Abstract
BACKGROUND:
Adolescent intermittent ethanol (AIE) exposure is an emerging risk factor for adult psychopathology such as anxiety disorders. Enhancer RNAs (eRNAs) are short non-coding RNAs transcribed from enhancer regions that regulate synaptic plasticity-associated gene expression including activity-regulated cytoskeleton-associated protein (Arc), but their role in AIE-induced anxiety susceptibility in adulthood is unknown.
METHODS:
Rats were exposed to ethanol (2 days on/off; AIE) or intermittent n-saline (AIS) during postnatal days (PND) 28–41 and allowed to grow to adulthood for analysis of behavior and biochemical measures. Some AIE and AIS rats were exposed to an acute challenge with ethanol in adulthood. Cohorts of alcohol-naïve adult rats were cannulated in the central nucleus of amygdala (CeA) and infused with either lysine demethylase 6B(Kdm6b) small interfering RNA (siRNA) or an antisense locked nucleic acid (LNA) oligonucleotide specific to Arc eRNA prior to behavioral and biochemical analysis.
RESULTS:
AIE adult rats display heightened anxiety and decreased Arc eRNA expression, which is regulated epigenetically through decreased KDM6B expression. This triggers condensed chromatin at the synaptic activity response element (SARE) site and promoter of the Arc gene, facilitating increased negative elongation factor (NELF) binding to the Arc promoter and decreasing Arc expression in the amygdala. Knockdown of Kdm6b or Arc eRNA expression in the CeA provokes anxiety in naïve adult rats and recapitulates the molecular and epigenetic phenotypes of AIE.
CONCLUSION:
These data suggest that eRNA regulation via epigenetic reprogramming in the amygdala, particularly at the Arc SARE site, contributes to adult anxiety after adolescent alcohol exposure.
Keywords: Adolescent alcohol, Amygdala, Anxiety, Arc, Enhancer RNA, epigenetic
INTRODUCTION
The adolescent brain undergoes structural and molecular changes during development (1–3). Both clinical and preclinical findings indicate that binge drinking during adolescence increases risk for alcohol use disorder (AUD) and affective disorders in adulthood (4–8). Patients diagnosed with AUD are more than twice as likely to have an anxiety disorder (7, 9), and preclinical models reveal that exposure to ethanol during adolescence increases anxiety-like behaviors and alcohol preference in adulthood (10–12). These behavioral changes are associated with neurochemical alterations including increased neuroimmune activation, decreased neurotrophin signaling, and altered epigenetic mechanisms (1, 13, 14).
Epigenetics refers to modifications to histone proteins and DNA itself that alter gene expression without altering the underlying DNA sequence (15). Additionally, non-coding RNAs can directly and indirectly regulate gene expression and serve as epigenetic modifiers (16). Adolescent intermittent ethanol (AIE) exposure causes long-lasting epigenetic alterations in the amygdala into adulthood specifically at the promoter regions of the synaptic plasticity-associated genes brain-derived neurotrophic factor (Bdnf) and activity-regulated cytoskeleton-associated protein (Arc), possibly explaining the decreased dendritic spine density and increased anxiety and alcohol intake seen in these animals as this brain region is crucial for affective regulation (10, 11). Interestingly, Arc transcription is tightly regulated by the synaptic activity response element (SARE) site located ~7kb upstream of the transcription start site (TSS) (17), and this site also regulates an RNA product known as the Arc enhancer RNA (eRNA) (18, 19). eRNAs are a recently discovered class of non-coding RNAs bidirectionally transcribed from active enhancer regions, and some of these RNAs have functional roles in the regulation of target gene expression (19, 20). Arc eRNA functions to decoy the negative elongation factor (NELF) protein away from the promoter of Arc, allowing for poised RNA polymerase II (Pol II) to begin elongating and transcribing Arc mRNA (18, 21). The epigenetic regulation of Arc eRNA in the amygdala and its role in AIE-induced anxiety-like behavior is currently unknown.
Several epigenetic marks at histone 3 lysine 27 (H3K27) and H3K4 are critical for regulating enhancer sequences and eRNA expression (19, 22, 23). H3K27ac is rapidly recruited to enhancers of immediate-early genes (IEGs) including Arc in response to neuronal activity, leading to eRNA synthesis that precedes mRNA transcription (18, 24). Additionally, H3K27 trimethylation (H3K27me3) is a repressive mark present at bivalent promoters (25) and poised enhancers (26). The Polycomb repressive complex 2 (PRC2) adds methyl groups to H3K27, while lysine demethylase 6A (KDM6A) and KDM6B are responsible for H3K27me2/3 demethylation (27, 28). KDM6B complexes with members of the cyclic AMP-response element binding protein (CREB) pathway such as CREB binding protein (CBP) to activate expression of activity-regulated genes in response to neuronal depolarization (29, 30). We recently observed that AIE exposure decreases CBP via deficits in H3K9/14 acetylation in the amygdala (31). We hypothesize that KDM6B and CBP may epigenetically regulate amygdala eRNA expression to drive changes in gene expression and anxiety susceptibility after AIE in adulthood. We therefore explored whether AIE produces an enduring impact on amygdaloid histone modifications at the Arc SARE enhancer site and promoter region, thus altering higher-order chromatin interactions leading to aberrant Arc expression, synaptic remodeling, and anxiety susceptibility using an animal model.
METHODS AND MATERIALS
Male Sprague-Dawley adolescent rats [postnatal days (PND) 28–41] were exposed to ethanol (2g/kg intraperitoneal, 2 days on/off; AIE) or intermittent n-saline (AIS) and allowed to mature into adulthood (10,11). A cohort of AIE and AIS adult rats was also challenged with an acute dose of ethanol (2g/kg) or n-saline. All rats were used for anxiety measurement and biochemical analysis in the amygdala. In mechanistic experiments, alcohol-naïve adult rats were cannulated targeting the central nucleus of amygdala (CeA) and infused with either Kdm6b small interfering RNA (siRNA) or an antisense locked nucleic acid (LNA) oligonucleotide specific to Arc eRNA and subjected to anxiety measures followed by molecular analysis in the amygdala. Detailed methods, materials, and statistical analyses are provided in Supplemental Materials.
