Abstract
Integrins mediate cell adhesion and transmit mechanical and chemical signals to the cell interior. Various mechanisms deregulate integrin signaling in cancer, empowering tumor cells with the ability to proliferate without restraint, to invade through tissue boundaries, and to survive in foreign microenvironments. Recent studies have revealed that integrin signaling drives multiple stem cell functions, including tumor initiation, epithelial plasticity, metastatic reactivation, and resistance to oncogene- and immune-targeted therapies. Here, we discuss the mechanisms leading to the deregulation of integrin signaling in cancer and its various consequences. We place emphasis on novel functions, determinants of context dependency, and mechanism-based therapeutic opportunities.
Introduction
Since their discovery in the late 1980s, the integrins have taken center-stage among receptors involved in cell adhesion and signaling, and extensive studies have documented their multifarious functions in tumorigenesis. Upon binding to the extracellular matrix (ECM), the integrins organize the cytoskeleton and activate intracellular signaling, regulating complex cellular behaviors, including survival, proliferation, migration, and various cell fate transitions (Giancotti and Ruoslahti, 1999; Hynes, 1992). A paramount function of integrins is to impart positional control on the action of cytokine and growth factor receptors so as to coordinate development, regeneration, and various repair processes (Danen and Yamada, 2001; Giancotti and Tarone, 2003). Exemplifying this control, integrins and receptor tyrosine kinases (RTKs) need to be jointly engaged to ensure optimal activation of pro-mitogenic and pro-survival signaling through the Ras-extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathways.
Because many prevalent oncogenic mutations deregulate intracellular signaling downstream of both integrins and RTKs (e.g., Ras), it has been initially argued that neoplastic cells are no longer dependent on integrin signaling (Schwartz, 1997). However, genetic and biochemical studies have indicated that the integrins function not merely by buttressing mitogenic and survival signaling but also more directly control diverse aspects of cancer development, ranging from tumor initiation and initial invasion to metastatic reactivation of dormant disseminated tumor cells (Desgrosellier and Cheresh, 2010; Giancotti, 2013; Guo and Giancotti, 2004). We here discuss the origins and consequences of deregulated integrin signaling in cancer with an emphasis on new functions—such as mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance—and we illustrate emergent therapeutic opportunities.
Overview of Integrin Signaling
The integrins comprise a family of 24 heterodimeric receptors, which mediate adhesion to a variety of extracellular matrix components and, in some cases, to counter-receptors on other cells (Figure 1A; see Humphries et al., 2006 for ligand binding-specificity of integrins). Large allosteric changes couple ligand binding to the ectodomain of the integrin with the recruitment of the cytoskeletal protein talin to the intracellular portion of the integrin β subunit. Hence, ligand binding triggers integrin association with the actin cytoskeleton via talin and, conversely, intracellular signaling pathways impinge on MRL proteins (RIAM and lamellipodin) to promote talin binding to the cytoplasmic domain of the integrin β subunit and thus integrin activation (Figure 1B). Because of these properties, the integrins behave as allosteric bidirectional signaling machineries (Hynes, 2002). Ligand-bound integrins engage the actin network via talin and additional cytoskeletal linker proteins, leading to integrin clustering and the ensuing activation of focal adhesion kinase (FAK) and SRC family kinases (SFKs). Organization of the actin cytoskeleton and kinase signaling pathways impinge on prominent pro-mitogenic/pro-survival signaling pathways and their transcriptional outputs, including the Ras-ERK, PI3K/AKT, and YAP/TAZ pathways (Box 1).
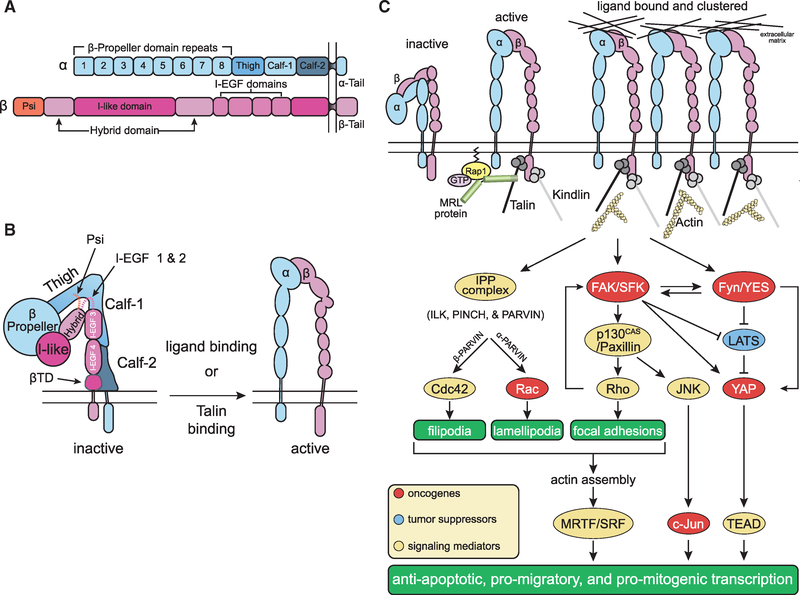
Figure 1. Integrin-Mediated Signal Transduction.
(A) Domain organization and structure of a generic integrin. The α and β subunits have large extracellular domains and short cytoplasmic domains. Exceptions to this generic domain structure include the a subunits of leukocyte integrins (αL, αM, and αX) and those of collagen-binding β1 integrins, which have an I domain inserted between β propeller domains 2 and 3. When present, the I domain participates in ligand binding together with the I-like domain in the extracellular portion of the β subunit. In addition, the β4 integrin is also structurally variant as it possesses a large and unique cytoplasmic domain with two pairs of type III fibronectin-like repeats and connects with the keratin, not the actin, cytoskeleton at hemidesmosomes.
(B) Allostery-driven bidirectional signaling. The β propeller in the N-terminal portion of the α subunit combines with the I-like and hybrid domain in the corresponding portion of the β subunit to form the ligand binding pocket and the head piece of the integrin. Inactive integrins exhibit a closed conformation (“are bent at their knees”): the ligand binding pocket possesses low affinity for ligand and faces toward the plasma membrane and the “legs” (α subunits Calf-1 and −2; β subunit I-EGF3, I-EGF4 and the membrane-proximal tail domain βTD), transmembrane and cytoplasmic domains are adjoined (left). Talin binding to the β subunit cytoplasmic domain triggers large conformational changes that include an extension of the legs and a separation of the heterodimeric subunits at the level of the transmembrane and cytoplasmic domains. Ligand binding to partially active integrins can induce the same conformational changes, reinforcing integrin activation. Active integrins are extended and possess a ligand binding-competent headpiece exposed toward the extracellular space and a β cytotail bound to talin (right). These mechanisms ensure that talin binding induces integrin activation and, conversely, ligand binding induces talin binding and hence association with the actin cytoskeleton.
(C) Integrin activation, clustering, and autonomous signaling. Current models suggest that GTP-Rap1 induces MRL proteins, such as RIAM and lamellipodin, to activate talin, which in turn binds to the integrin β subunit cytoplasmic domain, separating it from the corresponding portion of the α subunit. Talin binding thus triggers the large conformational changes that lead to integrin activation. Both talin and kindlin can connect to the actin cytoskeleton and promote integrin clustering, initiating robust intracellular signaling. In addition to organizing the cytoskeleton and thereby impinging on myocardin-related transcription factor (MRTF)/serum response factor (SRF), integrin signaling contributes to the activation of AP-1, inhibition of FOXO, and activation of YAP/TEAD. While the integrins impinge on these transcription factors predominantly through activation of MRTF, c-Jun, and YAP, receptor tyrosine kinases potently activate c-Fos and inhibit FOXO through the Ras-ERK and PI3K-AKT pathways (see Figure 2). Oncogenes are shown in red, tumor suppressors in blue, and mediators of integrin signaling in yellow.
Box 1. Integrin Signaling Redux.
Biophysical studies have illustrated the large allosteric changes that enable ligand binding and the initial connection of integrins to the actin cytoskeleton (Luo et al., 2007). But the ensuing events, which presumably occur at nascent focal adhesions, are less well understood. Cytoskeletal linker proteins, such as talin, vinculin, and kindlin, and the scaffold/adaptor integrin-linked kinase (ILK) connect integrins, either directly or indirectly, to actin filaments (Qin and Wu, 2012; Wolfenson et al., 2013). In particular, activated talin is key to the formation of the molecular clutch that enables the transmission of mechanical forces across integrin adhesion sites and it collaborates with kindlin to mediate integrin clustering (Sun et al., 2016; Ye et al., 2014) (Figure 1C). Both events are critical for the activation of FAK and the recruitment and activation of SFKs. As a consequence of these events and the recruitment of ILK, the Rho family GTPases Cdc42, Rac, and Rho undergo local activation, coordinating the assembly of filopodia, lamellipodia, and focal adhesions, respectively (Wolfenson et al., 2013). Ultimately, the organization of the actin cytoskeleton promotes transcription through myocardin-related transcription factors (MRTFs), which are co-activators for the serum response factor (SRF) (Miralles et al., 2003) (Figure 1C). In growth factor-stimulated cells, SRF cooperates with the ternary complex factor subfamily of ETS transcription factors to drive the expression of immediate-early genes, such as FOS, illustrating a major mechanism for integration of integrin and growth factor signals.
Upon activation of FAK and in response to tension exerted across integrin adhesion sites, p130CAS undergoes substantial conformational changes and its substrate domain unfolds, making several tyrosine residues available for phosphorylation by SFKs and for binding of Crk (Mitra and Schlaepfer, 2006; Roca-Cusachs et al., 2012). This oncoprotein in turn promotes activation of JNK and, again in conjunction with growth factor stimulation, AP-1-dependent transcription, driving proliferation and migration (Lopez-Bergami et al., 2010) (Figure 1C). With and without the assistance of FAK, SFKs phosphorylate additional substrates, impinging on multiple pathways, including the Ras-ERK and PI3K-AKT pathways. These pathways exert their pro-survival and pro-mitogenic roles by inducing the phosphorylation of cytoplasmic targets as well as by inducing AP-1 and repressing FOXO-mediated transcription in the nucleus. Furthermore, integrin signaling through FAK/SFK activates YAP (Kim and Gumbiner, 2015; Si et al., 2017), and FAK continues to convey antiapoptotic signals as it is internalized in endosomes and can enter the nucleus to promote degradation of p53 and to induce transcription of cytokine genes (Alanko and Ivaska, 2016; Kleinschmidt and Schlaepfer, 2017). Both mechanisms contribute to expand the repertoire of transcriptional events controlled by integrins (Figure 1C).
The signaling pathways activated by integrins and RTKs are extensively interconnected at various levels to support anchorage-dependent cell survival and proliferation (Figure 2). Notably, many growth factors bind to the ECM, and the geometric arrangement of integrin and growth factor binding sites in the ECM enables simultaneous engagement of their cognate receptors on the plasma membrane (Hynes, 2009). In addition, galectins and specialized plasma membrane domains, such as caveolin microdomains, regulate the nucleation of integrin and RTK signaling complexes and the activation of palmitoylated SFKs, such as Fyn and YES, and hence the activation of the signaling adaptor Shc (Giancotti and Tarone, 2003; Lajoie et al., 2009). Figure 2A depicts some of the integrin-RTK interactions that have been characterized and emphasizes the role of integrins in activating SFKs, and thus inducing phosphorylation of the P loop and activation of RTKs. Reciprocal effects have also been described, such as the epidermal growth factor receptor (EGFR)- or ErbB2-mediated phosphorylation of FAK or integrin β4 (Giancotti and Tarone, 2003).
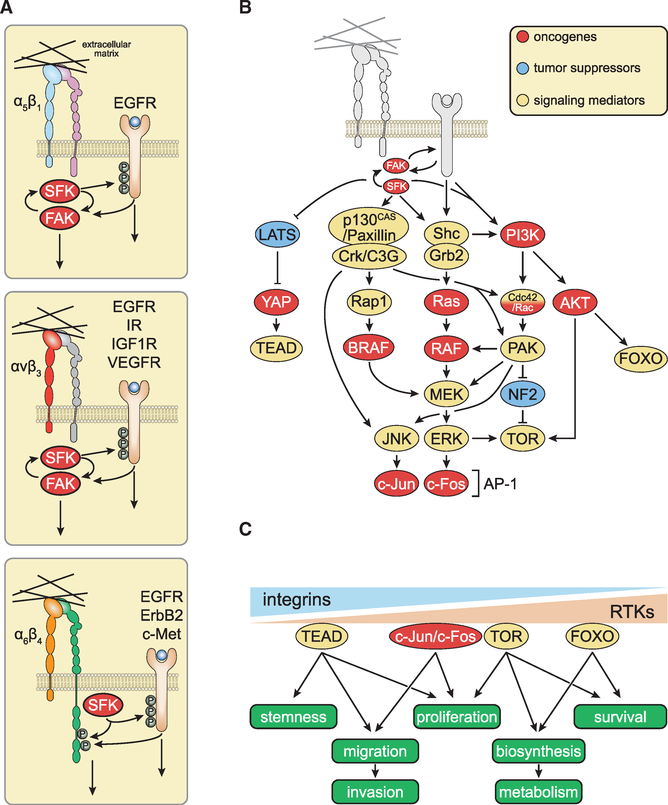
Figure 2. Joint Integrin-Receptor Tyrosine Kinase Signaling and Mechanotransduction.
