene expression in colonic epithelium; cecal SCFA concn; oxidoreduction potential in cecal contents; colonic crypt depth; total cells/crypt in colon; expression of differentiation proteins of secretory lineage (KLF-4, ChgA); Muc2 production in colonic epithelium; colonic mucin glycosylation | 182 |
| NA (2 species) B. thetaiotaomicron VPI-5482 + B. longum NCC2705 |
|
Human | Mouse (SW) |

|
3 | Standard diet (Purina LabDiet 5K67) | NR | NR (10 days) | Fecal bacterial content; metabolites in feces and urine | 86 |
| NA (2, 8, and 9 species) 2 species: B. thetaiotaomicron + D. piger 8 species: B. thetaiotaomicron, B. caccae, B. ovatus, E. rectale, Marvinbryantia formatexigens, C. aerofaciens, E. coli, Clostridium symbiosum 9 species: 8 species + D. piger |
2 species: 8 species:  9 species: 
|
Human | Mouse (NMRI) |

|
4–20 | Irradiated low-fat/high-plant-polysaccharide or high-fat/high-simple-sugar diet ad libitum, HF/HS diet with modified sulfate concn (600-fold range), or HF/HS diet supplemented with chondroitin sulfate | M | 7–8 wk (2 wk) | Fecal bacterial relative abundance; fecal metatranscriptome; gene expression of D. piger; gene expression of mouse proximal colon; cecal metabolites | 42 |
| NA (14 species) + viruslike particles C. aerofaciens ATCC 25986, B. caccae ATCC 43185, B. ovatus ATCC 8483, B. thetaiotaomicron VPI-5482 + 7330, Bacteroides uniformis ATCC 8492, Bacteroides vulgatus ATCC 8482, Bacteroides cellulosilyticus WH2, Parabacteroides distasonis ATCC 8503, C. scindens ATCC 35704, C. symbiosum ATCC 14940, C. spiroforme DSM 1552, D. longicatena DSM 13814, E. rectale ATCC 33656, R. obeum ATCC 29174 |
|
Human | Mouse (C57BL/6J) |

|
5 | Autoclaved low-fat/high-plant-polysaccharide diet (B&K) ad libitum | NR | 8 wk (46 days) | Gut barrier and immune function; overall health status; body wt and adiposity; no. of CD4+ and CD8+ T cells in spleens and MLN; fecal bacterial content and viral abundance; genetic changes upon viral attack (phage resistance); bacterial contents of proximal and distal small intestines, cecum, and colon; prophage activation | 45 |
| NA (14 species) B. ovatus DSM 1896, Bacteroides uniformis DSM 8492, B. thetaiotaomicron DSM 2079, B. caccae DSM 19024, Barnesiella intestinihominis YIT11860, Roseburia intestinalis 14610 (L1-82), E. rectale DSM 17629 (A1-86), F. prausnitzii DSM 17677 (A2-165), Marvinbryantia formatexigens DSM 14469 (I-52), C. symbiosum DSM 934, C. aerofaciens DSM 3979, E. coli HS, A. muciniphila DSM 22959 Muc, D. piger ATCC 29098 |
|
Human | Mouse (SW) |

|
Total of 51 | Autoclaved standard fiber-rich (15% dietary fiber), fiber-free, or prebiotic (addition of purified soluble glycans) diet ad libitum | Both | 8–9 wk (54 days) | Microbial composition in feces, cecum, colonic lumen, and mucus layer; bacterial CAZyme expression in cecum; mucin-specific transcripts in B. caccae, A. muciniphila, and B. thetaiotaomicron; cecal microbial enzyme activities; levels of SCFAs and organic acids; colonic mucus layer thickness; colonic expression of mucus-production-related genes; no. of goblet cells in colon; histopathology; body wt; fecal lipocalin; colon length; cecal transcriptome; after infection with C. rodentium, histological scores of cecum and colon, area of inflamed tissue in cecum, survival, ascending and descending colon and rectum, adherent C. rodentium bacteria in colon | 43 |
Abbreviations: NA, consortium name not available/applicable; AS CR, Academy of Sciences of the Czech Republic; ChgA, chromogranin A; KLF-4, Kruppel-like factor 4; LP, lamina propria; MLN, mesenteric lymph nodes; NR, not reported; SCFAs, short-chain fatty acids; HF/HS, high fat/high sugar; CAZyme, carbohydrate-active enzyme; s3, species 3.
Two different strains tested are counted as one species. Strains were not always reported. Pathogenic species, in the case of an infection model, are not included.
The colonization time includes the time from colonization (time zero in the case of transfer of microbiota to offspring) until and including the time of sacrifice or the end of experimental (e.g., dietary) manipulations, in cases where this is clearly stated in the paper. If age is given and animals are colonized at birth, the age is included in the colonization time.
Study outcomes are reported for the animals colonized with the defined community of interest.
See reference 7.
 , Firmicutes;
, Firmicutes;
 , Bacteroidetes;
, Bacteroidetes;
 , Actinobacteria;
, Actinobacteria;
 , Proteobacteria;
, Proteobacteria;
 , Verrucomicrobia;
, Verrucomicrobia;
 , other.
, other.
The color codes from left to right in the illustration are as follows:
 , stomach;
, stomach;
 , duodenum;
, duodenum;
 , jejunum;
, jejunum;
 , ileum;
, ileum;
 , cecum;
, cecum;
 , colon;
, colon;
 , rectum;
, rectum;
 , feces.
, feces.
TABLE 3.
Studies using defined communities in nonrodents to study host-microbe interactions in vivo (n = 6)a
| Consortium (no. of speciesb) | Division of phylae | Strain source | Host species (strain) | Part of the gut studiedf | No. of animals/group | Chow | Sex | Age (collection time[s]c) | Study outcomesd | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Bristol (3 and 4 species), modified ASF (6 and 7 species) 3 species: Lactobacillus amylovorus DSM 16698T, Clostridium glycolicum, and Parabacteroides sp. (ASF519) 4 species: 3 species + R. intestinalis 6 species: Clostridium sp. (ASF356), Lactobacillus sp. (ASF360), Lactobacillus animalis (ASF361), E. plexicaudatum (ASF492), Parabacteroides sp. (ASF519), and Propionibacterium sp. 7 species: 6 species + Staphylococcus sp. or Bacillus sp. |
3 species: 4 species:  6 species: 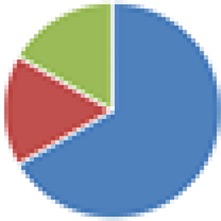 7 species: 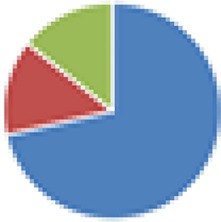
|
Pig | Pig (commercial hybrid and Babraham) |

|
2–6 | Evaporated milk | NR | 0–17 days (14–21 days after 1st inoculation) | Presence of bacteria and mean total bacterial content in proximal and distal jejunum, terminal ileum, cecum, and colon; serum immunoglobulin concn | 52 |
| Bristol (3 species) | Pig | Pig ([Great York × Pie] × ‘Dalland’ cross) |

|
6 | Pasteurized sow colostrum (first hours), ad libitum milk replacer diet (days 0–4), moist diet (remaining) | NR | Neonates (26–37 days) | Relative OR51E1 expression in jejunum | 59 | |
| Bristol (3 species) | Pig | Pig ([Great York × Pietrain] × ‘Dalland’ cross) |

|
6 | Sow serum or pasteurized sow colostrum, followed by ad libitum milk replacer diet (days 0–4), followed by a control diet or medium-chain-fatty-acid diet | NR | 1 day (2–3 wk) | Oxyntic mucosa transcriptome | 60 | |
| DMF (7 and 8 species) 7 species: Bifidobacterium adolescentis, B. longum, B. thetaiotaomicron, E. faecalis, L. brevis, Streptococcus bovis, and C. clostridioforme 8 species: DMF + E. coli Nissle |
7 species: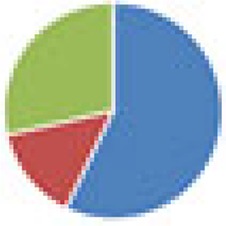 8 species: 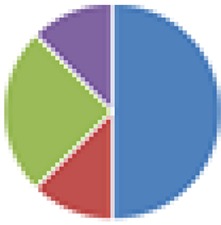
|
Pig | Pig (Landrace × Yorkshire × Duroc cross-bred) |

|
3–6 | NR | NR | 7 days (35 days) | Fecal virus shedding; mean duration of diarrhea; diarrhea severity and % of diarrhea; gene expression levels of ChgA, Muc2, PCNA, SOX9, and villin in jejunal intestinal epithelial cells | 62 |
| Pig | Pig (NR) |

|
3–5 | NR | NR | 5 days (14–35 days) | Bacterial content in rectum, duodenum, jejunum, ileum, colon, and feces/rectal swabs; diarrhea and virus shedding after virulent human rotavirus challenge | 63 |
NR, not reported; ChgA, chromogranin A.
Two different strains tested are counted as one species. Strains were not always reported. Pathogenic species, in the case of an infection model, are not included.
The colonization time includes the time from colonization (time zero in the case of transfer of microbiota to offspring) until and including the time of sacrifice or the end of experimental (e.g., dietary) manipulations, in cases where this is clearly stated in the paper. If age is given and animals are colonized at birth, the age is included in the colonization time.
Study outcomes are reported only for the animals colonized with the defined community of interest.
 , Firmicutes;
, Firmicutes;
 , Bacteroidetes;
, Bacteroidetes;
 , Actinobacteria;
, Actinobacteria;
 , Proteobacteria;
, Proteobacteria;
 , Verrucomicrobia;
, Verrucomicrobia;
 , other.
, other.
The color codes from left to right in the illustration are as follows:
 , stomach;
, stomach;
 , duodenum;
, duodenum;
 , jejunum;
, jejunum;
 , ileum;
, ileum;
 , cecum;
, cecum;
 , colon;
, colon;
 , rectum;
, rectum;
 , feces.
, feces.
(Altered) Schaedler Flora
In 1965, Russel W. Schaedler colonized GF mice with a defined microbial community composed of strains isolated from normal mice, to study the fate of the bacteria in the gastrointestinal tract (GIT) and their effect on cecum size. With respect to these parameters, it turned out that the Schaedler flora (SF) was able to, at least partially, normalize the cecum size of GF mice in comparison with animals raised under conventional conditions (9). The defined microbial population was supplied to animal vendors to serve as a community that could limit infection of ex-GF rodents with opportunistic pathogens. Schaedler developed several different bacterial cocktails over time. In 1978, Roger P. Orcutt set out to standardize and improve the SF flora, but in view of the monitoring costs, the total number of bacterial species was limited to eight. Orcutt made a selection of bacterial species (altered Schaedler flora [ASF]) based on their representation and stable colonization in the murine gut, their ease of identification (morphologically), and their presence in or interference with isolator contaminants. For instance, the cocci and spore-forming, blunt-ended rods were eliminated, which represented the majority of isolator contaminants. Also, the amount of facultative anaerobes was limited, as they outgrew aerobic isolator contaminants and thus impeded the ability to detect the latter (13). The ASF consists of six Firmicutes (Clostridium species [ASF356], Lactobacillus intestinalis or Lactobacillus acidophilus [ASF360], Lactobacillus murinus or Lactobacillus salivarius [ASF361], Eubacterium plexicaudatum [ASF492], Pseudoflavonifractor sp. [ASF500], and Clostridium sp. [ASF502]), one Bacteroidetes species (Parabacteroides distasonis [ASF519]), and one Deferribacteres species (Mucispirillum schaedleri [ASF457]).
The ASF has been used multiple times as a reference or minimal defined microbiota, and its applications were extensively reviewed elsewhere (14). Several studies involving ASF in mice (or other animals) reported its effect on host parameters (Tables 1 to 3). The list is probably not exhaustive, given the wide application of ASF mice as a control or minor population in studies, which makes these studies harder to identify.
The applications of ASF in rodents varied from wild-type strains (mostly C57BL/6 but also C3H/HeN and Swiss Webster mice) to models prone to diseases, including irritable bowel disease (IBD) (15–17), type I diabetes (18), or colorectal cancer (19). The ASF lacks Proteobacteria, a phylum shared by mice and humans, whereas some researchers introduced Proteobacteria to ASF mice, such as Oxalobacter formigenes (20) and Escherichia coli (21). Other studies included only selected members of the ASF, because not all members were found to successfully colonize the murine cecum (18) or to test the levels of colonization resistance of different combinations of ASF members (22). Overall, the application of ASF for the study host-microbe interactions has been quite diverse, regarding host strain, gut region of interest, and host parameters studied.