RESULTS
AIE exposure decreases synapse number and expression of Arc mRNA and eRNA in the adult amygdala
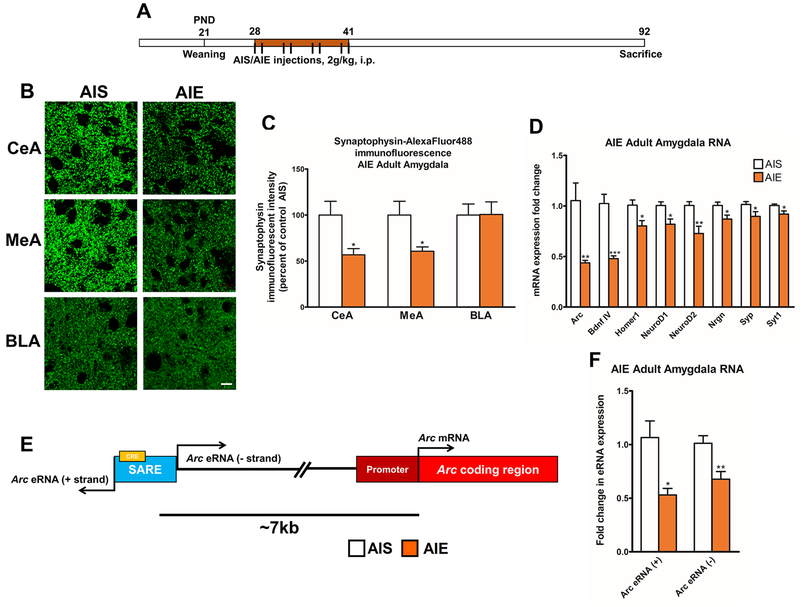
To measure changes in synapse number after AIE (Figure 1A) in the adult amygdala, we quantified synaptophysin (Syp)-immunostaining, which labels presynaptic terminals (32), in the CeA, medial nucleus of amygdala (MeA), and basolateral amygdala (BLA) in adulthood after AIE exposure. AIE adult rats show decreased synaptophysin immunoreactivity in the CeA (p<0.05) and MeA (p<0.05), but not the BLA, compared to adolescent intermittent saline (AIS) exposed adult rats (Figure 1B, C, S1). In order to explore the transcriptional mechanisms underlying the synaptic alterations, we measured the expression of several genes involved in synaptic plasticity and maintenance (32), finding that AIE significantly (p<0.05–0.001) decreased Arc, Bdnf exon IV, Homer1, NeuroD1, NeuroD2, neurogranin (Nrgn), Syp, and synaptotagmin 1 (Syt1) mRNA levels in the adult amygdala (Figure 1D). We generated a gene network with GeneMANIA (33) to prioritize highly interactive genes (Figure S2A) for detailed epigenetic studies. Arc is the ‘hub gene’ in our network of synaptic plasticity-associated genes downregulated after AIE in adulthood (Figure S2B, C). Studies have indicated that the Arc SARE site (Figure 1E) is highly conserved and involved in the fine-tuning of Arc mRNA transcription in response to environmental stimuli (17, 24, 34). We found that levels of Arc eRNA (+) and Arc eRNA (−) are significantly (p<0.05–0.01) decreased in the AIE adult amygdala compared to AIS adult rats (Figure 1F).
Figure 1. Adolescent intermittent ethanol (AIE) exposure leads to synaptic deficits and decreased Arc eRNA and mRNA expression in the amygdala at adulthood.
A) Schematic of AIE (or adolescent intermittent saline [AIS]) treatment.
B) Representative photographs showing synaptophysin (Syp)-immunofluorescent staining visualized with confocal microscopy in the central nucleus of the amygdala (CeA), medial nucleus of the amygdala (MeA), and basolateral amygdala (BLA) of AIS and AIE animals (scale bar = 10 μm).
C) Bar diagram showing quantification of Syp-immunofluorescent staining. Data represent mean ± SEM [n = 5 rats in each group, CeA (t (8) =2.65, *p<0.05) and MeA (t (8) =2.51, *p<0.05) by Student’s t-test].
D) mRNA analysis of synaptic plasticity-associated genes in amygdala tissue obtained from AIS and AIE adult rats. Each gene represents an independent but not repeated measure. Data represent mean ± SEM derived from n = 5–8 rats in each group [Arc, t(9)=3.85, **p<0.01; Bdnf exon IV, t (9)=6.21, ***p<0.001; Homer1, t (11)=2.79, *p<0.05; NeuroD1, t(11)=2.81, *p<0.05; NeuroD2, t(11)=3.27, **p<0.01; Nrgn (neurogranin), t (11)=2.58, *p<0.05; Syp, (t(14)=2.18, *p<0.05; Syt1 (synaptotagmin 1), t(14)=2.46, *p<0.05 by Student’s t-test).
E) Schematic representation of the Arc gene, including the synaptic activity response element (SARE) site, which contains a cAMP response element (CRE) for CREB binding and transcribes enhancer RNA (eRNA) transcripts.
F) RNA analysis of Arc eRNA expression in the amygdala of AIS and AIE adult rats at baseline. Data represent mean ± SEM derived from n=5–6 rats in each group [Arc eRNA (+) strand, t (9) =2.98, p<0.05); Arc eRNA (−) strand, t (9) =5.35, p<0.01) by Student’s t-test].
We next investigated the epigenetic regulation of Arc transcription in the adult amygdala after AIE. We found increased H3K27me3 occupancy at the Arc SARE site and five other sites across the Arc promoter and gene body in the amygdala of AIE adult rats compared to AIS rats (Figure S3A, B). H3K27me3 occupancy was not altered between the groups at the most distal site in intron 2 of the Arc gene body (Figure S3B). We found that mRNA levels of Kdm6b, but not Kdm6a or other members of the PRC2, were decreased in the AIE adult amygdala compared to AIS rats (Figure S3C).
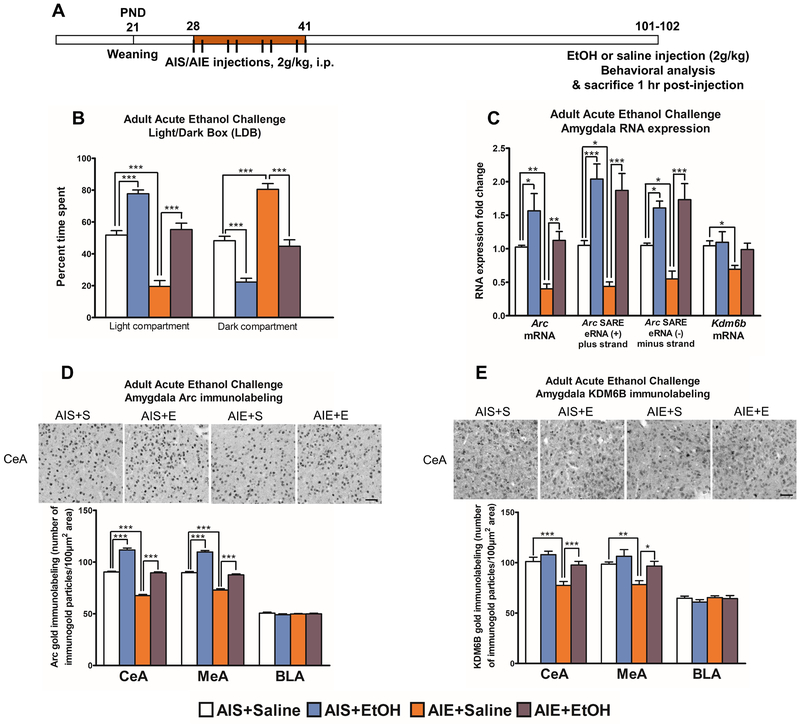
Acute alcohol in adulthood reverses AIE-induced anxiety-like behavior and Arc eRNA & mRNA transcriptional changes in the amygdala
Previous work indicates that acute ethanol exposure (2g/kg) in adulthood in AIE rats decreases anxiety-like behavior in the elevated plus maze (EPM) (10). We extended this finding using the same ethanol exposure paradigm (Figure 2A) but a different anxiety measurement, the light/dark box (LDB). We found that AIE rats spend more time than AIS rats in the dark compartment and less time in the light compartment (p<0.001), indicative of an anxiety-like phenotype, which was attenuated by acute ethanol challenge in adulthood (Figure 2B). Acute ethanol challenge (2g/kg) also leads to anxiolytic-like effects in AIS rats. We found a main effect of acute alcohol exposure on total ambulation in the LDB as a measure of general activity and saw a significant (p<0.05) increase in AIE animals exposed to acute alcohol compared to AIE animals exposed to saline (Figure S4A).
