Abstract
In September 2015, CDC was notified of a suspected outbreak investigation of lymphogranuloma venereum (LGV) cases by the Michigan Department of Health and Human Services (MDHHS). CDC offered support with a laboratory-developed PCR test for LGV. This note describes the laboratory workflow and procedures used for the laboratory confirmation of LGV infection.
Keywords: LGV, Chlamydia trachomatis, Laboratory testing, PCR
Summary
This note describes laboratory procedures for LGV confirmation at the CDC STD Laboratory during a 2015/ 2016 investigation of a cluster of cases among men who have sex with men.
Introduction
Lymphogranuloma venereum (LGV) is a sexually transmitted infection caused by invasive Chlamydia trachomatis (CT) serovars L1, L2a, L2b and L3. LGV proctitis is caused by L2 strains primarily in men who have sex with men (MSM) and is often associated with HIV infection (1). On September 23, 2015, the Michigan Department of Health and Human Services (MDHHS) contacted the CDC for assistance with investigating a suspected LGV outbreak, as recently reported (2). Prior to this, the CDC laboratory had last assisted with LGV laboratory confirmation in 2004/ 2005 when CDC was alerted to LGV cases among MSM in the Netherlands (3) and later also in the United States. Since then, new laboratory assays for LGV confirmation have been developed. The purpose of this note is to document the current laboratory support CDC can offer, in order to accelerate the laboratory response to future LGV outbreaks or case investigations. The need for such information was brought to our attention repeatedly during the outbreak.
Methods and Findings
Because FDA-approved, commercial nucleic acid amplification tests (NAATs) cannot distinguish CT LGV strains from non-LGV strains, specimens from probable LGV cases in Michigan were referred to the CDC LRRB (Laboratory Reference and Research Branch), i.e., the CDC STD Laboratory. Specimens were tested using an in-house, molecular test for probable LGV cases.
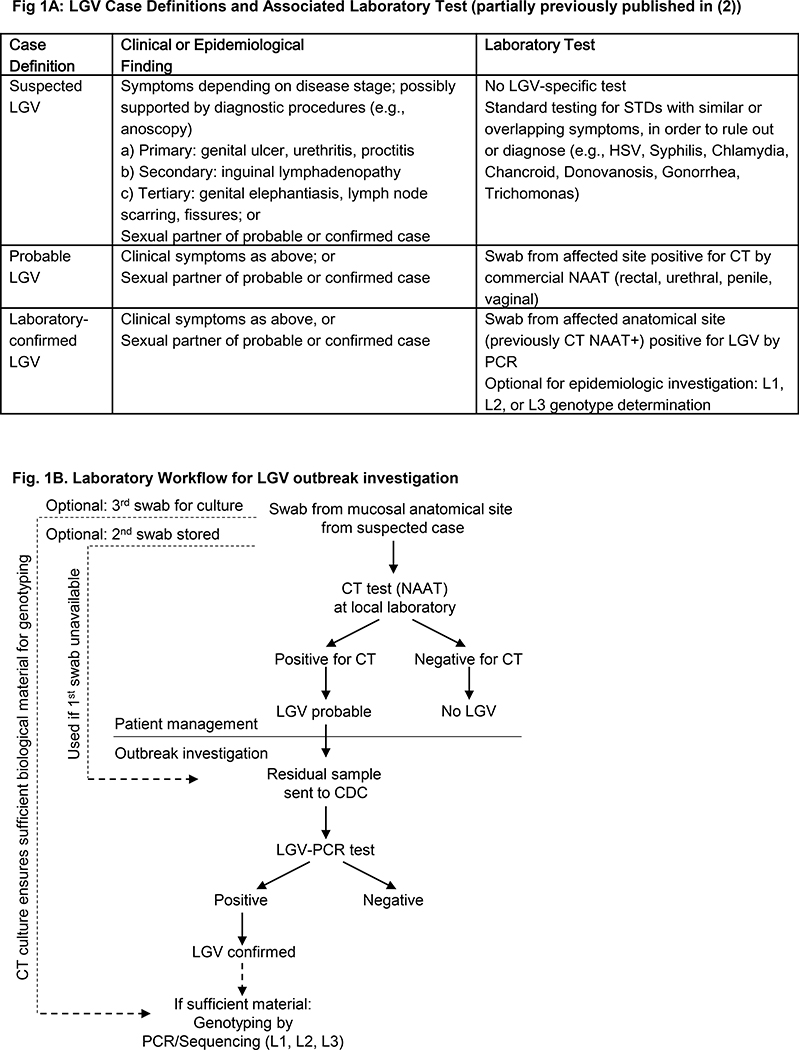
Prior to CDC laboratory confirmation of LGV, patients were managed in accordance with CDC treatment guidelines (4), and with the aid of working case definitions developed by MDHHS and CDC (2). Figure 1A summarizes suspected, probable, and confirmed LGV case definitions, and lists the different laboratory tests associated with the definitions. In brief, a suspected LGV case was defined as a patient who presented with clinical symptoms consistent with LGV infection (e.g., genital ulcer, lymphadenopathy, or proctitis) but had no LGV-specific testing. Typically, the treating provider who suspected LGV infection first ordered routine STD laboratory testing to diagnose or rule out other STDs with overlapping symptoms, such as Herpes Simplex Virus (HSV) or Syphilis. The CDC-recommended laboratory tests for the detection of genitourinary CT infections are commercial NAATs using specimens from a variety of different sources such as vaginal or endocervical swabs or first-catch urine from women, or urethral swabs or first-catch urine from men (5). If routine STD testing identified other infections, appropriate treatment was initiated while LGV was further investigated.
Figure 1:
A. Schematic representation of suspected, probable, and confirmed case definitions and associated laboratory tests used in the 2015–2016 LGV cluster investigation. Abbreviations are explained in the manuscript. Note: CT serology may be used to support other laboratory findings; however, it was not used. B. Schematic workflow and specimen movement in the 2015–2016 LGV outbreak investigation.