Although the ASF has been used multiple times as a reference microbiota and has aided in the establishment of other defined microbiota, such as Oligo-MM and the Bristol microbiota, its representability of the normal gut microbiota has been criticized (23), as discussed below.
Oligo-MM
Another defined community of murine microbiota, Oligo-MM12, was constructed in an attempt to provide full colonization resistance against Salmonella enterica serovar Typhimurium (22). Twelve strains were selected to represent the five most prevalent and abundant phyla of the laboratory mouse intestine, i.e., the Firmicutes members Acutalibacter muris, Flavonifractor plautii, Clostridium clostridioforme, Blautia coccoides, Clostridium innocuum, Lactobacillus reuteri, and Enterococcus faecalis; the Bacteroidetes members Bacteroides caecimuris, and Muribaculum intestinale; the Actinobacteria member Bifidobacterium longum subsp. animalis; the Proteobacteria member Turicimonas muris; and the Verrucomicrobia member Akkermansia muciniphila. Colonization resistance of ASF mice or mice colonized with Oligo-MM12 and/or (a subset of) ASF strains was compared to that of conventional mice. ASF was used as a reference because of its wide usage in gnotobiotic mouse research. Oligo-MM12 mice conferred increased, but not full, resistance compared to mice colonized with a subset of ASF strains with and without Oligo-MM. Functional genomic analysis of Oligo-MM and whole ASF revealed that both consortia together cover 66.6% of the KEGG modules of a conventional mouse microbiota. The addition of three facultative anaerobes (E. coli, Streptococcus danieliae, and Staphylococcus xylosus), underrepresented in Oligo-MM12, increased coverage and furthermore conferred full colonization resistance (22). C57BL/6 mice stably colonized with Oligo-MM12 have been designated stable defined moderately diverse microbiota mice (sDMDMm2). The designers of Oligo-MM12 stressed the importance of expanding the amount of available mouse-derived strains, as initiated recently (24), in favor of the design of functionally defined and simplified microbial consortia for application in gnotobiotic animals (22). Because Oligo-MM12 was found to lack the enzymatic pathway to carry out 7α-dehydroxylation, an important bile acid transformation step, the addition of Clostridium scindens (a 7α-dehydroxylating bacterium) was tested in another study. This modification normalized large intestinal bile acid composition in mice, which was accompanied by colonization resistance against Clostridium difficile and decreased intestinal pathology (25). Finally, Oligo-MM12 served as a defined reference microbiota to verify the significant difference between the bacterial compositions in the large intestinal outer mucus layer and the lumen (26), but host parameters were not assessed. Note that the latter two studies that applied Oligo-MM12 left out the three additional facultative anaerobes that were found to be crucial for full colonization resistance.
SIHUMI(x)
Because the ASF was found to poorly represent the dominant intestinal bacteria and ASF mice hardly differed from GF mice in a key set of microbial biochemical activities (23) (Midtvedt criteria [see below]), a simplified human intestinal microbiota (SIHUMI) was established in rats to provide a highly standardized animal model to study host-microbe interactions. Species were selected according to their prevalence in humans, their fermentative capacity, the availability of their genomic sequence, and their ability to stably colonize the rodent gut. SIHUMI(x) includes four Firmicutes (Anaerostipes caccae, Lactobacillus plantarum, Blautia producta, and Clostridium ramosum), one Bacteroidetes species (Bacteroides thetaiotaomicron), one actinobacterium (B. longum), and one proteobacterium (E. coli). All seven members successfully colonized the rat intestinal tract, and total bacterial numbers in fecal samples did not differ from those in human feces. The amount of short-chain fatty acids (SCFAs) produced, however, was dramatically smaller than that in humans, probably owing to the smaller number of species. An eighth species was added to the consortium (SIHUMIx), Clostridium butyricum, which led to increased butyrate production. All members of the SIHUMIx consortium were successfully transferred to offspring. Dietary interventions varying in fiber and fat contents resulted in responses (partially) reflecting those observed in mice and humans (27).
In other studies, SIHUMIx served as a resident community to study the effect of the addition or removal of species. For instance, the inclusion of A. muciniphila, a mucin-degrading commensal, was found to worsen intestinal inflammation induced by S. Typhimurium in mice (28). The same researchers recently showed, however, that in a colitis-prone mouse model colonized with SIHUMI, A. muciniphila did not induce or exacerbate intestinal inflammation (29). In two other studies, the polyamine-producing organism Fusobacterium varium was added to the low-polyamine-producing SIHUMIx in mice, which disclosed that gut morphology was not affected by either increased putrescine concentrations (30) or higher levels of other polyamines and SCFAs (31). Additionally, the mechanism underlying the obesogenic potential of C. ramosum in a SIHUMIx-associated animal model was further investigated by including or excluding this bacterium in SIHUMIx-associated mice fed a high- or a low-fat diet. The increased body fat deposition in the presence of C. ramosum was suggested to be due to the upregulation of small intestinal glucose and fat transporters (32). It should be noted that although SIHUMI was originally established in rats, all other studies applied the community in mice.
Toward a Normal Model Gut Microbiota
Since the generation of the Schaedler flora in the 1960s, other defined gut microbiotas have been developed in an attempt to normalize GF animals or generate animal models harboring a bacterial community representative of the human gut microbiome. During the 1970s, Syed et al. aimed to normalize GF mice with respect to cecum size, cecal numbers of E. coli cells, histology of the intestinal tract, and the development of a mucosa-associated microbiota in the stomach and large intestine (33). A mixture of 50 strictly anaerobic organisms (later designated “N-strains” [34]) and 70 facultative anaerobes (“F-strains”) was found to generate a normal mouse phenotype, whereas less-complex bacterial communities led to intermediate phenotypes with respect to the parameters studied, including cecum size, cecal E. coli levels, GIT histology, and the development of a mucosa-associated microbiota in the stomach and large intestine (33). The exact taxonomic classification of the species within the F- and N-strains was limited by a lack of characterization at that time (33). It was considered likely that a number of the isolates used were identical. Based on morphology and fatty acid production, the total numbers of different strains were estimated to be rather on the order of 35 (N-strains) and 60 (F-strains) (34). The N-strains alone could not control the E. coli population and cecum size when associated with mice fed a crude instead of refined diet, but this could be restored by additional association with the F-strains (34). The F-strains were exploited as an indigenous gut microbiota to investigate E. coli plasmid transfer in vivo (35), but other studies using the N- or F-strains could not be identified.
At the end of the 1970s, the use of the UW-GL (University of Wisconsin Gnotobiote Laboratory flora) was reported, which was used as the intestinal microbiota of heterozygous athymic mice (36). This defined bacteriome consisted of nine Gram-positive species from the genera Lactobacillus, Bacillus, Clostridium, and Corynebacterium (37) and additionally two Gram-negative species that were not further specified (36). It was used to study colonization resistance against Candida albicans (37) and Clostridium botulinum (36). The latter study compared UW-GL with other defined microbiotas, including ASF and a partial UW-GL. Whereas death rates significantly dropped compared to those of GF mice, only the complete UW-GL fully prevented C. botulinum infection (36). The use of the UW-GL microbiota has not been reported since.
Logically, the conception of a healthy or “normal” microbiota is dependent on the available knowledge on conventional animals and/or healthy human subjects, and thus, the composition varies per study. While testing the effect of bacterial species on intestinal IgA immune system development, Moreau et al. paid specific attention to communities of Clostridium species, which were considered a dominant microbiota of the digestive tract of adult conventional mice (38). In studies using defined communities with human-derived gut bacteria, species were selected based on their prevalence in (healthy) human feces (39, 40) and/or their representation of the major three or four dominant phyla of the human gut microbiota (40–42). Next to the designers of Oligo-MM12, only a few studies acknowledged the presence of five phyla (including Verrucomicrobia) of the human gut microbiota. A recently designed 14-membered synthetic microbiota that collectively possessed important core metabolic capabilities was applied to study in vivo foraging of host-derived mucus glycoproteins during fiber deprivation (43). Similarly, other studies took into account the functional capabilities of species. For instance, one study included species that are able to break down complex dietary polysaccharides not accessible to the host (B. thetaiotaomicron, Bacteroides ovatus, and Bacteroides caccae), to consume oligosaccharides and simple sugars (Eubacterium rectale, Marvinbryantia formatexigens, Collinsella aerofaciens, and E. coli), to ferment amino acids (Clostridium symbiosum and E. coli), or to remove the end products of fermentation by reducing sulfate (Desulfovibrio piger) or generating acetate (Blautia hydrogenotrophica) (41). This community has been frequently exploited to study host-microbe interactions or microbe-microbe interactions by the same research group or adopted by others albeit in different combinations ranging from 8 to 15 species (40, 42, 44–50). Recently, a more diverse, complex defined community comprising no fewer than 92 species was developed (51). The consortium consisted of phylogenetically diverse, human-derived bacterial strains, which had previously been cultured and sequenced. It also included strains representing species that were demonstrated to be age and/or growth discriminatory in models of microbiota development during the first years of life. Of all strains, 44 comprised a core group that could be detected in fecal samples of all colonized mice, independent of dietary intervention (51). No host parameters, however, were assessed in this study.
Remaining inclusion criteria for defined communities are the availability of the genomic sequence and the cultivability of the species. Obviously, both criteria make each individual species more easily traceable. If the entire genetic repertoire of the defined community is known, gene expression of the whole community as well as its individual members can easily be assessed (28, 40), and their function can be more precisely predicted. Interestingly, although the ASF has been used for over 50 years, publications on the replication of the four extremely-oxygen-sensitive ASF members on a defined medium are still lacking (14).
Defined Communities in Nonrodents
The above-discussed defined microbiotas were either isolated from rodents or applied to them. Laycock et al. stressed the need for a well-established intestinal colonization microbiota for pigs, given the high representability of these animal models in early immune development studies (52): in pigs, there is no transfer of maternal immunoglobulin G in utero (53, 54) and a poorly developed mucosal system in neonates (55). Furthermore, pigs are genetically more similar to humans than mice (56), and their digestive physiology is comparable to ours (57). Colonization of germfree piglets with ASF members turned out to be largely unsuccessful, and only the most consistently colonizing ASF member (Parabacteroides sp.) was incorporated into the novel “Bristol” microbiota. Additional strains were selected based on their representation of the major phylogenetic groups in gut sections of 12- to 18-week-old pigs and either their ability to grow on a wide range of metabolic carbohydrate structures (Roseburia intestinalis) or their presence in unweaned pigs (Clostridium glycolicum and Lactobacillus amylovorus). Except for R. intestinalis, the novel microbiota successfully colonized the GIT after administration to germfree piglets, with high clinical safety and an expected increase in serum immunoglobulin levels (52). The Bristol microbiota was exploited by other researchers as a simplified starter microbiota to study additional effects of a complex microbiota on early-life microbiota development (58), the intestinal expression of a butyrate-sensing olfactory receptor (59), and the gastric transcriptome (60). Note that in the latter three studies, the piglets were not maintained in a sterile environment, hampering comparison of the effects of the Bristol microbiota on host parameters between studies. A different 10-membered porcine gut microbiota, originally designed as a competitive-exclusion culture for pigs, was used to investigate antibody repertoire development in ex-germfree newborn piglets (61). Another “defined commensal microflora” (DMF) included seven porcine bacterial species and had a composition similar to that of the ASF. Species were originally isolated from the cecal contents of 6-week-old healthy pigs and administered to germfree pigs to evaluate the interactions between intestinal commensals, antibiotics, probiotics, and human rotavirus. This model was primarily applied as a model commensal gut microbiota of neonates (62, 63).
OTHER DEFINED COMMUNITIES IN VIVO
Apart from the defined communities as models for the normal (human) gut microbiome to study host-microbe interactions, other kinds of communities have been composed for application in gnotobiotic animals. These communities, however, are not listed in Tables 1 to 3, and their application goes beyond the scope of this review, as they did not aim to represent the normal microbiota. For instance, these communities include disease-specific consortia, e.g., IBD related (15, 64–67). Others are age-specific, such as the human baby microbiota (68–70), the DMF (62, 63), and a recently developed Bifidum-dominated model consortium (71). Finally, some communities were developed for therapeutic or probiotic purposes. A well-studied and globally marketed multispecies probiotic is the bacterial cocktail VSL#3, which was recently characterized at the genomic level and has been used to treat various gastrointestinal disorders (72–74). Other communities were designed to treat infections (among others, C. difficile infection [CDI] [75–77] and colitis [78]) or to facilitate recovery from cholera (79). Two remarkable applications of defined communities, which were not per se meant to model the normal human gut microbiota, are discussed in more detail below.