Figure 2. Adult ethanol challenge reverses AIE-induced anxiety-like behaviors and KDM6B and Arc expression deficits in the adult amygdala.
A) Schematic of adolescent intermittent ethanol (AIE) or saline (AIS) treatment followed by adult exposure to acute ethanol challenge (2 g/kg, intraperitoneal injection).
B) Light/dark box (LDB) exploration test for anxiety measures in AIS and AIE adult rats exposed to an acute challenge of either ethanol or saline. Data represent mean ± SEM derived from n=9–10 rats in each group. ***p<0.001 by two-way ANOVA [main effect of both AIE treatment (F1,33=67.3, p<0.001) and acute ethanol exposure (F1,33=85.4, p<0.001)] followed by post-hoc Tukey test.
C) RNA analysis of Arc enhancer RNA (eRNA), Arc mRNA, and Kdm6b mRNA transcripts in the amygdala of rats exposed to either AIS or AIE exposed to acute ethanol or saline in adulthood. Data represent mean ± SEM derived from n=5–6 rats in each group. *p<0.05, **p<0.01, ***p<0.001 by two-way ANOVA [Arc mRNA levels, AIE treatment (F1,20=12.6, p<0.01), acute alcohol exposure (F1,20=17.8, p<0.001); Arc eRNA (+), AIE exposure (F1,20=4.93, p<0.05), acute alcohol exposure (F1,20=47.3, p<0.001); Arc eRNA (−), acute alcohol exposure (F1,19=35.0, p<0.001), interaction between AIE treatment and acute alcohol exposure (F1,19=4.44, p<0.05), Kdm6b mRNA levels, AIE treatment (F1,20=4.90, p<0.05)] followed by post-hoc Tukey test.
D) Representative photomicrographs of gold immunolabeling showing Arc-positive cells in the central nucleus of amygdala (CeA) and quantification of gold-immunolabeling for Arc protein in the CeA, medial nucleus of amygdala (MeA) and basolateral amygdala (BLA) of AIE and AIS adult rats exposed to acute ethanol (E) or saline (S) challenge in adulthood (scale bar = 50 μm). Data represent mean ± SEM (n=5–6 rats in each group). ***p<0.001 by two-way ANOVA [CeA, AIE exposure (F1,19=349.1, p<0.001), acute ethanol challenge (F1,19=327.1, p<0.001); MeA, AIE exposure (F1,19=272.0, p<0.001), acute ethanol challenge (F1,19=217.3, p<0.001)] followed by post-hoc Tukey test.
E) Representative photomicrographs of gold immunolabeling showing KDM6B-positive cells in the CeA and quantification of gold-immunolabeling for KDM6B protein in the CeA, MeA, and BLA of AIE and AIS rats exposed to acute ethanol (E) or saline (S) in adulthood (scale bar = 50 μm). Data represent mean ± SEM derived from n=6 rats in each group. *p<0.05, **p<0.01, ***p<0.001 by two-way ANOVA [CeA, AIE treatment (F1,20=19.9, p<0.001), acute ethanol exposure (F1,20=12.4, p<0.01), MeA, AIE exposure (F1,20=10.5, p<0.01), acute ethanol exposure (F1,20=8.00, p<0.05)] followed by post-hoc Tukey test.
We then determined whether the transcriptional deficits of Arc eRNA and mRNA in the AIE adult amygdala returned to baseline after an acute ethanol challenge in adulthood. Post-hoc analysis after two-way ANOVA (Figure 2C) revealed that both (−) and (+) strand transcripts of Arc eRNA (p<0.05) and Arc mRNA (p<0.01) levels are decreased in the adult amygdala of AIE rats and are increased by acute ethanol in both AIS (p<0.05–0.001) and AIE rats (p<0.01–0.001). Post-hoc comparison after two-way ANOVA (Figure 2C) shows that Kdm6b mRNA is decreased (p<0.05) in the amygdala of AIE rats compared to AIS rats but returns to levels similar to AIS control rats after adult ethanol challenge in AIE rats (Figure 2C).
We next analyzed Arc protein and mRNA levels using gold immunolabeling and in situ PCR histochemical procedures, respectively. Post-hoc analysis after two-way ANOVA shows that, in both the CeA and the MeA, AIE+ Saline rats show decreased Arc mRNA and protein levels in the adult amygdala compared to AIS+ Saline rats (p<0.001; Figure 2D, S4B). Additionally, acute ethanol challenge in adulthood leads to increased Arc mRNA and protein expression in both AIS+ EtOH and AIE+ EtOH rats compared to saline-exposed rats from AIS and AIE groups (p<0.001), respectively (Figure 2D, S4B). Our in-situ PCR data confirms the mRNA findings of decreased Arc mRNA in the AIE adult amygdala as measured by qPCR (Figure 2C). We also performed gold immunolabeling to measure KDM6B protein levels in AIS and AIE animals exposed to adult acute ethanol challenge (Figure 2E). Post-hoc analysis after two-way ANOVA indicates that KDM6B is decreased in AIE+ Saline rats in the CeA (p<0.001) and MeA (p<0.01) compared to AIS+ Saline rats, which returns to levels similar to AIS controls after acute ethanol exposure. KDM6B protein levels in the BLA did not significantly change between the groups (Figure 2E).
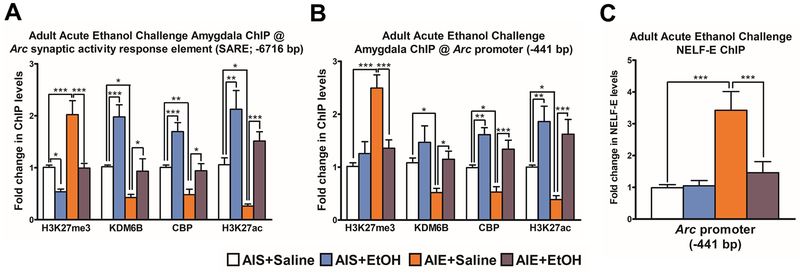
Epigenetic changes at the Arc SARE site and promoter in the amygdala correspond with Arc eRNA transcript levels
We investigated the occupancy of several epigenetic marks at the Arc SARE site by chromatin immunoprecipitation (ChIP) assay (Figure 3A). Post-hoc testing after two-way ANOVA revealed that repressive H3K27me3 is decreased (p<0.05), and the activating marks KDM6B (p<0.001), CBP (p<0.001), and H3K27 acetylation (H3K27ac) (p<0.01) are increased, at the Arc SARE in the amygdala of AIS+ EtOH rats compared to AIS+ Saline rats. Interestingly, H3K27me3 (p<0.001) is increased and KDM6B (p<0.05), CBP(p<0.01), and H3K27ac (p<0.05) are decreased at the Arc SARE site in the amygdala of AIE+ Saline rats, and these four epigenetic marks return to control-like levels after adult ethanol challenge (Figure 3A). We investigated chromatin occupancy at the Arc promoter (Figure 3B) and found increased occupancy of H3K27me3 along with decreased occupancy of KDM6B, CBP and H3K27ac at the Arc promoter site in the amygdala of AIE+ Saline rats compared to AIS+ Saline rats. Changes in occupancy of these epigenetic marks return to control-like levels in the amygdala of AIE adult rats after acute ethanol challenge (Figure 3B).
