Abstract
Mono-methylindoles (MMI) were described as agonists and/or antagonists of the human aryl hydrocarbon receptor (AhR). Here, we investigated the effects of MMI on AhR-CYP1A pathway in human hepatocytes and HepaRG cells derived from human progenitor hepatic cells. All MMI, except of 2-methylindole, strongly induced CYP1A1 and CYP1A2 mRNAs in HepaRG cells. Induction of CYP1A genes was absent in AhR-knock-out HepaRG cells. Consistently, CYP1A1 and CYP1A2 mRNAs and proteins were induced by all MMIs (except 2-methylindole), in human hepatocytes. The enzyme activity of CYP1A1 was inhibited by MMIs in human hepatocytes and LS180 colon cancer cells in a concentration-dependent manner (IC50 values from 1.2 μM to 23.8 μM and from 3.4 μM to 11.4 μM, respectively). Inhibition of CYP1A1 activity by MMI in human liver microsomes was much weaker as compared to that in intact cells. Incubation of parental MMI with human hepatocytes either diminished (4-methylindole, 6-methylindole) or enhanced (7-methylindole) their agonist effects on AhR in AZ-AHR reporter cells. In conclusion, overall effects of MMI on AhR-CYP1A pathway in human cells comprise the induction of CYP1A genes through AhR, the inhibition of CYP1A catalytic activity and possibly the metabolic transformation causing loss or gain of AhR agonist activity of parental compounds.
Keywords: Microbial catabolites, Entero-hepatic axis, Aryl hydrocarbon receptor, Tryptophan, Methylindoles
GRAPHICAL ABSTRACT

1. Introduction
Aryl hydrocarbon receptor (AhR) is a central regulator of many physiologic and pathophysiologic processes and is a fundamental receptor involved in responses to xenobiotics and microbial products (Roager and Licht, 2018). At the molecular level, AhR acts as a ligand-activated transcriptional factor that binds response elements in DNA in the form of heterodimer with ARNT (AhR nuclear translocator), which is referred to as canonical signaling. Indeed, there are non-canonical (atypical) pathways described for AhR and those comprise some functions that include ARNT-independent effects (Wilson et al., 2013). Regardless, a major and unique AhR transcriptional target gene is CYP1A1 and a primary target used to assess AhR function (Hankinson, 2016; Mescher and Haarmann-Stemmann, 2018). CYP1A is also involved in the metabolism of many xenobiotics, but also in biotransformation of microbial tryptophan catabolite 3-methylindole (Lanza and Yost, 2001).
Ligands of AhR comprise wide spectrum of structurally unrelated compounds, including anthropogenic xenobiotics (e.g. polychlorinated biphenyls, polychlorinated dioxins), natural xenobiotics (e.g. flavonoids, anthocyanidins), endogenous intermediates (e.g. eicosanoids, tetrapyrroles, kynurenine) and compounds produced by intestinal microbiota (e.g. tryptophan catabolites), by pulmonary pathogens (e.g. pyocyanin) or UV irradiation in skin (indolocarbazoles) (Stejskalova et al., 2011). Different AhR ligands cause differential physiological and cellular effects, which is a general phenomenon related to ligand-receptor interaction and selective receptor-effector coupling. It is recognized that endogenous ligands of AhR have desirable biological effects while xenobiotic ligands of AhR do the opposite. Given the complex roles of AhR in human physiology and patho-physiology, the therapeutic (Roman et al., 2018) and chemopreventive targeting of AhR (Mescher and Haarmann-Stemmann, 2018) are emerging issues. A virtual black-box remains to decipher the roles for intestinal microbiome-produced AhR ligands, which may be considered both xenobiotics and endobiotics, and may have dual effects on human health. Indeed, AhR has been reported to participate in regulation of intestinal innate and mucosal immunity and that AhR activity can be modulated via intestinal indole-based microbiota-produced metabolites (Schiering et al., 2017). Several compounds, having an indole core in their structure, are AhR ligands. Besides xenobiotics, they are food-born substances, including those produced by the intestinal microbiota. The examples are diindolylmethane (Chen et al., 1998), indole-3-carbinol (Chen et al., 1996), indole-3-aldehyde (Rothhammer et al., 2016), skatole (3-methylindole) (Rasmussen et al., 2016) or indole itself, which is human AhR specific ligand (Hubbard et al., 2015). We have recently described series of methyl- and methoxy-indoles as novel ligands of AhR, acting in a context-dependent manner as full agonists, partial agonists or antagonists (Stepankova et al., 2018). In the context of methylation of simple indoles, structure-function would be crucial to know for developing skatole-like mimics as potential lead or drug-like AhR ligands, e.g. for use in the therapy of inflammatory bowel disease.
Here we report a follow-up study, with focus on the effects of simple mono-methylindoles (MMI) on AhR activation dependence in cell lines and the expression and catalytic activity of CYP1A1 in human hepatic and LS180 colon cancer in vitro models.
2. Materials and methods
2.1. Chemicals and reagents
4-Me-indole (97% purity as determined by supplier) was obtained from Energy Chemical (Shanghai, China). All other mono-methylated indoles (purity from 97% to 99% as determined by supplier), foetal bovine serum, hygromycin B, dimethylsulfoxide (DMSO) were purchased from Sigma Aldrich (Prague, Czech Republic). Reporter lysis buffer was purchased from Promega (Hercules, CA, USA) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was from Ultra Scientific (North Kingstown, RI, USA). All other chemicals used in this research were of the highest quality commercially available.
2.2. Cell lines
Stably transfected reporter gene AZ-AHR cell line was described previously (Novotna et al., 2011). Human Caucasian colon adenocarcinoma cell line LS180 was purchased from European Collection of Cell Cultures (ECACC No. 87021202). Both AZ-AHR and LS180 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum, 4mM L-glutamine, 1% non-essential amino acids and 1 mM sodium pyruvate.
AHR knock-out (AHR-KO) and control 5 F Clone HepaRG cells were obtained from Sigma Aldrich (Prague, Czech Republic). The cells were cultured using ISOM medium as recommended by manufacturer.
Primary human hepatocyte cultures used in this study were of two origins: (i) Short-term primary human hepatocytes in monolayer batch Hep200565 (female, 21 years, Caucasian), Hep200570 (male, 64 years, unknown ethnicity), Hep200571 (male, 77 years, unknown ethnicity) and Hep220993 (female, 76 years, Caucasian) were purchased from Biopredic International (Rennes, France). (ii) Primary human hepatocytes from multiorgan donor LH75 (female, 78 years, Caucasian) were prepared at Faculty of Medicine, Palacky University Olomouc. Liver tissue was obtained from Faculty Hospital Olomouc, Czech Republic, and the tissue acquisition protocol followed the requirements issued by “Ethical Committee of the Faculty Hospital Olomouc, Czech Republic” and Transplantation law #285/2002 Coll. Primary human hepatocyte cultures were maintained in serum-free cultivation medium.