Therapeutic Communities
Although the concept is not new and was pioneered 30 years ago (75), the interest in fecal transplantations has recently increased, and the avenue of synthetic microbiotas as stool substitutes has been suggested (80). A particular example of such a stool substitute is microbial ecosystem therapeutic 1 (MET-1), designed as a synthetic stool mixture to treat recurrent CDI. Sixty-two species were recovered from the stool of a healthy 41-year-old female donor, of which 33 species were selected that were sensitive to a range of antimicrobials and were easy to culture. Two CDI patients that were “rePOOPulated” with MET-1 returned to their normal bowel pattern within a few days and remained symptom-free for at least 6 months. The use of a synthetic stool mixture has several advantages over conventional stool transplants: (i) the bacterial composition is known, controllable, and reproducible; (ii) a pure consortium is more stable than stool; (iii) the formulation is safe, owing to the lack of viruses and pathogens; and (iv) the administered organisms can be selected based on their sensitivity to antimicrobials, which further enhances safety (77). Some of these benefits also strengthen the use of defined communities in host-microbe interaction research. Notably, the application of MET-1 as a defined community in GF animals, instead of antibiotic-treated animals, was limited to one study, in which it was used as a healthy, Firmicutes-rich microbiota to study colitis susceptibility and host immune responses (81).
In contrast to the use of a defined synthetic community, the anaerobically cultivated human intestinal microflora (ACHIM) was derived from a fecal sample from a healthy Western donor that has been maintained in anaerobic culture for more than 20 years now and has been applied in fecal microbiota transplantation (82). Although the microbiota is regularly checked for the absence of pathogenic organisms and multiple CDI patients have been treated successfully with this cultured microbiota transplant from a single donor (82), its composition is not controllable.
Instead of starting with a certain disease or phenotype and generating a defined community to treat this condition, as is true for MET-1 and ACHIM, researchers recently tested different defined bacterial communities to generate various phenotypes in mice and to identify the strains responsible for the observed phenotypic variation. By administering GF mice one of 94 different, defined bacterial consortia of species randomly drawn from the culture collection, strains that modulated adiposity, intestinal metabolite composition, and the immune system were identified. According to those authors, a similar approach could be applied to identify and characterize next-generation probiotics or combinations of pre- and probiotics (83).
Minimal Communities
Another category of defined communities is formed by minimal communities. Essentially, all defined microbial communities are minimal in the sense that they are not as complex as microbiota in vivo. Nonetheless, some studies exploited even-more-simplified defined consortia, i.e., with a limited amount of species or clearly lacking certain functions, to study host-microbe interactions in general. This is exemplified by biassociation studies involving single members of (dominant) phyla. In a recent study, GF mice were colonized with B. thetaiotaomicron, as a prominent member of the adult human gut microbiota, plus one of three probiotic strains (B. longum, Bifidobacterium animalis, or Lactobacillus casei) to study microbe-microbe and host-microbe interactions (84). In the same laboratory, gnotobiotic mice were colonized with bacteria from the two dominant phyla in the adult human distal gut microbiota: Firmicutes and Bacteroidetes. Based on their prominence in culture-independent surveys in the distal human gut, the pattern of representation of carbohydrate-active enzymes in their glycobiomes, and E. rectale’s ability to generate butyrate as a major end product of fermentation, a “marriage was arranged” between E. rectale and B. thetaiotaomicron. This reductionist approach provided information on microbe-microbe interactions, the microbial response to host diet, and the microbial effects on host physiology (e.g., the upregulation of the production of [mucin] glycans by the host) (85).
Despite the value of minimal communities for studying microbe-microbe and host-microbe interactions, a study of mice colonized with another simplified microbiota (B. thetaiotaomicron and B. longum) clearly demonstrated that the simple microbiota could not reconstitute the metabolomic complexity of a humanized microbiota, i.e., derived from human donors (86). Nevertheless, Tables 2 and 3 include some minimal communities, because of their representation of major phyla of the human gut microbiota or relevant application to the study of host parameters.
CRITICAL EVALUATION OF DEFINED COMMUNITIES IN VIVO: THE MICROBIOTA
In the sections above, we provide an objective description of defined microbial communities that have been applied in in vivo models to study host-microbe interactions. The next section discusses the representability of these communities, focusing on their design criteria and sources (murine versus human). Additionally, comparisons are made between simple versus complex and bottom-up- versus top-down-constructed communities. Suggestions for the future design of defined communities representing the normal intestinal microbiota are provided as well.
How Representative Are Defined Microbiota Models of a Normal Microbiota?
The development of defined communities representative of the human gut microbiota raises the issue, “What defines a normal microbiota?” Among the included studies that aimed to design a representative gut microbiota, different selection criteria were used. The representation of the major phyla and various metabolic capacities have been frequently put forward. A meta-analysis was performed to compare the composition of the core mouse gut microbiome (based on five different mouse models, i.e., varying in age, phenotype, and sampling site) with that of the human gut microbiome (based on 16 individuals) (87). Apart from the differences within the mouse microbiota, Bacteroidetes and Firmicutes were clearly the most dominant phyla in all samples (together 87% to 97%). (87) The same is true for the compositions of the well-established defined communities ASF, SIHUMI(x), and Oligo-MM12 (75% to 87.5%). Similar to most murine microbiotas included in the meta-analysis, however, the ASF and SIHUMI(x) lack Verrucomicrobia, which were found among the five most abundant phyla in human and some murine samples (87). In that sense, Oligo-MM12, originally designed to represent the murine microbiota, is compositionally more complete than SIHUMI(x), which was meant to represent the human microbiota. The frequently used ASF also lacks Actinobacteria and Proteobacteria, which are abundant in both murine and human samples (87–89). Similarly, a large part of the other defined communities discussed here (Tables 1 to 3) did not include representatives of all five major phyla of the human microbiota, with some not including even one of the two most prominent phyla. Note that species selection has been based mostly on the microbiota composition of Western individuals.
Furthermore, community design has been limited by the availability of genomes and cultivability of strains. In the case of the ASF, the number of species was limited for financial reasons, i.e., taking into account the monitoring costs. Nevertheless, this community has been frequently used in gnotobiotic animal models. The assumption that ASF mice can be regarded as conventional mice with respect to their gut microbiota has been criticized (23). Several functional activities in fecal materials from ASF mice were analyzed and compared to those in samples from GF and conventional rodents and other mammalian species, including humans. The five biomarkers investigated, the so-called Midtvedt criteria (i.e., conversion of cholesterol to coprostanol, conversion of bilirubin to urobilinogens, degradation of β-aspartylglycine, degradation of mucin, and absence of fecal tryptic activity [23]), are claimed to reflect host-bacterium interactions, independent of the intestinal localization of the bacteria involved and the kind of species. With regard to these criteria, fecal samples from ASF mice showed patterns more resembling GF rather than conventional mice (23), which complemented previous results demonstrating an abnormal microbiota in specific-pathogen-free (SPF) mice (90). Although this could be due to one of the limitations of the ASF, i.e., its low diversity, ASF mice were shown to be immunologically, reproductively, and metabolically similar to conventional mice (23). The Midtvedt criteria were also used to assess the suitability of SIHUMI(x) as a model microbiome. SIHUMI(x)-associated rats shared four criteria with conventional rats, three of which, however, were less pronounced (27).
A major difference between the ASF and a consortium such as SIHUMI(x) is the fact that the latter involves human-derived bacterial strains. Most members of recently developed communities, except for Oligo-MM, are of human origin as well. This may be obvious, given the fact that although their microbiotas are similar at the division (superkingdom) level, 85% of the microbial genera and species detected in mice are not found in humans (91). Although, qualitatively, humans and mice share a largely similar core, their intestinal microbiotas are quantitatively very different (87). On the other hand, the development of small intestinal immune maturation was found to be host specific, with humanized mice more closely resembling GF mice than mice associated with a murine microbiota (92). This host specificity might also, at least partially, explain the unsuccessful colonization of piglets with ASF (52). Additionally, humanized rodent models were claimed to have been utilized mainly for short-term biomedical research studies (14). Questions remain regarding how human-derived bacteria would adapt during long-term colonization and vertical transmission in murine hosts (14, 93, 94) and, thus, which kind of microbiota would be most reliable to study host-microbe interactions using murine hosts. The maximum colonization time reported in the studies discussed here (Tables 1 to 3) was less than 1 year. With respect to vertical transmission, stability after transfer to offspring has been addressed mainly for murine microbiotas only (ASF [95] and Oligo-MM [22]). Within the humanized defined communities, SIHUMI(x) is an exception, of which bacterial concentrations in the cecum were verified between founder rats as well as their offspring. At the age of 8 weeks, SIHUMIx-treated rats harbored similar bacterial levels as their founders but not at 2 weeks (except for E. coli) (27).
Simplified versus Complex Communities
The distinction between minimal communities, with two or three members, and larger defined communities is not black-and-white. For instance, the ASF, initially used as a microbiota to standardize mouse models, slowly adopted the role of a minimal community instead of one representing the normal microbiota of mice. Nonetheless, the simplicity of a defined community also has some advantages over more-complex communities. The limited nature of the ASF should, as proposed by Brand et al., allow investigators to evaluate the in vivo effect of the removal or addition of bacterial species on mucosal homeostasis and colonization dynamics or, potentially, factorial interactions of the community (14). Indeed, some of the studies discussed here (Tables 1 to 3) used only a subset of the ASF species or added species to already established defined communities, including the ASF and SIHUMI(x). Additionally, one- and two-member communities could be applied to model aspects of a more complete microbiota, such as depletion of certain dietary compounds or metabolites (86). Finally, as discussed above, a simplified consortium makes each species traceable, as opposed to a very complex community (28, 40).
On the other hand, complex communities might more closely resemble the normal human gut microbiota and are more likely to confer colonization resistance to opportunistic pathogens, which has been a frequently mentioned criterion in the studies described above. In the 1980s, Freter and coworkers formulated the nutrient niche theory, which states that a certain bacterium can successfully colonize a host only if it is able to use a specific limiting nutrient more efficiently than its competitors (96). This implies that colonization resistance correlates with community complexity, as supported by several studies (22, 36, 97). Freter et al.’s theory was corroborated in a recent study in which the relative abundances of each species of a 10-membered community were correctly predicted based on the concentrations of individual dietary ingredients (41). The theory assumes, however, an environment in which bacterial growth is balanced and nutrients are perfectly mixed, whereas in reality, bacteria are metabolically flexible (i.e., they have the ability to switch nutrient sources), and nutrient levels in the gut are spatiotemporally heterogeneous (reviewed in references 98 and 99).
Metabolic flexibility was hardly addressed in the studies discussed in this review. Some researchers ensured the inclusion of species in a defined community that, as a whole, was able to thrive on a wide range of nutrients. Once established in vivo, however, the behavior of the community was addressed seldomly or only for a single species. This could be due to the fact that most of the included studies focused primarily on the effects of the whole microbiota or a subset of species on the host (host-microbe interactions) rather than the exact nutrient niche occupation by its separate species (microbe-microbe interactions). Exceptional is a recent study that quantified the in vivo responses of both mucin specialists (A. muciniphila and Barnesiella intestinihominis) and mucin generalists (B. caccae and B. thetaiotaomicron) upon fiber deprivation (43). A fiber-deficient diet stimulated the expansion and activity of the mucus-degrading bacteria, promoting epithelial access and pathogen-induced colitis (43).
With respect to spatiotemporal heterogeneity, Oligo-MM12 was used to verify that the bacterial compositions in the large intestinal outer mucus layer and the intestinal lumen are significantly different (26). Due to extensive mucus shedding and mixing in the lumen, however, the differences may be relatively small (98). Indeed, it was recently shown that, on a microscale level, the proximal colon should be viewed as a partially mixed bioreactor rather than a clearly compartmentalized gut section with spatially segregated communities. A next step would be to quantify the distribution of nutrients and metabolites and the role of host factors such as diet, gut motility, and mucus composition (48). Vice versa, it would be interesting to study the effects of spatial organization on relevant host parameters, which unfortunately were not addressed in the latter study. Those authors admitted that the 15-membered community used may not be complex enough to demonstrate stronger spatial associations with food particles, host cells, and mucus (48), reinforcing, all in all, the need for more-complex communities.
Both metabolic flexibility and spatiotemporal heterogeneity allow for increased community diversity, which is thought to be crucial for ecosystem robustness (98). Defined communities enable the precise investigation of both concepts, but on the other hand, the question remains regarding whether they can be made sufficiently complex to properly address these issues.