Figure 3. Adult ethanol challenge reverses AIE-induced chromatin remodeling at the Arc SARE site and promoter.
A) Chromatin immunoprecipitation (ChIP) analysis of H3K27me3, KDM6B, CREB binding protein (CBP) and acetylated H3K27 (H3K27ac) at the synaptic activity response element (SARE) site of the Arc gene in the amygdala of adolescent intermittent ethanol (AIE) or saline (AIS) adult rats exposed to acute ethanol or saline. Data represent mean ± SEM derived from n=5–6 rats in each group.*p<0.05, **p<0.01, ***p<0.001 by two-way ANOVA [H3K27me3 occupancy, AIE treatment (F1,20=25.5, p<0.001), acute ethanol exposure(F1,20=26.6, p<0.001); KDM6B occupancy, AIE treatment (F1,18=27.8, p<0.001), acute ethanol exposure F1,18=22.3, p<0.001); CBP occupancy, AIE treatment (F1,19=25.3, p<0.001), acute ethanol exposure (F1,19=20.5, p<0.001); H3K27ac occupancy, F1,19=10.2, p<0.01), acute ethanol exposure (F1,19=27.8, p<0.001) at the Arc SARE site] followed by post-hoc Tukey test.
B) ChIP analysis of H3K27me3, KDM6B, CBP, and H3K27ac at the Arc promoter (−441 bp) in the amygdala of AIS of AIE adult rats exposed to acute ethanol or saline. Data represent mean ± SEM derived from n=5–8 rats in each group. *p<0.05, **p<0.01, ***p<0.001 by two-way ANOVA (H3K27me3, AIE treatment (F1,24=17.7, p<0.001), acute ethanol exposure (F1,24=5.7, p<0.05), AIE x acute ethanol interaction (F1,24=13.5, p<0.01); KDM6B, AIE treatment (F1,26=5.3, p<0.05), acute ethanol exposure (F1,26=7.0, p<0.05); CBP, AIE treatment (F1,19=8.9, p<0.01), acute ethanol exposure (F1,19=33.6, p<0.001); H3K27ac, AIE treatment (F1,19=4.7, p<0.05), acute ethanol exposure (F1,19=28.3, p<0.001)] followed by post-hoc Tukey test.
C) ChIP analysis of the E subunit of negative elongation factor (NELF-E) at the Arc promoter region (−441 bp) in the amygdala of AIS of AIE adult rats exposed to acute ethanol or saline in adulthood. Data represent mean ± SEM (n=6 rats in each group). ***p<0.001 by two-way ANOVA [AIE treatment (F1,20=16.2, p<0.001), acute ethanol exposure (F1,20=7.2, p<0.05), and the interaction between the two factors (F1,20=8.2, p<0.05)] followed by post-hoc Tukey test.
Arc eRNA (−) binds to the RNA recognition domain of the NELF-E subunit to decoy the NELF complex away from the Arc promoter and allow Pol II to transcribe Arc mRNA (18). Therefore, we measured the occupancy of NELF-E at the Arc promoter in AIS and AIE adult rats exposed to an acute ethanol challenge (Figure 3C). NELF-E occupancy at the Arc promoter site (−441 bp) is increased in the amygdala of AIE adult rats (p<0.001) and normalized after acute ethanol exposure (Figure 2C).
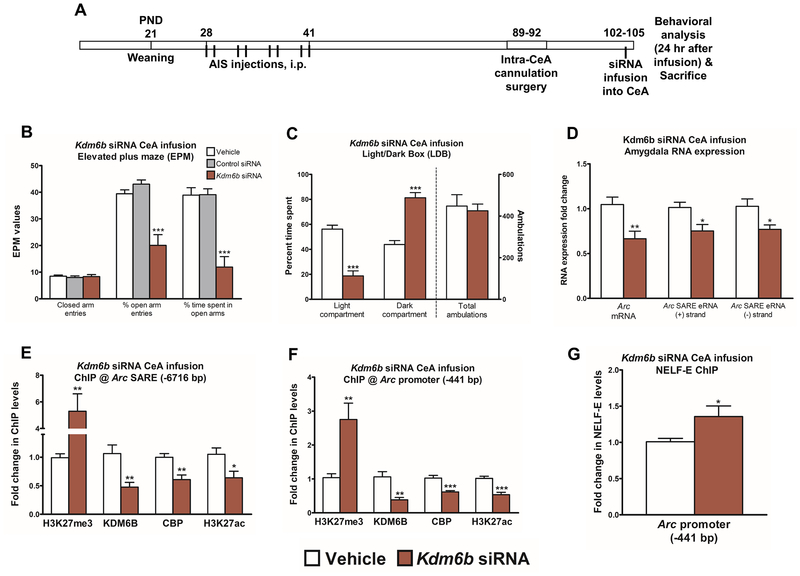
Kdm6b siRNA infusion in the CeA provokes anxiety-like behavior
To determine the direct contribution of Kdm6b-medated chromatin remodeling and regulation of Arc eRNA and mRNA expression in the amygdala to anxiety-like behaviors, we cannulated alcohol-naïve AIS control rats in the CeA and infused either control or Kdm6b siRNA. Anxiety-like behaviors were measured using both the EPM and LBD (Figure 4A) in these rats 24 hrs after a single siRNA infusion in separate batches of rats. Kdm6b siRNA infusion into the CeA provoked anxiety-like behavior in the EPM as demonstrated by significant (p<0.001) decrease in percentage of open arm entries and percent of time spent in the open arm compared to rats infused with vehicle (iFect solution) or control siRNA (Figure 4B). There were no differences in closed arm entries as a measure of general activity of rats. Due to the lack of effect of the control siRNA, we did not test this group in subsequent experiments. Kdm6b siRNA infusion additionally leads to increased time spent in the dark compartment (p<0.001) and decreased time spent in the light compartment (p<0.001) in the LDB compared to vehicle-infused AIS rats without altering general activity (total ambulation) (Figure 4C). These data suggest that Kdm6b siRNA infusion into the CeA in AIS adult rats leads to anxiety-like behavior in multiple behavioral tests, thus mimicking AIE-induced anxiety-like behaviors in adulthood.
Figure 4. Kdm6b siRNA infusion into the CeA provokes anxiety-like behavior and decreases expression of Arc eRNA and mRNA.