(A) Membrane-proximal mechanisms underlying joint integrin-RTK signaling. Integrins buttress mitogenic signaling by RTKs at multiple levels. At or near the plasma membrane, integrin-activated SRC family kinases (SFKs) induce the phosphorylation of the P loop of RTKs, priming them for ligand-induced activation. The RTKs in turn induce phosphorylation of focal adhesion kinase (FAK) or the signaling domain of the β4 integrin. These elements recruit distinct subsets of signaling enzymes and adaptors, refining the specificity of individual partner RTKs.
(B) Major membrane-distal interconnections between integrin and RTK signaling pathways. Integrins and RTKs cooperate to activate FAK, SFKs, and PI3K. The FAK-SFK complex phosphorylates and activates p130CAS and paxillin, thereby mediating activation of JNK and––in cells expressing Rap1––of BRAF. Several mechanisms ensure joint regulation of Ras and PI3K by integrins and RTKs.
(C) Integrins and RTKs regulate multiple cellular functions of normal and transformed cells through additive, co-dependent, or synergistic mechanisms.
Additional mechanisms contribute to integrate integrin and RTK signaling. For example, the inositol phosphatase SHIP2 acts as a scaffold to enable recruitment of the fibroblast growth factor receptor (FGFR) to focal adhesions, promoting activation of SFKs and hyperphosphorylation of FRS2. This mechanism contributes to prolong ERK activation in response to FGF (Fafilek et al., 2018). Furthermore, and perhaps of equal importance, integrins and growth factor receptors jointly activate key downstream signaling components, such as Shc, PI3K, Rac, and MEK, in an additive, co-dependent, or synergistic manner (Figure 2B). As a result, optimal activation of many downstream targets, such as AP-1 (c-Jun/c-Fos) and target of rapamycin (TOR), require simultaneous ligation of integrins and RTKs, although to differing degrees depending on the effector. For example, YAP and FOXO seem to be activated predominantly by integrins and RTKs, respectively. Through these mechanisms, integrins and RTKs cooperate to regulate stemness, migration/invasion, proliferation, biosynthesis, metabolism, and survival (Figure 2C). In normal cells, flux through the major signaling pathways jointly activated by integrins and RTKs is limited by strong negative feedback mechanisms, which adjust the amplitude and frequency of signaling to the physiological needs of tissue development and repair, and act as a barrier to tumor development (Avraham and Yarden, 2011; Chandarlapaty, 2012).
Adding complexity to the integrin signaling system, several integrin-specific signaling connections have emerged. These include integrin associations with one or another RTK, with specific tetraspanins, syndecans, uPAR, CD44, and direct integrin recruitment of signaling components (Cantor et al., 2008; Danen and Yamada, 2001; Hemler, 2003; Kugler et al., 2003; Morgan et al., 2007). In a particularly striking example of specialization, the αvβ6 and αvβ8 integrins directly bind to the RGD sequence in latency-associated peptide (LAP) (transforming growth factor β [TGF-β] pro-peptide) and, by pulling on it, provoke the release of active TGF-β from the matrix-bound large latent complex (Munger and Sheppard, 2011). Since these integrins participate in the activation of TGF-β, they may, through this mechanism, inhibit the proliferation of cells sensitive to the growth inhibitory role of TGF-β. Because several integrins can bind to the same matrix ligand, these forms of specialization may provide a mechanism for differential cellular responses to the same integrin ligand (Giancotti, 2000).
Mechanisms Driving Deregulation of Integrin Signaling in Cancer
Changes in Matrix Composition/Structure and Integrin Expression
Changes in the ECM and the repertoire of integrins on tumor cells contribute to deregulate integrin signaling in cancer. At the onset of frank malignancy, carcinoma cells dissociate from one another and from adjacent normal cells, and dissolve the underlying basement membrane through decreased production and increased proteolysis of many of its components. Cancer cells subsequently recruit various host cells within the tumor microenvironment (TME), including fibroblasts, angiogenic endothelial cells, and immune cells, to aid their invasive growth (Egeblad et al., 2010a). The ECM, which is jointly produced and deposited in the extracellular space by tumor cells and host cells, undergoes profound changes in composition, structure, and mechanical properties during tumor progression (Lu et al., 2012). In breast cancers, collagen I becomes highly linearized, as if it were under tension, and oriented either tangentially or perpendicular to the tumor-stroma interface (Provenzano et al., 2008). Moreover, hypoxia-inducible factor 1 induces production of lysyl oxidase (LOX), which crosslinks type I collagen and other fibrillar collagens, increasing the stiffness of the tumor matrix and enhancing integrin signaling and proliferation (Erler et al., 2006; Levental et al., 2009) (Figure 3).
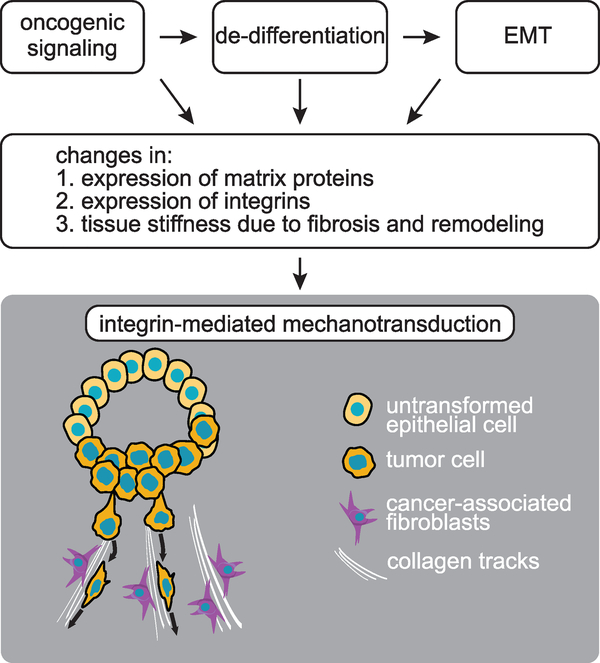
Figure 3. Origin and Consequences of Integrin-Mediated Mechanotransduction in Cancer.
Oncogenic signaling and changes in cell fate contribute to deregulate integrin expression and function in cancer. Changes in matrix composition and structure enable integrins to transmit integrated biochemical and mechanical signals to the interior of cancer cells.
Although fibrotic changes may pose a barrier to invasion, tumor cells create pathways through rigid matrices by producing matrix metalloproteases and other matrix remodeling enzymes. Matrix proteolysis also results in the release of growth factors and cytokines, such as vascular endothelial growth factor (VEGF) and active TGF-β, that collectively provoke neo-angiogenesis and facilitate tumor cell intravasation (Egeblad et al., 2010b). The upstream mechanisms that control remodeling of the ECM during tumor progression are not well defined. However, since the genes encoding matrix proteins and remodeling enzymes are not commonly deleted or amplified in primary cancers, it is likely that oncogene-induced changes in transcription, epigenetic reprogramming to a less-differentiated state, and the epithelial-mesenchymal transition (EMT) jointly contribute to the altered composition of tumor matrix (Figure 3).
Acting at the onset of tumorigenesis and prior to invasion, neoplastic conversion profoundly alters the level of expression of individual integrins, modifying the integrin repertoire on cancer cells and provoking changes in integrin signaling that sustain neoplastic transformation (Giancotti and Ruoslahti, 1990; Plantefaber and Hynes, 1989). Oncogenic signaling is a major driver of these changes (Figure 3). Consistently, activated KRas and BRAF induce the expression of the pro-tumorigenic α6 and β3 integrin subunits through activation of ERK in pancreatic cancer and melanoma (Woods et al., 2001), whereas loss of the tumor suppressive microRNA miR-25 produces the same effect by reversing silencing of the corresponding mRNAs in prostate cancer (Zoni et al., 2015). Providing a particularly relevant case in point, “gain-of-function” TP53 mutations, which are found in many tumor types, disrupt integrin-mediated adhesion to the basement membrane and induce invasion through dominant negative inhibition of p63 (Muller et al., 2009). One of the major effects of inhibition of p63 is the upregulation of the Rab-coupling protein, which increases recycling of the α5β1 integrin and the EGFR to invasive protrusions, promoting joint integrin/RTK signaling (Jacquemet et al., 2013; Muller et al., 2009). In addition, at least in ovarian cancer, mutant p53 acts through the same integrin to induce expression of the EMT transcription factor TWIST1 and to promote the formation of tumor cell clusters, which intercalate in the mesothelium and outgrow into peritoneal tumors (Iwanicki et al., 2016). Finally, oncogenic signaling can lead to changes in integrin expression by inducing dedifferentiation and EMT, as first demonstrated by early studies on TGF-β signaling (Ignotz and Massague, 1987) (Figure 3). However, not all of the effects of oncogenic mutations on integrins facilitate tumor progression. For example, it has been suggested that MYC inhibits invasion and metastasis in breast cancer by downregulating αvβ3 (Liu et al., 2012). These examples illustrate the striking effect of oncogenic context on integrin expression and signaling.
The effects of remodeled tumor matrices and deregulated integrin signaling may be far reaching. Transcriptomic analysis of a transplantation model of skin cancer identified a tumor progression signature enriched in matrix proteins, integrins, and AP-1 transcription factors. Provocatively, antibody-mediated blockade of β1 integrins inhibited tumor progression in this model, suggesting that tumor matrices exert their effect through integrin signaling and AP-1 (Reuter et al., 2009). Moreover, comparison of metastatic and non-metastatic variants of human breast cancer cells implanted in mice revealed both stromal and tumor cell-derived protein signatures, and the latter were found to comprise matrix proteins implicated in distinct steps of metastasis (Naba et al., 2014). In particular, tenascin-C (TNC) was shown to be an integral component of the metastatic niche molded by breast cancer cells during lung colonization (Oskarsson et al., 2011), and later studies using a proteomic approach expanded the role of this matrix protein to lung cancer metastasis (Gocheva et al., 2017). These results suggest that ECM proteins produced by both tumor cells and stromal cells contribute to alter integrin signaling to promote invasive growth and metastasis.
Rewiring of the Integrin Signaling Network in Cancer
Building on the notion of integrin-specific signaling, we made the hypothesis that certain integrins promote tumorigenesis, while others do not exert this effect or they may even suppress tumorigenesis (Guo and Giancotti, 2004). In agreement with this hypothesis, several studies have implicated αvβ3 and α6β4 integrins as positive regulators of tumorigenesis (Desgrosellier and Cheresh, 2010; Guo and Giancotti, 2004). This function likely arises from the ability of both integrins to cooperate with oncogenic RTKs. For example, the α6β4 integrin combines with the EGFR, ErbB2 (also known as HER2 for human EGFR2, encoded by ERBB2) and c-Met RTKs to amplify oncogenic signaling in several carcinomas (Guo et al., 2006; Mariotti et al., 2001; Trusolino et al., 2001). Similarly, the αvβ3 integrin combines with the platelet-derived growth factor β (PDGF-β) receptor and enhances its signaling capacity in PDGF-overproducing glioblastomas (GBMs) (Schneller et al., 1997; Sturm et al., 2014). One shared mechanism of action originates from the ability of these integrins to induce SFKs to phosphorylate the P loop in RTKs, sustaining their activation. Conversely, activated RTKs coopt the cytoplasmic domain of β4 or signaling adaptors downstream of αvβ3 to amplify their signaling output (Giancotti, 2007; Giancotti and Tarone, 2003) (Figure 2A). In fact, genetic studies have demonstrated that ErbB2 coopts the β4 integrin to disrupt epithelial adhesion and polarity and to promote invasion and hyperproliferation during mammary tumorigenesis (Guo et al., 2006). These findings suggest that extensive bidirectional crosstalk between certain integrins and their companion RTKs sustains oncogenesis in certain contexts.
Other integrins may support or oppose tumorigenesis independently of RTKs. For example, a recent study has identified a dependency of myxofibrosarcoma on the collagen-binding integrin α10β1. Interestingly, this integrin activates Rac-PAK through the adaptor TRIO and mammalian TOR through the substrate adaptor RICTOR—notably TRIO and RICTOR are coamplified in a fraction of these cancers (Okada et al., 2016). Moreover, given their roles in activation of TGF-β, the αvβ5, αvβ6, and αvβ8 integrins may inhibit cell proliferation in tumor cells that retain an intact cytostatic response to TGF-β and induce invasion and metastasis in other oncogenic contexts. In fact, it is well established that mutations of the TGF-β target genes CDKN2B and CDKN1A debilitate TGF-β’s cytostatic program and empower TGF-β signaling to implement its pro-invasive program, often through an EMT (David and Massague, 2018). Intriguingly, at least αvβ6 can switch from activation of TGF-β to promotion of invasion and metastasis in tumor cells expressing Eps8, an activator of Rac (Tod et al., 2017).