Bottom-Up versus Top-Down Approaches
One way to obtain a more complex model community is to start with a complex sample, e.g., human stool, and narrow the number of species down via one or more enrichment steps, e.g., by culturing on selective media (top-down approach [100]) or using fermentation models. Tables 1 to 3 include only a few examples with regard to normal microbiota (Oligo-MM12 [22, 40]). The majority of the studies listed in Tables 1 to 3 used a bottom-up approach, in which single previously cultured and characterized strains are combined into a synthetic bacterial community, e.g., based on selection criteria mentioned above, and administered to germfree animals. An advantage of the latter method is the known composition of the microbiota, as emphasized above. A drawback, however, is formed by the risk that the desired phenotype (in this case a normalized host) cannot be entirely recapitulated (100).
Future Design
Probably a more important question is whether a normal microbiota actually exists. In the 1970s, Freter and Abrams concluded that significant fluctuations occur in the normal microbiota and that there is “no such a thing as a reproducible and precisely definable ‘normal enteric flora’.” Instead, they considered the F-strain collection most optimal for use as a microbiota representing a “state which is sometimes found in ‘normal’ individuals” (34). Clearly, the concept of the normal microbiota has changed over time and has evolved with the development of techniques to sequence the human gut microbiome, with increased insight into its composition, dynamics, and function. Recently, researchers aimed to draw the compositional functional core of the human gut microbiota, or the core microbiome. They emphasized that the gut microbiome should be considered a complex landscape, with both common and individual characteristics and alternative stable states with respect to composition, structure, and function (101). They listed a top set of 50 bacterial genus-like taxa that are part of the phylogenetic core, a common core of bacterial taxa shared by the majority of (adult Western) human individuals, based on data from previous studies (101–103). This core may include keystone species, whose roles are crucial for ecosystem structure and function, for instance, the breakdown of carbon sources to support the growth of other core members (104, 105). Mapping this core, including its keystone species, and comparing it with diseased microbiota could increase our understanding of a normal microbiota and facilitate the design of a defined community representative of a healthy human gut microbiota. Next to the phylogenetic core, increased insight into the minimal intestinal metagenome (106) and the active functional core (107) within the human gut ecosystem might provide new criteria for assessing the “normality” of a designed defined community. The paradigm seems to shift from rather black-box-like measures, such as the Midtvedt criteria, to actually understanding the function of the gut microbiota and the contribution of its individual species. Subsequently, this approach could allow a more thorough comprehension and more accurate design of age-, region-, and disease-specific defined communities.
Although this review primarily focuses on bacterial communities, it should be mentioned that the human (gut) microbiome also includes fungi, archaea, microeukaryotes, and many viruses, mainly bacteriophages. A study from 1980 included a “yeast fungus” in a defined hexaflora, but the specific role of this microbe was not addressed (108). One of the few studies in this area addressed the interaction between the murine host, an archaeon (Methanobrevibacter smithii), and a bacterium (B. thetaiotaomicron) (109). In addition, the same research group designed a gnotobiotic animal model with a simplified defined gut community to study phage-bacterial host dynamics (45). In parallel with the healthy gut microbiome, researchers recently mapped the healthy gut phageome (110), but this field is still in its infancy. It is reasonable to assume that with increasing insight into the role of nonbacterial gut microbes in host-microbe interactions, the design of defined microbial communities becomes more representative of the whole human gut microbiome.
CRITICAL EVALUATION OF DEFINED COMMUNITIES IN VIVO: THE HOST
Next to the discussion of the exact composition of the defined microbial community, the selection of the host animal to study host-microbe interactions is critical. Rodents are the most commonly used mammalian models in which defined communities have been applied. The suitability of rodents as models for the human host was extensively reviewed elsewhere (10) and goes beyond the scope of this review. In summary, murine intestines are anatomically, histologically, and physiologically very similar to human intestines, but sizes, metabolic rates, and dietary habits differ largely, leading to qualitative and quantitative differences in microbial composition (10). With respect to the gnotobiotic models discussed in this review, there are some additional discrepancies to be mentioned. The high value of using gnotobiotic animals as models of humans, i.e., their known composition and controllability, seems to be weakened by poor control of host parameters known to influence the human gut microbiome, such as diet, genotype, sex, part of the gut studied, age, and immune system.
Host Parameters Influencing the Microbiota
Diet is a complex and strong determinant of gut microbiota composition (reviewed in references 111 and 112). The individual species levels of a 10-membered defined community in mice fed diets systematically varying in protein, fat, polysaccharide, and simple sugar contents were assessed in order to develop a model to predict the variation in species abundance. Next, the model was validated with 48 random combinations and concentrations of four ingredients selected from a set of eight human baby foods. Approximately 60% of the variation in species abundance could be explained by the known concentrations of pureed foods (41). This study exemplified the application of defined communities to systematically assess the response of individual gut members to various food components, which are, moreover, typical of the human diet. Clearly, a standardized diet of a laboratory animal is different from that of humans, which varies per region, season, individual taste, and even day. Some studies listed in Tables 1 to 3 incorporated a previously developed prototypic “Western-style” diet (27, 32, 39, 42, 46, 85), containing large amounts of saturated and unsaturated fats and carbohydrates commonly used as human food additives (i.e. sucrose, maltodextrin, and/or cornstarch). A lack of standardization in laboratory animal feeding protocols, however, was emphasized previously, for instance, with respect to diet composition and texture (113), and indeed, diets used by studies discussed here are highly variable (Tables 1 to 3). Moreover, in ∼40% of the studies, the diet was not clearly defined or was not even reported, which is alarming given the large impact of diet on the gut microbiome.
The choice of mouse genotype also varied per study (Tables 1 to 3), although an effect of host genotype on microbiota composition was established within species (114–118). These results were corroborated by studies with defined communities such as ASF (119) and SIHUMI(x) (64). Additionally, colonization of different mouse strains with SIHUMI(x) demonstrated host-specific cecal levels of polyamines and SCFAs (31). In mice associated with B. longum and B. thetaiotaomicron, host genetic background was found to affect the overall transcriptome of the latter bacterium but not the expansion of the bacterial substrate range of this bacterium (84). Obviously, defined communities allow the careful investigation of such host-dependent effects, but validation of host-microbe interactions in a wide range of host strains seems crucial before drawing of conclusions and extrapolation to humans.
Although reports on the effect of gender have been contradictive (106, 117, 120–124), it might be a crucial determinant in gut microbiota composition and/or behavior. In turn, the commensal microbiota was shown to affect sex hormone levels (125, 126). Sex differences in gut microbiota composition were recently comprehensively investigated in 89 common inbred mouse strains. After excluding confounding by host genetics, diet, age, or cage effects, the researchers detected gender-specific differences in taxon abundances and diet responses. These differences could be partially explained by sex hormones (127). Among the studies discussed here (Tables 1 to 3), one reported differences in metabolic profiles in urine and plasma between both sexes, but no explanation was put forward (39). In an older study, male mice were found to be more susceptible to death after C. botulinum infection, which could be explained by their coprophagic behavior or a more general higher susceptibility to disease (36). In contrast, other studies reported an absence of gender-specific effects on, for instance, levels of Oxalobacter formigenes colonizing ASF mice (20) or the assembly of a synthetic microbiota (43). Whereas some studies discussed here (Tables 1 to 3) reported the use of a mixed-gender population, others included only one gender (n = 12 of 53 studies), in which male animals were more often used than female animals (nine versus three). Remarkably, the establishment of SIHUMI(x) was verified in both genders, whereas the effect of dietary fiber was tested in male rats, and the effect of a high-fat diet was investigated in female rats (27). A similar discrepancy was found in a study that assessed the effects of five fermented milk product strains on human female twins but male gnotobiotic animals. Although microbiota responses were more or less similar in both species (40), such a gender mismatch may complicate translation. Finally, not all studies clearly reported the gender used per experiment, and approximately half of the studies did not report animal gender at all. This too may hinder data reproduction and, more importantly, translation.
Defined communities allow the quantitative comparison of microbial compositions along the GIT, within and between models. ASF-associated mice were used to quantitatively demonstrate that the microbiota of the colon is poorly reflected in fecal samples (95). Relative abundances of species were also different between feces (rectal swabs) and colon in pigs colonized with a defined microbiota (63). In rats colonized with SIHUMI(x), however, bacterial concentrations in the cecum, colon, and feces were similar (27). Additionally, increases in relative abundances of mucin-degrading bacteria in the cecum and colon upon switching to a fiber-free diet were reflected in feces (43). In a mouse model associated with a 12-membered community, individual bacterial levels were also similar between feces and cecum (46). These conflicting results could be explained by various factors, including host, community composition, and sampling time. Irrespective of the actual difference between GIT sites, it is disappointing that some other studies relied solely on fecal bacterial content. In a study applying a 92-membered community, for instance, not even half of the members could be detected in feces. Other species may have established themselves in different regions of the gut, but this was beyond the scope of that paper (51). Nevertheless, due the invasiveness of sampling, systematic studies comparing colonic and fecal bacterial contents are lacking in humans as well (99, 112). The variation in GIT sites looked at by the studies included in Tables 1 to 3 makes it difficult to compare the colonization pattern of the defined communities to that of natural colonization. Apart from differences along the GIT, capturing the transversal heterogeneity within one compartment may be crucial for properly modeling and understanding host-microbe interactions, as discussed above.
The age at which animals are colonized was quite variable among the studies, including animals bred with the desired defined community as opposed to GF animals colonized with the community of interest to create a gnotobiotic animal model. In the latter case, animals are inoculated at various time points among studies, whereas the timing of microbial colonization was demonstrated to impact, among others, immune maturation (128, 129), mucosal homeostasis (130), and gut-brain axis communication in mice (131). Moreover, as discussed above, colonization times of animals in studies discussed here (Tables 1 to 3) were limited. Nevertheless, some studies confirmed the stability of their defined community of interest over time and even over generations, which should be sufficient to draw conclusions within a specific colonization time window. This, however, does not allow one to infer any information on the long-term effects of colonization.
A last factor determining gut microbiota composition and behavior is the immune system, which in turn is influenced by, among others, the above-mentioned factors and the gut microbiota itself. Looking at the studies discussed here (Tables 1 to 3), several researchers investigated immunological parameters such as serum immunoglobulin levels and the presence of (subsets of) immunological cells in the gut. Nevertheless, due to the complexity of the immune system, it is difficult to quantify and compare the model hosts used with respect to immunological parameters. The key findings on the interactions of gut microbiota members and their products with the immune system have recently been reviewed elsewhere (100). Those authors emphasized the value of minimal microbiomes and subsequent standardized (animal) models. Determining the effects of a specific gut microbiota on the host could help to identify host-microbe interactions that shape the immune system (100). Most studies discussed in this review did not make a distinction between the contributions of each specific microbe to immunological effects observed.
The advantages and the levels of controllability of gnotobiotic research, as well as its pitfalls in practice, as outlined above, are summarized in Table 4.
TABLE 4.
Advantages and pitfalls of gnotobiotic animal models in comparison with human research, with respect to the factors influencing intestinal microbiota composition or behaviora
| Factor | Advantage(s) (vs human research) | Pitfalls in practice |
|---|---|---|
| Inoculum (defined community) | Controllable composition (healthy vs diseased microbiota [e.g., missing keystone species], human vs animal derived) | Animal microbiome ≠ human microbiome; difficulties in defining a healthy or normal microbiota; host-specific selection of microbiota |
| Diet | Controllable composition, timing, amt (tailored to human diet [region, age, and season, etc.]) | Lack of standardization in laboratory animal feeding protocols; not always reportedb |
| Host genotype | Controllable; genetic changes possible (ability to introduce disease) | Validation of host-microbe interactions in multiple strains needed before extrapolation to humans; animal genotype ≠ human genotype |
| Sex | Controllable | Only one gender investigatedb; not always reportedb |
| Part of the gut | Ability to measure bacterial levels in virtually all intestinal parts; ability to capture transversal heterogeneity | Anatomy and physiology different from humans; variations in relative abundances per gut region different per modelb; focus on specific gut regions or feces onlyb |
| Colonization time | Controllable | Long-term effects not studiedb ; animals not always colonized starting at birthb; stability over generations not always confirmedb |
| Immune system | Controllable at start/birth | Uncontrollable in long-term studies, especially locally; complex, determined by internal and external factors; not quantified or quantifiableb |
Validation of In Vivo Models
As emphasized above, differences exist between humans and animals, not only limited to their intestinal microbiota. In line with the question of what defines a normal or healthy intestinal microbiota, one could ask, “When is the animal model sufficiently representative of the human situation?” With regard to the studies discussed here (Tables 1 to 3), diverse host criteria are applied. For the models exploiting a murine microbiota, validation is relatively easy. Most researchers aimed to normalize GF hosts to conventionally raised animals, thereby focusing on host parameters such as cecal size or weight (9, 22, 33, 34). With respect to humanized mice, validation is more complicated, but some studies made an effort. For instance, total bacterial numbers in feces and fecal SCFA levels between humans and SIHUMI(x)-associated rats were evaluated, and a previously reported increase in the abundance of Erysipelotrichaceae upon a high-fat diet in humans was mirrored in SIHUMI(x)-associated animals (27). Other host parameters (e.g., immune system or other systemic parameters), however, were not taken into account. Similarly, validation was lacking in other studies applying SIHUMI(x), in which, moreover, mice were used instead of rats (28, 30–32).
