Abstract
Background
The benefits of statins in the prevention of primary and secondary atherosclerotic cardiovascular (CV) disease events have been well documented. Suboptimal adherence is a persistent problem associated with increased CV events and increased healthcare utilization. Proportion of days covered (PDC) is widely used to measure medication adherence, and provides a single value that does not adequately depict different adherence behavior patterns. Group-based trajectory modeling has been used to identify adherence patterns (or trajectories) over time. The identification of characteristics unique to each pattern can help in the early identification of patients who are likely to be poor adherents and can inform the development of interventions.
Objectives
To identify distinct trajectories of statin adherence in patients enrolled in a Medicare Advantage plan and the sociodemographic and clinical predictors associated with each trajectory.
Methods
Patients were included in the study if they were continuously enrolled in a Medicare Advantage plan between 2013 and 2016 and had a statin prescription between January 2015 and June 2015. We observed each patient for 360 days and computed the monthly PDC. The monthly PDC was incorporated into a group-based trajectory model to provide distinct patterns of adherence. Using group-based trajectory modeling, the patients were categorized into groups based on their adherence patterns. Multinomial logistic regression was performed to identify the sociodemographic and clinical factors associated with each group.
Results
A total of 7850 patients were included in the analysis and were categorized into 4 distinct groups based on statin adherence—rapid discontinuation (7.8%), gradual decline (16.8%), gaps in adherence (17.2%), and high or nearly perfect adherence (58.2%). Significant predictors of being placed into one or more of the low-adherence trajectories compared with the high-adherence trajectory included sex, age, low-income subsidy, language, Charlson Comorbidity Index score, statin intensity, and 90-day refills.
Conclusions
The predictors identified in this study provide valuable insight into patient characteristics that increase the risk for statin nonadherence, which has the potential to inform targeted interventions. Identifying patient trajectories can inform the future development of protocols to individualize appropriate interventions for these patients.
Keywords: adherence, cardiovascular disease, elderly patients, nonadherence, predictors of statin adherence, statin therapy, trajectory modeling
Medication adherence is defined as the extent to which a patient's medication-taking behavior corresponds with the agreed recommendations from the healthcare provider.1 The medical literature reports varying levels of medication adherence among patients, which ranges widely from 20% to more than 90%, depending on factors such as disease type, duration of adherence measurement, medication regimen complexity, and patient age.2–9 Poor medication adherence has been shown to result in suboptimal outcomes, including disease progression, increased costs, and subpar benefit from medications, which can result in long-term complications.2–9 In the United States, 33% to 69% of all medication-related hospital admissions can be attributed to poor medication adherence, resulting in an estimated cost of $100 billion annually.5 Given the impact of nonadherence on healthcare outcomes and costs, coupled with an increasing prevalence of chronic diseases, an understanding of the patient characteristics that increase the risk for medication nonadherence has major public health implications.10
KEY POINTS
-
▸
Suboptimal adherence to statin therapy is associated with increased cardiovascular events and excessive healthcare utilization.
-
▸
Variations in the patterns of patient adherence can influence patient prognosis and should be considered when designing medication adherence interventions.
-
▸
This retrospective study used group-based trajectory modeling to identify patterns of adherence to statin therapy and the predictors associated with each pattern among older adults.
-
▸
A total of 7850 patients in a Medicare Advantage plan were grouped into 4 adherence cohorts—rapid discontinuation, gradual decline, gaps in adherence, and high/nearly perfect adherence.
-
▸
Women were more likely to be in the low-adherence group than in the high-adherence trajectory as were those receiving low-income subsidy.
-
▸
Patients aged 71 to 75 years were more likely than younger or older patients to be in the perfect-adherence group, as were patients with a 90-day refill.
-
▸
The identified predictors characterize traits related to adherence and nonadherence, which can guide the development of interventions to improve patient adherence.
Cardiovascular disease (CVD) is the leading cause of death in the United States and increases the disease burden, especially in adults aged >65 years.11,12 Of the 85.6 million American adults who were estimated to have CVD between 2009 and 2012, 43.7 million were estimated to be aged ≥60 years.13 However, because of high medication complexity, multiple illnesses, and an increased risk for side effects and cognitive decline in older adults, adherence is a specific concern in this population.8,14
Statins are the most frequently prescribed lipid-lowering agents, with extensive clinical trial data demonstrating their benefits in the primary prevention of CVD, lowering the rate of subsequent events in secondary prevention and in the reduction of cardiovascular mortality.15–17 The benefits of statins in reducing mortality and cardiovascular events are also seen in older adults.18
Despite the known benefits of statin therapy, studies report high discontinuation rates for these drugs, with approximately 50% of patients discontinuing statin treatment within 1 year and more patients doing so over time.19–22 The most frequently reported reasons for poor adherence to or discontinuation of statin treatment include adverse effects, the perception that treatment is unnecessary, and cost-related factors.23
The medication proportion of days covered (PDC) is often used as a measure of patient adherence to therapy, and the relationship between PDC for statins and clinical outcomes has been clearly demonstrated using administrative claims data.24,25 PDC is calculated based on pharmacy claims and is reported as a single value over a predetermined period, which results in the same PDC values for patients with variable medication adherence patterns.25,26
Medication adherence is a complex behavior of patient's beliefs, illness, and other environmental factors. Consequently, dichotomizing patients' behavior as adherent versus nonadherent can result in loss of valuable information that can aid and inform the development of interventions. Variations in the patterns (or trajectories) of patient adherence can influence patient prognosis and should be considered when designing medication adherence interventions.25,26
Group-based trajectory modeling has been used as an alternative to map medication adherence over time using administrative data, given that this type of modeling identifies longitudinal trajectories of adherence patterns over a period of time.25,26 This method entails identifying groups of patients with similar medication adherence patterns over time using pharmacy refill data and can help to depict the longitudinal adherence behavior. Patients within each trajectory have a similar adherence pattern and therefore may have similar characteristics.