2.3. Reporter gene assay in AZ-AHR cells
AZ-AHR cells were seeded in 96-well plates and allowed to attach and stabilize for 24 h. Thereafter, AZ-AHR cells were incubated for 24 h with culture media aspired from human hepatocytes cultures, which were before that incubated for 0 h and 24 h with vehicle (DMSO; 0.1% v/v), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; 5nM), and test MMI (200 μM). Then, cells were lysed and luciferase activity was measured using Tecan Infinite M200 plate luminometer (Schoeller Instruments, Czech Republic). Experiments were performed in triplicates and data are expressed as fold induction over negative control (DMSO; 0.1% v/ v).
2.4. mRNA isolation and quantitative reverse transcriptase polymerase chain reaction
Total RNA was isolated using TRI Reagent® (Sigma Aldrich, Prague, Czech Republic). The concentration and purity of total RNA was determined spectrophotometrically. 1000 ng of total RNA was used for cDNA synthesis using M-MuLV reverse transcriptase (New England Biolabs, Ipswich, MA, USA). The reverse transcription was performed in 42 °C for 60 min using Random Primers 6 (New England Biolabs, Ipswich, MA, USA) and diluted in ratio 1:4 by PCR grade water. The quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed on the Lightcycler 480 II using the LightCycler ® 480 Probes Master (Roche Diagnostic Corporation, Prague, Czech Republic). The levels of CYP1A1, CYP1A2 and GAPDH mRNAs were determined using the Universal Probe Library probes (UPL; Roche Diagnostic Corporation, Prague, Czech Republic) in combination with specific primers as described previously (Kubesova et al., 2016). The following protocol was used: an activation step at 95 °C for 10 min was followed by 45 cycles of PCR (denaturation at 95 °C for 10 s; annealing with elongation at 60 °C for 30 s). All measurements were performed in triplicates and the gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a house-keeping gene. Data were processed using delta-delta Ct method and expressed as fold induction over negative control (DMSO).
2.5. Simple western blotting by Sally Sue™
Total protein extracts were prepared using non-denaturizing ice-cold lysis buffer (150 mM
NaCl; 10 mM Tris pH 7.2; 0.1% (w/v) SDS; 1% (v/v) Triton X-100; anti-phosphatase cocktail; anti-protease cocktail; 1% (v/v) sodium deoxycholate; 5 mM EDTA). The concentration of proteins was determined using Bradford reagent.
All reagents, capillaries and 384-well plates used for the simple western by Sally Sue™ were purchased from ProteinSimple (San Jose, CA, USA) and handled according to the manufacturer’s instructions. CYP1A1 (mouse monoclonal, sc-393979, A-9, dilution 1:100) and CYP1A2 (mouse monoclonal, sc-53614, 3B8C1, dilution 1:1000)primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), β-actin (mouse monoclonal; 3700S, 8H10D10, dilution 1:1000) primary antibody was obtained from Cell Signalling Technology (Denvers, Massachusetts, USA). Target proteins were detected by conjugation of specific primary antibodies with horseradish-conjugated secondary antibody followed by reaction with chemiluminescent substrate. Data were analysed using the Compass Software version 2.6.5.0 by ProteinSimple. For the purposes of quantification, signals were normalized to β-actin as a loading control and expressed as fold induction over negative control (DMSO). Note: The presented data are not recorded gels but intensity bands generated by ProteinSimple software from automated capillary electrophoresis analyses.
2.6. CYP1A1 enzyme activity in human liver microsomes
XTreme 200 Pooled Human liver microsomes were obtained from XenoTech (Lenexa, KS, USA). The CYP1A1 enzyme activity was determined using P450-Glo™ CYP1A1 (Luciferin-CEE) Assay system from Promega (Hercules, CA, USA). The reaction was performed using 96-well white plates and the luminescence signal was recorded by Tecan Infinite M200 Pro plate reader (Schoeller Instruments, Prague, Czech Republic). The amount of produced luminescence is directly proportional to CYP1A1 activity. Reaction mixtures were buffered with 100 mM K2PO4 (pH 7.4) and contained test compound, human liver microsomes (HLM, 1 mg/ml), specific enzyme substrate (Luciferin-CEE) and NADPH regenerating system. Tested MMI were assayed in concentrations between 0.1 μM and 200 μM. Each reaction was performed in duplicates at 37 °C, and two independent measurements were performed for each replicate. Inhibition of CYP1A1 catalytic activity was evaluated by plotting remaining percentage of activity against concentration of tested compound.
2.7. Single concentration inactivation assay in human liver microsomes
A single concentration of MMI (200 μM) and vehicle (DMSO) was pre-incubated for 30 min at 37 °C, with HLM (in amount 10-fold higher than required for the assay; 10mg/mL) containing NADPH regenerating system. Thereafter, an aliquot from pre-incubation was incubated for additional 30 min in the buffer containing Luciferin-CEE. In parallel, a single concentration (200 μM) of MMI was pre-incubated in the absence of NADPH regenerating system, which was added as late as into the secondary incubation. The percentage of inhibition, expressing time-dependent inhibition, observed following the pre-incubation was calculated using the equation below, where R is response of the metabolite (Atkinson et al., 2005
2.8. CYP1A1 enzyme activity in LS180 cells and primary human hepatocytes
The P450-Glo™ CYP1A1 cell-based assay from Promega (Hercules, CA, USA) was used to determine CYP1A1 catalytic activity in cultured adherent cells. The amount of produced luminescence was directly proportional to CYP1A1 activity. Experiments were performed in two layouts: (i) Assessing CYP1A1 induction and inhibition by MMI: Cells were incubated for 24 h with MMI in concentrations ranging from 0.1 μM to 200 μM, vehicle (DMSO; 0.1% v/v) and TCDD (5 nM). Thereafter, enzyme substrate luciferin-CEE was applied for additional 3 h; (ii) Assessing inhibition of TCDD-induced CYP1A1 by MMI: Cells were incubated for 24 h with TCDD (5 nM). Thereafter, enzyme substrate luciferin-CEE together with MMI was applied for additional 3 h.
Two independent experiments were performed, and in each, incubations were in duplicates. Net luminescence signals were calculated by subtracting background luminescence values (no-cell control) from treated and untreated values. Induction of CYP1A1 catalytic activity was evaluated by plotting fold induction against MMI concentrations.
2.9. Metabolism of mono-methylindoles in human hepatocytes by liquid chromatography / mass spectrometry (LC/MS)
Three cultures of primary human hepatocytes (LH75, Hep200565, Hep200570) were incubated for 24 h with MMI in 200 μM concentration. Thereafter, culture media were aspired and used for reporter gene assay in AZ-AHR cells (vide supra) and for LC/MS analyses. Briefly, a slightly modified method of previously reported one (Zhang et al., 2014) was used for separation of metabolites using the UHPLC Dionex UltiMate 3000 (Thermo Fisher Scientific) coupled with the LCQ Fleet™ Ion Trap Mass Spectrometer (Thermo) using the electrospray ionization in positive ionization mode. The mobile phase consisted of water supplemented with 0.1% trifluoroacetic acid (TFA) and acetonitrile supplemented with 0.1% of TFA. During the 18 min analysis, the mobile phase gradient was used starting with 95% of water for 1 min, followed by the linear gradient to 15 min down to 5% of water, followed by the rapid linear gradient to 18 min reconstituting the starting 95% of water. The stable mobile phase flow of 0.5 mL/min was applied at the reverse-phase chromatographic column Agilent Poroshell 120 EC-C18, 4.6 × 50 mm, 2.7 μm particle size. The PDA detector was set to the detection wavelengths of 254 ± 4nm, 210 ± 4nm, 225 ± 4nm (used for quantification of residual levels of MMI) and 350 ± 4 nm and the MS analyser was set to detect the mass range of 50–2000 m/z. The sample volume applied to the column was 10 μl in all cases. All samples were analysed in three parallel determinations and the LC/MS data were analysed using the Thermo Excalibur 2.2 Qual Browser (Thermo).