A better example was recently described in a study in which the effect of a fermented milk product on both humans and gnotobiotic mice humanized with a 15-membered microbiota was tested. The proportional representation of the intestinal bacterial species and genes and metabolic changes upon the introduction of the probiotic strains were hardly different between mice and humans, but the researchers also acknowledged the limitations of their gnotobiotic animal model with respect to translatability (40). In most other studies (Tables 1 to 3), control groups were limited to conventionally raised and GF animals or animals with a control treatment, for which translatability of the results to the human situation remains speculation.
DEFINED COMMUNITIES IN VITRO
As opposed to in vivo models, the use of defined communities to study host-microbe interactions in vitro has been limited so far, although the development of sophisticated in vitro model systems is advancing rapidly. In this section, we discuss in vitro models in which defined communities have been applied or could be applied to study host-microbe interactions. A distinction is made between models focused on the microbiota (e.g., composition and characteristics) and those that were designed to realistically represent the human host in vitro. Figure 1 summarizes all existing in vitro models of the human host and microbiota, illustrating how their interactions can be studied by combining advanced in vitro cell-based systems with defined communities. Ultimately, the goal is to combine the best of both worlds.
FIG 1.
In vitro models of the human gut and gut microbiota. Models are organized from bottom to top, with the most representative and complex at the top and the most controllable and traceable, with respect to host parameters or microbial species, at the bottom.
Modeling the Intestinal Microbiota In Vitro
The use of fermentation models has proven successful in modeling the intestinal microbiota in vitro, ranging from short-term batch incubations to multicompartmental continuous systems. As discussed above, most defined communities applied in vivo (Tables 1 to 3) were constructed bottom-up, by selecting species based on their function, prevalence, or other criteria. Alternatively, communities can be composed top-down by inoculating GIT-mimicking chemostats with human feces. Well-known examples of these chemostats, such as the MacFarlane-Gibson three-stage continuous-culture system, (M-)SHIME [(mucosal) Simulator of the Human Intestinal Microbial Ecosystem], EnteroMix, the Lacroix model, and TIM-2 (TNO Intestinal Model 2) have been extensively reviewed elsewhere (132–134). The high reproducibility, stability, and complexity of bacterial communities cultured in chemostats (135, 136) have allowed the development and application of representative communities of the human intestinal microbiota in vitro. Most of these models, however, did not include a host component. The host-microbiota interaction (HMI) module comprised a promising exception in which feces from a healthy volunteer were first fed into an adapted SHIME system, with fluid compartments mimicking the stomach, small intestine, and ascending colon. Subsequently, the SHIME effluent was exposed to an artificial mucus layer, separated by a semipermeable membrane from a compartment containing Caco-2 cells. This module allowed the coculture of bacteria with enterocytes for up to 48 h (137), which is discussed in more detail below.
Modeling the Host In Vitro
With respect to well-established defined communities, the probiotic cocktail VSL#3 and the fecal transplant substitute MET-1 have been tested on various human or animal intestinal cell lines (Caco-2, T84, and HT-29) (e.g., see references 138–140). In most studies, however, the use of bacterial lysates or conditioned media was preferred over live bacteria (e.g., see references 72 and 141–144), because the (mainly anaerobic) gut bacteria cannot survive under the aerobic conditions needed for intestinal cell culture. In these two-dimensional (2D) models, the interaction with the immune system or other tissues cannot be studied. Although the direct effect of VSL#3 was tested on spleen and dendritic cells (145, 146), tissue-tissue interactions were lacking in these models. This problem can be (partially) solved in transwell coculture models, in which bacteria, mucosal immune cells, and intestinal epithelial cells can be studied together (147). A transwell model with an apical anaerobic compartment enabled the coculture of an anaerobe bacterium with an intestinal cell line to study host-microbe interactions (148). Still, these cell lines lacked their tissue-specific context, including all major types of epithelial cells (e.g., goblet cells, enterocytes, enteroendocrine cells, and Paneth cells) organized in crypts and villi. Moreover, as cell lines are tumor-derived, their epithelial characteristics are affected. These issues have been overcome by the development of gut organoids, self-organizing three-dimensional (3D) epithelial structures derived from intestinal stem cells (149) or human pluripotent stem cells (150). The use of organoids to study host-microbe interactions was reviewed elsewhere (151). The closed structure of organoids, in which the lumen is sealed with epithelial cells and a mucus layer, may facilitate the establishment of hypoxia in the core lumen (151). The anaerobic pathogen C. difficile survived for up to 12 h within organoids, but luminal oxygen levels still ranged from 5% to 15%, which may be tolerated by specific strains of C. difficile only (152). More recently, researchers developed an organ culture system for the mouse intestine, in which the stromal and hematopoietic components of the normal intestine were preserved ex vivo. The device supported the survival and growth of both anaerobic and aerobic microbiotas, allowing the investigation of their effects on neuronal parameters (153).
Coculture of defined microbial communities with human cells in transwells, organoids, or organ culture systems has been limited, probably owing to the static nature of these models. More-advanced in vitro models to study host-microbe interactions have been developed (as recently reviewed in reference 133), only a few of which have hitherto allowed the coculture of multiple bacteria with intestinal cells or cell lines.
Organ-on-a-chip technology is an emerging concept within biomedical research, to replace conventional cell culture and animal testing. Organ-on-a-chip devices are microfluidic devices in which cells are cultured with organ-relevant spatiotemporal chemical gradients and dynamic mechanical cues, thereby aiming to reconstitute the structural tissue arrangements and functional complexity of living organs in vitro (154). Several gut-on-a-chip devices have already been developed (155–158), only one in which multiple intestinal bacteria were successfully cultured (158). In this device, two channels simulating the gut lumen and a blood vessel are separated by a membrane coated with extracellular matrix and Caco-2 cells (158). As opposed to cell monolayers and organoids, the gut-on-a-chip is a dynamic model: shear stress and gut peristalsis are mimicked by continuous medium flow and stretching/relaxing of the membrane, respectively. Interestingly, these environmental cues stimulated Caco-2 cells to undergo differentiation into four types of intestinal epithelial cells, organized in 3D villus-like structures (159). Also, the successful incorporation of endothelial cells and peripheral blood mononuclear cells was demonstrated (160). The authors of that study claimed the successful cultivation of a single bacterium “on chip” (Lactobacillus rhamnosus) for more than 1 week (158) and the eight-membered VSL#3 for at least 96 h (160). However, the viability of the probiotic bacteria was based solely on imaging, and which species exactly succeeded in “colonizing” the crypts was not exactly determined. The growth of anaerobic bacteria in this device has not yet been reported.
In contrast, another recent study reported the successful coculture of the strictly anaerobic bacterium B. caccae with L. rhamnosus and Caco-2 cells. In this microfluidic-based model mimicking the human gut, HuMiX, bacteria were grown in a separate, anoxic compartment (161). Similarly, the HMI module allowed the investigation of bacteria for up to 48 h under microaerophilic conditions. Fluorescence in situ hybridization (FISH) analysis revealed the presence of strictly anaerobic bifidobacteria in the upper part of the mucus layer and the positioning of Faecalibacterium prausnitzii at the oxic-anoxic interphase (137). In both the HuMiX and HMI modules, however, a mucin-coated attachment membrane prevented direct or natural contact between host and microbe. Moreover, as opposed to the gut-on-a-chip, gut peristalsis was not mimicked, and the formation of the main epithelial cell types or crypts was not reported in these models (137, 161).
A promising development in gut-on-a-chip technology is the incorporation of 2D organoids, which grow in a plane rather than in clumps, in the chip device (156), combining the advantages of organoids (tissue differentiation) with those of gut-on-a-chip technology (controllable flow, mechanical cues, and tissue-tissue interaction). To date, the cultivation of a defined intestinal microbiota in this device has not yet been reported.
Validation of In Vitro Models
In comparison with animal models, validation of in vitro models is even more challenging. The cellular processes studied in transwells, organoids, or gut-on-a-chip devices cannot be readily validated in human subjects. On the other hand, however, such sophisticated in vitro models enable the investigation of processes that cannot be readily studied in humans, increasing our understanding of the molecular mechanisms of certain bacterial compounds or products. Furthermore, they allow the elimination of potentially confounding factors present in in vivo models, such as the immune system. At the same time, this is also one of the major drawbacks of the above-mentioned in vitro models: as opposed to in vivo models, they lack a systemic component, whereas the impact of the gut microbiota on human health extends beyond the GIT. The emergence of organ-on-a-chip technologies has led to the concept of a “human-on-a-chip” (162), but its implementation in research is still at an early stage. Nevertheless, the road to such a human-on-a-chip may be just as interesting. “Rebuilding” the human body through assembly of its separate parts (lung-on-a-chip, gut-on-a-chip, and kidney-on-a-chip, etc.) might increase our understanding of these building blocks and their contribution to the whole.
CONCLUSIONS AND FUTURE OUTLOOK
Our understanding of the human gut microbiome has rapidly grown over the past decades, which has definitely supported the design of defined communities representative of the human gut microbiome. Whereas defined communities were initially aimed at normalizing germfree hosts to conventionalized mice, they could be a valuable tool to study host-microbe interactions, because of their controllability and traceability. For the same reasons, defined communities have a high potential for therapeutic application. In this review, however, we show that these rationally designed consortia have been applied in in vivo models that are not entirely representative of the human host environment. Next to the obvious and frequently discussed differences between mice and humans, we also discuss that the power of gnotobiotic animals has been further undermined by poor control of the host parameters known to affect gut microbiota composition and behavior.
Simultaneously with the increasing knowledge on the human gut microbiota, the implementation of more-advanced in vitro models of the human gut is accelerating, with the development of stem-cell-derived organoids and gut-on-a-chip approaches. Although the research is still in its infancy, these systems might partially replace the use of animal models. This development is beneficial not only for ethical and, in the long term, financial reasons but also from a scientific perspective. Human-inspired in vitro systems allow us to model and capture host-microbe interactions at a more fundamental and controlled level.
Both the design of defined communities and in vitro models of the gut have not yet reached their plateau. The former can be improved, via either bottom-up or top-down approaches. Key is further expanding our knowledge about the intestinal microbiome in health and disease, in which the NIH Human Microbiome Project and the European MetaHit project have played a crucial role (106, 163) (bottom-up). The characterization of the gut microbiota and genome sequences facilitates the in silico prediction of host-microbe interactions through constraint-based genome-scale metabolic modeling (164) or other types of mathematical modeling (165) and, subsequently, the in silico design of representative defined communities (bottom-up). Further exploring our whole microbiome, including phages, fungi, and archaea, will revolutionize the design of microbial communities as well (bottom-up). Finally, the increased ability to reproducibly culture the microorganisms in human feces in vitro using well-established fermentation technologies (135, 166) may open the avenue to study human feces-derived, functionally enriched defined communities at a more personalized level (top-down). In this way, both health- and disease-related microbiotas can be easily reproduced. The same level of personalization can be obtained on the host side. For instance, the implementation of 2D organoids from patient-derived induced pluripotent stem cells in in vitro systems, such as the gut-on-a-chip, can lead to highly personalized screening devices.
All in all, these models will provide a basis for the rational development and screening of novel therapies targeting intestinal diseases, ranging from anti-, pre-, and probiotics to manipulate existing gut microbiota to therapeutic microbes (167), fecal microbiota transplantation (168), and stool substitutes (77).
ACKNOWLEDGMENTS
This research was partly funded by The Netherlands Organization for Scientific Research (NWO) in the framework of the Building Blocks of Life program (737.016.003), a Gravitation grant (SIAM 024.002.002), and the National Roadmap for Large-Scale Research Facilities (NRGWI.obrug.2018.005).
We declare no relevant conflicting financial interests.
Biographies

Janneke Elzinga is a Ph.D. candidate at the Laboratory of Microbiology in Wageningen, The Netherlands. She obtained a bachelor’s degree in Biomedical Sciences and a master’s degree in Molecular Mechanisms of Disease, both at Radboud University in Nijmegen, The Netherlands. She has been working in the field of microbiology since March 2017, with a particular interest in keystone species of the human intestinal microbiota and in vitro models of the human gut to study host-microbe interactions. The application of these models may facilitate a better understanding of the molecular mechanisms underlying host-microbe interactions, with the potential to develop (personalized) therapeutic strategies.