In 2014, 15% of the US population was aged ≥65 years, which demonstrates an increasing prevalence of older adults over time.27 As a result of increased medication complexity and multiple comorbidities, older adults (age >65 years) may have a higher risk for medication nonadherence.28 The objectives of this study were to identify the trajectories of adherence to statins over a 1-year period among patients enrolled in a Medicare Advantage plan and the clinical and sociodemographic predictors of falling into each trajectory. The 4 trajectories identified in this study will be utilized to customize a motivational interview-based intervention, in collaboration with a Medicare Advantage plan, to improve medication adherence.
Methods
This retrospective cohort study was based on data from members enrolled in a Texas-based Medicare Advantage plan. This particular data source was chosen given that patients who are enrolled in the Medicare Advantage plan are predominantly adults aged >65 years and are at high risk for CVD, as well as the potential to follow them prospectively for future development of an intervention. The study population consisted of members who were continuously enrolled in the Medicare Advantage plan between January 2013 and June 2016.
The data contained membership and demographic information, as well as medical and pharmacy claims for all the patients. The medical claims include information on all inpatient and outpatient claims, as well as on diagnoses and on procedures. The pharmacy files used included patient and drug identification information, prescription fill dates, days of supply, quantity dispensed, and dosing information.
All medical and pharmacy claims were available for the enrolled patients between January 2013 and June 2016. The patient identification period was from January 2015 to June 2015, so that all patients had at least 1 year of follow-up data available for the measurement of statin adherence. The first statin prescription in the identification period was defined as the index prescription, and the date of the prescription was defined as the index date. The baseline characteristics and comorbidities were identified in the 2-year period before the index date (ie, the preindex period). A 2-year preindex period was used to best capture the chronic comorbidities. Each patient was followed for 1 year after the index date.
Inclusion and Exclusion Criteria
Patients who had continuous enrollment in the health plan between January 2013 and June 2016 and had a statin prescription in the patient identification period were included. Patients with conditions that are symptoms of poor statin tolerance, including liver disease, myalgia, or skeletal muscle diseases diagnosis, in the 2-year preindex period were excluded, because poor statin tolerance could lead to treatment discontinuation or could affect adherence in the follow-up period.29,30 Given that these patients were at high risk for intolerance, it is unlikely that their adherence could be enhanced through motivational interviewing interventions. Patients who are receiving a combination of drugs, which includes a statin as an index prescription, were also excluded to ensure that adherence to a statin was not confounded by the patient adherence to any other medication.30 Being diagnosed with dementia in the same time frame was also an exclusion criterion, because dementia-related cognitive impairment can lead to nonadherence and to involvement of caregivers' support.31 Finally, patients with statin intolerance, defined as a diagnosis of myopathy during the follow-up period, were also excluded.30
Adherence Measurement
Medication adherence was measured for 360 days after the index date using pharmacy claims. PDC was used to measure adherence and was also calculated separately for each 30-day period after the index date. During each of the 12 consecutive 30-day assessments, the PDC was dichotomized, with a PDC of ≥0.8 considered adherent based on the threshold, which has been widely used in the literature and by the Centers for Medicare & Medicaid Services (CMS) quality measures.32,33
The 12 dichotomized monthly indicators of statin adherence were then used in a logistic group-based trajectory model. The trajectory model uses several multinomial logistic regression equations simultaneously, estimating the probability of membership in each group and the probability of being adherent as a function of time. The model used between 1 and 5 adherence groups, using second-order polynomials of time to estimate the probability of being adherent. Based on the Bayesian information criterion, as well as the clinical relevance of the identified trajectories, one model was selected for further evaluation for the predictors of falling into each trajectory.25,26
The effect of the addition of groups on the Bayesian information criterion value was assessed incrementally. The logarithm of twice the difference in Bayesian information criterion values between the complex model (with more groups) and the simpler model (with fewer groups) was assessed stepwise. If the value of the logarithm of difference was more than 2, the more complex model was concluded to have greater prediction power than the simpler model.34
Predictors of Adherence Trajectory
After a set of adherence trajectory patterns was selected, the predictors associated with falling into a particular adherence trajectory group were estimated by using multinomial logistic regression, with the distinct trajectory groups used as the dependent variable. The trajectory group with consistent monthly adherence to statins (ie, the adherent group) was considered as the reference to which other groups were compared.
The independent variables included sociodemographic and clinical variables. The demographic variables were sex, age (<65, 65-70, 71-75, or >75 years), language (English vs other), and subsidy level (low-income subsidy vs no subsidy). The prescribing physician specialty was grouped as primary care versus specialist.
The variables for statin utilization included statin type, intensity, days supply of the index refill (90 days vs other), statin use for primary versus secondary prevention, and prevalent use of statins. Statin type was categorized as lipophilic (atorvastatin, simvastatin, and lovastatin) or hydrophilic (pravastatin, rosuvastatin, and fluvastatin). Statin intensity was categorized as low, moderate, or high based on major guidelines.35 Statin use was considered to be secondary prevention if the patient had a diagnosis of acute coronary syndrome, ischemic stroke, peripheral vascular disease, coronary artery disease, myocardial infarction, angina, or a revascularization procedure in the 2-year period before index date.36 A patient was considered a prevalent user of statin therapy if the patient had a prescription for statin(s) in the 6 months before the index date.
The comorbidities that were controlled for included diabetes, hypertension, HIV, congestive heart failure, as well as the number of hospitalizations in the past year. To further control for the illness severity in the cohort, the CMS risk score and Deyo's Charlson Comorbidity Index (CCI) were evaluated. The CCI is constructed based on International Classification of Diseases, Ninth Revision, Clinical Modification codes and assigns weights to major clinical conditions, which can be obtained from medical claims data.37,38 The CMS risk score accounts for medication burden and disease severity and is calculated based on data taken from a large pool of beneficiaries to estimate the average predicted costs for each of the component factors (eg, age, sex, low-income status, individual disease groups).39
All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc; Cary, NC) at an a priori significance level of 0.05.