2.10. Statistics
In order to determine significantly different results over negative control (vehicle; DMSO; 0.1%), one-way analysis of variance (ANOVA) followed by Dunnett's test was applied. The result was considered significant if the p-value was lower than 0.05. Prior to the ANOVA, the normality of data was checked using the Shapiro-Wilk normality test. The IC50 calculations were determined using the nonlinear regression fitting by least-square method. The D’Agostino-Pearson omnibus normality test was applied and strict convergence criteria were selected to verify the normality of the data. The R-squared value was checked in all of the calculations and did not drop below 0.7. All the calculations were performed using GraphPad Prism version 8.0 for Windows (GraphPad Software, La Jolla, CA).
3. Results
3.1. Mono-methylindoles induce CYP1A1 and CYP1A2 mRNAs in HepaRG cell by AhR-dependent mechanism
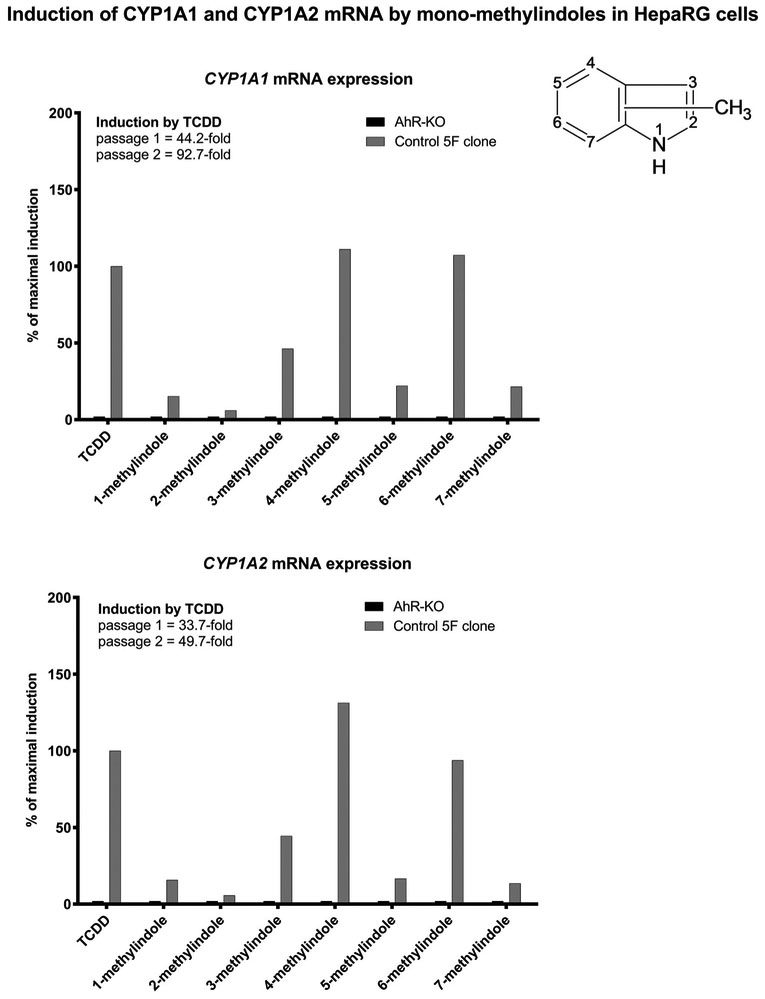
In the first series of experiments, we examined the effects of MMI on the expression of CYP1A1 and CYP1A2 mRNAs, which are prototypical AhR-dependent genes (Fig. 1). We used progenitor hepatic cells HepaRG that are highly competent for induction studies of xenobiotic-metabolizing genes. Control clone (5 F) and AhR knock-out variant of HepaRG cells (AhR-KO) were incubated for 24 h with vehicle (DMSO; 0.1%), model CYP1A inducer dioxin (TCDD; 5 nM) and test MMI (200 μM). The expression of CYP1A genes was determined by the means of RT-PCR. In two consecutive passages of 5 F-HepaRG cells, dioxin strongly induced CYP1A1 (44-fold and 93-fold) and CYP1A2 (34-fold and 50-fold) mRNA. Derivatives 4-methylindole and 6-methyl indole induced CYP1A genes with efficacy similar to that of TCDD (100%). Modest but variable levels of CYP1A induction was achieved by 1-methylindole (15%), 3-methyl indole (~50%), 5-methylindole (~20%) and 7-methylindole (~20%), while 2-methylindole was nearly inactive. MMI did not induce CYP1A1 mRNA in AhR-KO-HepaRG cells (Fig. 1).
Fig. 1.
Effects of MMI on the expression of CYP1A1 and CYP1A2 mRNAs in HepaRG cells. AhR knock-out (AHR-KO) and control 5 F Clone of HepaRG cells were incubated for 24 h with the vehicle (DMSO; 0.1% v/v), dioxin (TCDD; 5nM) and mono-methylindoles (MMI; 200 μM). The expression of CYP1A1 and CYP1A2 mRNAs was determined by RT-PCR in two consecutive cell passages (n = 2) and the data were normalized to GAPDH mRNA level. RT-PCR analyses were carried out in triplicates (technical replicates).