John van der Oost (1958) has been leader of the Bacterial Genetics group in the Laboratory of Microbiology at Wageningen University since 1995. Initially, research mainly focused on unraveling unique features of central metabolic pathways in bacteria and archaea, revealing many novel enzymes and their regulation. In 2005, he was appointed Full Professor; in 2013, he was elected as an EMBO member; and in 2017, he was elected as a member of the Royal Dutch Academy for Arts and Sciences (KNAW). In the last decade, he used NWO grants (VICI-2005, TOP-2010/2015, and Gravitation-2017) to establish a successful research line on prokaryotic antiviral defense systems (CRISPR-Cas and prokaryotic Argonaute). This has provided an excellent basis for development of unprecedented genome-editing tools that currently find applications in biotechnology and molecular medicine (gene therapy).

Willem M. de Vos studied Biochemistry and obtained a Ph.D. at Groningen University, The Netherlands, partly done at the Max Planck Institute for Molecular Genetics in Berlin, Germany. Subsequently, he spent a postdoc in Reading, United Kingdom, and became Molecular Genetics Group manager at NIZO, Ede, The Netherlands. For over 30 years he has been a Professor at Wageningen University, The Netherlands, where he holds the Chair of Microbiology, and he serves as Professor of Human Microbiomics at the Medical Faculty of the University of Helsinki, Finland, where he chairs the Research Program Human Microbiome. His research aims to understand and exploit microbes using molecular, (meta)genomics, and systems approaches. For a dozen years, his research interest has been focused on the human intestinal tract microbiota and its relation with health and disease.

Hauke Smidt studied Biotechnology at the Technical University of Braunschweig, Germany, and obtained his Ph.D. from Wageningen University. Following a postdoc position at the University of Washington, Seattle, WA, he rejoined the Laboratory of Microbiology at Wageningen University to head the Molecular Microbial Ecology group. In 2008, he was appointed Visiting Professor at Nanjing Agricultural University, and since 2010, he has held a Personal Chair in Complex Microbial Ecosystems at Wageningen University. His research focuses on the integrated application of innovative cultivation and functional genomics-based methods to study the composition and activity of intestinal tract microbiota in humans and farm and model animals, as well as their interaction with their host, in relation to host nutrition and health. Further interest lies in the evolution and spread of antibiotic-resistant bacteria and their genes, following a OneHealth philosophy that links environmental, human, and animal health.
REFERENCES
- 1.Bocci V. 1992. The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect Biol Med 35:251–260. doi: 10.1353/pbm.1992.0004. [DOI] [PubMed] [Google Scholar]
- 2.Lynch SV, Pedersen O. 2016. The human intestinal microbiome in health and disease. N Engl J Med 375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 3.Rajilić-Stojanović M, de Vos WM. 2014. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederberg J. 2000. Infectious history. Science 288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 5.De Roy K, Marzorati M, Van den Abbeele P, Van de Wiele T, Boon N. 2014. Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities. Environ Microbiol 16:1472–1481. doi: 10.1111/1462-2920.12343. [DOI] [PubMed] [Google Scholar]
- 6.Lagier J-C, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, Dubourg G, Durand G, Mourembou G, Guilhot E, Togo A, Bellali S, Bachar D, Cassir N, Bittar F, Delerce J, Mailhe M, Ricaboni D, Bilen M, Dangui Nieko NPM, Dia Badiane NM, Valles C, Mouelhi D, Diop K, Million M, Musso D, Abrahao J, Azhar EI, Bibi F, Yasir M, Diallo A, Sokhna C, Djossou F, Vitton V, Robert C, Rolain JM, La Scola B, Fournier P-E, Levasseur A, Raoult D. 2016. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol 1:16203. doi: 10.1038/nmicrobiol.2016.203. [DOI] [PubMed] [Google Scholar]
- 7.Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD. 2016. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson GR, Trexler PC. 1971. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut 12:230–235. doi: 10.1136/gut.12.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaedler RW, Dubos R, Costello R. 1965. Association of germfree mice with bacteria isolated from normal mice. J Exp Med 122:77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugenholtz F, de Vos WM. 2018. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75:149–160. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TL, Vieira-Silva S, Liston A, Raes J. 2015. How informative is the mouse for human gut microbiota research? Dis Model Mech 8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundberg R, Toft MF, August B, Hansen AK, Hansen CH. 2016. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes 7:68–74. doi: 10.1080/19490976.2015.1127463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trexler PC, Orcutt RP. 1999. Development of gnotobiotics and contamination control in laboratory animal science, p 121–128. In American Association for Laboratory Animal Science (ed), 50 years of laboratory animal science. American Association for Laboratory Animal Science, Memphis, TN. [Google Scholar]
- 14.Brand MW, Wannemuehler MJ, Phillips GJ, Proctor A, Overstreet AM, Jergens AE, Orcutt RP, Fox JG. 2015. The altered Schaedler flora: continued applications of a defined murine microbial community. ILAR J 56:169–178. doi: 10.1093/ilar/ilv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB, Simmons HC. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest 98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. 1997. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun 65:3126–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natividad JMM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD, Verdu EF. 2012. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1−/−;Nod2−/− mice. Inflamm Bowel Dis 18:1434–1446. doi: 10.1002/ibd.22848. [DOI] [PubMed] [Google Scholar]
- 18.Wen L, Ley RE, Volchkov PV, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. 2008. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, Godfrey V, Heise MT, Threadgill DS, Han A, Swenberg JA, Threadgill DW, Bultman SJ. 2014. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov 4:1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Ellis ML, Dowell AE, Kumar R, Morrow CD, Schoeb TR, Knight J. 2016. Response of germfree mice to colonization by Oxalobacter formigenes and altered Schaedler flora. Appl Environ Microbiol 82:6952–6960. doi: 10.1128/AEM.02381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hapfelmeier S, Lawson MAE, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R III, McCoy KD, Macpherson AJ. 2010. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brugiroux S, Beutler M, Pfann C, Garzetti D, Ruscheweyh HJ, Ring D, Diehl M, Herp S, Lötscher Y, Hussain S, Bunk B, Pukall R, Huson DH, Münch PC, McHardy AC, McCoy KD, MacPherson AJ, Loy A, Clavel T, Berry D, Stecher B. 2016. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat Microbiol 2:16215. doi: 10.1038/nmicrobiol.2016.215. [DOI] [PubMed] [Google Scholar]
- 23.Norin E, Midtvedt T. 2010. Intestinal microflora functions in laboratory mice claimed to harbor a “normal” intestinal microflora. Is the SPF concept running out of date? Anaerobe 16:311–313. doi: 10.1016/j.anaerobe.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Lagkouvardos I, Pukall R, Abt B, Foesel BU, Meier-Kolthoff JP, Kumar N, Bresciani A, Martinez I, Just S, Ziegler C, Brugiroux S, Garzetti D, Wenning M, Bui TP, Wang J, Hugenholtz F, Plugge CM, Peterson DA, Hornef MW, Baines JF, Smidt H, Walter J, Kristiansen K, Nielsen HB, Haller D, Overmann J, Stecher B, Clavel T. 2016. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol 1:16131. doi: 10.1038/nmicrobiol.2016.131. [DOI] [PubMed] [Google Scholar]
- 25.Studer N, Desharnais L, Beutler M, Brugiroux S, Terrazos MA, Menin L, Schürch CM, McCoy KD, Kuehne SA, Minton NP, Stecher B, Bernier-Latmani R, Hapfelmeier S. 2016. Functional intestinal bile acid 7α-dehydroxylation by Clostridium scindens associated with protection from Clostridium difficile infection in a gnotobiotic mouse model. Front Cell Infect Microbiol 6:191. doi: 10.3389/fcimb.2016.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, Stolp B, Stein JV, Stecher B, Sauer U, McCoy KD, Macpherson AJ. 2015. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun 6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker N, Kunath J, Loh G, Blaut M. 2011. Human intestinal microbiota: characterization of a simplified and stable gnotobiotic rat model. Gut Microbes 2:25–33. doi: 10.4161/gmic.2.1.14651. [DOI] [PubMed] [Google Scholar]
- 28.Ganesh BP, Klopfleisch R, Loh G, Blaut M. 2013. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One 8:e74963. doi: 10.1371/journal.pone.0074963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ring C, Klopfleisch R, Dahlke K, Basic M, Bleich A, Blaut M. 25 September 2018. Akkermansia muciniphila strain ATCC BAA-835 does not promote short-term intestinal inflammation in gnotobiotic interleukin-10-deficient mice. Gut Microbes doi: 10.1080/19490976.2018.1511663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slezak K, Hanske L, Loh G, Blaut M. 2013. Increased bacterial putrescine has no impact on gut morphology and physiology in gnotobiotic adolescent mice. Benef Microbes 4:253–266. doi: 10.3920/BM2012.0047. [DOI] [PubMed] [Google Scholar]
- 31.Slezak K, Krupova Z, Rabot S, Loh G, Levenez F, Descamps A, Lepage P, Doré J, Bellier S, Blaut M. 2014. Association of germ-free mice with a simplified human intestinal microbiota results in a shortened intestine. Gut Microbes 5:176–182. doi: 10.4161/gmic.28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woting A, Pfeiffer N, Loh G, Klaus S, Blaut M. 2014. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. mBio 5:e01530-14. doi: 10.1128/mBio.01530-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syed SA, Abrams GD, Freter R. 1970. Efficiency of various intestinal bacteria in assuming normal functions of enteric flora after association with germ-free mice. Infect Immun 2:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freter R, Abrams GD. 1972. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun 6:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freter R, Freter RR, Brickner H. 1983. Experimental and mathematical models of Escherichia coli plasmid transfer in vitro and in vivo. Infect Immun 39:60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells CL, Sugiyama H, Bland SE. 1982. Resistance of mice with limited intestinal flora to enteric colonization by Clostridium botulinum. J Infect Dis 146:791–796. doi: 10.1093/infdis/146.6.791. [DOI] [PubMed] [Google Scholar]
- 37.Helstrom PB, Balish E. 1979. Effect of oral tetracycline, the microbial flora, and the athymic state on gastrointestinal colonization and infection of BALB/c mice with Candida albicans. Infect Immun 23:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreau MC, Ducluzeau R, Guy-Grand D, Muller MC. 1978. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun 21:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezzonico E, Mestdagh R, Delley M, Combremont S, Dumas ME, Holmes E, Nicholson J, Bibiloni R. 2011. Bacterial adaptation to the gut environment favors successful colonization: microbial and metabonomic characterization of a simplified microbiota mouse model. Gut Microbes 2:307–318. doi: 10.4161/gmic.18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, Chervaux C, Knights D, Lozupone CA, Knight R, Duncan AE, Bain JR, Muehlbauer MJ, Newgard CB, Heath AC, Gordon JI. 2011. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med 3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faith JJ, McNulty NP, Rey FE, Gordon JI. 2011. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science 333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. 2013. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci U S A 110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EC. 2016. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339.e21–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. 2009. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes A, Wu M, McNulty NP, Rohwer FL, Gordon JI. 2013. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc Natl Acad Sci U S A 110:20236–20241. doi: 10.1073/pnas.1319470110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNulty NP, Wu M, Erickson AR, Pan C, Erickson BK, Martens EC, Pudlo NA, Muegge BD, Henrissat B, Hettich RL, Gordon JI. 2013. Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol 11:e1001637. doi: 10.1371/journal.pbio.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, McNulty NP, Rodionov DA, Khoroshkin MS, Griffin W, Cheng J, Latreille P, Kerstetter RA, Terrapon N, Henrissat B, Osterman AL, Gordon JI. 2015. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350:aac5992. doi: 10.1126/science.aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mark Welch JL, Hasegawa Y, McNulty NP, Gordon JI, Borisy GG. 2017. Spatial organization of a model 15-member human gut microbiota established in gnotobiotic mice. Proc Natl Acad Sci U S A 114:E9105–E9114. doi: 10.1073/pnas.1711596114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugahara H, Odamaki T, Fukuda S, Kato T, Xiao JZ, Abe F, Kikuchi J, Ohno H. 2015. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep 5:13548. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim B, Zimmermann M, Barry NA, Goodman AL. 2017. Engineered regulatory systems modulate gene expression of human commensals in the gut. Cell 169:547.e15–558.e15. doi: 10.1016/j.cell.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW, Barratt MJ, Giannone RJ, Hettich RL, Osterman AL, Gordon JI. 2017. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med 22:eaal4069. doi: 10.1126/scitranslmed.aal4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laycock G, Sait L, Inman C, Lewis M, Smidt H, van Diemen P, Jorgensen F, Stevens M, Bailey M. 2012. A defined intestinal colonization microbiota for gnotobiotic pigs. Vet Immunol Immunopathol 149:216–224. doi: 10.1016/j.vetimm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen TV, Yuan L, Azevedo MSP, Jeong K, Gonzalez A-M, Saif LJ. 2007. Transfer of maternal cytokines to suckling piglets: in vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet Immunol Immunopathol 117:236–248. doi: 10.1016/j.vetimm.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butler JE, Sinkora M. 2007. The isolator piglet: a model for studying the development of adaptive immunity. Immunol Res 39:33–51. doi: 10.1007/s12026-007-0062-7. [DOI] [PubMed] [Google Scholar]
