Abstract
In this issue of Immunity, Conche et al. (2009) define an antigen-independent signaling pathway that is dependent on cyclic adenosine monophosphate and extracellular signal-regulated kinase and T cells for subsequent T cell antigen receptor signaling.
Investigators studying lymphocyte activation often simplify the stimulation process to their experimental advantage. Those pursuing biochemical pathways crosslink the T cell antigen receptor (TCR) and various coreceptor molecules with monoclonal antibodies before cell lysis and protein isolation. Those who wish to visualize the molecular process of activation drop cells on antibody-covered surfaces or planar lipid bilayers doped with appropriate molecular ligands. These and other simplifications have taught us much, but the more physiological cell-cell contact of activation has many secrets to reveal about the events both preceding and following receptor engagement. A study by Conche et al. (2009) in the current issue of Immunity elegantly combines biochemical and molecular imaging approaches to elucidate an adhesion-mediated signaling cascade that occurs prior to TCR ligation, sensitizes the signaling environment, and leads to more robust signaling once the TCR is engaged (Conche et al., 2009).
Adhesion of T cells to artificial substrates or to dendritic cells (DCs) in the absence of peptide-MHC complexes triggers a number of intracellular events that act in concert to prime the intracellular signaling environment for subsequent TCR ligation. Adhesion via integrins triggers recruitment of signaling and cytoskeletal proteins to the point of contact (Doucey et al., 2003; Revy et al., 2001). Adhesion also triggers recruitment of phosphatidylinositol 4,5-bisphosphate (PIP2) to the plasma membrane and increases intracellular calcium stores (Randriamampita et al., 2003). The culmination of these events, coined adhesion-induced T cell priming or AITCP (Randriamampita et al., 2003), prepares the T cell so that, when stimulated via the TCR, it signals more robustly than does a T cell that did not receive adhesion signals. The goal of the study by Conche et al. (2009) was to define at a molecular level the intracellular signaling pathways responsible for AITCP.
Conche et al. (2009) made a number of “educated leaps” in elucidating a cyclic adenosine monophosphate (cAMP)- and extracellular signal-regulated kinase (ERK)-dependent pathway required for AITCP. They first investigated the role of ERK signaling and showed indeed that AITCP requires ERK phosphorylation. Surprisingly, however, adhesion-based ERK activation was not mediated by its upstream kinase MAP-ERK kinase (MEK), but rather by the inhibition of an ERK phosphatase. At this point, Conche et al. (2009) made their first leap and sought a potential role for cAMP elevation in AITCP, because ERK and cAMP “crosstalk” in a number of systems. By using a FRET-based probe that tracks protein kinase A (PKA) activity, they showed that there was a transient rise in PKA (and presumably cAMP) activity within 1 min of T cell adhesion that returned tobaselinewithin10min. Byusingacombination of cAMP analogs and a photoactivatable cAMP, the authors showed that a transient, but not sustained, rise in cAMP could substitute for adhesion in “priming” T cells, and that both the timing and amplitude of the cAMP signal were important for maximal TCR-mediated signaling. Furthermore, inhibition of cAMP signaling blocked adhesion-induced ERK activation, showing that ERK was downstream of cAMP in AITCP.
To mechanistically tie cAMP and ERK signaling together, Conche et al. (2009) made their second leap and investigated the role of HePTP, an ERK phosphatase that can be phosphorylated and inactivated by PKA downstream of TCR signaling (Nika et al., 2004). Knockdown of HePTP increased basal ERK activation and substituted for adhesion in potentiating TCR-induced calcium flux. These data suggested that HePTP could be the physiologic target of cAMP-PKA in AITCP. Conche et al. (2009) then returned to imaging studies to validate their signaling model in vivo. The interaction of T cells with DCs in the absence of antigen showed transient increases in PKA activity by FRET and specific phospho-ERK staining at the T-DC interface. Both signals were sensitive to cAMP pathway inhibition. Furthermore, simultaneous live-cell imaging of PKA activity and calcium flux showed that the transient rise in cAMP preceded the calcium signal, giving further evidence that the cAMP signal is an initiating event after T-DC contact. Finally, they showed that in the presence of antigen, simultaneous blocking of adenylate cyclase (AC) and PKA reduced calcium flux, NF-AT trans-location, and CD69 expression. Thus, Conche et al. (2009) proposed a model that upon T cell-DC contact and prior to TCR ligation, there was a transient rise in cAMP-PKA signaling that led to ERK activation via PKA-mediated inactivation of the ERK phosphatase HePTP (Figure 1, left).