3.2. Mono-methylindoles induce CYP1A1 and CYP1A2 mRNAs and proteins in primary human hepatocytes
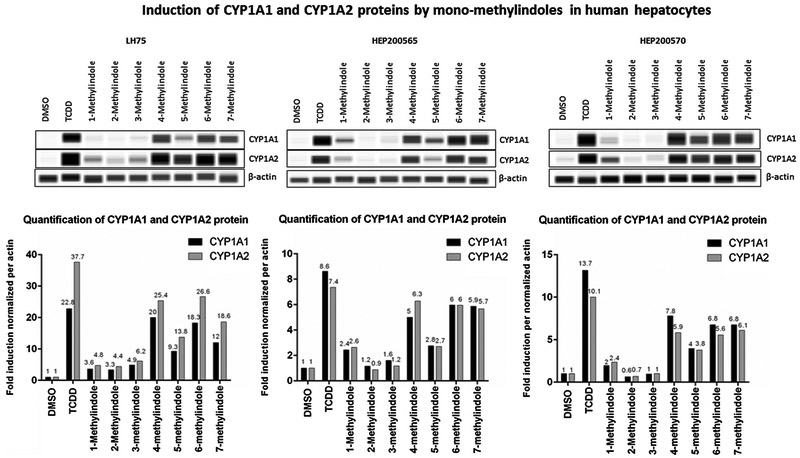
We also examined the effects of MMI on the expression of CYP1A1 and CYP1A2 mRNAs and proteins in primary human hepatocytes cultures, obtained from three different donors of liver tissue (Figs. 2 and 3). Human hepatocytes were incubated for 24 h with vehicle (DMSO; 0.1%), dioxin (TCDD; 5nM) and MMI (200 μM). The expression of CYP1A genes was determined by the means of RT-PCR. The levels of CYP1A1 and CYP1A2 proteins were determined by quantitative automated western-blot analyzer SallySue. Dioxin strongly induced CYP1A1 and CYP1A2 mRNAs and proteins in all three human hepatocytes cultures. The effects of MMI were highly consistent between individual human hepatocytes cultures and the changes in the expression of CYP1A1 and CYP1A2 mRNAs were followed by the same trend at the levels of CYP1A1 and CYP1A2 proteins. Strong inducers of CYP1A1 and CYP1A2 were MMI methylated at positions 4, 5, 6 and 7. Modest induction of CYP1A was achieved by 1-methylindole and 3-methylindole, while 2-methylindole had no effect (Figs. 2 and 3). The effects of MMI on CYP1A expression in human hepatocytes are in accordance with those observed in HepaRG cells. The exception was 7-methylindole, which was modest CYP1A inducer in HepaRG cells (Fig. 1) and in LS180 intestinal cells (Stepankova et al., 2018), but strong inducer in all human hepatocytes cultures (Fig. 2). Such a behavior indicates possible metabolic enhancement of 7-methylindole AhR-activity by human hepatocytes.
Fig. 2.
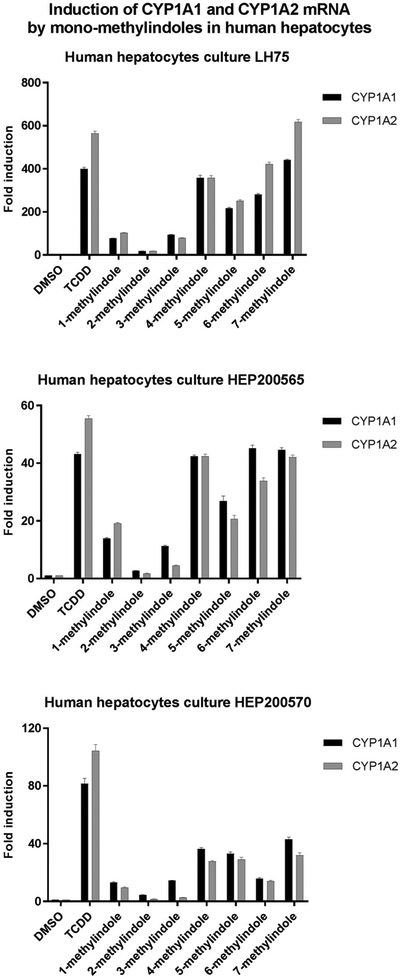
Effects of MMI on the expression of CYP1A1 and CYP1A2 mRNAs in primary human hepatocytes. Primary human hepatocytes cultures, obtained from three liver tissue donors (n = 3) (LH75, HEP200565, HEP200570) were incubated for 24 h with the vehicle (DMSO; 0.1% v/v), dioxin (TCDD; 5nM) and mono-methylindoles (MMI; 200 μM). The expression of CYP1A1 and CYP1A2 mRNAs was determined by RT-PCR and the data were normalized to GAPDH mRNA level. Data in each hepatocytes culture are expressed as the means ± SD from triplicate RT-PCR measurements (technical replicates).
Fig. 3.
Effects of MMI on the expression of CYP1A1 and CYP1A2 proteins in primary human hepatocytes. Primary human hepatocytes from three donors (n = 3) (cultures LH75, HEP200565, HEP200570) were incubated for 24 h with the vehicle (DMSO; 0.1% v/v), dioxin (TCDD; 5 nM) and mono-methylindoles (MMI; 200 μM). The levels of CYP1A1 and CYP1A2 proteins were determined by quantitative automated western-blot analyzer SallySue. Upper panels: Records of CYP1A1, CYP1A2 and β-actin proteins from SallySue software: Lower panels: Bar graphs show quantified CYP1A1 and CYP1A2 proteins, normalized to β-actin levels.
Note: The presented data are not recorded gels but intensity bands generated by ProteinSimple software from automated capillary electrophoresis analyses.
Note: The presented data of CYP1A1, CYP1A2 and β-actin proteins are not recorded gels but intensity bands generated by ProteinSimple software from automated capillary electrophoresis analyses.
3.3. Effects of mono-methylindoles on CYP1A1 catalytic activity in cultured human hepatocytes and LS180 cells
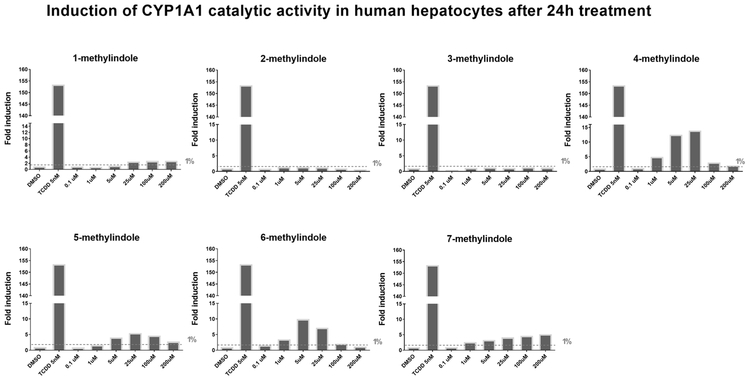
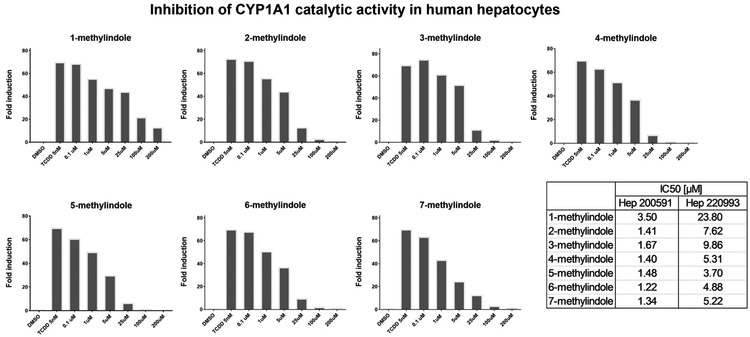
Since prototypical functional endpoint of AhR-dependent gene induction is the expression of CYP1A1 enzyme, we assayed the effects of MMI on CYP1A1 catalytic activity in cell cultures, using primary human hepatocytes and intestinal LS180 cells. Importantly, measuring CYP1A1 catalytic activity comprises the information about the amount (induction or degradation) and catalytic activity (inhibition) of CYP1A1 enzyme. Therefore, experiments were performed in two layouts: (i) Assessing CYP1A1 induction and inhibition: Cells were incubated for 24 h with MMI (0.1 μM - 200 μM); thereafter, enzyme substrate luciferin-CEE was applied for additional 3 h (Fig. 4; Fig. 6); (ii) Assessing inhibition of TCDD-induced CYP1A1: Cells were incubated for 24 h with TCDD (5 nM). Thereafter, enzyme substrate luciferin-CEE together with MMI (0.1 μM - 200 μM) was applied for additional 3 h (Fig. 5; Fig. 7). The induction of CYP1A1 enzyme activity by TCDD in human hepatocytes cultures Hep200571 and Hep220993 was 11-fold and 153-fold, respectively. No induction was observed in human hepatocytes incubated with 2-methylindole and 3-methylindole. Concentration-dependent induction of CYP1A1 activity was attained by 1-methylindole and 7-methylindole, but the relative efficacy was very weak (~2%) as compared to TCDD. Inverse U-shaped, concentration-dependent profiles of CYP1A1 induction, peaking between 5 μM and 25 μM, were observed for 4-methylindole, 5-methylindole and 6-methylindole (Fig. 4). Catalytic activity of CYP1A1 was concentration-dependently inhibited by all tested MMI, in human hepatocytes pre-incubated for 24 h with TCDD, with IC50 values ranging from 1.2 μM to 23.8 μM (Fig. 5).