The term “probable LGV infection” was used when CT was detected with a commercial NAAT assay on a swab specimen from the affected anatomical site (Fig. 1A). Treating providers most often collected one anorectal swab although collecting three swabs at the visit if LGV is suspected is optimal in order to allow for ample material for testing (see Fig. 1B, and see below). The commercial NAAT for CT detection was performed at a local laboratory. These tests are currently not FDA-approved for extragenital specimens. Thus, medical providers needed to identify local laboratories with CLIA certification that had validated these tests for use on extragenital specimens, as almost all of the major laboratories have done. Almost all probable LGV cases from Michigan in 2015 were diagnosed from male anorectal swab specimens, with a few notable exceptions of penile swabs (2). Of note, urine NAATs from male patients were often negative for Chlamydia during the Michigan outbreak (2) even in those patients who were later confirmed with LGV, indicating that a negative CT urine test could not rule out CT or LGV infection at other anatomical sites, as has been previously reported (6). Patient treatment was initiated for all probable LGV cases.
While patient management was underway locally, CDC provided assistance to MDHHS to investigate the suspected outbreak. The CDC laboratory’s only specimen acceptance criteria were that the case was a probable LGV case and that the specimen had tested positive by a commercial CT NAAT. Initially, residual swab specimens in commercial NAAT transport medium were submitted to the CDC laboratory for confirmation of LGV by PCR. Subsequently, CDC also provided AssayAssure (Sierra Molecular) nucleic acid transport medium for specimen collection in addition to accepting residual specimens in commercial CT NAAT transport medium. Fig. 1B graphically depicts specimen and laboratory workflow and acceptance criteria for CDC laboratory testing. To communicate acceptance criteria and shipping instructions for specimens, the CDC laboratory wrote a letter to potential sample submitters that was included in the MDHHS Health Alert Network (HAN) notification on October 22, 2015 (7). The CDC letter can be found in supplemental digital content 1. The documents specified submission details for samples from probable cases to the CDC. The letter stated that only specimens from probable LGV cases should be sent to CDC. Furthermore, we explained that the test had been validated using rectal and anal specimens, and was not well established for other specimens such as genital ulcers, skin lesions, or bubo aspirates, and other samples. We indicated that transport media for all commercially available NAATs for CT were compatible with our test, and our willingness to send out transport media upon request. Specimen were to be sent preferably frozen at −20 oC, or at all other temperatures recommended for the NAAT transport media of choice. We gave detailed instructions on CDC’s centralized electronic specimen and data submission system which requires submission with approval of local State or Local Public Health Laboratories (use CDC Form 50.34, found at http://www.cdc.gov/laboratory/specimen-submission/form.html, and test order CDC-10192, after contact with LRRB at ajp7@cdc.gov). Lastly, we explained expected results as: a) confirmation of LGV (includes genotypes L1, L2, or L3), b) absence of LGV with or without confirmatory detection of C. trachomatis, or c) inconclusive results due to insufficient sample quality as indicated by internal assay control performance.