A) Schematic representation of Kdm6b siRNA infusion directly into the central nucleus of amygdala (CeA) of adolescent intermittent saline (AIS)-exposed control adult rats.
B) Elevated plus maze (EPM) exploration test for anxiety-like behaviors in adult rats infused with Kdm6b siRNA, control siRNA or vehicle (iFect solution) in the CeA. Data represent mean ± SEM (n=6 rats in each group). ***p<0.001 by one-way ANOVA [percentage of open arm entries (F2,15=21.8, p<0.001) and percent of time spent in the open arm (F2,15=26.3, p<0.001)] followed by post-hoc Tukey test.
C) Light/dark box (LDB) exploration test for anxiety-like behaviors in adult control rats infused with Kdm6b siRNA or vehicle (iFect solution) in the CeA. Data represent mean ± SEM (n=8 rats in each group). ***p<0.001 by Student’s t-test [time spent in the dark compartment (t (14) =−7.25, p<0.001), time spent in the light compartment (t (14) =7.25, p<0.001)].
D) RNA analysis of Arc enhancer RNA [eRNA (+) and (−) strand] and Arc mRNA transcripts in the amygdala of AIS adult rats infused with Kdm6b siRNA or vehicle (iFect solution) in the CeA. Data represent mean ± SEM (n=7–8 rats in each group). *p<0.05, **p<0.01 by Student’s t-test [eRNA plus (t (14) =2.82, p<0.05) and minus strand (t (14) =2.69, p<0.05) transcripts of Arc eRNA; Arc mRNA (t (12) =3.25, p<0.01)].
E) Chromatin immunoprecipitation (ChIP) analysis of H3K27me3, KDM6B, CREB binding protein (CBP) and acetylated H3K27 (H3K27ac) at the synaptic activity response element (SARE) site of the Arc promoter in the amygdala of AIS adult rats infused with Kdm6b siRNA or vehicle (iFect solution) in the CeA. Data represent mean ± SEM (n=7–8 rats in each group). *p<0.05, **p<0.01, ***p<0.001 by Student’s t-test [H3K27me3 occupancy (t (13) =−3.08, p<0.01), KDM6B (t (13) =3.54, p<0.01), CBP (t (14) =3.77, p<0.01), and H3K27ac (t (14) =2.59, p<0.05) occupancy].
F) ChIP analysis of H3K27me3, KDM6B, CBP, and H3K27ac at the Arc promoter (−441 bp) site in the amygdala of adult AIS rats infused with Kdm6b siRNA or vehicle (iFect solution) in the CeA. Data represent mean ± SEM (n=7–8). **p<0.01, ***p<0.001 by Student’s t-test [H3K27me3 occupancy (t (13) =−3.26, p<0.01), KDM6B (t (13) =4.21, p<0.01), CBP (t (14) =4.56, p<0.001), and H3K27ac (t (14) =5.03, p<0.001) occupancy].
G) ChIP analysis for the E subunit of negative elongation factor (NELF-E) at the Arc promoter (−441 bp) in the amygdala of rats infused with Kdm6b siRNA or vehicle (iFect solution) in the CeA. Data represent mean ± SEM (n=7–8 rats in each group). *p<0.05 by Student’s t-test [t (13) =−2.39, p<0.05].
Kdm6b siRNA infusion produces significantly (p<0.01) decreased Kdm6b mRNA levels in the amygdala, but no change in Cbp and Kdm6a mRNA levels, compared to vehicle-infused controls (Figure S5A). We additionally performed confocal microscopy in control rats to colocalize KDM6B protein within the CeA, MeA, and BLA with NeuN and GFAP and found that KDM6B is predominately colocalized with NeuN, but not GFAP, in the CeA, MeA, and BLA (Figure S5B), indicating that this protein is expressed at relatively high levels in neuronal populations. We have previously shown that siRNA infusion with iFect solution penetrates neurons (35), and we therefore suggest the possibility that Kdm6b siRNA exerts its main effects in neuronal cell populations in the CeA.
Kdm6b siRNA infusion decreases Arc eRNA & mRNA transcription via epigenetic changes at the SARE site
We examined whether Arc eRNA transcripts were altered by Kdm6b siRNA infusion. Both the plus and minus strand transcripts of Arc eRNA were significantly (p<0.05) decreased in the amygdala of Kdm6b siRNA-infused rats (Figure 4D). Additionally, Arc mRNA, but not Bdnf exon IV and Syp mRNA (Figure S5A), was decreased (p<0.01) in the amygdala of Kdm6b siRNA-infused rats (Figure 4D). We next performed ChIP followed by qPCR for H3K27me3, KDM6B, CBP, and H3K27ac occupancy at the Arc SARE site and promoter in rats infused with Kdm6b siRNA directly into the CeA. Kdm6b siRNA infusion into the CeA leads to significantly increased (p<0.01) H3K27me3 occupancy and decreased (p<0.05–0.01) KDM6B, CBP, and H3K27ac occupancy at the Arc SARE site in the amygdala compared to vehicle-exposed rats (Figure 4E). A similar pattern of epigenetic modifications was observed at the Arc promoter site (Figure 4F).
Due to the epigenetically-encoded decrease in Arc eRNA in these rats, we tested whether NELF-E binding was altered in Kdm6b siRNA-infused rats in the amygdala. We observed increased (p<0.05) NELF-E occupancy at the Arc promoter region, corresponding with decreased Arc eRNA and mRNA expression, in Kdm6b siRNA-infused rats as compared with vehicle-infused rats (Figure 4G). We used the same primers (Figure S6A) used to amplify ChIP pulldown from KDM6B and CBP that we used to amplify the Arc eRNA (−) RNA product to determine whether the epigenetic changes seen at the SARE site might be solely responsible for regulating the Arc eRNA (−) transcript in our experiments (Figure S6B). We did not find any alterations in KDM6B and CBP occupancy at the Arc eRNA (−) transcribed region (Figure S6A, B), suggesting that epigenetic changes at the SARE site are likely responsible for the regulation of Arc eRNA expression.
Inhibition of Arc eRNA (−) in the CeA mimics AIE-induced anxiety-like behaviors and decreases Arc mRNA expression
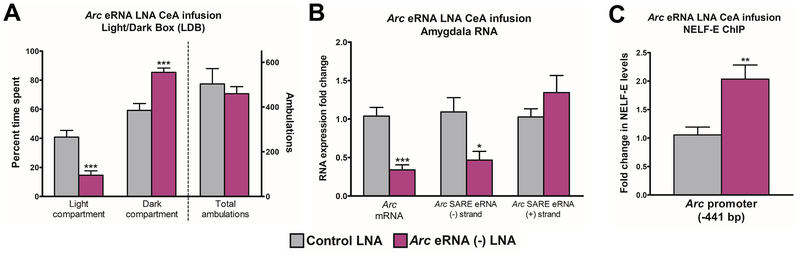
We infused a custom-designed Arc eRNA (−) antisense LNA or a control LNA oligonucleotide into the CeA of alcohol-naïve control adult rats twice per day for two days. On the third day (approximately 16 hrs after the last LNA infusion) we measured anxiety-like behaviors in the LDB. Interestingly, we observed anxiety-like behaviors in rats infused with Arc eRNA (−) LNA in the CeA (Figure 5A), as represented by decreased percentage of time spent in the light compartment (p<0.001) and increased percentage of time spent in the dark compartment (p<0.001) compared to control LNA-infused rats. There were no alterations in total ambulation between the groups, indicating that general activity levels were unaltered (Figure 5A).