Fig. 4.
Induction of CYP1A1 catalytic activity in primary human hepatocytes by mono-methylindoles. Primary human hepatocytes from two donors (n = 2) (cultures Hep200571 and Hep220993) were incubated with mono-methylindoles (0.1 μM, 1 μM, 5 μM, 25 μM, 100 μM, 200 μM), TCDD (5 nM) and vehicle (DMSO; 0.1% v/v) for 24 h. Specific CYP1A1 substrate luciferin-CEE was then applied to the cells for additional 3 h. Net luminescence signals were calculated by subtracting background luminescence values (no-cell control) from treated and untreated values. Induction of CYP1A1 catalytic activity was evaluated by plotting fold induction against MMI concentrations. Incubations were performed in two parallel wells (technical duplicates). Bar graph shows data from culture Hep220993.
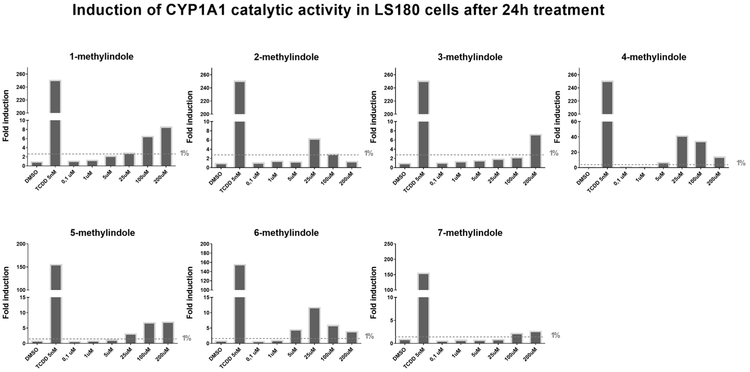
Fig. 6.
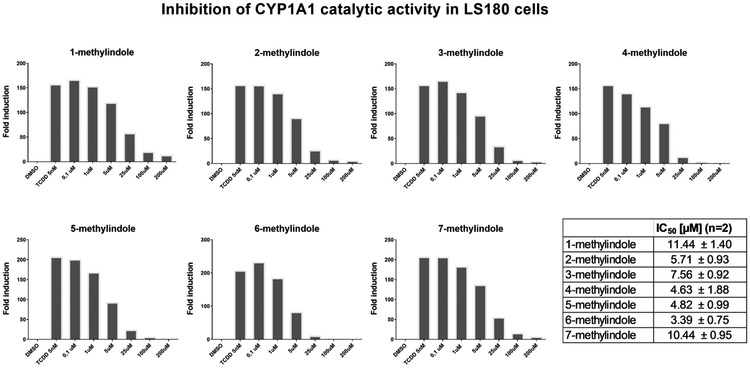
Induction of CYP1A1 catalytic activity in LS180 cells by mono-methylindoles. Intestinal LS180 cells were plated and stabilized for 24 h. Thereafter, cells were incubated with mono-methylindoles (0.1 μM, 1 μM, 5 μM, 25 μM, 100 μM, 200 μM), TCDD (5 nM) and vehicle (DMSO; 0.1% v/v) for 24 h. Specific CYP1A1 substrate luciferin-CEE was then applied to the cells for additional 3 h. Net luminescence signals were calculated by subtracting background luminescence values (no-cell control) from treated and untreated values. Induction of CYP1A1 catalytic activity was evaluated by plotting fold induction against MMI concentrations. Two independent experiments were performed, and in each, incubations were in duplicates.
Fig. 5.
Inhibition of CYP1A1 catalytic activity in primary human hepatocytes by mono-methylindoles. Primary human hepatocytes from two donors (n = 2) (cultures Hep200571 and Hep220993) were pre-incubated with 5 nM TCDD for 24 h. Thereafter, mono-methylindoles (0.1 μM, 1 μM, 5 μM, 25 μM, 100 μM, 200 μM) in the mixture with specific CYP1A1 substrate luciferin-CEE were applied to the cells for 3 h. Incubations were performed in two parallel wells (technical duplicates). Bar graph shows data from culture Hep220993. The values of IC50 were calculated using data from both cultures and inserted in the figure (bottom right).
Fig. 7.
Inhibition of CYP1A1 catalytic activity in LS180 cells by mono-methylindoles. Intestinal LS180 cells were plated and stabilized for 24 h, prior to the incubation with 5 nM TCDD for additional 24 h. Thereafter, mono-methylindoles (0.1 μM, 1 μM, 5 μM, 25 μM, 100 μM, 200 μM) in the mixture with specific CYP1A1 substrate luciferin-CEE were applied to the cells for 3 h. Two independent experiments (biological replicates) were performed, and in each, incubations were in duplicates (technical replicates). The values of IC50 were calculated and inserted in the figure (bottom right).
The induction of CYP1A1 catalytic activity in LS180 cells by TCDD ranged from 150-fold to 250-fold as compared to vehicle-treated cells. All tested MMI induced CYP1A1 catalytic activity in LS180 cells, yielding distinct concentration-response profiles. Concentration-dependent pattern was observed for 1-methylindole, 3-methylindole, 5- methylindole and 7-methylindole. In contrast, inverse U-shape profiles were observed for 2-methylindole, 4-methylindole and 6-methylindole, peaking at 25 μM concentration. Maximal relative efficacies of MMI ranged from 2% to 25%, as compared to 5 nM TCDD (Fig. 6). All tested MMI inhibited concentration-dependently CYP1A1 catalytic activity in TCDD-pre-incubated LS180 cells, with IC50 values ranging from 3.4 μM to 11.4 μM (Fig. 7).