Integrin-specific signals play a role even in cancers initiated by common genetic alterations, such as mutations of KRAS, BRAF, PTEN, and PIK3CA, which should in principle alleviate the requirement for joint integrin-RTK signaling. In fact, genes encoding integrin-specific signaling components are often mutated in cancer types that harbor these common mutations. The prototype is PTK2, encoding FAK, that is amplified or overexpressed in about 40% of ovarian cancers (Cancer Genome Atlas Research Network, 2011) and 25% of invasive breast cancers (Cancer Genome Atlas Network, 2012b; Pylayeva et al., 2009), and in metastatic castration-resistant prostate cancers (Cancer Genome Atlas Research Network, 2011; Kumar et al., 2016; Robinson et al., 2015). SRC, encoding the FAK’s partner kinase SRC, is amplified or overexpressed in about 10% of colorectal cancer (Cancer Genome Atlas Network, 2012a). Moreover, DLC1, encoding a RhoGAP, is frequently deleted in hepatocellular carcinoma (Xue et al., 2008), and activating mutations in RAC1 and RAC2 have been identified in several cancer types (Kawazu et al., 2013). Finally, integrins negatively regulate Merlin, encoded by the tumor suppressor NF2, via PAK-mediated phosphorylation (Okada et al., 2005). Once phosphorylated, this FERM domain protein no longer accumulates in the nucleus to inhibit the E3 ubiquitin ligase CRL4DCAF1 (Li et al., 2010). CRL4DCAF1 can in turn induce the ubiquitylation and degradation of LATS, leading to activation of YAP (Li et al., 2014). In addition, SRC can directly phosphorylate and inhibit LATS, also contributing to the activation of YAP (Si et al., 2017) (Figure 4). Intriguingly, FAK is a FERM domain protein and, similarly to Merlin, accumulates in the nucleus to regulate gene expression in a kinase-independent fashion (Lim et al., 2008; Serrels et al., 2015). In many of the above scenarios, deregulation of integrin signaling provides a competitive advantage to tumor cells without directly impinging on joint integrin-RTK signaling.
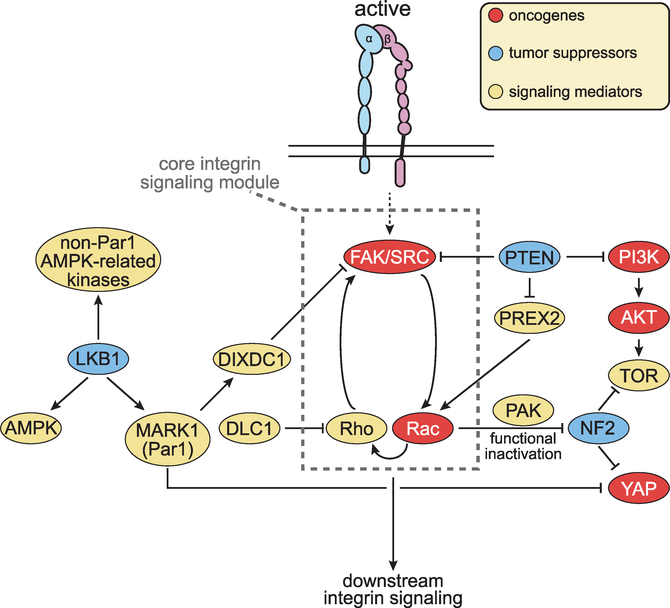
Figure 4. Rewiring of the Integrin Signaling Network in Cancer.
Alterations in oncogenes and tumor suppressors deregulate integrin signaling in cancer. Oncogenic receptor tyrosine kinases and protumorigenic integrins collaborate to activate FAK-SFK signaling. Mutations in LKB1, MARK1, and DIXDC1 remove an inhibitory constraint. Mutations in PTEN exert the same effect by activating FAK as well as Rac. In addition to reinforcing activation of Rho and hence FAK, Rac activates PAK and functionally suppresses NF2. DLC1 opposes activation of Rho. Additional mechanisms enable specific integrins to promote or suppress tumorigenesis, often in a context-dependent manner.
Finally, several oncogenic mutations may function upstream of integrin signaling. For example, although the tumor suppressor PTEN inhibits cell proliferation by dephosphorylating PIP3 and suppressing PI3K signaling, its ability to restrain cell migration and invasion depends on tail domain-mediated suppression of the Rac GEF PREX2 (Mense et al., 2015) and on dephosphorylation and inhibition of FAK (Tamura et al., 1998). In support of the pathological relevance of the latter mechanism, recent studies have shown that FAK is overactivated and promotes survival signaling in PTEN null T cell acute lymphoblastic leukemias (T-ALL); notably, protein-phosphorylation but not lipid phosphorylation-defective mutants of PTEN reverse FAK activation and induce apoptosis in these leukemias (You et al., 2015). Another case in point is LKB1, which is inactivated in the inherited cancer disorder, Peutz-Jeghers syndrome, and in about 25% of non-small-cell lung cancers (NSCLC). LKB1 controls apico-basal polarity and metabolism by phosphorylating a wide spectrum of substrates (Hardie, 2013). Phosphorylation and activation of AMP-activated protein kinase leads to suppression of mTORC1 and growth inhibition, while phosphorylation of MARK1 (Par1) governs apico-basal polarity and activates the Hippo tumor suppressor pathway (Martin-Belmonte and Perez-Moreno, 2011; Mohseni et al., 2014). Intriguingly, MARK-mediated phosphorylation of the scaffolding protein DIXDC1 stabilizes focal adhesions and thus prevents SFK-mediated activation of ERK and induction of the EMT-inducing transcription factor Snail (Goodwin et al., 2014). These observations suggest that inactivation of PTEN or LKB1 promotes invasion and metastasis by activating integrin signaling (Figure 4).
Genetic Studies Demonstrate a Pro-oncogenic Role for Integrin Signaling
Genetic studies have demonstrated that integrin activation and signaling promotes and, in some cases, is required for tumor initiation and progression. Expression of a constitutively active β1 integrin subunit in the basal layer of the mouse epidermis promotes neoplastic conversion of papillomas to squamous cell carcinomas in a chemical carcinogenesis model (Ferreira et al., 2009). Conversely, mutation of integrin cytoplasmic NPXY motifs, which mediate talin and kindlin binding and integrin activation, interferes with this process, suggesting that integrin activation is required for invasive growth (Meves et al., 2011) (Figure 5A). Additional genetic studies have indicated that integrins contribute to tumorigenesis through their signaling capacity. This concept is most vividly illustrated by classical studies, demonstrating that FAK and the β4 integrin are necessary for tumor initiation and progression in mouse models of mammary and skin tumorigenesis, albeit through distinct signaling mechanisms and in the context of distinct cooperating oncogenic mutations.
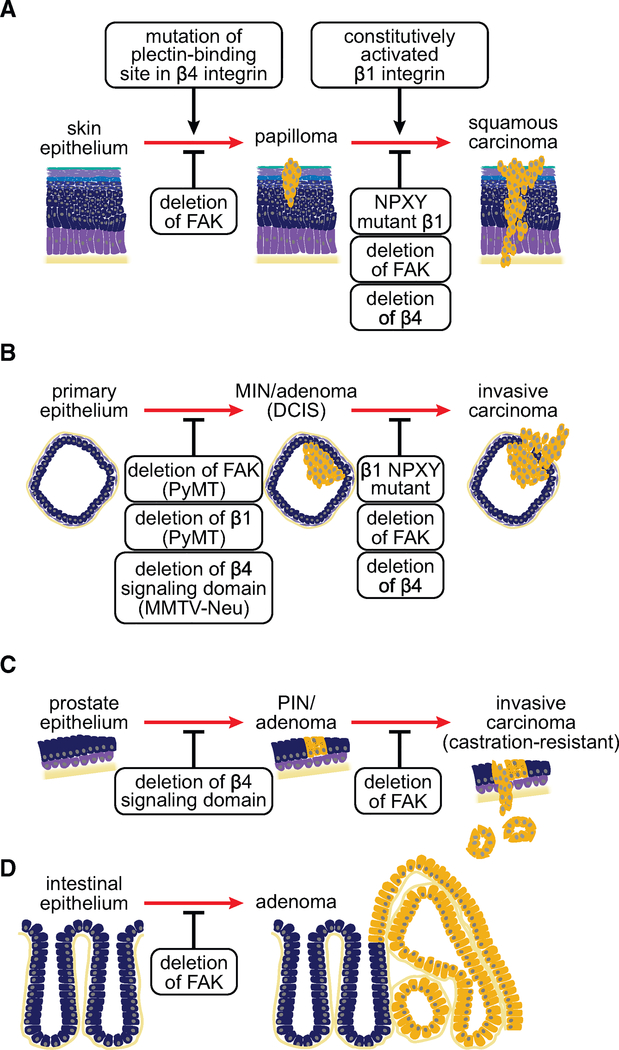
Figure 5. Genetics Underlying Integral Roles of Integrins and FAK in Cancer Development and Progression.
Genetic studies in mice revealed integrins, in particular β4, and FAK are necessary for tumor initiation and progression in models of skin (A), breast (B), prostate (C), and intestinal (D) cancers, yet the mechanisms underlying these dependencies vary between the models. DCIS, ductal carcinoma in situ; MIN, mammary intraepithelial neoplasia; PIN, prostatic intraepithelial neoplasia.
Deletion of Fak blocks skin papilloma formation in a chemical carcinogenesis model and, when the deletion is induced after the formation of papillomas, it blocks their conversion to squamous cell carcinomas. These effects were ascribed to both reduced migration/invasion and reduced survival (McLean et al., 2004) (Figure 5A). Notably, expression of v-Ha-ras (viral ras G12V mutant) in anogenital epithelium blocks apoptosis and accelerates the formation of squamous cell carcinomas initiated by inactivation of the TGF-β receptor gene Tgfbr2. This enables FAK-SRC signaling to block differentiation and prompt invasion (Guasch et al., 2007; Schober and Fuchs, 2011). In transplantation experiments, human keratinocytes lacking the β4 integrin resist transformation induced by v-Ha-ras and inhibition of nuclear factor κB (NF-κB) (Dajee et al., 2003). Interestingly, whereas linkage of the β4 integrin to the keratin cytoskeleton prevents p53 and Smad4 loss-mediated expansion of benign skin tumors, β4 signaling is necessary for the outgrowth of squamous cell carcinomas initiated by the subsequent introduction of v-Ha-ras (Raymond et al., 2007) (Figure 5A). These results suggest that β4, which signals independently of FAK, can have a tumor suppressive or an oncogenic role in skin epithelium depending on the genetic background.
Genetic deletion experiments in mouse models of breast cancer have documented a requirement for both FAK and integrin signaling during tumor initiation and progression. Deletion of Itgb1 encoding the β1 integrin or Fak inhibits tumor initiation and delays tumor progression in the mammary epithelium of mice expressing the polyomavirus middle T-antigen (PyMT), which activates SRC and PI3K (Lahlou et al., 2007; White et al., 2004). Deletion of Fak using a more efficient MMTV-Cre line causes instead a complete blockage of PyMT-initiated mammary tumor formation: the few tumors that arise in this model originate from cells that have escaped Cre-mediated recombination, providing strong evidence that Fak is necessary for mammary tumorigenesis in this system (Pylayeva et al., 2009) (Figure 5B). Mechanistic experiments have linked these effects to the ability of FAK to recruit SFKs—thereby inducing phosphorylation of p130CAS and recruitment of Crk (Pylayeva et al., 2009)—and to sustain the self-renewal capability of cancer stem cells (Luo et al., 2009). Intriguingly, deletion of Fak inhibits tumor initiation or progression only to a small extent in MMTV-Neu mice, which express an activated form of ErbB2 (Lahlou et al., 2012). In contrast, targeted deletion of the signaling domain of the β4 integrin impairs the expansion of mammary intraepithelial neoplasia lesions and their progression to invasive adenocarcinomas to a large extent in these mice (Guo et al., 2006) (Figure 5B). Mechanistic studies revealed that the mutation uncouples β4 from ErbB2, blocking phosphorylation of c-Jun and activation of STAT3. Whereas c-Jun promotes tumor cell hyperproliferation, STAT3 is required for disruption of epithelial adhesion and polarity in this model (Guo et al., 2006). These studies indicate that FAK and the β4 integrin contribute to tumor initiation and progression through distinct signaling mechanisms and their effect depends on the oncogenic background.
Additional studies have documented a role for integrin signaling in mouse models of other tumor types. Targeted inactivation of the β4 signaling domain suppresses prostate tumor growth and progression in Pb-TAg mice, which lack functional p53 and Rb in their prostatic epithelium. In addition, this mutation suppresses Pten loss-driven prostate tumorigenesis in tissue recombination experiments. Mechanistic studies traced these defects to an inability of signaling defective β4 to transactivate ErbB2 and c-Met in prostate tumor progenitors and promote their self-renewal capability (Yoshioka et al., 2013). Instead, deletion of Fak specifically inhibits progression to castrationresistance and metastasis in Pb-TAg mice (Slack-Davis et al., 2009) (Figure 5C). In intestinal epithelium, inactivation of Apc and hence induction of Wnt signaling drives the formation of adenomas, in part through expression of Myc (Figure 5D). Genetic analysis revealed that Myc exerts this effect by activating FAK and AKT-mTOR signaling (Ashton et al., 2010). In a mouse model of NSCLC driven by activated Kras and loss of Cdkn2a, activation of ERK disrupts negative regulation of Rho by p190-RhoGAP. FAK is the primary target of Rho in this model, as silencing or pharmacological inhibition of either Rho or FAK induces growth arrest and apoptosis and hence tumor regression (Konstantinidou et al., 2013). Moreover, consistent with the identification of FAK as a target of PTEN, FAK is overactivated in mouse models of Pten mutant T-ALL and contributes to its development through activation of NF-κB (You et al., 2015). Finally, an in vivo RNA interference screen has identified a requirement for the αvβ3 integrin during myeloid leukemia stem cell engraftment of the bone marrow. Depletion of β3 impaired homing and induced differentiation in this model (Miller et al., 2013). These findings document a requirement for specific integrin signals during neoplastic conversion and subsequent invasive growth in carcinomas and during engraftment in leukemias.