Figure 5. Arc eRNA (−) LNA oligonucleotide infusion into the CeA provokes anxiety-like behaviors and decreases Arc mRNA in alcohol-naïve rats.
A) Light/dark box (LDB) exploration test for anxiety-like behaviors in adult alcohol-naïve control rats infused with a locked nucleic acid (LNA) anti-sense oligonucleotide directed toward the minus strand of the Arc enhancer RNA [Arc eRNA (−)] or control LNA in the central nucleus of amygdala (CeA). Data represent mean ± SEM (n=7 rats in each group). ***p<0.001 by Student’s t-test [percentage of time spent in the light compartment (t (12) =4.69, p<0.001), percentage of time spent in the dark compartment (t (12) =−4.69, p<0.001)].
B) RNA analysis in the amygdala of rats infused with Arc eRNA (−) LNA in the CeA. Data represent mean ± SEM (n=6–7 rats in each group), *p<0.05, ***p<0.001 by Student’s t-test [Arc eRNA (−) strand mRNA (t (12) =2.88, p<0.05), Arc mRNA (t (12) =5.31, p<0.001)].
C) Chromatin immunoprecipitation (ChIP) analysis for the E subunit of negative elongation factor (NELF-E) at the Arc promoter (−441 bp) in the amygdala of rats infused with Arc eRNA (−) LNA in the CeA. Data represent mean ± SEM (n=7 rats in each group). **p<0.01 by Student’s t-test [(t (12) =−3.45, p<0.01)].
We measured the status of Arc eRNA transcripts (to confirm LNA knockdown) and Arc mRNA in the amygdala of rats infused with Arc eRNA (−) LNA in the CeA (Figure 5B). Arc eRNA (−) LNA infusion significantly decreased Arc eRNA (−) in the amygdala when compared to control LNA-infused rats (p<0.05; Figure 5B), indicating that the Arc eRNA (−) LNA knockdown was effective. There was no effect on Arc eRNA (+) expression between the two groups. Interestingly, Arc mRNA (Figure 5B), but not Bdnf exon IV and Syp mRNA (Figure S7A), transcription was significantly decreased (p<0.001) in the amygdala of rats infused with Arc eRNA (−) LNA compared to control LNA-infused rats (Figure 5B).
Finally, we investigated whether NELF-E occupancy at the Arc promoter was altered following infusion of the Arc eRNA (−) LNA into the CeA using the ChIP assay. Arc eRNA (−) LNA infusion directly into the CeA led to a significant (p<0.01) increase in NELF-E occupancy at the Arc promoter region in the amygdala compared to control LNA-infused rats (Figure 5C). Previous in vitro studies of eRNA function have shown that knockdown of eRNA transcripts can cause locus-specific chromatin remodeling (36), potentially representing a genomic effect of the probe rather than antisense inhibition of the eRNA transcript. To investigate this, we measured KDM6B and CBP occupancy at the Arc SARE site and promoter as well as H3K27ac levels at the Arc SARE site, Arc eRNA (−) transcribed region, and Arc promoter in the amygdala of rats infused with Arc eRNA (−) LNA or control LNA. We found no differences in the occupancy of these epigenetic marks between groups (Figure S7B–D), suggesting that the inhibition of Arc eRNA (−) by the LNA oligonucleotide occurs post-transcriptionally.
DISCUSSION
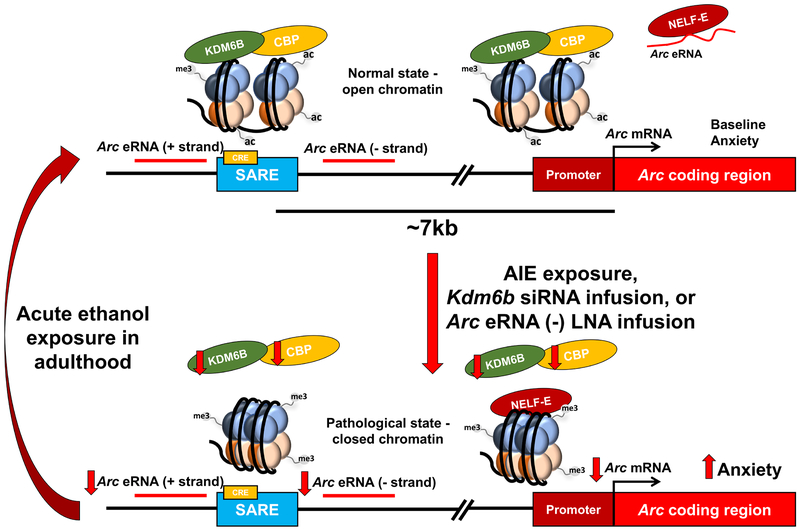
Here, we show for the first time that deficits in KDM6B-mediated epigenetic reprogramming lead to long-lasting reductions in Arc eRNA and mRNA expression after AIE exposure in the adult amygdala, corresponding to decreased synapse number in the CeA and MeA and heightened anxiety-like behaviors. We show that H3K27me3 occupancy is increased and KDM6B, CBP, and H3K27ac occupancy is decreased at the SARE site of the Arc gene in AIE adult rats, which is the likely site of bidirectional transcriptional suppression of Arc eRNA expression. Kdm6b siRNA infusion into the CeA is sufficient to provoke anxiety-like behaviors in control rats and decreases Arc eRNA and mRNA expression via decreased KDM6B and CBP occupancy, the associated increased H3K27me3 and decreased H3K27ac at the SARE site, and a subsequent increase in NELF-E binding to the Arc promoter. Lastly, direct knockdown of Arc eRNA (−) in the CeA of control rats leads to anxiety-like behaviors, increased NELF-E occupancy at the Arc promoter, and decreased Arc mRNA expression in the amygdala. Our data suggests that epigenetic reprogramming due to deficits in KDM6B suppresses eRNA transcription and possibly involved in the risk for anxiety due to adolescent alcohol exposure. It also provides evidence that KDM6B and CBP may form a complex in vivo in the amygdala to regulate neuronal gene transcription and synaptic function via epigenetic modifications. Furthermore, Arc eRNA expression in the CeA directly regulates anxiety-like behaviors (Figure 6).
Figure 6. Adolescent intermittent ethanol (AIE) leads to adult anxiety susceptibility via epigenetic reprogramming at the Arc synaptic activity response element (SARE) site and decreased Arc enhancer RNA (eRNA) in the amygdala.