3.4. Enzyme kinetic of CYP1A1 in human liver microsomes by mono-methylindoles
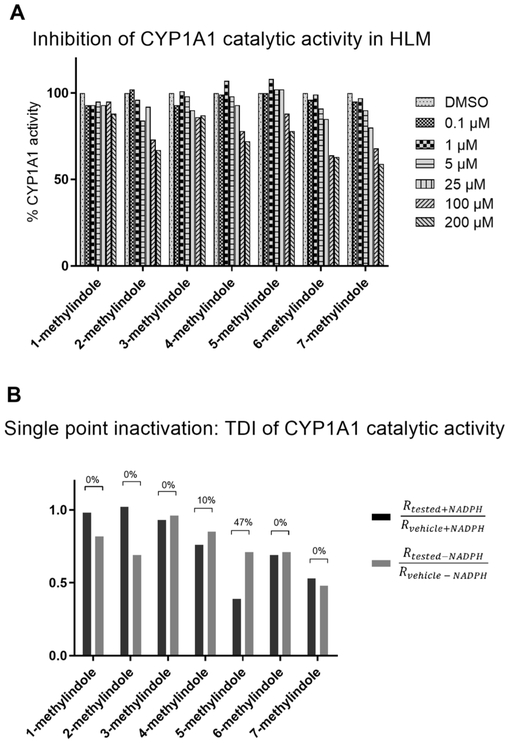
Inhibition of CYP1A1 activity by MMI in HLM was assessed using specific enzyme substrate Luciferin-CEE. Tested MMI were used in concentrations 0.1 μM, 1 μM, 5 μM, 25 μM, 100 μM and 200 μM. With exception of 1-methylindole, all MMI caused concentration-dependent inhibition of CYP1A1 activity. However, these effects were rather moderate and IC50 values were not reached even at 200 μM concentrations of MMI (Fig. 8A). We also performed a single concentration inactivation assay, using 200 μM concentration of MMI. This assay allows uncovering time-dependent inhibition (TDI) of CYP1A1, which refers to a change in potency during an in vitro incubation. We observed TDI for 4-methylindole (10%) and 5-methylindole (47%) (Fig. 8B). Potential mechanisms may include the formation of inhibitory metabolites or mechanism-based inhibition.
Fig. 8.
Effects of mono-methylindoles on catalytic activity of CYP1A1 in human liver microsomes. Inhibition of CYP1A1 activity by MMI in HLM was assessed using specific enzyme substrate Luciferin-CEE. Two independent experiments were performed, with measurements in duplicates. Panel A: MMI were used in concentrations 0.1 μM, 1 μM, 5 μM, 25 μM, 100 μM and 200 μM. Inhibition of enzyme activity was expressed as the mean ± SD and bar graph shows per cents of remaining activity relative to the control (100%, without MMI). Panel B: Single point inactivation assay, using 200 μM concentration of MMI. Bar graph shows the percentage of inhibition, expressing time-dependent inhibition (TDI) (for details see Materials and Methods).
3.5. Agonist activity of mono-methylindoles at AhR is altered after pre-incubation with human hepatocytes
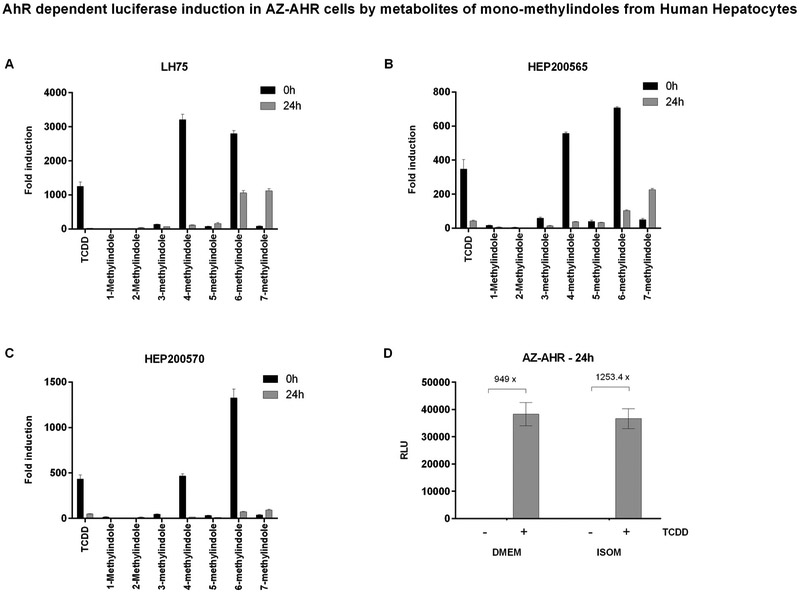
Indoles undergo cytochrome P450-mediated oxidative metabolic transformation, as documented in literature (Gillam et al., 2000) or in our microsomal time-dependent inhibition assay (Fig. 8). Therefore, we collected culture media from human hepatocytes incubated for 0 h and 24 h with MMI, and we subjected these samples to reporter gene assay in AZ-AHR cells to find out, whether biotransformation alters AhR agonist activity of parental MMI. Firstly, we proved that the application of ISOM culture medium from human hepatocytes does not influence the responsivity of AZ-AHR cells to AhR agonists as compared to common DMEM medium (Fig. 9D). Pre-incubation of 4-methylindole and 6-methylindole with three different human hepatocytes cultures (LH75, Hep200565, Hep200570) led to drastic diminution of AhR-agonist activity of these two derivatives. In lesser extent, AhR-agonist activity of 3-methylindole also dropped. In contrast, 7-methylindole, which is weak agonist of AhR, gained strong AhR-agonist activity after the incubation with human hepatocytes (Fig. 9). These data reveal that hepatic cells transform MMI into metabolites with altered AhR-agonist activities.
Fig. 9.
Agonist activities of MMI metabolites at AhR in reporter gene assay. Primary human hepatocytes cultures (LH75, Hep200565, Hep200570) were incubated for 0 h and 24 h with mono-methylindoles (200 μM). Thereafter, culture medium from hepatocytes cultures was collected and applied to AZ-AHR cells for 24 h (Panels A, B, C). AZ-AHR cells were lysed and luciferase activity was measured. Data are expressed as a fold induction of luciferase activity over vehicle-treated cells and they are the mean ± SD from triplicates. Panel D: Responsivity of AZ-AHR to TCDD (24 h; 5 nM) in DMEM and ISOM culture media.