New Functions: Stemness, Epithelial Plasticity, and Metastasis
Integrin Signaling Promotes Self-Renewal and Expansion of Cancer Stem Cells
Specialized ECM niches and integrin signals support the function of normal adult stem cells and their neoplastic derivatives (Plaks et al., 2015). In skin epithelium, β1 integrins mediate adhesion of basal keratinocytes to the underlying basement membrane and govern stem cell renewal by regulating cell-cycle progression and by determining the axis of polarity for asymmetric cell division (Lechler and Fuchs, 2005; Taddei et al., 2008). In contrast, activation of TGF-β by the αvβ6 integrin induces basal keratinocytes to reduce their proliferative rates, restricting the size of the progenitor compartment and reducing skin tumor susceptibility (Rognoni et al., 2014). Prospective identification studies have indicated that additional integrins, such as α6β1, α6β4, and αvβ3, are highly expressed in normal and cancer stem cells. In fact, the α6 (CD49f) and β3 (CD61) subunits are commonly used as markers to identify luminal progenitors in the mouse mammary gland and in their ErbB2-transformed derivatives (Lo et al., 2012; Visvader and Stingl, 2014); the α6 and β4 (CD104) subunits can be used to sort bipotential progenitors in the normal mouse prostate and in mouse models of prostate cancer (Lawson and Witte, 2007; Yoshioka et al., 2013); and the β4 subunit (CD104) is enriched in alveolar progenitors in the mouse lung and tumor-initiating cells in a KRas-driven model of NSCLC (Chapman et al., 2011; Zheng et al., 2013). These observations potentially implicate the α6 integrins (α6β1 and α6β4) and the αvβ3 integrin in positive regulation of stemness.
Emerging evidence indicates that cancer stem cells coopt niche-integrin signals to fuel their expansion (Figure 6). In both GBMs and skin tumors, production of the pro-angiogenic cytokine VEGF induces the formation of a perivascular niche for cancer stem cell (CSC) expansion. In addition to inducing neoangiogenesis, VEGF also binds to VEGF receptor 2 (VEGFR2) and its co-receptor neuropilin 1 on CSCs, stimulating their self-renewal in an autocrine fashion (Beck et al., 2011; Calabrese et al., 2007; Hamerlik et al., 2012). Intriguingly, the CSCs in both types of tumors express high levels of the laminin-binding α6β1 integrin, which not only facilitates adhesion to the abluminal surface of the endothelial basement membrane but also transmits self-renewal signals through FAK (Lathia et al., 2010; Schober and Fuchs, 2011). Seemingly more immature subpopulations of GBM and esophageal carcinoma stem cells depend instead on the laminin-binding integrin α7β1 for activation of FAK and invasive outgrowth (Haas et al., 2017; Ming et al., 2016) (Figure 6A).
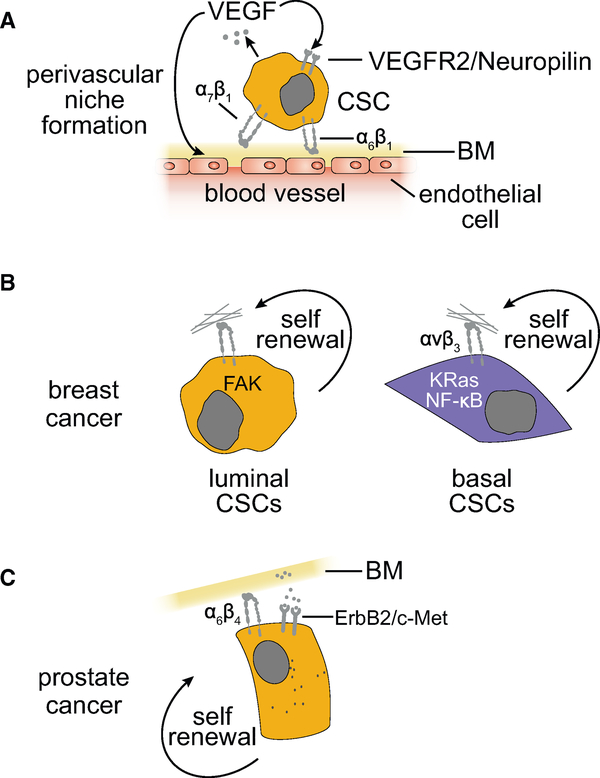
Figure 6. Specific Integrin Signaling Mechanisms Contribute to the Self-Renewal of Cancer Stem Cells in Different Tumor Types.
(A) The cancer stem cells in several cancer types adhere to the endothelial basement membrane (BM) at perivascular niches via α6β1 or α7β1 integrins and undergo self-renewal in response to activation of FAK.
(B and C) Shown are major integrin signaling mechanisms that enable epithelial BMs to support breast (B) and prostate (C) cancer stem cells.
In triple-negative breast cancer (TNBC), the α6β1 integrin associates with the VEGFR2-neuropilin 2 complex to promote activation of FAK and ERK and hence transcription of Hedgehog pathway components (Goel et al., 2012, 2013) (Figure 6A). Mesenchymal-like breast CSCs express elevated levels of an α6 cytoplasmic domain splice variant. When paired with β1, this splice variant promotes activation of TAZ, inducing a self-renewal and tumor-initiation program that includes overproduction of its ligand laminin 511 (laminin-10) (Chang et al., 2015; Goel et al., 2014). Providing further support for a specific role of FAK in stemness, genetic or pharmacological inhibition of FAK suppresses the self-renewal capacity of luminal breast CSCs (Luo et al., 2013). In contrast, inhibition of the αvβ3 integrin impairs expression of Slug and hence tumor-initiation capacity independently of FAK in basal breast CSCs (Desgrosellier et al., 2014). Mechanistic studies in carcinoma stem cells resistant to EGFR inhibitors have linked the ability of αvβ3 to promote stemness to activation of KRas and signaling to NF-κB (Seguin et al., 2014) (Figure 6B). Finally, targeted deletion of the signaling domain of β4, which also pairs with α6, reduces the self-renewal capacity of prostate tumor progenitors and the expansion of their transit-amplifying derivatives by impairing ErbB2 and c-Met signaling (Yoshioka et al., 2013) (Figure 6C). These findings suggest that multiple integrin signaling mechanisms contribute to the self-renewal and expansion capacity of CSCs in a context-dependent manner.
Even leukemia stem cells, which are usually considered to be anchorage-independent, depend on integrin signaling. Genetic deletion of CD98, which amplifies integrin activation, interferes with the establishment of acute myelogenous leukemia (AML) stem cells in their niche and suppresses their propagation (Bajaj et al., 2016). In addition, the leukemia stem cells in MLL-AF9 AML depend on αvβ3, which binds to osteopontin produced by niche cells in the remodeled hematopoietic compartment (Miller et al., 2013).
In conclusion, it appears that, depending on tumor type and possibly cell-of-origin and genetic background, the α6 subunit-containing integrins, α6β1 and α6β4, and the αvβ3 integrin, respond to niche signals to sustain self-renewal and tumor initiation. It remains to be established whether CSCs are more dependent on integrin signaling compared with normal stem cells or they are dependent on specific integrin signaling pathways/mechanisms that can be safely targeted therapeutically.
Roles of Integrins in Disruption of Epithelial Adhesion, EMT, and Invasion
The transition from ductal carcinoma in situ or adenoma to invasive carcinoma is driven by a discrete series of adhesive changes. At the onset of this process, individual cancer cells or groups of cancer cells detach from adjacent normal cells and the underlying basement membrane by remodeling or dissolving E-cadherin-dependent junctions and integrin-mediated adhesions, including, if present, α6β4-nucleated hemidesmosomes. Upon losing apico-basal polarity, tumor cells acquire a motile phenotype and, in response to oncogenic signaling, produce elevated levels of MMPs and plasmin. Focal degradation of the basement membrane finally enables tumor cells to penetrate into the underlying interstitial stroma of the tissue (Bonnans et al., 2014; Guo and Giancotti, 2004) (Figure 3). Cancer cells may implement these changes through a partial EMT (pEMT), independently of transcriptional changes, or through a canonical EMT, which involves complex changes in gene expression and is linked to cancer stemness (Box 2) (Figure 7).
Box 2. The Epithelial-Mesenchymal Transition and Stemness.
In many cases, cancer cells undergo local invasion by highjacking the transcriptional developmental program referred to as epithelial-mesenchymal transition (EMT) (Nieto et al., 2016). In addition to promoting invasion, passage through the EMT enables cancer cells to avoid apoptosis when deprived of matrix adhesion and shields them from immune recognition. Most intriguingly, tumor cells that have acquired mesenchymal traits exhibit enhanced stemness, suggesting that EMT and stemness are molecularly linked in support of metastatic dissemination and outgrowth (Lambert et al., 2017). The origin of this link is currently unclear. However, it is possible that in some cases the cancer cell-of-origin is a mesenchymal-like stem cell and thus its transformed derivative retains mesenchymal traits (Figure 7, left), whereas in other cases the cell-of-origin is a more differentiated cell that dedifferentiates to produce a mesenchymal-like cancer stem cell (Figure 7, right). Often, however, carcinoma cells can acquire motile and invasive properties without undergoing the complex epigenetic and transcriptional changes necessary for the acquisition of a mesenchymal cell fate, most notably the transcriptional repression of the E-cadherin gene (Grunert et al., 2003). For example, oncogenic RTKs, such as ErbB2 and c-Met, induce phosphorylation of components of E-cadherin adhesive junctions leading to reduced epithelial adhesion and polarity and increased migration (Guo et al., 2006). Since epithelial cells with such plastic junctions exhibit a mesenchymal morphology, this process has been referred to as a partial EMT (pEMT) (O’Brien et al., 2004). As discussed in the main text, tumor cells that have progressed only partially through a canonical EMT and thus possess hybrid epithelial/mesenchymal (E/M) traits arise through a different process and possess a distinct phenotype.
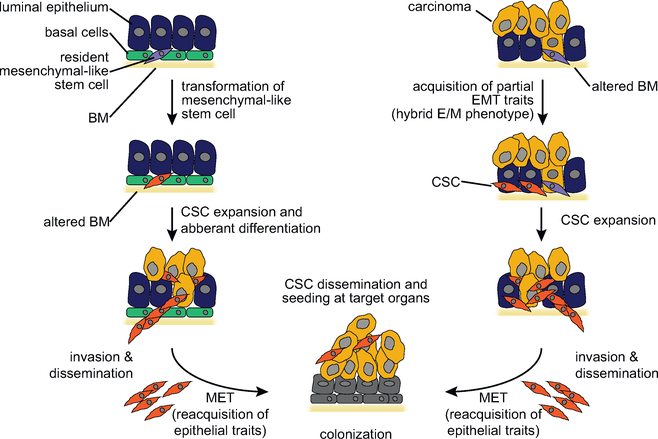
Figure 7. EMT and Stemness.
Models for the role of the EMT in stemness and metastatic colonization of carcinomas. Cancer stem cells may possess mesenchymal traits because they originate from the transformation of mesenchymal-like stem cells or the de-differentiation of neoplastic epithelial progenitors. Cancer stem cells with mesenchymal traits produce aberrantly differentiated epithelial progeny at the primary site and, because of their elevated invasive capacity, disseminate through the bloodstream and seed target organs (left). In a non-mutually exclusive model, the cancer stem cells possess epithelial traits but undergo a “partial” EMT in response to micro-environmental cues and thereby acquire the capacity to invade and disseminate (right). It is currently unresolved whether cancer stem cells with mesenchymal traits have to re-acquire epithelial traits (mesenchymal-epithelial transition [MET]) in order to outgrow into macroscopic metastases (bottom).
Integrin signals participate in both canonical and pEMT. During the latter process, integrin signaling through FAK and SFKs remodels E-cadherin-dependent junctions through direct phosphorylation of components of the E-cadherin-β-catenin complex. Furthermore, changes in integrin expression and usage facilitate cancer cell migration and invasion through molecular mechanisms similar to those operating in normal cells (Guo and Giancotti, 2004; Murphy and Courtneidge, 2011). Cancer cells that have undergone a pEMT, and thus retain plastic E-cadherin adhesions, are more likely to undergo collective cell migration, in which proteolysis is activated only in the immediate surroundings of the leader cells (Friedl et al., 2012). Solitary migrating cancer cells exhibit instead either an ameboid or a mesenchymal-like mode of migration and invasion, depending on the strength of integrin-mediated adhesion at the leading edge, the specific Rho GTPase deployed to derive traction, and the 3D architecture of the matrix (Sanz-Moreno and Marshall, 2010). The ameboid migration of isolated cancer cells through interstitial matrices may proceed independently of β1 integrins as demonstrated for leukocytes (Lammermann et al., 2008).