Results
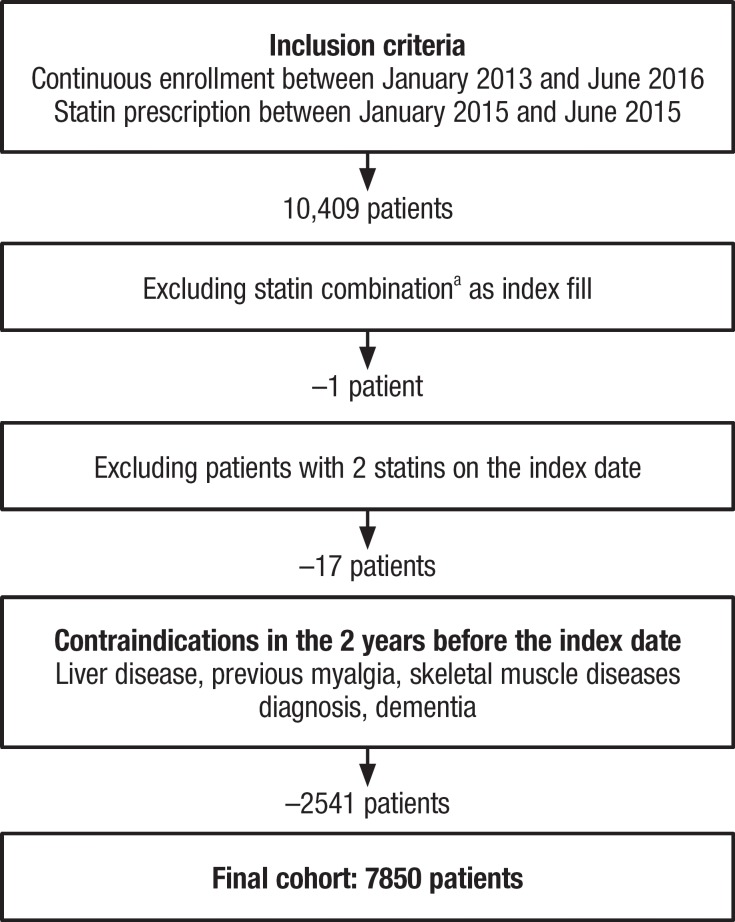
The data contained 10,409 continuously enrolled patients with a statin prescription in the identification period (ie, the index statin). The final cohort after exclusions comprised 7850 patients (Figure 1). The baseline characteristics of the cohort are presented in Table 1. The patients' mean age was 71 years, approximately 55% were female, and approximately 66% were receiving moderate-intensity statins at the index date.
Figure 1. Flow Diagram of Patient Selection.
aStatin plus another drug.
Table 1.
Baseline Demographic and Clinical Characteristics of the Overall Cohort and by Trajectory
| Baseline demographics | Total patients (N = 7850) | Rapid decline (N = 611; 7.78%) | Gradual decline (N = 1317; 16.78%) | Gaps in adherence (N = 1354; 17.25%) | Perfect adherence (N = 4568; 58.19%) | P valuea |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male, N (%) | 3516 (44.79) | 253 (41.41) | 553 (41.99) | 601 (44.39) | 2109 (46.17) | .014 |
| Female, N (%) | 4334 (55.21) | 358 (58.59) | 764 (58.01) | 753 (55.61) | 2459 (53.83) | |
| Age | ||||||
| <65 yrs, N (%) | 1169 (14.89) | 114 (18.66) | 182 (13.82) | 230 (16.99) | 643 (14.08) | .017 |
| 65-70 yrs, N (%) | 2736 (34.85) | 211 (34.53) | 454 (34.47) | 485 (35.82) | 1586 (34.72) | |
| 71-75 yrs, N (%) | 2047 (26.08) | 138 (22.59) | 354 (26.88) | 333 (24.59) | 1222 (26.75) | |
| >75 yrs, N (%) | 1898 (24.18) | 148 (24.22) | 327 (24.83) | 306 (22.61) | 1117 (24.45) | |
| Health plan | ||||||
| Low-income subsidy, N (%) | 3994 (50.88) | 298 (48.77) | 736 (55.88) | 694 (51.26) | 2251 (49.28) | <.001 |
| No subsidy, N (%) | 3856 (49.12) | 313 (51.23) | 581 (44.12) | 660 (48.74) | 2317 (50.72) | |
| Language | ||||||
| English, N (%) | 5788 (73.73) | 453 (74.14) | 917 (69.63) | 952 (70.31) | 3466 (75.88) | <.001 |
| Other, N (%) | 2062 (26.27) | 158 (25.86) | 400 (30.37) | 402 (29.69) | 1102 (24.12) | |
| Prescriber specialty | ||||||
| Primary care, N (%) | 5959 (75.97) | 444 (72.79) | 986 (74.92) | 1006 (74.3) | 3523 (77.19) | .019 |
| Specialty, N (%) | 1885 (24.03) | 166 (27.21) | 330 (25.08) | 348 (25.7) | 1041 (22.81) | |
| Statin type | ||||||
| Lipophilic, N (%) | 5591 (71.22) | 444 (72.67) | 935 (70.99) | 947 (69.94) | 3265 (71.48) | .597 |
| Hydrophilic, N (%) | 2259 (28.78) | 167 (27.33) | 382 (29.01) | 407 (30.06) | 1303 (28.52) | |
| Statin intensity | ||||||
| Low, N (%) | 1295 (16.5) | 99 (16.2) | 242 (18.38) | 191 (14.11) | 763 (16.7) | .002 |
| Moderate, N (%) | 5201 (66.25) | 401 (65.63) | 844 (64.09) | 887 (65.51) | 3069 (67.18) | |
| High, N (%) | 1354 (17.25) | 111 (18.17) | 231 (17.54) | 276 (20.38) | 736 (16.11) | |
| Refill type | ||||||
| 90-day, N (%) | 5817 (74.1) | 387 (63.34) | 1028 (78.06) | 881 (65.07) | 3521 (77.08) | <.001 |
| Other, N (%) | 2033 (25.9) | 224 (36.66) | 289 (21.94) | 473 (34.93) | 1047 (22.92) | |
| Mail-order prescription | ||||||
| Yes, N (%) | 7786 (99.18) | 608 (99.51) | 1307 (99.24) | 1333 (98.45) | 4538 (99.34) | .01 |
| No, N (%) | 64 (0.82) | 3 (0.49) | 10 (0.76) | 21 (1.55) | 30 (0.66) | |
| Statin use type | ||||||
| Primary prevention, N (%) | 428 (5.45) | 49 (8.02) | 80 (6.07) | 74 (5.47) | 225 (4.93) | .01 |
| Secondary prevention, N (%) | 7422 (94.55) | 562 (91.98) | 1237 (93.93) | 1280 (94.53) | 4343 (95.07) | |
| Statin user | ||||||
| New, N (%) | 1466 (18.68) | 332 (54.34) | 297 (22.55) | 397 (29.32) | 440 (9.63) | <.001 |
| Prevalent, N (%) | 6381 (81.32) | 279 (45.66) | 1020 (77.45) | 957 (70.68) | 4128 (90.37) | |