3.6. Metabolism of mono-methylindoles in primary human hepatocytes
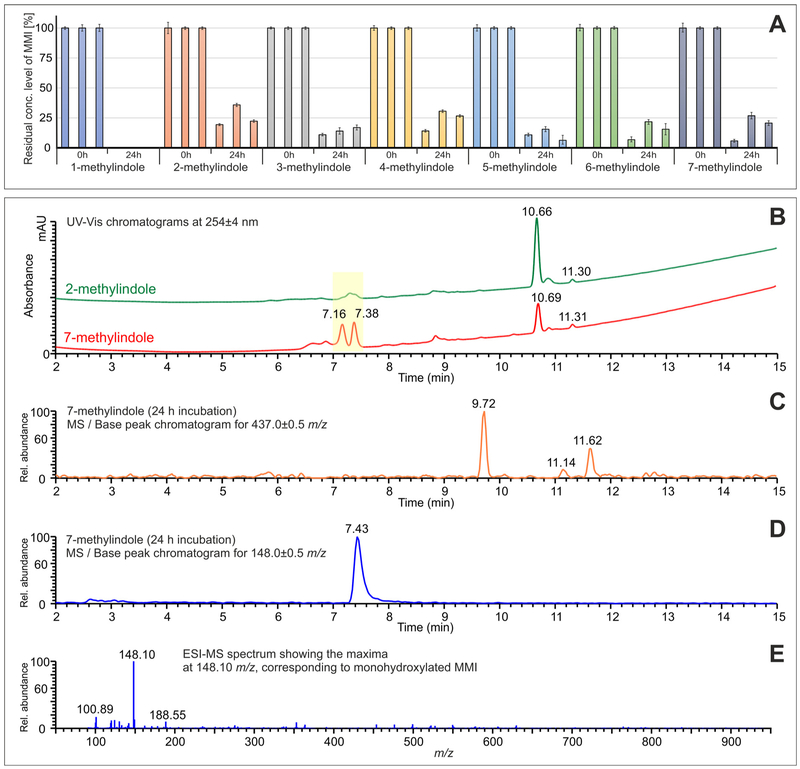
In final series of experiments, we performed liquid chromatography / mass spectrometry (LC/MS) analyzes of culture media from three human hepatocytes cultures incubated with MMI. The concentration of parental MMI in culture media drastically dropped after incubation with human hepatocytes; in the samples treated with 1-methylindole, no residual MMI was detected after 24 h. In case of the samples treated with other MMI, the residual concentrations of MMI were in the range of 6.3–35.9 % of the initial 200 μM concentration and apparent differences were observed between the three cultures of used hepatocytes (Fig. 10A). The causes for such a decrease of MMI in media may comprise metabolic transformation, but also active uptake by human hepatocytes, degradation in media or non-specific binding. We also aimed to identify the classes of putative MMI metabolites, employing the means of selective molecular mass search, based on the previously published metabolic studies (Zhang et al., 2014; Lanza et al., 1999). In our specific case, we were able to identify two major types of metabolites in the cell culture media after 24 h of incubation. The first set of mass-ionization peaks identified represents the various derivatives of mono-oxidated MMI, such as hydroxylated or monomethyl-oxindole derivatives, which were detected in the region of tR = 7.0–7.5 min and were represented by the molecular peak at 148.10 m/z in the mass spectrum (Fig. 10D and E), corresponding to the mono-protonated species. In this region, we observed substantial differences between the chromatograms obtained for specific MMI. The most substantial differences were observed between the chromatograms obtained for 2-methylindole and 7-methylindole (see the highlighted region in Fig. 10B), while the peak at tR =7.16 min was observed in the chromatogram for 7-methylindole only. The second set of mass-ionization peaks identified comprised GSH-conjugated MMI derivatives, observed at tR ≈ 9.70 min, 11.15 min, and 11.60 min (Fig. 10C), represented by the molecular peak at 437.13 m/z in the mass spectra, corresponding to the [GS–MMI+H]+ species.
Fig. 10.
Liquid Chromatography / Mass Spectrometry analyzes of mono-methylindoles metabolites. Panel A: The quantitative evaluation of residual MMI levels in culture media after 24 h incubation with human hepatocytes (cultures LH75, Hep200565, Hep200570); Panel B: The comparison of UV/Vis chromatograms for the samples of 2-methylindole and 7-methylindole showing the significant differences in the region of mono-oxidated metabolites (highlighted in yellow); Panel C: The base-peak chromatogram for the mass range of 437.0 ± 0.5 m/z showing the GSH-conjugated metabolites of 7-methylindole at tR =9.72 min, 11.14 min, and 11.62 min; Panel D: The base-peak chromatogram for the mass range of 148.0 ± 0.5 m/z showing the broad peak of mono-oxidated metabolites of 7-methylindole at tR =7.43 min; Panel E: Mass spectrum, corresponding to the mono-oxidated species of 7-methylindole with the maxima at 148.10 m/z, i.e. [MMI+H]+ species.
4. Discussion
Compounds with an indole ring in their structure, including xenobiotics, endogenous compounds or microbial products, have been demonstrated as ligands of human AhR (Hubbard et al., 2015). A wide array of indole-based AhR-active substances is produced by intestinal microbiota from dietary precursors, such as glucobrassicin, and from essential amino acid tryptophan that originates mainly from cleavage of dietary proteins. For instance, skatole (3-methylindole), which is produced by decarboxylation of indole-3-acetate by human intestinal microbiome (Roager and Licht, 2018), was recently described as a partial agonist of human AhR (Rasmussen et al., 2016). As a prelude to further discovery of such indole derivatives, we have systematically investigated the entire series of mono-methylated indoles as ligands for AhR-CYP1A activation and antagonism. The effects overall of MMI on AhR-CYP1A pathway in human cells comprise the induction of CYP1A genes through AhR, the inhibition of CYP1A catalytic activity and possibly the metabolic transformation causing loss or gain of AhR agonist activity of parental compounds.
The relative efficacy of individual MMI at AhR differed substantially. For example, 4-methylindole and 6-methylindole were approx. 25 times more efficacious AhR agonists as compared to 3-methylindole (skatole), while 2-methylindole was nearly inactive at AhR (Stepankova et al., 2018). Given the fact that very subtle change in chemical structure of MMI, such as a shift in methyl moiety at indole ring, results in dramatic impact on MMI activity at AhR, we performed current follow-up study. Firstly, we show that MMI induce CYP1A1 and CYP1A2 genes in human hepatic progenitor cells HepaRG, by AhR-dependent mechanism, which is supported by the loss of CYP1A induction in AhR knock-out HepaRG cells. MMI also induced CYP1A1 and CYP1A2 mRNAs and proteins in primary cultures of human hepatocytes. Relative efficacies of MMI in HepaRG cells and human hepatocytes were similar to those observed previously for CYP1A1 in intestinal cancer cells LS180 (Stepankova et al., 2018), with one exception. While 7-methylindole was weak inducer of CYP1A1 in LS180 and HepaRG, it was very strong inducer of CYP1A genes in three cultures of human hepatocytes. Since primary human hepatocytes are fully equipped with xenobiotic-metabolizing apparatus, the plausible explanation for strong gain of capability to induce CYP1A genes by 7-methylindole might be its metabolic conversion to highly AhR-active product. We observed substantially increased activation of AhR in transgenic reporter gene cells AZ-AHR by culture media from human hepatocytes incubated 24 h with 7-methylindole as compared to media containing 7-methylindole without pre-incubation, which corroborates the hypothesis about metabolic activation of 7-methylindole. In contrast to gain of AhR activity of 7-methylindole, we observed loss of AhR agonist activity of 4-methylindole after incubation with human hepatocytes. Drastic drop of MMI concentrations in culture media after 24 h incubation with human hepatocytes indicates either metabolic degradation or active cellular uptake of parental MMI. By the means of LC/MS analyses, we observed in the cell culture media the formation of different derivatives of mono-oxidated MMI (mono-hydroxylated MMI or mono-methyl-oxindole derivatives) and GSH-conjugates of MMI. Significant differences were observed in the amount and types of mono-oxidated metabolites in the samples. Conjugation with large polar molecule of glutathione and/or the introduction of hydroxyl group in indole skeleton could compromise AhR agonist activity of MMI, the latter being observed also by other authors (Heath-Pagliuso et al., 1998). Speculatively, this could be a case of AhR agonist activity loss for 4-methylindole. On the other hand, formation of mono-methyl-oxindoles might lead to the formation of condensed products, analogical to indigo and indirubin, which are prominent AhR agonists. This could be the reason for gain of AhR agonist activity by 7-methylindole. Collectively, distinct metabolic signatures for individual MMI might result in no change, loss or gain of AhR agonist activity.