Integrin signaling exerts its most evident effect during a canonical EMT. The αv integrins, in particular αvβ6 and αvβ8, activate TGF-β, which in turn promotes expression of mesenchymal matrix proteins and their cognate integrin receptors, including also αv integrins. Furthermore, integrins and TGF-β receptors sustain each other’s expression and jointly modulate gene expression, providing additional mechanisms for positive reinforcement (Margadant and Sonnenberg, 2010). Transcriptional repression of E-cadherin during an EMT is sufficient to induce expression of the neuronal cell adhesion molecule, which interacts with Fyn in lipid rafts, leading to activation of FAK, assembly of focal adhesions, and cell migration (Lehembre et al., 2008). Illustrating the complexity and interdependence of migratory and invasion mechanisms, inactivation of β1 integrins leads to compensatory upregulation of the αvβ3 integrin and activation of TGF-β signaling in E-cadherin-positive TNBC cells. Increased TGF-β signaling in turn disrupts the feedback loop that represses Zeb2, inducing an EMT and disrupting collective cell migration (Parvani et al., 2013; Truong et al., 2014).
Progression through a canonical EMT requires complex epigenetic and transcriptional changes, proceeding through intermediary metastable stages (Tam and Weinberg, 2013). Emerging evidence suggests that these intermediary stages are more reversible and more conducive to metastatic colonization compared with a full mesenchymal state (Lambert et al., 2017; Nieto et al., 2016). It seems likely that the metastasis-initiating cells reside in one such intermediary stage, facilitating their dissemination but also their reversion to an epithelial state upon outgrowth and differentiation in the target organ (Giancotti, 2013; Lambert et al., 2017). Intriguingly, a recent study has identified the β4 integrin and CD44 as markers of breast CSCs residing in a hybrid epithelial/mesenchymal (E/M) state (Bierie et al., 2017). This finding suggests that other β4-positive CSCs, such as those of the prostate and lung (Chapman et al., 2011; Yoshioka et al., 2013; Zheng et al., 2013), also possess hybrid E/M traits and metastatic capacity. Further studies will be required to determine if the abundance of hybrid E/M carcinoma cells in primary carcinomas and among circulating cancer cells (CTCs) correlates with early metastatic relapse in patients.
Integrin-Mediated Mechanotransduction
Endowed with mesenchymal traits, many invasive tumor cells cooperate with cancer-associated fibroblasts (CAFs) to produce a rigid tumor matrix. Both types of cells deposit fibrillar collagens and other matrix proteins in their vicinity and reorient and crosslink individual collagen fibers in characteristic manners (Bonnans et al., 2014; Schedin and Keely, 2011). The consequences of a rigid matrix in tumors are far reaching. Many integrin complexes are able to sense the rigidity of the matrix and reinforce the strength of the adhesive bonds that they form with it through vinculin-dependent reinforcement of the adhesion clutch and formation of catch bonds with their ligands. Parallel clustering of the above force-transducing bonds reinforces mechanotransduction, enhancing activation of FAK (Sun et al., 2016). Moreover, invasive cancer cells often bear bulky glycoproteins on their surface, which create an incompressible ring around integrins and hence increase the tensile force applied to integrin ligand bonds, facilitating integrin clustering and signaling (Paszek et al., 2014).
When breast cancer cells are placed in a rigid 3D matrix, Rho signaling and integrin-mediated mechanotransduction, acting in a feedforward positive reinforcement cycle, drive disruption of epithelial adhesion and polarity and induce invasion (Paszek et al., 2005). Consistently, embedding fibrosarcoma cells or mesenchymal-like carcinoma cells in high-density fibrillar collagen induces the formation of MT1-MMP-containing invadopodia and invasion through a signaling pathway requiring the collagen-binding integrin α2β1 and kindlin 2 (Artym et al., 2015). In addition to promoting invasion, FAK-Rho signaling controls CSC self-renewal and overproliferation through AP-1 and YAP/TAZ (Assoian and Klein, 2008; Cordenonsi et al., 2011; Zanconato et al., 2015). Supporting a functional role of mechanotransduction in cancer, LOX-mediated crosslinking of collagen fibers and the ensuing formation of focal adhesions and activation of PI3K promote mammary tumor progression in MMTV-Neu mice (Levental et al., 2009). In luminal breast cancers, part of these effects may be due to the induction of miR-18a, which targets the homeobox protein A9, leading to inactivation of PTEN (Mouw et al., 2014). In a particularly striking example of integrins functioning at the nexus of mechanotransduction and malignancy, integrin-FAK mechanosignaling was shown to promote GBM mesenchymal- and stem-like phenotypes and increased invasion and therapeutic resistance in a feedforward mechanism linked to a bulky glycocalyx and rigid matrix (Barnes et al., 2018).
The role of integrin-mediated mechanotransduction in cancer may not be limited to activation of FAK/SFK signaling. As mentioned earlier, the αvβ5, αvβ6, and αvβ8 integrins activate TGF-β by pulling on LAP. Intriguingly, the type I and II TGF-β receptors accumulate at the periphery of focal adhesions, where tensile forces are diminished, suggesting that intermediate levels of tension facilitate TGF-β receptor signaling (Rys et al., 2015). As suggested earlier, the outcome of this process—cytostasis versus invasion and metastasis—may depend on whether cancer cells possesses mutations that disable TGF-β-mediated growth inhibition (David and Massague, 2018). Moreover, mechanical forces transmitted by the cytoskeleton and acting on the nucleus during integrin-mediated adhesion and spreading, or during cyclical stretching of the matrix, can activate the myocardin-related transcription factor (MRTF)-serum response factor (SRF) complex and even modify the organization of the chromatin to facilitate epigenetic silencing by the polycomb repressive complex 2 (Baarlink et al., 2013; Le et al., 2016). Future studies will be required to address the importance of these long-range mechanosensitive connections in cancer. To target integrin-dependent mechanotransduction in cancer, it will be important to further delineate the core mechanisms through which this process promotes tumorigenesis.
Multiple Integrin Signals Foster Development of the Tumor Microenvironment
From initial disruption of the basement membrane at the primary site to metastatic colonization of target organs, heterotypic interactions of tumor cells with elements of the TME dominate various steps of the metastatic cascade. These interactions drive neoangiogenesis, intravasation, dissemination, and homing and outgrowth in target organs (Egeblad et al., 2010a). Not surprisingly, signals transmitted by integrins and growth factor and cytokine receptors regulate the development of the various cellular systems of the tumor microenvironment during these transitions.
Several studies have addressed the role of integrins in angiogenesis, particularly αv integrins. Although the interpretation of the results of genetic and pharmacological inactivation has not been straightforward, the preponderance of the evidence suggests that the αv integrins promote tumor angiogenesis in a context-dependent manner (Robinson and Hodivala-Dilke, 2011). The α6β4 integrin may exert a similar context-dependent pro-angiogenic effect (Guo et al., 2006; Nikolopoulos et al., 2004). In contrast, signaling by the endothelial α3β1 integrin negatively regulates tumor angiogenesis by decreasing endothelial cell expression of VEGFR2 (da Silva et al., 2010). Further highlighting the complexity of the system, endothelial cell-specific inactivation of FAK blocks tumor angiogenesis, but deletion of a single allele of Fak promotes this process, suggesting that the effect of FAK on tumor angiogenesis may be non-linear and dose dependent (Kostourou et al., 2013; Tavora et al., 2010). Finally, the α4β1 integrin, which binds to fibronectin and to the counter-receptor VCAM1, has been linked to lymphangiogenesis and tumor cell colonization of loco-regional lymph nodes (Garmy-Susini et al., 2010). In addition to promoting the development of tumor lymphatics by binding to subendothelial fibronectin, α4β1 captures VCAM1-expressing metastatic cells in lymph nodes, prompting subsequent dissemination to distant organs (Garmy-Susini et al., 2013).
Signals from integrins also affect the behavior of other elements of the tumor microenvironment. Integrin-mediated tyrosine phosphorylation of caveolin induces activation of Rho and enables CAFs to acquire a contractile phenotype and activate mechanotransduction. The ensuing remodeling of the tumor stroma favors directional migration, invasion, and metastasis (Goetz et al., 2011). In fact, squamous carcinoma cells often move in collectives but follow the extracellular matrix tracks created by CAFs (Gaggioli et al., 2007). Inflammatory stimuli converge on PI3Kγ to activate the α4β1 integrin in myeloid-derived suppressor cells, promoting the recruitment and extravasation of these pro-inflammatory and immune suppressive cells into tumors (Schmid et al., 2011). In contrast, M2-like tumor-associated macrophages use the αvβ3 integrin to bind to periostin (POSTN) and home to the GBM stem cell niche (Zhou et al., 2015). In breast cancer, these immune cells secrete CCL18, which activates FAK and the FAK-related kinase PYK2 by binding to the G protein-coupled receptor PITPNM3 on cancer cells (Chen et al., 2011a). Given the complexity of the heterotypic cellular interactions involved, it is likely that future studies will reveal additional integrin signaling mechanisms involved in shaping the tumor microenvironment during tumor progression.
Integrin Functions during Hematogenous Dissemination and Initial Seeding of Target Organs
Hematogenous dissemination is likely to be the major mechanism that enables cancer cells to seed metastatic outgrowths in distant organs. Often aided by the invasive ability conferred upon them by the EMT, cancer cells traverse the stromal matrix and gain access to the blood circulation by exploiting discontinuities of the tumor vasculature induced by macrophage-derived VEGF (Harney et al., 2015). Sequential adhesive interactions and paracrine signaling between CTCs, platelets, leukocytes, and endothelial cells have emerged as critical determinants of hematogenous dissemination and initial seeding of target organs (Labelle and Hynes, 2012). Cooperating with other classes of adhesion receptors, the integrins play a crucial role in these processes.
Primary tumors shed individual cancer cells and cancer cell clusters into the circulation, the latter presumably originating from collective intravasation and possessing high metastatic capacity. Surprisingly, both isolated CTCs and CTC clusters can exhibit epithelial or mesenchymal markers (Aceto et al., 2014). In pancreatic cancer, single-cell RNA sequencing has revealed that CTCs harbor a distinctive extracellular matrix gene expression pattern, including high levels of SPARC, and functional studies have linked SPARC to invasion and metastasis (Ting et al., 2014). In estrogen receptor-negative breast cancer, aggressive subpopulations within primary tumors depend on a FOXQ1-instigated autocrine loop, involving α4 chain-containing laminins (laminins 411 and 421) and β1 integrins, for their proliferation and ability to seed metastatic lesions in multiple organs (Ross et al., 2015). These results suggest that the most aggressive CTCs may express extracellular matrix proteins that favor their subsequent homing and outgrowth in the microenvironment of target organs (Figure 8A).
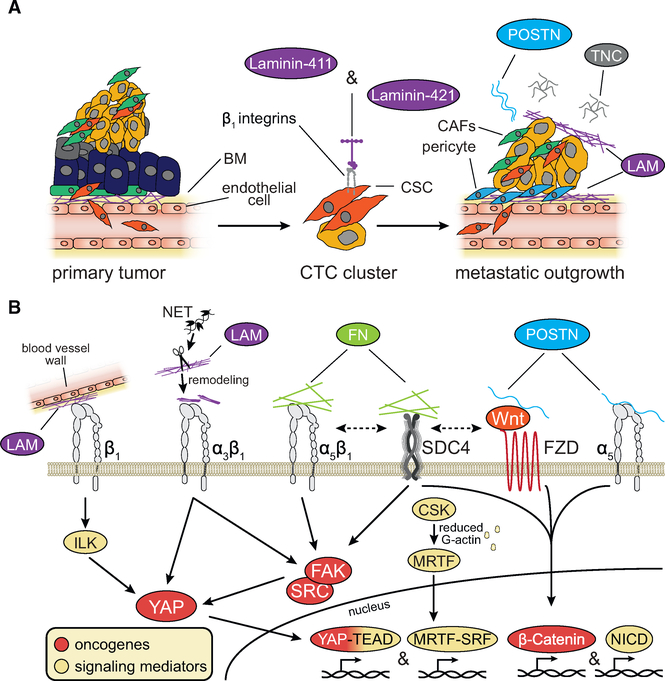
Figure 8. Integrin Signaling in Tumor Initiation and Re-initiation at Metastatic Sites.
(A) Secretion of specific laminin (LAM) isoforms facilitates carcinoma initiation and re-initiation at metastatic sites by activating β1 integrin signaling. Tenascin-C (TNC) and periostin (POSTN) are also key stromal elements promoting metastatic reactivation.
(B) Stromal elements and cancer cells cooperate to deposit the extracellular matrix of metastatic niches through several mechanisms including neutrophil extracellular trap (NET) remodeling. LAM, fibronectin (FN), POSTN, and TNC––acting through integrins, Syndecan-4 (SDC4), and the Wnt receptor Frizzled (FZD)––activate signaling pathways involved in metastatic outgrowth.