| Comorbidities | ||||||
| Diabetes | ||||||
| Yes, N (%) | 4482 (57.1) | 316 (51.72) | 758 (57.56) | 788 (58.2) | 2620 (57.36) | .044 |
| No, N (%) | 3368 (42.9) | 295 (48.28) | 559 (42.44) | 566 (41.8) | 1948 (42.64) | |
| Hypertension | ||||||
| Yes, N (%) | 6751 (86) | 503 (82.32) | 1121 (85.12) | 1160 (85.67) | 3967 (86.84) | .015 |
| No, N (%) | 1099 (14) | 108 (17.68) | 196 (14.88) | 194 (14.33) | 601 (13.16) | |
| Congestive heart failure | ||||||
| Yes, N (%) | 832 (10.6) | 61 (9.98) | 150 (11.39) | 132 (9.75) | 489 (10.7) | .532 |
| No, N (%) | 7018 (89.4) | 550 (90.02) | 1167 (88.61) | 1222 (90.25) | 4079 (89.3) | |
| HIV | ||||||
| Yes, N (%) | 18 (0.23) | 4 (0.65) | 2 (0.15) | 4 (0.3) | 8 (0.18) | .111 |
| No, N (%) | 7832 (99.77) | 607 (99.35) | 1315 (99.85) | 1350 (99.7) | 4560 (99.82) | |
| Hyperlipidemia | ||||||
| Yes, N (%) | 7306 (93.07) | 547 (89.53) | 1215 (92.26) | 1260 (93.06) | 4284 (93.78) | .008 |
| No, N (%) | 544 (6.93) | 64 (10.47) | 102 (7.74) | 94 (6.94) | 284 (6.22) | |
| CMS score, mean (SD) | 1.20 (0.85) | 1.17 (0.93) | 1.25 (0.90) | 1.19 (0.88) | 1.20 (0.82) | .083 |
| Charlson Comorbidity Index | ||||||
| 0, N (%) | 6654 (84.76) | 524 (85.76) | 1118 (84.89) | 1180 (87.15) | 3832 (83.89) | .064 |
| 1-2, N (%) | 1146 (14.6) | 81 (13.26) | 189 (14.35) | 166 (12.26) | 710 (15.54) | |
| ≥3, N (%) | 50 (0.64) | 6 (0.98) | 10 (0.76) | 8 (0.59) | 26 (0.57) | |
| Mean (SD) | 0.25 (0.67) | 0.26 (0.84) | 0.24 (0.66) | 0.21 (0.59) | 0.26 (0.66) | .077 |
| Frequency of hospitalization in the previous year | ||||||
| None, N (%) | 7342 (93.53) | 554 (90.67) | 1234 (93.7) | 1274 (94.09) | 4280 (93.7) | .042 |
| 1-2 times, N (%) | 485 (6.18) | 53 (8.67) | 77 (5.85) | 76 (5.61) | 279 (6.11) | |
| >2 times, N (%) | 23 (0.29) | 4 (0.65) | 6 (0.46) | 4 (0.3) | 9 (0.2) | |
| Mean proportion of days covered | 0.77 (0.23) | 0.23 (0.08) | 0.65 (0.13) | 0.62 (0.15) | 0.92 (0.06) | <.001 |
P values represent results from chi-square/ANOVA tests.
ANOVA indicates analysis of variance; CMS, Centers for Medicare & Medicaid Services; SD, standard deviation.
Adherence Trajectories
The models' graphs with 1 to 5 adherence groups are available in the Appendix Figure (see www.AHDBonline.com) and the Bayesian information criterion for each of the models is presented in Table 2. Based on the Bayesian information criterion and the expected patterns from real-world practice and literature, the model with 4 adherence groups was used for further analysis to identify predictors of adherence.
Table 2.
Bayesian Information Criterion Values of Trajectory Models Limited to Different Number of Groups
| Groups in trajectory modeling, N | Bayesian information criterion | Akaike information criterion | Log (2∆ Bayesian information criterion) |
|---|---|---|---|
| 1 | –52449 | –52438 | |
| 2 | –46113 | –46089 | 4.10 |
| 3 | –45101 | –45063 | 3.30 |
| 4 | –44692 | –44621 | 2.91 |
| 5 | –44550 | –44484 | 2.45 |
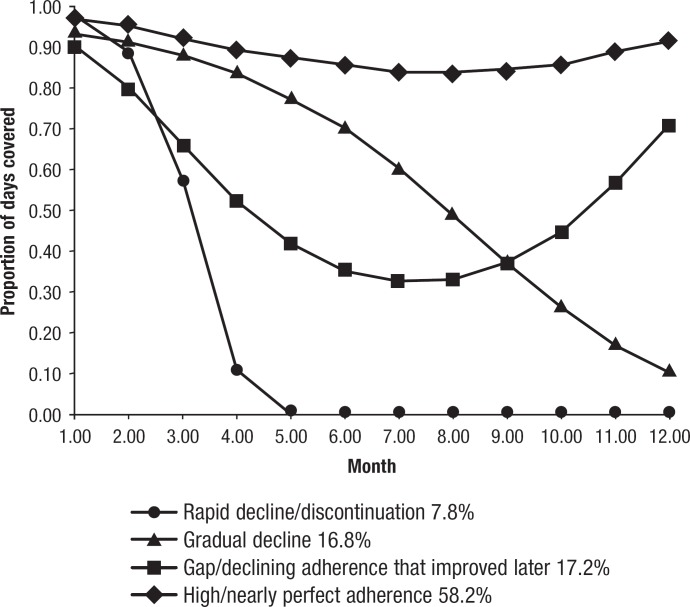
When 4 adherence trajectories were considered, the groups, as shown from bottom to top in Figure 2, included Group 1, which had rapid decline or discontinuation (7.8%); Group 2, which had a gradual decline over time (16.8%); Group 3, which had gaps in adherence that improved over time (17.3%); and Group 4, which had nearly perfect adherence (58.2%). The mean PDC across each adherence group is shown in Table 1. The 4 groups had significantly different mean PDCs over the follow-up period.