- 55.Rothkotter HJ, Ulbrich H, Pabst R. 1991. The postnatal development of gut lamina propria lymphocytes: number, proliferation, and T and B cell subsets in conventional and germ-free pigs. Pediatr Res 29:237–242. doi: 10.1203/00006450-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Wernersson R, Schierup MH, Jørgensen FG, Gorodkin J, Panitz F, Stærfeldt H-H, Christensen OF, Mailund T, Hornshøj H, Klein A, Wang J, Liu B, Hu S, Dong W, Li W, Wong GKS, Yu J, Wang J, Bendixen C, Fredholm M, Brunak S, Yang H, Bolund L. 2005. Pigs in sequence space: a 0.66× coverage pig genome survey based on shotgun sequencing. BMC Genomics 6:70. doi: 10.1186/1471-2164-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez LM, Moeser AJ, Blikslager AT. 2015. Porcine models of digestive disease: the future of large animal translational research. Transl Res 166:12–27. doi: 10.1016/j.trsl.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jansman AJM, Zhang J, Koopmans SJ, Dekker RA, Smidt H. 2012. Effects of a simple or a complex starter microbiota on intestinal microbiota composition in caesarean derived piglets. J Anim Sci 90(Suppl 4):433–435. doi: 10.2527/jas.53850. [DOI] [PubMed] [Google Scholar]
- 59.Priori D, Colombo M, Clavenzani P, Jansman AJ, Lalles JP, Trevisi P, Bosi P. 2015. The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, co-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS One 10:e0129501. doi: 10.1371/journal.pone.0129501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trevisi P, Priori D, Motta V, Luise D, Jansman AJM, Koopmans SJ, Bosi P. 2017. The effects of starter microbiota and the early life feeding of medium chain triglycerides on the gastric transcriptome profile of 2- or 3-week-old cesarean delivered piglets. J Anim Sci Biotechnol 8:82. doi: 10.1186/s40104-017-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butler JE, Sun J, Weber P, Navarro P, Francis D. 2000. Antibody repertoire development in fetal and newborn piglets. III. Colonization of the gastrointestinal tract selectively diversifies the preimmune repertoire in mucosal lymphoid tissues. Immunology 100:119–130. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paim FC, Langel SN, Fischer DD, Kandasamy S, Shao L, Alhamo MA, Huang HC, Kumar A, Rajashekara G, Saif LJ, Vlasova AN. 2016. Effects of Escherichia coli Nissle 1917 and ciprofloxacin on small intestinal epithelial cell mRNA expression in the neonatal piglet model of human rotavirus infection. Gut Pathog 8:66. doi: 10.1186/s13099-016-0148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang HC, Vlasova AN, Kumar A, Kandasamy S, Fischer DD, Deblais L, Paim FC, Langel SN, Alhamo MA, Rauf A, Shao L, Saif LJ, Rajashekara G. 2018. Effect of antibiotic, probiotic, and human rotavirus infection on colonisation dynamics of defined commensal microbiota in a gnotobiotic pig model. Benef Microbes 9:71–86. doi: 10.3920/BM2016.0225. [DOI] [PubMed] [Google Scholar]
- 64.Eun CS, Mishima Y, Wohlgemuth S, Liu B, Bower M, Carroll IM, Sartor RB. 2014. Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10−/− mice. Infect Immun 82:2239–2246. doi: 10.1128/IAI.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 66.Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. 2003. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol 133:38–43. doi: 10.1046/j.1365-2249.2003.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66:5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin FPJ, Dumas ME, Wang Y, Legido-Quigley C, Yap IKS, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC, Sprenger N, Fay LB, Kochhar S, Van Bladeren P, Holmes E, Nicholson JK. 2007. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol 3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin FPJ, Wang Y, Sprenger N, Yap IKS, Lundstedt T, Lek P, Rezzi S, Ramadan Z, Van Bladeren P, Fay LB, Kochhar S, Lindon JC, Holmes E, Nicholson JK. 2008. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol 4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin F-PJ, Sprenger N, Yap IKS, Wang Y, Bibiloni R, Rochat F, Rezzi S, Cherbut C, Kochhar S, Lindon JC, Holmes E, Nicholson JK. 2009. Panorganismal gut microbiome-host metabolic crosstalk. J Proteome Res 8:2090–2105. doi: 10.1021/pr801068x. [DOI] [PubMed] [Google Scholar]
- 71.Luk B, Veeraragavan S, Engevik M, Balderas M, Major A, Runge J, Luna RA, Versalovic J. 2018. Postnatal colonization with human “infant-type” Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS One 13:e0196510. doi: 10.1371/journal.pone.0196510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caballero-Franco C, Keller K, Simone C, Chadee K. 2007. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292:G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 73.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. 2005. A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 74.Douillard FP, Mora D, Eijlander RT, Wels M, de Vos WM. 2018. Comparative genomic analysis of the multispecies probiotic-marketed product VSL#3. PLoS One 13:e0192452. doi: 10.1371/journal.pone.0192452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tvede M, Rask-Madsen J. 1989. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 333:1156–1160. doi: 10.1016/S0140-6736(89)92749-9. [DOI] [PubMed] [Google Scholar]
- 76.Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C, Deakin LJ, Pickard DJ, Duncan SH, Flint HJ, Clark TG, Parkhill J, Dougan G. 2012. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog 8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. 2013. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘rePOOPulating’ the gut. Microbiome 1:3. doi: 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 79.Hsiao A, Ahmed AMS, Subramanian S, Griffin NW, Drewry LL, Petri WA Jr, Haque R, Ahmed T, Gordon JI. 2014. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 515:423–426. doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Vos WM. 2013. Fame and future of faecal transplantations—developing next-generation therapies with synthetic microbiomes. Microb Biotechnol 6:316–325. doi: 10.1111/1751-7915.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Natividad JM, Pinto-Sanchez MI, Galipeau HJ, Jury J, Jordana M, Reinisch W, Collins SM, Bercik P, Surette MG, Allen-Vercoe E, Verdu EF. 2015. Ecobiotherapy rich in Firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm Bowel Dis 21:1883–1893. doi: 10.1097/MIB.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 82.Norin E. 2015. Experience with cultivated microbiota transplant: ongoing treatment of Clostridium difficile patients in Sweden. Microb Ecol Health Dis 26:27638. doi: 10.3402/mehd.v26.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. 2014. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6:220ra11. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sonnenburg JL, Chen CTL, Gordon JI. 2006. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol 4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, Cantarel BL, Coutinho PM, Henrissat B, Crock LW, Russell A, Verberkmoes NC, Hettich RL, Gordon JI. 2009. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A 106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. 2013. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J 7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krych L, Hansen CHF, Hansen AK, van den Berg FWJ, Nielsen DS. 2013. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS One 8:e62578. doi: 10.1371/journal.pone.0062578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD, MetaHIT Consortium, Bork P, Wang J. 2014. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 89.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’Hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. 2016. Population-level analysis of gut microbiome variation. Science 352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 90.Wilson KH, Brown RS, Andersen GL, Tsang J, Sartor B. 2006. Comparison of fecal biota from specific pathogen free and feral mice. Anaerobe 12:249–253. doi: 10.1016/j.anaerobe.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. 2005. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. 2012. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. 2013. The long-term stability of the human gut microbiota. Science 341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarma-Rupavtarm RB, Ge Z, Schauer DB, Fox JG, Polz MF. 2004. Spatial distribution and stability of the eight microbial species of the altered Schaedler flora in the mouse gastrointestinal tract. Appl Environ Microbiol 70:2791–2800. doi: 10.1128/AEM.70.5.2791-2800.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freter R, Brickner H, Botney M, Cleven D, Aranki A. 1983. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun 39:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, Von Mering C, Macpherson AJ, Hardt WD. 2010. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog 6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pereira FC, Berry D. 2017. Microbial nutrient niches in the gut. Environ Microbiol 19:1366–1378. doi: 10.1111/1462-2920.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donaldson GP, Lee SM, Mazmanian SK. 2016. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clavel T, Gomes-Neto JC, Lagkouvardos I, Ramer-Tait AE. 2017. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol Rev 279:8–22. doi: 10.1111/imr.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shetty SA, Hugenholtz F, Lahti L, Smidt H, de Vos WM. 2017. Intestinal microbiome landscaping: insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol Rev 41:182–199. doi: 10.1093/femsre/fuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jalanka-Tuovinen J, Salonen A, Nikkila J, Immonen O, Kekkonen R, Lahti L, Palva A, de Vos WM. 2011. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One 6:e23035. doi: 10.1371/journal.pone.0023035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salonen A, Salojarvi J, Lahti L, de Vos WM. 2012. The adult intestinal core microbiota is determined by analysis depth and health status. Clin Microbiol Infect 18:16–20. doi: 10.1111/j.1469-0691.2012.03855.x. [DOI] [PubMed] [Google Scholar]
- 104.Ze X, Le Mougen F, Duncan SH, Louis P, Flint HJ. 2013. Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 4:236–240. doi: 10.4161/gmic.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trosvik P, de Muinck EJ. 2015. Ecology of bacteria in the human gastrointestinal tract—identification of keystone and foundation taxa. Microbiome 3:44. doi: 10.1186/s40168-015-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, et al. . 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolmeder CA, de Been M, Nikkilä J, Ritamo I, Mättö J, Valmu L, Salojärvi J, Palva A, Salonen A, de Vos WM. 2012. Comparative metaproteomics and diversity analysis of human intestinal microbiota testifies for its temporal stability and expression of core functions. PLoS One 7:e29913. doi: 10.1371/journal.pone.0029913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nielsen E, Friis CW. 1980. Influence of an intestinal microflora on the development of the immunoglobulins IgG1, IgG2a, IgM and IgA in germ-free BALB/c mice. Acta Pathol Microbiol Scand C 88:121–126. [DOI] [PubMed] [Google Scholar]
- 109.Samuel BS, Gordon JI. 2006. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A 103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manrique P, Bolduc B, Walk ST, van der Oost J, de Vos WM, Young MJ. 2016. Healthy human gut phageome. Proc Natl Acad Sci U S A 113:10400–10405. doi: 10.1073/pnas.1601060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salonen A, de Vos WM. 2014. Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol 5:239–262. doi: 10.1146/annurev-food-030212-182554. [DOI] [PubMed] [Google Scholar]
- 112.Zoetendal EG, de Vos WM. 2014. Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol 30:189–195. doi: 10.1097/MOG.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 113.Clavel T, Desmarchelier C, Haller D, Gérard P, Rohn S, Lepage P, Daniel H. 2014. Intestinal microbiota in metabolic diseases. Gut Microbes 5:544–551. doi: 10.4161/gmic.29331. [DOI] [PubMed] [Google Scholar]
- 114.Esworthy RS, Smith DD, Chu F-F. 2010. A strong impact of genetic background on gut microflora in mice. Int J Inflam 2010:986046. doi: 10.4061/2010/986046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gulati AS, Shanahan MT, Arthur JC, Grossniklaus E, von Furstenberg RJ, Kreuk L, Henning SJ, Jobin C, Sartor RB. 2012. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS One 7:e32403. doi: 10.1371/journal.pone.0032403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hildebrand F, Nguyen TLA, Brinkman B, Yunta RG, Cauwe B, Vandenabeele P, Liston A, Raes J. 2013. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol 14:R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kovacs A, Ben-Jacob N, Tayem H, Halperin E, Iraqi FA, Gophna U. 2011. Genotype is a stronger determinant than sex of the mouse gut microbiota. Microb Ecol 61:423–428. doi: 10.1007/s00248-010-9787-2. [DOI] [PubMed] [Google Scholar]