Upon release from CTCs, tissue factor produces thrombin and, hence, induces platelet activation and coagulation. Platelet- and fibrin-rich thrombi adhere to the αvβ3 integrin on CTCs, effectively shielding them from shear stress and from immune recognition by NK cells (Degen and Palumbo, 2012; Gay and Felding-Habermann, 2011). Cytokines produced by tumor cells, such as interleukin-8, attract neutrophils and induce their adhesion to tumor cell emboli via activation of β2 integrins (Huh et al., 2010). Whereas tumor cell emboli finally become arrested in the capillaries of target organs because of their size, small clusters of tumor cells and individual tumor cells adhere to the endothelial lining of the vessels through specific interactions mediated by integrins and other adhesion receptors, such as selectins and metadherin (Brown and Ruoslahti, 2004; Kim et al., 1999; Wang et al., 2004, 2005).
Systemic or local inflammatory cues contribute to the arrest of tumor cell emboli in target organs by inducing expression of the integrin ligands ICAM1 and VCAM1, and of selectins, in activated endothelial cells. Monocytes and macrophages become incorporated in vessel-occluding emboli because they are attracted by CCL2 and CCL5 produced by activated tumor cells and endothelial cells, respectively (Labelle and Hynes, 2012). Moreover, cytokines produced by primary tumors promote activation of FAK and expression of E-selectin in discrete vascular foci in the lung, prompting the arrest and eventual extravasation of both inflammatory cells and metastasis-initiating cells (Hiratsuka et al., 2011). Platelet-derived TGF-β and direct platelet-tumor cell contact synergistically activate the TGF-β/Smad and NF-κB pathways in cancer cells, increasing their invasive ability and thus favoring extravasation (Labelle et al., 2011). This latter process involves local degradation and integrin-mediated traversal of the endothelial basement membrane (Reymond et al., 2012).
Metastatic dissemination to the CNS may occur through distinct mechanisms because the blood-brain barrier naturally opposes this process. Recent evidence indicates that ALL cells use α6 integrins to bind to laminins on the abluminal surface of blood vessels, allowing these cancer cells to migrate on the external surface of blood vessels spanning vertebral or skullcap bone marrow into the subarachnoid space and cerebrospinal fluid. This mechanism enables ALL cells to circumvent the blood-brain barrier and colonize the CNS (Yao et al., 2018). Once established, brain metastases pose a formidable threat because many therapeutics cannot traverse an intact blood-brain barrier.
ECM Niches and Metastatic Colonization
Adaptation to the often-inhospitable microenvironment of the target organ is a major barrier to metastatic colonization and, arguably, a major determinant of metastatic dormancy (Giancotti, 2013). Experiments in mouse models support the notion that systemic signals produced by primary cancers, such as LOX secreted by hypoxic tumor cells, can instruct bone marrow-derived hematopoietic progenitor cells to initiate events leading to the formation of pre-metastatic perivascular niches in target organs (Erler et al., 2009; Kaplan et al., 2005). The formation of these niches is facilitated by the dedifferentiation of local pericytes and vascular smooth muscle cells, which resume proliferation and produce a fibronectin-rich matrix (Murgai et al., 2017). In pancreatic cancer and melanoma, tumor cell-derived exosomes contribute to these processes by a variety of mechanisms (Costa-Silva et al., 2015; Peinado et al., 2012). Moreover, it has been suggested that the integrin repertoire on tumor exosomes may contribute to the organotropism of metastasis: α6β4- and α6β1-bearing exosomes would home to the pericellular matrix of lung-resident epithelial cells and fibroblasts, whereas αvβ5-bearing exosomes would home to the pericellular matrix of Kupffer cells in the liver, both facilitating the delivery of pro-inflammatory and pro-migratory cues to their target cells in the pre-metastatic niche (Hoshino et al., 2015). The molecular basis of this exquisite specificity remains to be examined.
Increasing evidence indicates that the metastasis-initiating cells are similar to CSCs and they enter into dormancy and undergo reactivation in response to the same intracellular signaling pathways and transcriptional programs that regulate normal adult stem cells (Giancotti, 2013) (Figure 8B). Intriguingly, breast cancer cells need to adhere to the lung matrix through β1 integrins, form filopodium-like protrusions, and activate FAK in order to undergo reactivation from dormancy at metastatic sites (Shibue et al., 2012, 2013; Shibue and Weinberg, 2009). Similarly, whereas hepatocellular carcinoma cells enter into a quiescent state in a soft matrix, they resume proliferation upon interacting with a stiff matrix, which enables integrin β1-dependent activation of FAK (Schrader et al., 2011). Among matrix components, fibronectin may play an important role as a component of the pre-metastatic niche and facilitator of metastatic reactivation. In addition to binding to integrins and activating FAK, fibronectin engages the Syndecan-4/Frizzled-7 complex, facilitating Wnt signaling and stem cell expansion (Bentzinger et al., 2013). In contrast, TNC and POSTN promote metastatic reactivation by activating Wnt and Notch signaling (O’Connell et al., 2011; Oskarsson et al., 2011; Malanchi et al., 2011). This effect does not appear to require integrin signaling because, although both matrix proteins co-assemble with fibronectin and collagen fibrils, they interfere with integrin-mediated adhesion and signaling (Midwood and Schwarzbauer, 2002; Soikkeli et al., 2010). One possibility is that these anti-adhesive matrix proteins facilitate the presentation of Wnt and Notch ligands, as has been suggested for POSTN (Malanchi et al., 2011) (Figure 8B).
The signals that induce dormancy or eventually reactivation often emanate from perivascular niches. Upon infiltrating the brain and possibly other organs, TNBC cells associate with the abluminal surface of blood vessels and outgrow by vascular cooption (Valiente et al., 2014). L1-CAM-positive metastatic cells displace pericytes to adhere to and spread onto the vascular basement membrane; this process requires L1-CAM-mediated activation of β1 integrins and integrin-linked kinase. Subsequent activation of PAK induces activation of YAP and MRTF, which contribute to metastatic outgrowth (Er et al., 2018). Similarly converging on YAP, neutrophil extracellular traps promote cleavage of laminin and activation of the α3β1 integrin, potentially explaining the ability of inflammation to trigger metastatic reactivation (Albrengues et al., 2018) (Figure 8B). Intriguingly, the signals that induce dormancy and reactivation appear to be physically segregated along blood vessels: even if fully competent for metastatic reactivation, TNBC cells receive either inhibitory (TSP1, which inhibits TGF-β) or stimulatory (TGF-β and TGF-β-induced matrix proteins—such as TNC, fibronectin, POSTN, and versican) signals depending on whether they are in contact with a resting or a sprouting segment of the blood vessel, respectively (Ghajar et al., 2013). Additional integrin-mediated interactions and signals enable incipient micrometastases to recruit and coopt distinct supportive elements of the tumor microenvironment depending on the target organ. In the lung, macrophages expressing the α4β1 integrin engage the counter-receptor VCAM1, and hence activate survival signaling in newly infiltrated breast cancer cells (Chen et al., 2011b). In the bone, breast cancer cells exploit the same receptor-ligand pair to recruit osteoclast progenitors and instigate the vicious cycle of osteolytic bone metastasis (Lu et al., 2011). These findings suggest that complex adhesive and signaling interactions between metastasis-initiating cells and perivascular niches govern metastatic dormancy and reactivation.
A Double-Edged Sword: Therapeutic Barriers and Opportunities
Integrin Signaling Promotes Resistance to Therapy
Integrin signaling enhances the capacity of cancer cells to resist the deleterious effects of chemotherapy and radiotherapy. In various carcinomas, primary radiotherapy resistance arises from the ability of matrix-engaged β1 integrins to activate DNA repair and pro-survival signaling (Ahmed et al., 2018; Dickreuter et al., 2016; Eke et al., 2012). In GBM, resistance to temozolomide has been linked to the α5β1 integrin, which inhibits p53 signaling (Janouskova et al., 2012). In contrast, in T-ALL, resistance to doxorubicin has been reported to be mediated by α2β1 through activation of ERK (Naci et al., 2012). In chronic lymphocytic leukemia (CLL), engagement of the α4β1 integrin by stromal VCAM1 and activation of CXCR4 jointly activate survival signaling through the Syk tyrosine kinase. Accordingly, inhibition of Syk sensitizes CLL cells to fludarabine in vitro, pointing to the potential utility of combining Syk inhibitors with chemotherapy in CLL (Buchner et al., 2010).
Similar mechanisms also promote adaptive resistance to chemotherapy. Ovarian cancer cells that have been selected for their ability to grow in the presence of cisplatin produce elevated levels of collagen VI, and plating of naive cancer cells on collagen VI—but not collagen I—enhances their ability to survive in vitro when treated with the drug (Sherman-Baust et al., 2003). In contrast, ovarian cancer cells that have acquired resistance to taxanes during tumor growth in mice exhibit increased expression of the matrix proteins TGFBI, FN, and TSP2. Intriguingly, adhesion to TGFBI is sufficient to stabilize the microtubule cytoskeleton via FAK-Rho signaling and hence reduces the sensitivity of these cancer cells to taxanes (Ahmed et al., 2007). The mechanisms underlying integrin-mediated primary and adaptive resistance to chemotherapy are variegated, possibly reflecting the individual integrins involved in a particular tumor type and the mechanism of action of the drug.
More recent studies have indicated that integrin signaling also promotes resistance to molecularly targeted agents. Under standard culture conditions, inhibition of the laminin-binding integrins α6β4 and α3β1 sensitizes ErbB2-positive breast cancer cells to lapatinib and trastuzumab (Yang et al., 2010). In addition, breast cancer cells that have been selected for their resistance to both drugs exhibit activated FAK and SRC, and depletion of β1 or pharmacological inhibition of FAK inhibits the ability of these drug-resistant cells to grow in 3D Matrigel (Huang et al., 2011). These findings suggest that FAK kinase inhibitors or SRC kinase inhibitors may increase the clinical efficacy of drugs targeting ErbB2. Studies on ovarian cancer organoids harboring PIK3CA mutations suggest that cancer cells adhering to the basement membrane elicit complex adaptive strategies to counter targeted agents. FOXO-dependent transcription and CAP-independent translation upregulate the expression of IGF1R, EGFR, and ErbB2 in matrix-attached cancer cells, enabling their resistance to PI3K and TOR inhibitors (Muranen et al., 2012). Recent studies have linked resistance of breast, lung, and pancreatic cancer to the EGFR inhibitor erlotinib to αvβ3 integrin-mediated stemness. Notably, this effect depends on the ability of unliganded αvβ3 to complex with KRas and RalB to activate NF-κB, independently of FAK (Seguin et al., 2014). These findings suggest the possibility that distinct integrin signaling mechanisms drive resistance to targeted therapies in CSCs or other cancer cells.
Recent studies have revealed some of the complex signaling interactions that promote drug resistance in the tumor microenvironment. Endothelial cell-specific deletion of FAK and consequent inactivation of NF-κB impairs the secretion of pro-inflammatory cytokines that promote resistance to doxorubicin and radiotherapy in mouse xenograft models (Tavora et al., 2014). In addition, deletion or inhibition of FAK restores anti-tumor immunity in squamous cell carcinoma models by blocking secretion of CCL5 and recruitment of regulatory T cells (Serrels et al., 2015) or by decreasing the deposition of a desmoplastic stroma in pancreatic cancer models (Jiang et al., 2016). Interestingly, FAK inhibition restores sensitivity to double immune checkpoint therapy in the KPC mouse model of pancreatic cancer (Jiang et al., 2016). In melanoma models, treatment with the mutant BRAF inhibitor PLX4720 results in paradoxical activation of wild-type BRAF in melanoma-associated fibroblasts, leading to matrix remodeling and activation of β1/FAK/SRC signaling in melanoma cells (Hirata et al., 2015). In addition to implicating intercellular interactions mediated by integrins in drug resistance, these findings point to additional mechanisms through which inhibition of FAK can alleviate drug resistance in specific clinical settings.
Therapeutic Opportunities
Monoclonal antibodies and small molecules blocking ligand binding to the platelet αIIbβ3 integrin or the leukocyte α4 and β2 integrins have demonstrated remarkable clinical efficacy for the prevention of thrombosis or the treatment of inflammation, respectively (Ley et al., 2016). However, a number of factors have complicated the development of integrin-based therapeutics for cancer. First, the integrins play multifarious and not always positive roles in cancer, and it is difficult to tease out these roles in human cancer and develop appropriate biomarkers of sensitivity. Second, in contrast to the platelet and leukocyte integrins, most other integrins play redundant roles in both adhesion and signaling, making it difficult to block these processes with a single agent or without enabling compensatory upregulation of a non-targeted integrin with similar specificity and function. Finally, targeting simultaneously integrin-mediated adhesion and signaling may not be achievable without unacceptable toxicity, especially if one wants to target the common β1 subunit or even α6β4, which has a much more restricted tissue distribution but is critically involved in maintaining the integrity of the skin and upper gastrointestinal tracts (Dowling et al., 1996; Murgia et al., 1998). Indirect blockade of integrin function may be achievable in specific contexts to avoid toxicity, for example PI3K inhibitors reduce the expression of α6 integrin in ALL, thereby limiting CNS metastasis (Yao et al., 2018).