Figure 2. Adherence Groupings in the Trajectory Model Using 4 Groups.
Multinomial Regression
The baseline characteristics of the patients in each trajectory are shown in Table 1. There were significant differences in the demographic characteristics of patients across the 4 trajectories. A greater proportion of women were in the lower-adherence trajectories than in the perfect-adherence trajectory. The rapid-decline group had the highest percentage of patients who were new statin users compared with the other trajectories. Fewer patients who had comorbidities than those without comorbidities, including diabetes, hypertension, and hyperlipidemia, were included in the rapid-decline group.
The covariates significantly associated with each trajectory are shown in Table 3. Physician specialty, statin type, the presence of diabetes, hypertension, HIV, congestive heart failure, hyperlipidemia, and the frequency of hospitalization were not associated with being placed into any trajectory compared with the perfect-adherence group.
Table 3.
Multinomial Logistic Regression for the Trajectory Groups versus the Perfect-Adherence Group as Reference
| Variable | Rapid decline vs perfect adherence | Gradual decline vs perfect adherence | Gaps in adherence vs perfect adherence | |||
|---|---|---|---|---|---|---|
| aORa (95% CI) | P value | aORa (95% CI) | P value | aORa (95% CI) | P value | |
| Female vs male | 1.301 (1.081-1.565) | .0052 | 1.18 (1.039-1.342) | .0111 | 1.143 (1.004-1.297) | .0433 |
| Age-group | ||||||
| 65-70 yrs vs <65 yrs | 0.8 (0.609-1.051) | .109 | 1.072 (0.874-1.315) | .5028 | 0.883 (0.727-1.073) | .2111 |
| 71-75 yrs vs <65 yrs | 0.699 (0.523-0.933) | .0152 | 1.082 (0.877-1.335) | .4597 | 0.814 (0.664-0.997) | .0472 |
| >75 yrs vs <65 yrs | 0.863 (0.647-1.151) | .3148 | 1.101 (0.89-1.363) | .375 | 0.83 (0.674-1.021) | .0783 |
| Having low-income subsidy vs not | 1.02 (0.84-1.238) | .8437 | 1.201 (1.049-1.375) | .0081 | 0.99 (0.864-1.134) | .8862 |
| Other language vs English | 1.104 (0.89-1.369) | .381 | 1.282 (1.109-1.482) | .0008 | 1.346 (1.162-1.56) | <.0001 |
| Specialist physician vs nonspecialist | 1.164 (0.948-1.428) | .1467 | 1.112 (0.961-1.286) | .1527 | 1.113 (0.962-1.287) | .15 |
| Lipophilic vs hydrophilic statin | 1.063 (0.863-1.31) | .5643 | 0.994 (0.863-1.145) | .9339 | 0.917 (0.796-1.056) | .2286 |
| Statin intensity | ||||||
| Moderate vs low | 1.112 (0.859-1.44) | .4193 | 0.898 (0.757-1.066) | .2202 | 1.248 (1.036-1.502) | .0197 |
| High vs low | 1.313 (0.959-1.799) | .0897 | 1.034 (0.834-1.282) | .7585 | 1.585 (1.269-1.979) | <.0001 |
| 90-day vs other refill | 0.513 (0.423-0.623) | <.0001 | 1.008 (0.867-1.172) | .9161 | 0.535 (0.467-0.613) | <.0001 |
| Statin user type | ||||||
| Prevalent vs incident | 0.088 (0.073-0.107) | <.0001 | 0.365 (0.309-0.431) | <.0001 | 0.25 (0.213-0.293) | <.0001 |
| For secondary vs primary prevention | 1.086 (0.523-2.255) | .8163 | 1.097 (0.621-1.941) | .7449 | 1.034 (0.566-1.888) | .9074 |
| CMS risk score | 1.005 (0.88-1.146) | .9455 | 1.084 (0.994-1.183) | .0688 | 1.048 (0.957-1.148) | .3119 |
| Charlson Comorbidity Index | 0.99 (0.869-1.129) | .8751 | 0.943 (0.855-1.039) | .2364 | 0.883 (0.795-0.981) | .0228 |
| Diabetes (yes vs no) | 0.888 (0.733-1.076) | .2265 | 0.97 (0.848-1.109) | .6545 | 1.032 (0.901-1.182) | .6471 |
| Hypertension (yes vs no) | 0.885 (0.688-1.14) | .3446 | 0.893 (0.741-1.075) | .2325 | 0.976 (0.808-1.179) | .7991 |
| HIV (yes vs no) | 2.901 (0.771-10.916) | .1152 | 0.716 (0.149-3.435) | .6758 | 1.43 (0.414-4.942) | .5716 |
| CHF (yes vs no) | 0.941 (0.679-1.304) | .711 | 1.059 (0.852-1.316) | .6035 | 0.902 (0.718-1.134) | .3783 |
| Hyperlipidemia (yes vs no) | 0.899 (0.469-1.722) | .7475 | 0.859 (0.518-1.425) | .556 | 1.105 (0.648-1.887) | .7135 |
| Frequency of hospitalization in previous year | ||||||
| 1-2 times vs none | 1.369 (0.967-1.939) | .0763 | 0.907 (0.69-1.192) | .4831 | 0.858 (0.649-1.133) | .2803 |
| >2 times vs none | 1.947 (0.47-8.056) | .3577 | 1.975 (0.678-5.749) | .2121 | 1.219 (0.359-4.145) | .7507 |
Adjusted odds ratio in relation to all the other variables in the table.
aOR indicates adjusted odds ratio; CHF, congestive heart failure; CI, confidence interval; CMS, Centers for Medicare & Medicaid Services.