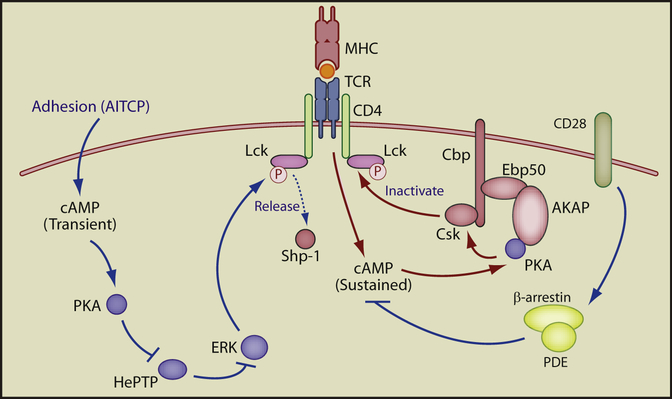
Figure 1. The Dual Roles of cAMP in T Cell Signaling.
Adhesion (AITCP) triggers a transient increase in cAMP-PKA activity. PKA then phosphorylates and inactivates the ERK phosphatase HePTP, leading to a transient rise in ERK activation (left). In contrast (right), TCR activation or other stimuli (diphtheria toxin, cholera enterotoxin, forskolin, PGE2, etc.) trigger strong, sustained cAMP-PKA activity. This degree of stimulation induces PKA phosphorylation and activation of the inhibitory kinase Csk on AKAP-Ebp50-Cbp scaffolded complexes. The action of TCR-inducted cAMP can be antagonized, however, by CD28 costimulation via recruitment of PDEs (far right). Both cAMP pathways may ultimately effect TCR signal transduction via, among other mechanisms, the modulation of Lck activity. cAMP pathways that enhance TCR signaling are shown in blue, those that diminish signaling are shown in red.
A number of questions arise, however, when one tries to integrate cAMP-mediated AITCP into our current understanding of TCR signal transduction. It is well established that cAMP-PKA can have a negative effect on TCR signaling. cAMP is generated via AC and is inactivated by phosphodiesterases (PDEs). Stimulation of the TCR increases localized cAMP concentrations, activating PKA that is bound to the cytoplasmic adaptor A-kinase anchoring protein (AKAP). AKAPs nucleate large signaling complexes that include PKA and the inhibitory kinase Csk and are required for maximal activation of Csk by PKA. Csk can then phosphorylate and inactivate Lck, inhibiting proximal TCR signaling. This inhibitory cAMP-PKA signaling is blocked, however, by costimulation via CD28. CD28 recruits PDEs via the adaptor β-arrestin, within proximity to the AKAP-scaffolded signalosome, allowing cAMP activity to be held in check (Ruppelt et al., 2007; Tasken and Stokka, 2006).
How does the unique positive role for cAMP reported by Conche et al. (2009) fit with the current understanding of cAMP-PKA as a negative regulator of TCR signaling? Conche et al. (2009) suggest that the answer may lie in the quality of the cAMP signal. The transient, low-amplitude response triggered by adhesion may be sufficient to inactivate HePTP but not to activate Csk, whereas sustained increases in cAMP can engage AKAP-scaffolded Csk complexes to inhibit T cell signaling (Figure 1, right; Rup-pelt et al., 2007). Alternatively, AITCP and TCR signaling may engage different pools of either adenylate cyclase or of cAMP effectors. If so, the local signaling environment could then exert tremendous control over the outcome of cAMP-PKA signaling. Furthermore, local control of cAMP can be directly exerted by PDE activity. PDE activity, in turn, is regulated through direct phosphorylation both positively by PKA and negatively by ERK (Tasken and Stokka, 2006). AKAPs can simultaneously interact with PKA and PDE (Asirvatham et al., 2004), which allows for rapid spatial and temporal regulation of cAMP activity. The localized control of cAMP signaling in both AITCP and TCR signaling, and how these competing signals may overlap, are important open questions.