While CDC accepted the residual swab sample from commercial CT NAAT testing, some medical providers found it difficult to have commercial laboratories retrieve residual specimens for shipping. To avoid these issues, collecting three swabs at the visit when LGV was suspected was optimal. The first swab was submitted for CT testing locally. The other two swabs were for epidemiologic investigation. The 2nd swab was typically stored until probable LGV infection was diagnosed, and then sent to CDC for LGV confirmation using CDC’s storage and shipping instructions as detailed in supplemental digital content 1. The third swab was used for local Chlamydia culture in order to facilitate genetic analysis (see below).
At the CDC laboratory, DNA was extracted from the commercial CT NAAT transport medium and a real-time quadriplex PCR test was performed as previously described (8). The test was previously shown to simultaneously detect LGV, non-LGV, or mixed infections in rectal specimens from the United Kingdom. In addition to LGV and non-LGV CT targets, the quadriplex assay is designed with two internal controls, i.e., a CT plasmid target for confirmation and the human RNase P gene for monitoring sample adequacy and PCR inhibition. The LGV-specific PCR or non-LGV-specific PCR is designed to target areas that are either encompassing or within the unique 36-base pair deletion region that only occurs in LGV strains of the polymorphic membrane protein H gene (pmpH). The quadriplex PCR identifies CT serovars A–K as non-LGV, and L1–L3 as LGV. The turnaround time was under seven business days in all cases. By September 2016, a total of 43 cases had been investigated in Michigan. Of those, 36 were identified as probable (based on commercial CT NAAT-positivity) and associated specimens were sent to CDC; the remaining seven were suspected cases. Of the 36 probable cases, 25 were confirmed as LGV. Eleven cases were not confirmed for LGV at CDC. Of those, three were negative for C. trachomatis at the CDC. It is possible that discrepant results were due to low copy number of targets, or sample integrity issues during collection, handling or shipping, and prior treatment before sample collection.
The CDC STD Laboratory also has the capacity to genotype LGV-positive samples, using PCR/ sequencing, and/or PCR/ restriction fragment length polymorphism (RFLP)(9). While this technique has not been fully modernized towards bioinformatics analysis of the whole genome due to limited specimen availability, it is still a potentially powerful tool for further epidemiologic investigation of transmission chains. In brief, sequencing the variable domain (VD)-2 of the ompA gene can distinguish most L-variants, as previously reported (9). CDC laboratory personnel suggested to collect an optional third swab sample for CT culture. CT culture is not a widespread skill set within laboratories, and thus was a barrier to the full investigation of many cases. However, in a subset of cases, the attending provider was able to work with CDC scientists and their local laboratories, and was able to culture CT. The specimen was placed in Bartels medium and cultured locally, then submitted to CDC for genotyping, either at the time of LGV confirmation request or once LGV was confirmed. This analysis revealed that of the 18 LGV cases with sufficient material for L-variant typing, 13 were L2b and 5 were L2f, indicating that the cluster of cases was not due to clonal spread of a single strain.
Discussion
This article describes the current molecular procedures and specimen acceptance criteria used by the CDC STD Laboratory for confirmation of LGV during an epidemiologic investigation of clustered cases. In the past, testing relied on culture or serology (3) (also reviewed in (10)). Culture is not practical in most settings and serologic assays are no longer recommended (5). Since 2014, CDC exclusively recommends using NAATs for CT diagnosis. PCR-based assays have also been developed for molecular epidemiologic investigation of LGV cases.