Since the αv integrins play non-essential roles in development but are involved in tumor growth and angiogenesis, considerable effort has been placed upon developing agents targeting these integrins. The agent that has most advanced through preclinical and clinical testing is the RGD-based peptide cilengitide. This compound was recently tested for clinical efficacy in combination with temozolomide radiochemotherapy in a phase III clinical study of GBM patients. Patients were enrolled based on MGMT methylation status, a biomarker predictive of positive response to temozolomide (Hegi et al., 2005). Despite encouraging results from initial phase I and II trials, the phase II CORE and phase III CENTRIC trials failed to meet overall survival endpoints (Stupp et al., 2014). The reasons contributing to the failure of cilengitide in GBM are not known. However, it has been shown that the αv and α5 integrins and their matrix binding partner fibronectin are all dispensable for tumor angiogenesis, raising questions about the ability of cilengitide to curb tumor angiogenesis (Murphy et al., 2015). Moreover, given the extensive biological heterogeneity of GBM and the potential for compensation and redundancy in the integrin system, it is likely that the αv integrins sustain disease progression only in a subset of these malignancies. In agreement with the latter hypothesis, a recent study has shown that αvβ3 specifically sustains the viability of patient-derived GBM stem cells of the proneural/classic subtype and linked this effect to the activation of YAP/TAZ and the expression of the glucose transporter Glut3 (Cosset et al., 2017).
Going forward, it may be useful to use temporally regulated genetic ablation in faithful mouse genetic models and in genotyped patient-derived xenografts to anticipate the possible effect of an integrin-based therapeutic on established tumors. On the basis of a positive outcome in a specific subset of cancer models, it may then be possible to develop and test integrin-targeted therapeutics in biomarker-driven clinical trials. However, we would argue that there is a need for more basic science in this area. For example, a better definition of the mechanisms enabling association of specific integrins with RTKs may lead to the development of monoclonal antibodies that block such association and the ensuing signaling without perturbing adhesion. It is possible that these agents will improve the efficacy of oncogene-targeted therapies in specific contexts. Targeting the tumor stroma and vasculature remains also a promising approach, given emerging evidence implicating additional integrins in these processes. For example, recent preclinical studies have uncovered the utility of targeting tumor-associated fibroblasts via inhibition of integrin α11 in NSCLC (Zhu et al., 2007) and bevacizumab-resistant vasculature in GBM via inhibition of β1 integrins (Carbonell et al., 2013). Furthermore, it has been shown that low doses of cilengitide can increase VEGFR recycling to the plasma membrane and promote, not inhibit, vascular growth (Reynolds et al., 2009; Wong et al., 2015). When used in combination with verapamil, low doses of cilengitide improve the delivery of chemotherapeutics into tumors, thus inhibiting tumor growth and metastasis (Reynolds et al., 2009; Wong et al., 2015). Finally, since chronic inflammation promotes tumorigenesis in some settings, it is possible that drugs targeting the α4 and β2 integrins may also find an indication in some cancer types.
Given the central role of the FAK/SFK complex in promoting invasive growth, considerable effort has been dedicated to developing FAK inhibitors. In vitro screening of a panel of cancer cell lines has suggested that NF2 mutant CSCs are selectively sensitive to the dual FAK and PYKγ inhibitor VS-4718 (formerly PND-1186). Consistently, this compound suppresses the growth of NF2 mutant mesothelioma xenografts when it is used after pemetrexed chemotherapy (Shapiro et al., 2014). However, a phase II trial of the selective FAK inhibitor defactinib in combination with standard-of-care pemetrexed in malignant pleural mesothelioma was halted due to poor performance irrespective of NF2 mutation status (NCT01870609). It must be noted that the mechanism through which loss of NF2 would lead to activation of FAK has not been defined, and so there may be better clinical settings than mesothelioma in which to test FAK inhibitors. Given the amplification of the gene encoding FAK in a substantial fraction of breast and ovarian cancers, it may make sense to test the efficacy of FAK inhibitors in these cancers, especially in combination with established oncogene-targeted agents. In addition, considering the ability of FAK to suppress anti-tumor immunity in squamous cell carcinoma and pancreatic cancer models (Jiang et al., 2016), it will be important to examine the ability of FAK inhibitors to increase sensitivity to immune checkpoint inhibition in patients affected by these cancer types. Finally, as suggested by studies in mouse models, inhibition of FAK may increase the efficacy of BRAF inhibitors in melanoma (Hirata et al., 2015). The mechanisms governing the immune and nonimmune microenvironment in cancer are being quickly elucidated, and we anticipate that the role of integrins and their downstream effectors will become increasingly appreciated in emerging therapeutic strategies embracing tumors as complex tissues.
Integrin overexpression in tumor cells and their vasculature have opened the door to integrin-targeted probes for non-invasive medical imaging and development of biomarkers. RGD-based 18F- and 68Ga-PET (positron emission tomography) was developed to exploit tumor-selective αv expression, where 18F-fluciclatide detects an array of solid cancers and is being explored clinically (Sharma et al., 2015). 68Ga-linked bombesin-RGD is in early development for co-targeting αv integrins and gastrin-releasing peptide receptor, with promising clinical efficacy in probing prostate tumors and metastases (Zhang et al., 2017). In the vein of theranostics, 124I-labelled RGD-coated nanoparticles bearing fluorescent dyes are efficacious multimodal PET/optical probes in preclinical models, conceptually broadening the possibilities for diagnostic and therapeutic payloads targeted to integrin-expressing tumors (Benezra et al., 2011). In addition, radiolabeled A20FMDV2 peptide, which is derived from the VP1 coat protein of foot-and-mouth-disease virus and targets αvβ6, was shown preclinically to target breast cancer cells, and preliminary clinical testing indicated a promising safety profile (Keat et al., 2018; Saha et al., 2010). RNAi and nanoparticle formulations are adding to the repertoire of integrin-targeting oncology therapeutics. siRNA-loaded nanoparticles targeting β3 integrins guided by RGD peptides elicit efficacy in xenograft models of TNBC (Parvani et al., 2015), and lipidoid-based nanoparticles containing αv- and β1-targeting small interfering RNAs (siRNAs) suppress hepatocellular carcinomas in vivo (Bogorad et al., 2014). Excitingly, the latter finding used the same underlying technology underpinning the recent approval of the first RNAi-based therapy (Adams et al., 2018).
Enormous progress has been made toward elucidating the role of integrin signaling in cancer. Given the number of integrins involved and the complexity of the signals that they deliver, it is not surprising that therapeutic development has somewhat lagged behind. We propose that going back to the drawing board to understand integrin dependency in various cancer types and develop appropriate biomarkers in genetically engineered and patient-derived xenograft mouse models will be necessary to develop truly effective agents blocking integrin signaling in cancer. Nascent and developing therapeutic and diagnostic technologies ranging from antibody-drug conjugates to nanoparticle-based delivery and RNA interference technology will support these mechanistically guided efforts.
ACKNOWLEDGMENTS
Integrin signaling work in the Giancotti laboratory is funded by NIH grants R35 CA197566 (to F.G.G.), P01 CA094060 (project no. 4 to F.G.G.), and by CPRIT Recruitment of Established Investigators Award RR160031. J.C. was partially supported by training grant T32 GM008539 during his graduate studies in the Giancotti laboratory.
DECLARATION OF INTERESTS
F.G.G. receives research support funding from Janssen Research & Development, LLC.
REFERENCES
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, et al. (2018). Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med 379, 11–21. [DOI] [PubMed] [Google Scholar]
- Ahmed AA, Mills AD, Ibrahim AE, Temple J, Blenkiron C, Vias M, Massie CE, Iyer NG, McGeoch A, Crawford R, et al. (2007). The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell 12, 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed KM, Pandita RK, Singh DK, Hunt CR, and Pandita TK (2018). β1-Integrin impacts Rad51 stability and DNA double-strand break repair by homologous recombination. Mol. Cell. Biol 38, 10.1128/MCB.00672-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanko J, and Ivaska J (2016). Endosomes: emerging platforms for integrin-mediated FAK signalling. Trends Cell Biol 26, 391–398. [DOI] [PubMed] [Google Scholar]
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Kuttner V, et al. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym VV, Swatkoski S, Matsumoto K, Campbell CB, Petrie RJ, Dimitriadis EK, Li X, Mueller SC, Bugge TH, Gucek M, and Yamada KM (2015). Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol 208, 331–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, et al. (2010). Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 19, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, and Klein EA (2008). Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 18, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, and Yarden Y (2011). Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol 12, 104–117. [DOI] [PubMed] [Google Scholar]
- Baarlink C, Wang H, and Grosse R (2013). Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340, 864–867. [DOI] [PubMed] [Google Scholar]
- Bajaj J, Konuma T, Lytle NK, Kwon HY, Ablack JN, Cantor JM, Rizzieri D, Chuah C, Oehler VG, Broome EH, et al. (2016). CD98-mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell 30, 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Kaushik S, Bainer RO, Sa JK, Woods EC, Kai F, Przybyla L, Lee M, Lee HW, Tung JC, et al. (2018). A tension-mediated glycocalyx-integrin feedback loop promotes mesenchymal-like glioblastoma. Nat. Cell Biol 20, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, et al. (2011). A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403. [DOI] [PubMed] [Google Scholar]
- Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, et al. (2011). Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Invest 121, 2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, and Rudnicki MA (2013). Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, Liu Donaher J, Reinhardt F, Chaffer CL, Keckesova Z, and Weinberg RA (2017). Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc. Natl. Acad. Sci. U S A 114, E2337–E2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad RL, Yin H, Zeigerer A, Nonaka H, Ruda VM, Zerial M, Anderson DG, and Koteliansky V (2014). Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat. Commun 5, 3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Chou J, and Werb Z (2014). Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol 15, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, and Ruoslahti E (2004). Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 5, 365–374. [DOI] [PubMed] [Google Scholar]
- Buchner M, Baer C, Prinz G, Dierks C, Burger M, Zenz T, Stilgenbauer S, Jumaa H, Veelken H, and Zirlik K (2010). Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood 115, 4497–4506. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. (2007). A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012a). Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012b). Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2011). Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JM, Ginsberg MH, and Rose DM (2008). Integrin-associated proteins as potential therapeutic targets. Immunol. Rev 223, 236–251. [DOI] [PubMed] [Google Scholar]
- Carbonell WS, DeLay M, Jahangiri A, Park CC, and Aghi MK (2013). beta1 Integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 73, 3145–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S (2012). Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, Ingerpuu S, Patarroyo M, Cao S, Lim E, et al. (2015). A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes Dev. 29, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, and Vu TH (2011). Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest 121, 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, Liu B, Deng H, Wang F, Lin L, et al. (2011a). CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 19, 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang XH, and Massague J (2011b). Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. (2011). The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772. [DOI] [PubMed] [Google Scholar]
- Cosset E, Ilmjarv S, Dutoit V, Elliott K, von Schalscha T, Camargo MF, Reiss A, Moroishi T, Seguin L, Gomez G, et al. (2017). Glut3 addiction is a druggable vulnerability for a molecularly defined subpopulation of glioblastoma. Cancer Cell 32, 856–868.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol 17, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva RG, Tavora B, Robinson SD, Reynolds LE, Szekeres C, Lamar J, Batista S, Kostourou V, Germain MA, Reynolds AR, et al. (2010). Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am. J. Pathol 177, 1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, and Khavari PA (2003). NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421, 639–643. [DOI] [PubMed] [Google Scholar]
- Danen EH, and Yamada KM (2001). Fibronectin, integrins, and growth control. J. Cell. Physiol 189, 1–13. [DOI] [PubMed] [Google Scholar]
- David CJ, and Massague J (2018). Contextual determinants of TGFbeta action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol 19, 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen JL, and Palumbo JS (2012). Hemostatic factors, innate immunity and malignancy. Thromb. Res 129 (Suppl 1), S1–S5. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, and Cheresh DA (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Lesperance J, Seguin L, Gozo M, Kato S, Franovic A, Yebra M, Shattil SJ, and Cheresh DA (2014). Integrin alphavbeta3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev. Cell 30, 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickreuter E, Eke I, Krause M, Borgmann K, van Vugt MA, and Cordes N (2016). Targeting of β1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene 35, 1353–1362. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, and Fuchs E (1996). beta4 Integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, and Werb Z (2010a). Tumors as organs: complex tissues that interface with the entire organism. Dev. Cell 18, 884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Rasch MG, and Weaver VM (2010b). Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol 22, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke I, Deuse Y, Hehlgans S, Gurtner K, Krause M, Baumann M, Shevchenko A, Sandfort V, and Cordes N (2012). beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Invest. 122, 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, et al. (2018). Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol 20, 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, and Giaccia AJ (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, and Giaccia AJ (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226. [DOI] [PubMed] [Google Scholar]
- Fafilek B, Balek L, Bosakova MK, Varecha M, Nita A, Gregor T, Gudernova I, Krenova J, Ghosh S, Piskacek M, et al. (2018). The inositol phosphatase SHIP2 enables sustained ERK activation downstream of FGF receptors by recruiting Src kinases. Sci. Signal 11, 10.1126/scisignal.aap8608. [DOI] [PubMed] [Google Scholar]
- Ferreira M, Fujiwara H, Morita K, and Watt FM (2009). An activating beta1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res. 69, 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Sahai E, Weiss S, and Yamada KM (2012). New dimensions in cell migration. Nat. Rev. Mol. Cell Biol 13, 743–747. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, and Sahai E (2007). Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol 9, 1392–1400. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Desgrosellier JS, Schmid MC, Foubert P, Ellies LG, Lowy AM, Blair SL, Vandenberg SR, Datnow B, et al. (2013). PI3Kalpha activates integrin alpha4beta1 to establish a metastatic niche in lymph nodes. Proc. Natl. Acad. Sci. U S A 110, 9042–9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL, et al. (2010). Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 70, 3042–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay LJ, and Felding-Habermann B (2011). Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. (2013). The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol 15, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG (2000). Complexity and specificity of integrin signalling. Nat. Cell Biol 2, E13–E14. [DOI] [PubMed] [Google Scholar]