Women were more likely to be placed into all 3 lower-adherence trajectories than into the perfect-adherence group (rapid decline, adjusted odds ratio [OR], 1.3; 95% confidence interval [CI], 1.1-1.6; gradual decline, OR, 1.2; 95% CI, 1.0-1.3; gaps in adherence, OR, 1.1; 95% CI, 1.0-1.3). Prevalent users of statin medications were less likely than incident users to be placed into any of the lower-adherence trajectories (rapid decline, OR, 0.08; 95% CI, 0.07-0.11; gradual decline, OR, 0.37; 95% CI, 0.31-0.43; gaps in adherence, OR, 0.25; 95% CI, 0.21-0.29).
Older patients (age 71-75 years vs <65 years) were less likely to be included in the lower-adherence trajectories, particularly the rapid-decline and gaps-in-adherence groups, than the perfect-adherence group (rapid decline, OR, 0.7; 95% CI, 0.5-0.9; gaps in adherence, OR, 0.8; 95% CI, 0.7-1.0). Having a primary language other than English was associated with greater odds of being classed into the gradual-decline and gaps-in-adherence trajectories than in the perfect-adherence trajectory (gradual decline, OR, 1.3; 95% CI, 1.1-1.5; gaps in adherence, OR, 1.4; 95% CI, 1.2-1.6).
Patients who had a low-income subsidy were more likely to be in the gradual-decline trajectory (OR, 1.2; 95% CI, 1.1-1.4). Patients who were receiving moderate- or high-intensity statins versus low-intensity statins were more likely to be in the gaps-in-adherence group (moderate vs low, OR, 1.3; 95% CI, 1.0-1.5; high vs low, OR, 1.6; 95% CI, 1.3-2.0). Patients with a higher CCI score were less likely to be in the gaps-in-adherence group (OR, 0.9; 95% CI, 0.8-1.0).
Discussion
This study followed patients from a Medicare Advantage plan over 1 year to identify groups with distinct patterns of adherence to statins. Up to 5 trajectory groups were tested, but 4 trajectories of adherence were eventually used to further identify the predictors of placing into each group. Several sociodemographic and patient factors were found to be predictors of being placed into each of the trajectories, most of which align closely with the existing literature.
Franklin and colleagues have identified patterns of adherence to statins using group-based trajectory modeling, but these studies included 6 groups for further outcome evaluation.25,26 The results of the trajectory modeling performed in our study align with those by Franklin and colleagues.
We took several factors into consideration to decide on using 4 adherence trajectory groups for further analysis. First, the rapid-decline group comprises 7.8% of the sample when the number of trajectories are limited to 4. When limiting the number to 5 trajectories, the rapid-decline group was further split into 2 smaller groups. On testing 6 groups, the perfect-adherence group was split into 2 smaller groups comprised of patients who were completely adherent and those who had nearly perfect adherence. These additional groups were comprised of very small percentages of the sample. Second, although further addition of groups beyond 4 led to significant decreases in Bayesian information criterion, the newer 2 groups were largely similar to the existing groups when considering the development and implementation of differentiated interventions.
Of the 7850 patients who were included in our sample, 57.5% placed into the high-adherence trajectory (ie, these patients consistently had a higher PDC during follow-up). A previous study showed that adherence to statins in elderly patients decreased over time after the initiation and proportion of nonadherent patients increased in a log-linear manner.8 This emphasizes the need to look at adherence as it varies over time rather than at a mean PDC value. Although the lower-adherence trajectories in our study also had lower mean PDCs, the pattern of each trajectory can identify possible barriers to adherence that are common within a particular trajectory, which will be critical for the development of interventions.
The significant predictors of placing into a certain adherence trajectory included sex, age, low-income subsidy, language, CCI, statin intensity, and 90-day refill. Because each group had a distinct adherence trajectory pattern, these predictors are more informative at understanding adherence than would be possible if patients were categorized based solely on mean PDC.
The results from the multinomial logistic regression suggest that women were more likely to be nonadherent to statins than men. This aligns with evidence from current research, which has repeatedly identified that women are less adherent to statins than men.40 Because our study included patients from a Medicare Advantage plan population, the mean age of women was >55 years, and they were therefore likely postmenopausal and at an increased risk for CVD.41 A review by Goldstein and colleagues summarized the sex-specific factors that contribute to nonadherence in women, some of which included a lack of provider awareness of CVD risk among women, an increased risk for side effects, and competing demands, such as family responsibilities.42 Therefore, tailored patient and provider interventions designed to increase awareness and address sex-specific barriers to adherence can be beneficial.
Of the age categories in our model, the age-group of 71 to 75 years had significantly greater odds of being placed in the adherent trajectory versus patients aged <65 years. A meta-analysis by Mann and colleagues showed a U-shaped association of age and adherence; the oldest (≥70 years) and youngest (<50 years) patients had lower adherence than the middle-aged (50-69 years) patients.20 Increasing age has been found to be associated with lower adherence in studies with mean ages >65 years.43 The decline in adherence in patients aged >75 years could be mediated by reasons such as cognitive changes, increased medication regimen complexity with age, and increased risk for side effects.44
Socioeconomic status and higher copayments have been associated with lower adherence.40,45 Aarnio and colleagues used group-based trajectory modeling to analyze the effect of socioeconomic inequalities on statin adherence, and showed that low socioeconomic position was a predictor of placing into poor-adherence trajectories.45 Our study results indicate that having low-income subsidy was associated with greater odds of placing into the gradual-decline group than into the perfect-adherence group, but no relationship was found for the other categories. The health plan used in this study has multiple statins in different tiers on its formulary, some of which carry little or no copay. Thus, it is less likely that the cost of statins alone, rather than the overall cost of healthcare, could be affecting statin adherence. More education and awareness regarding the importance of statins in this subgroup may emphasize the need to maintain adherence.