The activators of AhR and inducers of CYP1A genes are often the inhibitors of CYP1A enzymes. Indeed, this was also the case of MMI, which inhibited catalytic activity of CYP1A1 in cultured LS180 cells and human hepatocytes. Interestingly, the inhibition of CYP1A1 by MMI in human liver microsomes was much weaker as compared to hepatocytes or LS180 cells, suggesting formation of inhibitory metabolites in the cells. Another explanation could be a formation of metabolites with altered inhibitory capacity against CYP1A1, following the incubation with human hepatocytes (Figs. 4 and 5) and LS180 cells (Figs. 6 and 7). Hence, the measurements of CYP1A1 catalytic activity in cell cultures comprise mixed effects of parental MMIs and their metabolites. In addition, single concentration inactivation assay revealed time-dependent inhibition of CYP1A1 by 4-methylindole and 5-methylindole. Importantly, the overall effects of MMIs on CYP1A1 enzyme activity in cells cultured for 24 h (Figs. 4 and 6) comprise both inhibition of catalytic activity and increase of CYP1A1 amount by the gene induction, which may yield atypical dose-response profiles. Indeed, the sum effects of 4-methylindole and 6-methylindole in human hepatocytes and LS180 cells involve concentration-dependent increase on CYP1A1 amount (induction) and concentration-dependent inhibition of CYP1A1 activity, which results in inverse U-shaped profiles.
Overall, the effects of MMI on AhR-CYP1A signaling pathway involve agonist/antagonist effects at AhR and consequent changes in the expression of CYP1A genes, inhibition of CYP1A1 catalytic activity and the combined action of parental compounds and their metabolites. The data presented here might represent a cornerstone for development of highly efficacious AhR-active skatole-like scaffolds with potentially therapeutic use in inflammatory bowel disease.
Acknowledgments
Funding
We acknowledge financial support from Czech Science Foundation [19-00236S], the student grant from Palacky University in Olomouc [PrF-2019-003], the Operational Programme Research, Development and Education - European Regional Development Fund, the Ministry of Education, Youth and Sports of the Czech Republic [CZ.02.1.01/0.0/ 0.0/16_019/0000754], ICTR Pilot Award [AECOM], Broad Medical Research Program at Crohn’s & Colitis Foundation of America Grant [362520]; Department of Defense Partnering PI [PR160167 and R43DK105694 and P30DK041296], National Institute of Health [CA127231, CA161879, CA222469], Diabetes Research Center Grant [P30 DK020541] and Cancer Center Grant [P30CA013330].
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARNT
AhR nuclear translocator
- HLM
human liver microsomes
- MMI
mono-methyl-indole
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TDI
time-dependent inhibition
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Data availability statement
All data generated or analyzed during this study are included in this published article.
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Roager HM, Licht TR, 2018. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9 (1), 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Joshi AD, Elferink CJ, 2013. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J. Pharmacol. Exp. Ther. 345 (3), 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O, 2016. The role of AHR-inducible cytochrome P450s in metabolism of polyunsaturated fatty acids. Drug Metab. Rev. 48 (3), 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Haarmann-Stemmann T, 2018. Modulation of CYP1A1 metabolism: from adverse health effects to chemoprevention and therapeutic options. Pharmacol. Ther. 187, 71–87. [DOI] [PubMed] [Google Scholar]
- Lanza DL, Yost GS, 2001. Selective dehydrogenation/oxygenation of 3-methylindole by cytochrome p450 enzymes. Drug Metab. Dispos. 29 (7), 950–953. [PubMed] [Google Scholar]
- Stejskalova L, Dvorak Z, Pavek P, 2011. Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr. Drug Metab. 12 (2), 198–212. [DOI] [PubMed] [Google Scholar]
- Roman AC, et al. , 2018. The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol. Ther. 185, 50–63. [DOI] [PubMed] [Google Scholar]
- Schiering C, et al. , 2017. Feedback control of AHR signalling regulates intestinal immunity. Nature 542 (7640), 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, et al. , 1998. Aryl hydrocarbon receptor-mediated antiestrogenic and anti-tumorigenic activity of diindolylmethane. Carcinogenesis 19 (9), 1631–1639. [DOI] [PubMed] [Google Scholar]
- Chen I, Safe S, Bjeldanes L, 1996. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochem. Pharmacol. 51 (8), 1069–1076. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, et al. , 2016. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22 (6), 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MK, et al. , 2016. Skatole (3-Methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS One 11 (5) e0154629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, et al. , 2015. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 5 12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankova M, et al. , 2018. Methylindoles and methoxyindoles are agonists and antagonists of human aryl hydrocarbon receptor. Mol. Pharmacol. 93 (6), 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotna A, Pavek P, Dvorak Z, 2011. Novel stably transfected gene reporter human hepatoma cell line for assessment of aryl hydrocarbon receptor transcriptional activity: construction and characterization. Environ. Sci. Technol. 45 (23), 10133–10139. [DOI] [PubMed] [Google Scholar]
- Kubesova K, Travnicek Z, Dvorak Z, 2016. Pleiotropic effects of gold(I) mixed-ligand complexes of 9-deazahypoxanthine on transcriptional activity of receptors for steroid hormones, nuclear receptors and xenoreceptors in human hepatocytes and cell lines. Eur. J. Med. Chem. 121, 530–540. [DOI] [PubMed] [Google Scholar]
- Atkinson A, Kenny JR, Grime K, 2005. Automated assessment of time-dependent inhibition of human cytochrome P450 enzymes using liquid chromatography-tandem mass spectrometry analysis. Drug Metab. Dispos. 33 (11), 1637–1647. [DOI] [PubMed] [Google Scholar]
- Zhang CH, et al. , 2014. For a series of methylindole analogs, reactive metabolite formation is a poor predictor of intrinsic cytotoxicity in human hepatocytes. Toxicol. Res. 3 (3), 184–190. [Google Scholar]
- Gillam EM, et al. , 2000. Oxidation of indole by cytochrome P450 enzymes. Biochemistry 39 (45), 13817–13824. [DOI] [PubMed] [Google Scholar]
- Lanza DL, et al. , 1999. Specific dehydrogenation of 3-methylindole and epoxidation of naphthalene by recombinant human CYP2F1 expressed in lymphoblastoid cells. Drug Metab. Dispos. 27 (7), 798–803. [PubMed] [Google Scholar]
- Heath-Pagliuso S, et al. , 1998. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 37 (33), 11508–11515. [DOI] [PubMed] [Google Scholar]