So how do the positive and negative signals generated by cAMP modulate TCR-induced calcium signaling? One attractive target is the Src kinase Lck, which is sensitive to both positive and negative regulatory signals (Stefanova et al., 2003; Tasken and Stokka, 2006). Thus, transient cAMP signals could increase ERK activation, allowing ERK to phosphorylate Lck on Ser59, releasing Lck from negative regulation by the tyro-sine phosphatase Shp-1. More activated Lck might lead to enhanced calcium flux (Lovatt et al., 2006). Alternatively, sustained cAMP signaling increases Csk activity, allowing Csk to phosphorylate Lck on Tyr505 and causing the SH2 domain of Lck to fold back on itself, resulting in its inactivation (Figure 1). Whether either or both of these mechanisms, however, are relevant to cAMP signaling in vivo remains to be seen.
A number of questions also remain in understanding the generation of AITCP signals. Randriamampita et al. (2003) had previously reported that adhesion triggered recruitment of PIP2 to the plasma membrane and increased intra-cellular calcium stores. Although it is easy to understand how these changes enhance the intracellular signaling environment for maximal output, it is unclear how these signals are generated. Are they downstream of a cAMP-ERK pathway, or does adhesion trigger additional signals to account for these changes? This question remains open, as does the looming question of how cAMP is initially generated downstream of adhesion.
Finally, what is the in vivo consequence of AITCP? Conche et al. (2009) suggest that cAMP-dependent sensitization occurs in lymph nodes. If this is so, can manipulation of a cAMP-ERK signaling pathway modulate T cell responsiveness to low potency ligands, and could the ability to modulate immune responses be harnessed therapeutically? Although these questions seem much more difficult to address, they are critical for the in vivo validation of AITCP signaling, and to integrate this unique signaling pathway into our understanding of immune cell function.
REFERENCES
- Asirvatham AL, Galligan SG, Schillace RV, Davey MP, Vasta V, Beavo JA, and Carr DW (2004). J. Immunol 173, 4806–4814. [DOI] [PubMed] [Google Scholar]
- Conche C, Boulla G, Trautmann A, and Randriamampita C (2009). Immunity 30, this issue, 33–43. [DOI] [PubMed] [Google Scholar]
- Doucey MA, Legler DF, Faroudi M, Boucheron N, Baumgaertner P, Naeher D, Cebecauer M, Hudrisier D, Ruegg C, Palmer E, et al. (2003). J. Biol. Chem 278, 26983–26991. [DOI] [PubMed] [Google Scholar]
- Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, and Zamoyska R (2006). Mol. Cell. Biol 26, 8655–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nika K, Hyunh H, Williams S, Paul S, Bottini N, Tasken K, Lombroso PJ, and Mustelin T (2004). Biochem. J 378, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C, Boulla G, Revy P, Lemaitre F, and Trautmann A (2003). Eur. J. Immunol 33, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Revy P, Sospedra M, Barbour B, and Traut-mann A (2001). Nat. Immunol 2, 925–931. [DOI] [PubMed] [Google Scholar]
- Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpen O, and Tasken K (2007). J. Immunol 179, 5159–5168. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, and Germain RN (2003). Nat. Immunol 4, 248–254. [DOI] [PubMed] [Google Scholar]
- Tasken K, and Stokka AJ (2006). Biochem. Soc. Trans 34, 476–479. [DOI] [PubMed] [Google Scholar]