Receiving a moderate- or high-intensity index statin was associated with placing into the gaps-in-adherence group versus the perfect-adherence group. The guidelines recommend moderate- or high-intensity statins for the primary prevention of CVD in patients aged 40 to 75 years with ≥1 risk factors.35 Approximately 83% of the sample included in the analysis was receiving moderate- or high-intensity statins. In the elderly population, statin-related side effects may occur more often46; therefore, these patients may have lower adherence, which may improve by switching to a better-tolerated, lower-intensity statin.47 Although studies have shown that higher-intensity statins have greater effectiveness in reducing cardiovascular events,48,49 they have also been associated with modest reductions in adherence compared with lower-intensity statins.50
Studies have shown positive and negative effects of the number, severity, and type of comorbidities on adherence to medications.40,51,52 Our study did not show an association between adherence and the adjusted comorbidities, which have been known to be associated with adherence in the literature.40,51 However, patients with a higher CCI score were associated with lower odds of placing into the gaps-in-adherence group compared with the high-adherence group, which indicates that increased disease severity is associated with lower gaps in adherence. Prevalent users of statins were more likely to be adherent than new initiators of statin therapy.
Patients with a 90-day refill were more likely to be placed into the perfect-adherence group than into the rapid-decline or gaps-in-adherence groups. These results indicate that having a 3-month refill improves the overall 1-year adherence, but this may be artificial, because a single refill in the database indicates that the patient is adherent for 90 days.
We evaluated mail order as a part of the multinomial logistic model; however, only 64 (0.8%) patients had mail order pharmacy, and therefore, it was not added into the final model. In the model without mail order pharmacy, the patient age-group of 71 to 75 years was more likely to place into the perfect-adherence group than the gaps-in-adherence trajectory group, which was not observed when mail order was in the logistic model. Future studies with larger patient samples that use mail order are needed to investigate the effects of mail order on adherence in varying age-groups.
Limitations
This study has some limitations. The generalizability of the study findings may be limited to similar subpopulations. This study focused on older adults who were enrolled in a Texas-based Medicare Advantage plan.
The identified factors that affected adherence in this study may not be the same for younger patients or for patients residing in different geographic regions.
All the covariates that were analyzed as potential predictors of adherence trajectories were measured at baseline and were assumed to remain constant throughout the follow-up period. The follow-up period for adherence was limited to 1 year.
Using pharmacy claims to measure adherence does not capture the actual patient behavior of taking medications; however, it has been shown to be correlated with other adherence measures and clinical outcomes.5,25
The reasons for nonadherence to statins in our study could not be ascertained, because we used retrospective claims data for analysis. Future studies should evaluate other patient populations and should follow patient adherence for longer periods of time.
Conclusions
Extensive literature is available about the effectiveness of statins, as well as the inadequate adherence leading to suboptimal outcomes. Enhancing adherence is an integral step for improving outcomes, which result in an overall reduction in health services utilization. Adherence to chronic medications is a quality measure implemented by CMS to evaluate the health plan performance that is a constant concern for providers. The findings of this study provide valuable information that can be used by healthcare providers and by payers to improve statin adherence.
With group-based trajectory modeling, clinically relevant patterns of adherence (ie, trajectories) can be identified and can provide more insight to providers than a single mean value. Understanding the characteristics of patients that put them at risk for medication nonadherence has the potential to inform how best to intervene, which can instruct the future development of protocols and tools to better individualize interventions for patients requiring lipid-lowering therapy.
Appendix
Funding Source
This study was funded by Sanofi and Regeneron Pharmaceuticals.
Author Disclosure Statement
Ms Vadhariya had a 10-week internship with Regeneron Pharmaceuticals; Dr Fleming has received grants from Sanofi and Regeneron; Dr Johnson and Dr Essien have no conflicts of interest to report; Dr Serna and Dr Esse are employees of CareAllies; Dr Choi is an employee and shareholder of Sanofi; Ms Boklage is an employee and stockholder of Regeneron Pharmaceuticals; Dr Abughosh received a grant from Regeneron.
Contributor Information
Aisha Vadhariya, PhD Candidate, Department of Pharmaceutical Health Outcomes and Policy, University of Houston College of Pharmacy, TX.
Marc L. Fleming, Associate Professor, University of North Texas System College of Pharmacy, Houston.
Michael L. Johnson, Associate Professor, Department of Pharmaceutical Health Outcomes and Policy, University of Houston College of Pharmacy, TX.
E. James Essien, Professor, Department of Pharmaceutical Health Outcomes and Policy, University of Houston College of Pharmacy, TX.
Omar Serna, Clinical Operations Director at CareAllies, Houston.
Tara Esse, Clinical Program Manager at CareAllies, Houston.
Jeannie Choi, Former Director, Health Economics and Value Assessment, Sanofi.
Susan H. Boklage, Director, HEOR, Regeneron Pharmaceuticals, Tarrytown, NY.
Susan M. Abughosh, Associate Professor, Department of Pharmaceutical Health Outcomes and Policy, University of Houston College of Pharmacy..
References
- 1. Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 2. Butler RJ, Davis TK, Johnson WG, Gardner HH. Effects of nonadherence with prescription drugs among older adults. Am J Manag Care. 2011;17:153–160. [PubMed] [Google Scholar]
- 3. MacLaughlin EJ, Raehl CL, Treadway AK, et al. Assessing medication adherence in the elderly: which tools to use in clinical practice? Drugs Aging. 2005;22:231–255. [DOI] [PubMed] [Google Scholar]
- 4. van der Wal MHL, Jaarsma T. Adherence in heart failure in the elderly: problem and possible solutions. Int J Cardiol. 2008;125:203–208. [DOI] [PubMed] [Google Scholar]
- 5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 6. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–550. [DOI] [PubMed] [Google Scholar]
- 7. Murray MD, Morrow DG, Weiner M, et al. A conceptual framework to study medication adherence in older adults. Am J Geriatr Pharmacother. 2004;2:36–43. [DOI] [PubMed] [Google Scholar]
- 8. Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. [DOI] [PubMed] [Google Scholar]
- 9. Choudhry NK, Setoguchi S, Levin R, et al. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Heart disease fact sheet. www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_disease.htm. Accessed January 24, 2019.