The LGV PCR results were not used for patient management because our LGV test is not CLIA certified. We did not seek CLIA certification due to our limited laboratory capacity, and also due to lack of specimens within the US to validate our assay; however, our assay was validated using specimens from the UK (8). Very few cases have been brought to the attention of the CDC, and we received no requests for laboratory support in over a decade (between 2004 and 2015). Even if our test was CLIA certified, the turnaround time would not have been fast enough to meet patient management needs considering this was a potential outbreak situation. We made the decision to only accept specimens from probable cases and not from suspected cases. The goal was to limit the number of specimens arriving in our laboratory. This was done because our laboratory has limited capacity, and it was uncertain how large the outbreak was going to become.
We limited this note to laboratory processes, and did not attempt to discuss patient treatment issues such as whether patients should be treated with Azithromycin (recommended treatment for CT) or Doxycycline (recommended treatment for CT-LGV)(11). We acknowledge that availability of timely laboratory diagnostic test results to differentiate between the two could assist in these provider decisions. Commercial LGV diagnostic test development, and building increased laboratory diagnostic capacity locally would be beneficial for treatment decisions and for management of potential future outbreaks.
Supplementary Material
Acknowledgements
This work was funded by the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC. The authors wish to thank Deborah Richmond for her dedication to sending patient samples in exploratory media. For assistance with confirmatory LGV testing, readers are invited to contact the corresponding author.
Footnotes
List of Supplemental Digital Content
EKLGV_Labmethods_supplemental digital content.ppt
References
- 1.Van der Bij AK, Spaargaren J, Morre SA, et al. Diagnostic and clinical implications of anorectal lymphogranuloma venereum in men who have sex with men: a retrospective case-control study. Clin Infect Dis. 2006;42(2):186–94. [DOI] [PubMed] [Google Scholar]
- 2.de Voux A, Kent JB, Macomber K, et al. Notes from the Field: Cluster of Lymphogranuloma Venereum Cases Among Men Who Have Sex with Men - Michigan, August 2015-April 2016. MMWR Morb Mortal Wkly Rep. 2016;65(34):920–1. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Lymphogranuloma venereum among men who have sex with men--Netherlands, 2003–2004. MMWR Morb Mortal Wkly Rep. 2004;53(42):985–8. [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae−−2014. MMWR Recomm Rep. 2014;63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 6.de Vrieze NH, de Vries HJ. Lymphogranuloma venereum among men who have sex with men. An epidemiological and clinical review. Expert Rev Anti Infect Ther. 2014;12(6):697–704. [DOI] [PubMed] [Google Scholar]
- 7.Michigan Health Network Alert; 2015; Confirmed cases of Lymphogranuloma venereum in Michigan 10/22/2015; available: http://www.miregion7.com/alerts/confirmed-cases-of-lymphogranuloma-venerum-in-michigan; accessed 2/10/2016.
- 8.Chen CY, Chi KH, Alexander S, Ison CA, Ballard RC. A real-time quadriplex PCR assay for the diagnosis of rectal lymphogranuloma venereum and non-lymphogranuloma venereum Chlamydia trachomatis infections. Sex Transm Infect. 2008;84(4):273–6. [DOI] [PubMed] [Google Scholar]
- 9.Chen CY, Chi KH, Alexander S, et al. The molecular diagnosis of lymphogranuloma venereum: evaluation of a real-time multiplex polymerase chain reaction test using rectal and urethral specimens. Sex Transm Dis. 2007;34(7):451–5. [DOI] [PubMed] [Google Scholar]
- 10.Stoner BP, Cohen SE. Lymphogranuloma Venereum 2015: Clinical Presentation, Diagnosis, and Treatment. Clin Infect Dis. 2015;61 Suppl 8:S865–73. [DOI] [PubMed] [Google Scholar]
- 11.Leeyaphan C, Ong JJ, Chow EP, et al. Systematic Review and Meta-Analysis of Doxycycline Efficacy for Rectal Lymphogranuloma Venereum in Men Who Have Sex with Men. Emerg Infect Dis. 2016;22(10):1778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.