- 118.Toivanen P, Vaahtovuo J, Eerola E. 2001. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect Immun 69:2372–2377. doi: 10.1128/IAI.69.4.2372-2377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deloris Alexander A, Orcutt RP, Henry JC, Baker J Jr, Bissahoyo AC, Threadgill DW. 2006. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome 17:1093–1104. doi: 10.1007/s00335-006-0063-1. [DOI] [PubMed] [Google Scholar]
- 120.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lay C, Rigottier-Gois L, Holmstrøm K, Rajilic M, Vaughan EE, de Vos WM, Collins MD, Thiel R, Namsolleck P, Blaut M, Doré J. 2005. Colonic microbiota signatures across five northern European countries. Appl Environ Microbiol 71:4153–4155. doi: 10.1128/AEM.71.7.4153-4155.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Zhang M, Wei H, Chen Y, Lu H, Zuo J, Su M, Qiu Y, Jia W, Xiao C, Smith LM, Yang S, Holmes E, Tang H, Zhao G, Nicholson JK, Li L, Zhao L. 2008. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A 105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft H-JF, Dore J, Blaut M. 2006. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shastri P, McCarville J, Kalmokoff M, Brooks SPJ, Green-Johnson JM. 2015. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biol Sex Differ 6:13. doi: 10.1186/s13293-015-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 126.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity 39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. 2016. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hansen CHF, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, Tlaskalova-Hogenova H, Hansen AK. 2012. Patterns of early gut colonization shape future immune responses of the host. PLoS One 7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. 2012. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Aidy S, Hooiveld G, Tremaroli V, Backhed F, Kleerebezem M. 2013. The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microbes 4:118–124. doi: 10.4161/gmic.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.El Aidy S, Kunze W, Bienenstock J, Kleerebezem M. 2012. The microbiota and the gut-brain axis: insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Benef Microbes 3:251–259. doi: 10.3920/BM2012.0042. [DOI] [PubMed] [Google Scholar]
- 132.Venema K, van den Abbeele P. 2013. Experimental models of the gut microbiome. Best Pract Res Clin Gastroenterol 27:115–126. doi: 10.1016/j.bpg.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 133.von Martels JZH, Sadaghian Sadabad M, Bourgonje AR, Blokzijl T, Dijkstra G, Faber KN, Harmsen HJM. 2017. The role of gut microbiota in health and disease: in vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 44:3–12. doi: 10.1016/j.anaerobe.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Payne AN, Zihler A, Chassard C, Lacroix C. 2012. Advances and perspectives in in vitro human gut fermentation modeling. Trends Biotechnol 30:17–25. doi: 10.1016/j.tibtech.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 135.McDonald JAK, Schroeter K, Fuentes S, Heikamp-deJong I, Khursigara CM, de Vos WM, Allen-Vercoe E. 2013. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J Microbiol Methods 95:167–174. doi: 10.1016/j.mimet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 136.Van den Abbeele P, Grootaert C, Marzorati M, Possemiers S, Verstraete W, Gerard P, Rabot S, Bruneau A, El Aidy S, Derrien M, Zoetendal E, Kleerebezem M, Smidt H, Van de Wiele T. 2010. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl Environ Microbiol 76:5237–5246. doi: 10.1128/AEM.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marzorati M, Vanhoecke B, De Ryck T, Sadaghian Sadabad M, Pinheiro I, Possemiers S, Van den Abbeele P, Derycke L, Bracke M, Pieters J, Hennebel T, Harmsen HJ, Verstraete W, Van de Wiele T. 2014. The HMI module: a new tool to study the host-microbiota interaction in the human gastrointestinal tract in vitro. BMC Microbiol 14:133. doi: 10.1186/1471-2180-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krishnan M, Penrose HM, Shah NN, Marchelletta RR, McCole DF. 2016. VSL#3 probiotic stimulates T-cell protein tyrosine phosphatase-mediated recovery of IFN-γ-induced intestinal epithelial barrier defects. Inflamm Bowel Dis 22:2811–2823. doi: 10.1097/MIB.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martz SL, Guzman-Rodriguez M, He SM, Noordhof C, Hurlbut DJ, Gloor GB, Carlucci C, Weese S, Allen-Vercoe E, Sun J, Claud EC, Petrof EO. 2017. A human gut ecosystem protects against C. difficile disease by targeting TcdA. J Gastroenterol 52:452–465. doi: 10.1007/s00535-016-1232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Munoz S, Guzman-Rodriguez M, Sun J, Zhang YG, Noordhof C, He SM, Allen-Vercoe E, Claud EC, Petrof EO. 2016. Rebooting the microbiome. Gut Microbes 7:353–363. doi: 10.1080/19490976.2016.1188248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cinque B, La Torre C, Lombardi F, Palumbo P, Evtoski Z, Santini S, Falone S, Cimini A, Amicarelli F, Cifone MG. 2017. VSL#3 probiotic differently influences IEC-6 intestinal epithelial cell status and function. J Cell Physiol 232:3530–3539. doi: 10.1002/jcp.25814. [DOI] [PubMed] [Google Scholar]
- 142.Cinque B, La Torre C, Lombardi F, Palumbo P, Van Der Rest M, Cifone MG. 2016. Production conditions affect the in vitro anti-tumoral effects of a high concentration multi-strain probiotic preparation. PLoS One 11:e0163216. doi: 10.1371/journal.pone.0163216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. 2010. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A 107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trinchieri V, Laghi L, Vitali B, Parolin C, Giusti I, Capobianco D, Mastromarino P, De Simone C. 2017. Efficacy and safety of a multistrain probiotic formulation depends from manufacturing. Front Immunol 8:1474. doi: 10.3389/fimmu.2017.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mastrangeli G, Corinti S, Butteroni C, Afferni C, Bonura A, Boirivant M, Colombo P, Di Felice G. 2009. Effects of live and inactivated VSL#3 probiotic preparations in the modulation of in vitro and in vivo allergen-induced Th2 responses. Int Arch Allergy Immunol 150:133–143. doi: 10.1159/000218116. [DOI] [PubMed] [Google Scholar]
- 146.Mariman R, Tielen F, Koning F, Nagelkerken L. 2014. The probiotic mixture VSL#3 dampens LPS-induced chemokine expression in human dendritic cells by inhibition of STAT-1 phosphorylation. PLoS One 9:e0115676. doi: 10.1371/journal.pone.0115676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parlesak A, Haller D, Brinz S, Baeuerlein A, Bode C. 2004. Modulation of cytokine release by differentiated Caco-2 cells in a compartmentalized coculture model with mononuclear leucocytes and nonpathogenic bacteria. Scand J Immunol 60:477–485. doi: 10.1111/j.0300-9475.2004.01495.x. [DOI] [PubMed] [Google Scholar]
- 148.Ulluwishewa D, Anderson RC, Young W, McNabb WC, van Baarlen P, Moughan PJ, Wells JM, Roy NC. 2015. Live Faecalibacterium prausnitzii in an apical anaerobic model of the intestinal epithelial barrier. Cell Microbiol 17:226–240. doi: 10.1111/cmi.12360. [DOI] [PubMed] [Google Scholar]
- 149.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. 2009. Single Lgr5 stem cells build crypt villus structures in vitro without a mesenchymal niche. Nature 459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 150.Spence JR, Mayhew CN, Rankin SA, Kuhar M, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bartfeld S. 2016. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev Biol 420:262–270. doi: 10.1016/j.ydbio.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 152.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. 2015. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun 83:138–145. doi: 10.1128/IAI.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yissachar N, Zhou Y, Ung L, Lai NY, Mohan JF, Ehrlicher A, Weitz DA, Kasper DL, Chiu IM, Mathis D, Benoist C. 2017. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 168:1135.e12–1148.e12. doi: 10.1016/j.cell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. 2012. Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 155.Walsh DI III, Dydek EV, Lock JY, Carlson TL, Carrier RL, Kong DS, Cabrera CR, Thorsen T. 2018. Emulation of colonic oxygen gradients in a microdevice. SLAS Technol 23:164–171. doi: 10.1177/2472630317743425. [DOI] [PubMed] [Google Scholar]
- 156.Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zhang C, Rickner H, Richmond CA, Li H, Breault DT, Ingber DE. 2018. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 8:2871. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Villenave R, Wales SQ, Hamkins-Indik T, Papafragkou E, Weaver JC, Ferrante TC, Bahinski A, Elkins CA, Kulka M, Ingber DE. 2017. Human gut-on-a-chip supports polarized infection of coxsackie B1 virus in vitro. PLoS One 12:e0169412. doi: 10.1371/journal.pone.0169412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim HJ, Huh D, Hamilton G, Ingber DE. 2012. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 159.Kim HJ, Ingber DE. 2013. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb) 5:1130–1140. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 160.Kim HJ, Li H, Collins JJ, Ingber DE. 2016. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shah P, Fritz JV, Glaab E, Desai MS, Greenhalgh K, Frachet A, Niegowska M, Estes M, Jäger C, Seguin-Devaux C, Zenhausern F, Wilmes P. 2016. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat Commun 7:11535. doi: 10.1038/ncomms11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marx U, Walles H, Hoffmann S, Lindner G, Horland R, Sonntag F, Klotzbach U, Sakharov D, Tonevitsky A, Lauster R. 2012. ‘Human-on-a-chip’ developments: a translational cutting-edge alternative to systemic safety assessment and efficiency evaluation of substances in laboratory animals and man? Altern Lab Anim 40:235–257. [DOI] [PubMed] [Google Scholar]
- 163.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. 2009. The NIH Human Microbiome Project. Genome Res 19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van der Ark KCH, van Heck RGA, Martins Dos Santos VAP, Belzer C, de Vos WM. 2017. More than just a gut feeling: constraint-based genome-scale metabolic models for predicting functions of human intestinal microbes. Microbiome 5:78. doi: 10.1186/s40168-017-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bucci V, Xavier JB. 2014. Towards predictive models of the human gut microbiome. J Mol Biol 426:3907–3916. doi: 10.1016/j.jmb.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Macfarlane GT, Macfarlane S. 2007. Models for intestinal fermentation: association between food components, delivery systems, bioavailability and functional interactions in the gut. Curr Opin Biotechnol 18:156–162. doi: 10.1016/j.copbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 167.Cani PD, de Vos WM. 2017. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol 8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fuentes S, de Vos WM. 2016. How to manipulate the microbiota: fecal microbiota transplantation. Adv Exp Med Biol 902:143–153. doi: 10.1007/978-3-319-31248-4_10. [DOI] [PubMed] [Google Scholar]
- 169.Doolittle DJ, Butterworth BE, Sherrill JM. 1983. Influence of intestinal bacteria, sex of the animal, and position of the nitro group on the hepatic genotoxicity of nitrotoluene isomers in vivo. Cancer Res 43:2836–2842. [PubMed] [Google Scholar]
- 170.Jergens AE, Dorn A, Wilson J, Dingbaum K, Henderson A, Liu Z, Hostetter J, Evans RB, Wannemuehler MJ. 2006. Induction of differential immune reactivity to members of the flora of gnotobiotic mice following colonization with Helicobacter bilis or Brachyspira hyodysenteriae. Microbes Infect 8:1602–1610. doi: 10.1016/j.micinf.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 171.Jergens AE, Wilson-Welder JH, Dorn A, Henderson A, Liu Z, Evans RB, Hostetter J, Wannemuehler MJ. 2007. Helicobacter bilis triggers persistent immune reactivity to antigens derived from the commensal bacteria in gnotobiotic C3H/HeN mice. Gut 56:934–940. doi: 10.1136/gut.2006.099242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ivanov II, Frutos RDL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. 2009. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A 106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. 2010. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med 207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Geuking MB, Cahenzli J, Lawson MAE, Ng DCK, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. 2011. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 176.Natividad JMM, Hayes CL, Motta J-P, Jury J, Galipeau HJ, Philip V, Garcia-Rodenas CL, Kiyama H, Bercik P, Verdu EF. 2013. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol 79:7745–7754. doi: 10.1128/AEM.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mosconi I, Geuking MB, Zaiss MM, Massacand JC, Aschwanden C, Kwong Chung CKC, McCoy KD, Harris NL. 2013. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol 6:1157–1167. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 178.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. 2014. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil 26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 179.Peppercorn MA, Goldman P. 1972. Caffeic acid metabolism by gnotobiotic rats and their intestinal bacteria. Proc Natl Acad Sci U S A 69:1413–1415. doi: 10.1073/pnas.69.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ducluzeau R, Ladire M, Callut C, Raibaud P, Abrams GD. 1977. Antagonistic effect of extremely oxygen-sensitive clostridia from the microflora of conventional mice and of Escherichia coli against Shigella flexneri in the digestive tract of gnotobiotic mice. Infect Immun 17:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Šinkorová Z, Čapková J, Niederlová J, Štěpánková R, Šinkora J. 2008. Commensal intestinal bacterial strains trigger ankylosing enthesopathy of the ankle in inbred B10.BR (H-2k) male mice. Hum Immunol 69:845–850. doi: 10.1016/j.humimm.2008.08.296. [DOI] [PubMed] [Google Scholar]
- 182.Wrzosek L, Miquel S, Noordine M-L, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. 2013. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]