- 12. Centers for Disease Control and Prevention. Older persons' health. www.cdc.gov/nchs/fastats/older-american-health.htm. Accessed January 24, 2019.
- 13. Mozaffarian D, Benjamin EJ, Go AS, et al; for the American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2016 Update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. Erratum in: Circulation. 2016;133:e599. [DOI] [PubMed] [Google Scholar]
- 14. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. [DOI] [PubMed] [Google Scholar]
- 15. Cholesterol Treatment Trialists' Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cholesterol Treatment Trialists' Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. Errata in: Lancet 2005;366:1358; Lancet. 2008;371:2084. [DOI] [PubMed] [Google Scholar]
- 17. Delahoy PJ, Magliano DJ, Webb K, et al. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31:236–244. [DOI] [PubMed] [Google Scholar]
- 18. Afilalo J, Duque G, Steele R, et al. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysis. J Am Coll Cardiol. 2008;51:37–45. [DOI] [PubMed] [Google Scholar]
- 19. Abughosh SM, Kogut SJ, Andrade SE, et al. Persistence with lipid-lowering therapy: influence of the type of lipid-lowering agent and drug benefit plan option in elderly patients. J Manag Care Pharm. 2004;10:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann DM, Woodward M, Muntner P, et al. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44:1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep. 2013;15:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin I, Sung J, Sanchez RJ, et al. Patterns of statin use in a real-world population of patients at high cardiovascular risk. J Manag Care Spec Pharm. 2016;22:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choudhry NK, Glynn RJ, Avorn J, et al. Untangling the relationship between medication adherence and post–myocardial infarction outcomes: medication adherence and clinical outcomes. Am Heart J. 2014;167:51–58.e5. [DOI] [PubMed] [Google Scholar]
- 25. Franklin JM, Krumme AA, Tong AY, et al. Association between trajectories of statin adherence and subsequent cardiovascular events. Pharmacoepidemiol Drug Saf. 2015;24:1105–1113. [DOI] [PubMed] [Google Scholar]
- 26. Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51:789–796. Erratum in: Med Care. 2013;51:1029. [DOI] [PubMed] [Google Scholar]
- 27. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060: Population Estimates and Projections. Current Population Reports, P25–1143. Washington, DC: US Census Bureau; March 2015. [Google Scholar]
- 28. Yap AF, Thirumoorthy T, Kwan YH. Systematic review of the barriers affecting medication adherence in older adults. Geriatr Gerontol Int. 2016;16:1093–1101. [DOI] [PubMed] [Google Scholar]
- 29. Bays H, Cohen DE, Chalasani N, Harrison SA. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 suppl):S47–S57. [DOI] [PubMed] [Google Scholar]
- 30. Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: mechanisms and management. Diabetes Care. 2013;36(suppl 2):S325–S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith D, Lovell J, Weller C, et al. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS One. 2017;12:e0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pharmacy Quality Alliance. Adherence: PQA adherence measures. Updated August 28, 2018. www.pqaalliance.org/adherence-measures. Accessed May 12, 2019.
- 33. Centers for Medicare & Medicaid Services. Quality measures. Updated August 22, 2017. www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html. Accessed November 28, 2017.
- 34. Arrandale VH. An Evaluation of Two Existing Methods for Analyzing Longitudinal Respiratory Symptom Data. Master's thesis. Vancouver, Canada: University of British Columbia; 2006. [Google Scholar]
- 35. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(pt B):2889–2934. Errata in: J Am Coll Cardiol 2014;63(pt B):3024-3025; J Am Coll Cardiol. 2015;66:2812. [DOI] [PubMed] [Google Scholar]
- 36. Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC Expert Consensus Decision Pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:1785–1822. [DOI] [PubMed] [Google Scholar]
- 37. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 38. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 39. Centers for Medicare & Medicaid Services. Risk adjustment. 2017. www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors.html. Accessed January 29, 2018.
- 40. Mauskop A, Borden WB. Predictors of statin adherence. Curr Cardiol Rep. 2011;13:553–558. [DOI] [PubMed] [Google Scholar]
- 41. Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–1111. [DOI] [PubMed] [Google Scholar]
- 42. Goldstein KM, Zullig LL, Bastian LA, Bosworth HB. Statin adherence: does gender matter? Curr Atheroscler Rep. 2016;18:63. [DOI] [PubMed] [Google Scholar]
- 43. Kulkarni SP, Alexander KP, Lytle B, et al. Long-term adherence with cardiovascular drug regimens. Am Heart J. 2006;151:185–191. [DOI] [PubMed] [Google Scholar]
- 44. Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54(suppl 1):S57–S60. [DOI] [PubMed] [Google Scholar]
- 45. Aarnio E, Martikainen J, Winn AN, et al. Socioeconomic inequalities in statin adherence under universal coverage: does sex matter? Circ Cardiovasc Qual Outcomes. 2016;9:704–713. [DOI] [PubMed] [Google Scholar]
- 46. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tuohy CV, Dodson JA. Statins for primary prevention in older adults. American College of Cardiology. March 10, 2015. www.acc.org/latest-in-cardiology/articles/2015/03/10/07/46/statins-for-primary-prevention-in-older-adults. Accessed May 13, 2019.
- 48. Kim J, Lee HS, Nam CM, Heo JH. Effects of statin intensity and adherence on the long-term prognosis after acute ischemic stroke. Stroke. 2017;48:2723–2730. [DOI] [PubMed] [Google Scholar]
- 49. Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. Erratum in: N Engl J Med. 2006;354:778. [DOI] [PubMed] [Google Scholar]
- 50. Virani SS, Woodard LD, Akeroyd JM, et al. Is high-intensity statin therapy associated with lower statin adherence compared with low- to moderate-intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association cholesterol management guidelines. Clin Cardiol. 2014;37:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei MY, Ito MK, Cohen JD, et al. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7:472–483. [DOI] [PubMed] [Google Scholar]
- 52. Balkrishnan R, Rajagopalan R, Camacho FT, et al. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25:2958–2971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.