AIE produces long-lasting impacts on epigenetic reprogramming and decreases synapse-related transcriptional events in the amygdala leading to anxiety-like behaviors in adulthood. AIE increases the repressive epigenetic mark H3K27 trimethylation (H3K27me3) and decreases lysine demethylase 6B (KDM6B), CREB binding protein (CBP), and H3K27 acetylation (H3K27ac) at the Arc promoter and SARE site. The closed chromatin architecture at the SARE site is associated with decreased Arc eRNA expression, increased negative elongation factor (NELF) binding at the Arc promoter, and decreased Arc mRNA expression. These behavioral and epigenetic changes return to control-like levels after acute ethanol challenge in AIE adult rats. Notably, both Kdm6b siRNA infusion or locked nucleic acid (LNA) knockdown of Arc eRNA (−) strand directly in the central nucleus of amygdala leads to marked anxiety-like behavior, reduced Arc eRNA and mRNA expression, and increased NELF binding to the Arc promoter. Taken together, these results indicate that epigenetic alterations resulting in decreased Arc eRNA are critical for the increased risk of anxiety in adulthood after adolescent alcohol exposure.
AIE exposure leads to increased anxiety-like behavior in adulthood, confirming previous studies (11, 37), and this anxiety is reversed by acute alcohol challenge similar to our previous findings (10). Human epidemiological studies show a relationship between increased anxiety risk and early-life alcohol consumption (6, 38, 39). Patients diagnosed with alcohol dependence are more likely to have an additional anxiety disorder diagnosis, and early onset of anxiety disorders reliably predicts the age of first alcohol use (7, 9). Additionally, the presence of anxiety and/or depressive symptoms increases the likelihood of early alcohol dependence (6). Other groups have observed behavioral disinhibition in adult Wistar rats exposed to AIE, indicating that the lasting effects of adolescent alcohol exposure are dependent upon genetic background (40, 41). Notably, exposure to the same dosing regimen of alcohol in adulthood does not have lasting behavioral and physiological effects (42, 43), highlighting adolescence as a critical period sensitive to ethanol exposure (1, 13, 14).
The anxiety-like behavior induced by Kdm6b siRNA infusion into CeA indicates that this transcript is crucial for the expression of affective states via chromatin remodeling, thus mimicking the effects of AIE on KDM6B and related epigenetic processes at the Arc gene (Figure 6). Kdm6b siRNA infusion in the CeA did not alter the expression of Bdnf exon IV or Syp in control rats. These results suggest the possibility that Arc eRNA expression may be sensitive to Kdm6b reduction whereas reductions in other synaptic genes lacking activity-dependent enhancers may require changes in additional epigenetic regulators. KDM6B may lie downstream of BDNF action, as loss of KDM6B prevents BDNF-induced gene induction in cerebellar granule neurons (CGNs) (44). Importantly, several other genes involved in synaptic plasticity are also decreased, possibly due to globally condensed chromatin architecture in the amygdala after AIE. Previously, we have shown that AIE treatment causes increased histone deacetylase 2 (HDAC2) levels and decreased levels of a neuron-specific lysine-specific demethylase 1 (LSD1) isoform known as Lsd1+8a in the amygdala in adulthood, leading to altered histone modifications (H3K9ac and H3K9me2) both globally and at the promoter regions of Bdnf exon IV and Arc (10, 11). Taken together, our work indicates that alterations in KDM6B, HDAC2, CBP, and LSD1 create an imbalance between active and inactive chromatin states, particularly on H3K9 and H3K27 residues, in the adult amygdala after AIE. Other brain circuits, such as dopaminergic transmission in the prefrontal cortex (45), are likely involved in the lasting effects of AIE, but it should be noted that the negative affective states associated with alcohol addiction, such as anxiety, have been identified as promising phenotypes to utilize for AUD drug development (46, 47).
Enhancer RNAs were first discovered in large sequencing datasets and originally characterized as transcriptional noise or simply byproducts of active enhancer regions (19, 48). Comprehensive sequencing-based analyses indicate that eRNAs are relatively tissue-specific and brain region-specific, suggesting possible roles in cell fate determination and developmental processes (49, 50). However, some eRNAs do serve biological functions, such as the ability of Arc eRNA to bind to the RNA recognition site of NELF-E and cause the NELF complex to leave the chromatin so poised Pol II can begin actively transcribing Arc mRNA in vitro in neuronal culture cells (18). Our in vivo data in the CeA supports this mechanism of Arc expression. The chromatin modifications on both the SARE and promoter sites seen here may act in tandem to tightly regulate Arc mRNA transcription, although our data suggest the possibility that chromatin alterations at the SARE site are primarily responsible for the changes in Arc eRNA transcription seen in this study (Figure 6). The associations between Arc eRNA levels, NELF-E binding at the Arc promoter, and Arc mRNA levels across multiple models and perturbations would seem to suggest that, at least in the case of the Arc eRNA transcribed from the SARE site, this eRNA has a transcription-independent function (18). Therefore, some eRNAs may be functional entities while others simply have transcription-dependent functions, meaning that the act of transcribing the eRNA itself may be important for subsequent downstream activities such as transcription factor (TF) binding (20, 51). The act of eRNA transcription may also be important for maintaining enhancer-promoter looping, thus existing in a positive feedback loop to further promote target mRNA transcription. Our data involving Kdm6b siRNA infusion in the CeA suggest that Arc eRNA interacts with epigenetic machinery at the Arc promoter to decoy NELF and regulate Arc expression and anxiety-related phenotypes. Arc eRNA (−) inhibition by LNA infusion into the CeA increased NELF binding at the Arc promoter, decreased Arc mRNA expression, and provoked anxiety-like behaviors without altering the expression of Bdnf exon IV and Syp in the amygdala. Similarly, previous studies found that Arc eRNA inhibition decreased Arc mRNA expression without altering the expression of other immediate early genes (18). We also observed that Arc eRNA (−) inhibition by LNA did not alter the epigenetic dynamics at Arc SARE or promoter sites, suggesting that Arc expression may be regulated principally by eRNA-mediated decoying of NELF in this experimental condition (52). Mechanistic studies were performed in the CeA due to its critical involvement in AUD (10, 11, 35, 53), but this does not rule out the possibility that AIE-induced chromatin remodeling in the MeA may be involved in adult psychopathology. Therefore, future studies will increase the expression of Arc in both the CeA and MeA to investigate the similarities and differences in the function of specific amygdaloid nuclei in AIE-induced anxiety-like behaviors.
The present study provides novel evidence that epigenetically-regulated eRNA expression interacts with transcriptional machinery at the Arc gene to alter Arc expression in the CeA and orchestrate AIE-induced anxiety-like behaviors in adulthood (Figure 6). Future studies should continue to identify individual functional eRNAs, as well as novel functions of eRNAs as a group, as these molecules represent a new frontier of epigenetics and may play important roles in neuronal function and psychiatric disorders.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants UO1AA-019971, U24AA-024605 (Neurobiology of Adolescent Drinking in Adulthood project), RO1AA-010005, RO1AA-021662, P50AA-022538 (Center for Alcohol Research in Epigenetics), and by the Department of Veterans Affairs (Senior Research Career Scientist award) to SCP and a fellowship F30 AA024948 grant to EJK. Authors would like to thank Dr. Ritabrata Banerjee for his assistance with immunohistochemistry. Studies are part of PhD thesis of E.J.K. for his MD/PhD degree in the UIC Graduate College and College of Medicine. Portions of the data were presented at several scientific meetings including Society of Biological Psychiatry, American College of Neuropsychopharmacology, and Research Society on Alcoholism.
Footnotes
All authors report no potential conflicts of interest.
REFERENCES
- 1.Spear LP (2018): Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 19:197–214. [DOI] [PubMed] [Google Scholar]
- 2.Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T (2014): Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 1:549–558. [DOI] [PubMed] [Google Scholar]
- 3.Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, et al. (2013): Global epigenomic reconfiguration during mammalian brain development. Science. 341:1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC (2000): Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 157:745–750. [DOI] [PubMed] [Google Scholar]
- 5.Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. (2009): Underage alcohol use: summary of developmental processes and mechanisms: ages 16–20. Alcohol Res Health. 32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Veltman DJ, Beekman AT, et al. (2013): Depressive and anxiety disorders predicting first incidence of alcohol use disorders: results of the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. 74:1233–1240. [DOI] [PubMed] [Google Scholar]
- 7.Lai HM, Cleary M, Sitharthan T, Hunt GE (2015): Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Depend. 154:1–13. [DOI] [PubMed] [Google Scholar]
- 8.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. (2015): Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio-Stipec M, Angst J (1998): The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Compr Psychiatry. 39:176–184. [DOI] [PubMed] [Google Scholar]
- 10.Kyzar EJ, Zhang H, Sakharkar AJ, Pandey SC (2017): Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict Biol. 22:1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey SC, Sakharkar AJ, Tang L, Zhang H (2015): Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 82:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. (2013): Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 67:521–531. [DOI] [PubMed] [Google Scholar]
- 13.Crews FT, Vetreno RP, Broadwater MA, Robinson DL (2016): Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol Rev. 68:1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyzar EJ, Floreani C, Teppen TL, Pandey SC (2016): Adolescent alcohol exposure: burden of epigenetic reprogramming, synaptic remodeling, and adult psychopathology. Front Neurosci. 10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A (2009): An operational definition of epigenetics. Genes Dev. 23:781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi IA, Mehler MF (2011): Non-coding RNA networks underlying cognitive disorders across the lifespan. Trends Mol Med. 17:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, et al. (2009): Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci U S A. 106:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaukowitch K, Joo JY, Liu X, Watts JK, Martinez C, Kim TK (2014): Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. 56:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. (2010): Widespread transcription at neuronal activity-regulated enhancers. Nature. 465:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Notani D, Rosenfeld MG (2016): Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet. 17:207–223. [DOI] [PubMed] [Google Scholar]
- 21.Rajarajan P, Gil SE, Brennand KJ, Akbarian S (2016): Spatial genome organization and cognition. Nat Rev Neurosci. 17:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. (2010): Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 107:21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouzarides T (2007): Chromatin modifications and their function. Cell, 128:693–705. [DOI] [PubMed] [Google Scholar]
- 24.Tyssowski KM, DeStefino NR, Cho JH, Dunn CJ, Poston RG, Carty CE, et al. (2018): Different neuronal activity patterns induce different gene expression programs. Neuron. 98:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. (2007): Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 448:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zentner GE, Tesar PJ, Scacheri PC (2011): Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res. 21:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, et al. (2007): UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 449:731–734. [DOI] [PubMed] [Google Scholar]
- 28.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. (2006): Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 441:349–353. [DOI] [PubMed] [Google Scholar]
- 29.Palomer E, Carretero J, Benvegnu S, Dotti CG, Martin MG (2016): Neuronal activity controls Bdnf expression via Polycomb de-repression and CREB/CBP/JMJD3 activation in mature neurons. Nat Commun. 7:11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannister AJ, Kouzarides T (1996): The CBP co-activator is a histone acetyltransferase. Nature. 384:641–643. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Kyzar EJ, Bohnsack JP, Kokare DM, Teppen T, Pandey SC (2018): Adolescent alcohol exposure epigenetically regulates CREB signaling in the adult amygdala. Sci Rep. 8:10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. (2009): HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 459:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. (2010): The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38:W214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuchi M, Nakashima F, Tabuchi A, Shimotori M, Tatsumi S, Okuno H, et al. (2015): Class I histone deacetylase-mediated repression of the proximal promoter of the activity-regulated cytoskeleton-associated protein gene regulates its response to brain-derived neurotrophic factor. J Biol Chem. 290:6825–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC (2013): Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 73:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pnueli L, Rudnizky S, Yosefzon Y, Melamed P (2015): RNA transcribed from a distal enhancer is required for activating the chromatin at the promoter of the gonadotropin alpha-subunit gene. Proc Natl Acad Sci U S A. 112:4369–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC (2016): A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 221:4691–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birrell L, Newton NC, Teesson M, Tonks Z, Slade T (2015): Anxiety disorders and first alcohol use in the general population. Findings from a nationally representative sample. J Anxiety Disord. 31:108–113. [DOI] [PubMed] [Google Scholar]
- 39.Grant BF, Stinson FS, Harford TC (2001): Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 13:493–504. [DOI] [PubMed] [Google Scholar]
- 40.Gilpin NW, Karanikas CA, Richardson HN (2012): Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 7:e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehlers CL, Liu W, Wills DN, Crews FT (2013): Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 244:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT (2014): Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One. 9:e113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varlinskaya EI, Truxell E, Spear LP (2014): Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 48:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wijayatunge R, Liu F, Shpargel KB, Wayne NJ, Chan U, Boua JV, et al. (2018): The histone demethylase Kdm6b regulates a mature gene expression program in differentiating cerebellar granule neurons. Mol Cell Neurosci. 87:4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, et al. (2017): Binge-like alcohol exposure during adolescence disrupts dopaminergic neurotransmission in the adult prelimbic cortex. Neuropsychopharmacology. 42:1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koob GF, Mason BJ (2016): Existing and future drugs for the treatment of the dark side of addiction. Annu Rev Pharmacol Toxicol. 56:299–322. [DOI] [PubMed] [Google Scholar]
- 47.Pandey SC, Kyzar EJ, Zhang H (2017): Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology. 122:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen TH, Jacquier A, Libri D (2013): Dealing with pervasive transcription. Mol Cell. 52:473–484. [DOI] [PubMed] [Google Scholar]
- 49.Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, et al. (2014): An atlas of active enhancers across human cell types and tissues. Nature. 507:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao P, Lin P, Gokoolparsadh A, Assareh A, Thang MW, Voineagu I (2015): Coexpression networks identify brain region-specific enhancer RNAs in the human brain. Nat Neurosci. 18:1168–1174. [DOI] [PubMed] [Google Scholar]
- 51.Sigova AA, Abraham BJ, Ji X, Molinie B, Hannett NM, Guo YE, et al. (2015): Transcription factor trapping by RNA in gene regulatory elements. Science. 350:978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madabhushi R, Kim TK (2018): Emerging themes in neuronal activity-dependent gene expression. Mol Cell Neurosci. 87:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilpin NW, Herman MA, Roberto M (2015): The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 77:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.