- Giancotti FG (2007). Targeting integrin beta4 for cancer and anti-angiogenic therapy. Trends Pharmacol. Sci 28, 506–511. [DOI] [PubMed] [Google Scholar]
- Giancotti FG (2013). Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, and Ruoslahti E (1990). Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 60, 849–859. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, and Ruoslahti E (1999). Integrin signaling. Science 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, and Tarone G (2003). Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol 19, 173–206. [DOI] [PubMed] [Google Scholar]
- Gocheva V, Naba A, Bhutkar A, Guardia T, Miller KM, Li CM, Dayton TL, Sanchez-Rivera FJ, Kim-Kiselak C, Jailkhani N, et al. (2017). Quantitative proteomics identify tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc. Natl. Acad. Sci. U S A 114, E5625–E5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, Norum JH, Toftgard R, Shaw LM, and Mercurio AM (2014). Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 7, 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, Greiner DL, et al. (2013). GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol. Med 5, 488–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Pursell B, Standley C, Fogarty K, and Mercurio AM (2012). Neuropilin-2 regulates alpha6beta1 integrin in the formation of focal adhesions and signaling. J. Cell Sci. 125, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, et al. (2011). Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, and Shaw RJ (2014). An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol. Cell 55, 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert S, Jechlinger M, and Beug H (2003). Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol 4, 657–665. [DOI] [PubMed] [Google Scholar]
- Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, and Fuchs E (2007). Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, and Giancotti FG (2004). Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol 5, 816–826. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, and Giancotti FG (2006). beta 4 Integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489–502. [DOI] [PubMed] [Google Scholar]
- Haas TL, Sciuto MR, Brunetto L, Valvo C, Signore M, Fiori ME, di Martino S, Giannetti S, Morgante L, Boe A, et al. (2017). Integrin alpha7 is a functional marker and potential therapeutic target in glioblastoma. Cell Stem Cell 21, 35–50.e9. [DOI] [PubMed] [Google Scholar]
- Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J Jr., Fischer W, Lukas J, et al. (2012). Autocrine VEGF-VEGFR2-neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med 209, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG (2013). The LKB1-AMPK pathway-friend or foe in cancer? Cancer Cell 23, 131–132. [DOI] [PubMed] [Google Scholar]
- Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, and Condeelis JS (2015). Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 5, 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. (2005). MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med 352, 997–1003. [DOI] [PubMed] [Google Scholar]
- Hemler ME (2003). Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol 19, 397–422. [DOI] [PubMed] [Google Scholar]
- Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, and Sahai E (2015). Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 27, 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, and Jain RK (2011). Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc. Natl. Acad. Sci. U S A 108, 3725–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Park CC, Hilsenbeck SG, Ward R, Rimawi MF, Wang YC, Shou J, Bissell MJ, Osborne CK, and Schiff R (2011). beta1 Integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res. 13, R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SJ, Liang S, Sharma A, Dong C, and Robertson GP (2010). Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 70, 6071–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, and Humphries MJ (2006). Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2009). The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz RA, and Massague J (1987). Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell 51, 189–197. [DOI] [PubMed] [Google Scholar]
- Iwanicki MP, Chen HY, Iavarone C, Zervantonakis IK, Muranen T, Novak M, Ince TA, Drapkin R, and Brugge JS (2016). Mutant p53 regulates ovarian cancer transformed phenotypes through autocrine matrix deposition. JCI Insight 1, 10.1172/jci.insight.86829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet G, Green DM, Bridgewater RE, von Kriegsheim A, Humphries MJ, Norman JC, and Caswell PT (2013). RCP-driven alpha5beta1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J. Cell Biol 202, 917–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janouskova H, Maglott A, Leger DY, Bossert C, Noulet F, Guerin E, Guenot D, Pinel S, Chastagner P, Plenat F, et al. (2012). Integrin alpha5beta1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 72, 3463–3470. [DOI] [PubMed] [Google Scholar]
- Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, et al. (2016). Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med 22, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu M, Ueno T, Kontani K, Ogita Y, Ando M, Fukumura K, Yamato A, Soda M, Takeuchi K, Miki Y, et al. (2013). Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc. Natl. Acad. Sci. U S A 110, 3029–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keat N, Kenny J, Chen K, Onega M, Garman N, Slack RJ, Parker CA, Lumbers RT, Hallett W, Saleem A, et al. (2018). A microdose PET study of the safety, immunogenicity, biodistribution, and radiation dosimetry of (18)FFB-A20FMDV2 for imaging the integrin alphavbeta6. J. Nucl. Med. Technol 46, 136–143. [DOI] [PubMed] [Google Scholar]
- Kim NG, and Gumbiner BM (2015). Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J. Cell Biol 210, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Han HL, Varki NM, and Varki A (1999). Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am. J. Pathol 155, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt EG, and Schlaepfer DD (2017). Focal adhesion kinase signaling in unexpected places. Curr. Opin. Cell Biol 45, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou G, Ramadori G, Torti F, Kangasniemi K, Ramirez RE, Cai Y, Behrens C, Dellinger MT, Brekken RA, Wistuba II, et al. (2013). RHOA-FAK is a required signaling axis for the maintenance of KRAS-driven lung adenocarcinomas. Cancer Discov. 3, 444–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostourou V, Lechertier T, Reynolds LE, Lees DM, Baker M, Jones DT, Tavora B, Ramjaun AR, Birdsey GM, Robinson SD, et al. (2013). FAK-heterozygous mice display enhanced tumour angiogenesis. Nat. Commun 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler MC, Wei Y, and Chapman HA (2003). Urokinase receptor and integrin interactions. Curr. Pharm. Des 9, 1565–1574. [DOI] [PubMed] [Google Scholar]
- Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, Etzioni R, Bolouri H, Montgomery B, White T, et al. (2016). Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med 22, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, and Hynes RO (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, and Hynes RO (2012). The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Frame MC, and Muller WJ (2012). Focal adhesion kinase contributes to proliferative potential of ErbB2 mammary tumour cells but is dispensable for ErbB2 mammary tumour induction in vivo. Breast Cancer Res. 14, R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, and Muller WJ (2007). Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc. Natl. Acad. Sci. U S A 104, 20302–20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Goetz JG, Dennis JW, and Nabi IR (2009). Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J. Cell Biol 185, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, and Weinberg RA (2017). Emerging biological principles of metastasis. Cell 168, 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, and Sixt M (2008). Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, and Rich JN (2010). Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, and Witte ON (2007). Stem cells in prostate cancer initiation and progression. J. Clin. Invest 117, 2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HQ, Ghatak S, Yeung CY, Tellkamp F, Gunschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, and Wickstrom SA (2016). Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol 18, 864–875. [DOI] [PubMed] [Google Scholar]
- Lechler T, and Fuchs E (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre F, Yilmaz M, Wicki A, Schomber T, Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, et al. (2008). NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 27, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Rivera-Nieves J, Sandborn WJ, and Shattil S (2016). Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat. Rev. Drug Discov 15, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, et al. (2014). Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 26, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, et al. (2010). Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 140, 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, and Ilic D (2008). Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 29, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, and Bishop JM (2012). MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits. Nat. Cell Biol 14, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo PK, Kanojia D, Liu X, Singh UP, Berger FG, Wang Q, and Chen H (2012). CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene 31, 2614–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bergami P, Lau E, and Ronai Z (2010). Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat. Rev. Cancer 10, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, and Werb Z (2012). The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol 196, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X, et al. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BH, Carman CV, and Springer TA (2007). Structural basis of integrin regulation and signaling. Annu. Rev. Immunol 25, 619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S, Wicha MS, and Guan JL (2009). Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 69, 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Zhao X, Chen S, Liu S, Wicha MS, and Guan JL (2013). Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res. 73, 5591–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, and Huelsken J (2011). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89. [DOI] [PubMed] [Google Scholar]
- Margadant C, and Sonnenberg A (2010). Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, and Giancotti FG (2001). EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol 155, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, and Perez-Moreno M (2011). Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 12, 23–38. [DOI] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, and Frame MC (2004). Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18, 2998–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense SM, Barrows D, Hodakoski C, Steinbach N, Schoenfeld D, Su W, Hopkins BD, Su T, Fine B, Hibshoosh H, and Parsons R (2015). PTEN inhibits PREX2-catalyzed activation of RAC1 to restrain tumor cell invasion. Sci. Signal 8, ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves A, Geiger T, Zanivan S, DiGiovanni J, Mann M, and Fassler R (2011). Beta1 integrin cytoplasmic tyrosines promote skin tumorigenesis independent of their phosphorylation. Proc. Natl. Acad. Sci. U S A 108, 15213–15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood KS, and Schwarzbauer JE (2002). Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Mol. Biol. Cell 13, 3601–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PG, Al-Shahrour F, Hartwell KA, Chu LP, Jaras M, Puram RV, Puissant A, Callahan KP, Ashton J, McConkey ME, et al. (2013). In vivo RNAi screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell 24, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming XY, Fu L, Zhang LY, Qin YR, Cao TT, Chan KW, Ma S, Xie D, and Guan XY (2016). Integrin alpha7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat. Commun 7, 13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, and Treisman R (2003). Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342. [DOI] [PubMed] [Google Scholar]
- Mitra SK, and Schlaepfer DD (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol 18, 516–523. [DOI] [PubMed] [Google Scholar]
- Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, et al. (2014). A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat. Cell Biol 16, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, and Bass MD (2007). Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol 8, 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, et al. (2014). Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med 20, 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, et al. (2009). Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341. [DOI] [PubMed] [Google Scholar]
- Munger JS, and Sheppard D (2011). Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb. Perspect. Biol 3, a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, Gao S, Mills GB, and Brugge JS (2012). Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 21, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgai M, Ju W, Eason M, Kline J, Beury DW, Kaczanowska S, Miettinen MM, Kruhlak M, Lei H, Shern JF, et al. (2017). KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med 23, 1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, and Giancotti FG (1998). Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin beta4 cytoplasmic domain. EMBO J. 17, 3940–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, and Courtneidge SA (2011). The “ins” and “outs” of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol 12, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PA, Begum S, and Hynes RO (2015). Tumor angiogenesis in the absence of fibronectin or its cognate integrin receptors. PLoS One 10, e0120872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Lamar JM, Carr SA, and Hynes RO (2014). Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. Elife 3, e01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naci D, El Azreq MA, Chetoui N, Lauden L, Sigaux F, Charron D, Al-Daccak R, and Aoudjit F (2012). alpha2beta1 Integrin promotes chemoresistance against doxorubicin in cancer cells through extracellular signal-regulated kinase (ERK). J. Biol. Chem 287, 17065–17076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, and Thiery JP (2016). Emt: 2016. Cell 166, 21–45. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, and Giancotti FG (2004). Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 6, 471–483. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, and Mostov KE (2004). ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell 7, 21–32. [DOI] [PubMed] [Google Scholar]
- O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, et al. (2011). VEGF-A and tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. U S A 108, 16002–16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lee AY, Qin LX, Agaram N, Mimae T, Shen Y, O’Connor R, Lopez-Lago MA, Craig A, Miller ML, et al. (2016). Integrin-alpha10 dependency identifies RAC and RICTOR as therapeutic targets in high-grade myxofibrosarcoma. Cancer Discov. 6, 1148–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, and Giancotti FG (2005). Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J. Cell Biol 171, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, and Massague J (2011). Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med 17, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvani JG, Galliher-Beckley AJ, Schiemann BJ, and Schiemann WP (2013). Targeted inactivation of beta1 integrin induces beta3 integrin switching, which drives breast cancer metastasis by TGF-beta. Mol. Biol. Cell 24, 3449–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvani JG, Gujrati MD, Mack MA, Schiemann WP, and Lu ZR (2015). Silencing beta3 integrin by targeted ECO/siRNA nanoparticles inhibits EMT and metastasis of triple-negative breast cancer. Cancer Res. 75, 2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, et al. (2014). The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. [DOI] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Kong N, and Werb Z (2015). The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber LC, and Hynes RO (1989). Changes in integrin receptors on oncogenically transformed cells. Cell 56, 281–290. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, and Keely PJ (2008). Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, and Giancotti FG (2009). Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J. Clin. Invest 119, 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, and Wu C (2012). ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr. Opin. Cell Biol. 24, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K, Kreft M, Song JY, Janssen H, and Sonnenberg A (2007). Dual Role of alpha6beta4 integrin in epidermal tumor growth: tumor-suppressive versus tumor-promoting function. Mol. Biol. Cell 18, 4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JA, Ortiz-Urda S, Kretz M, Garcia J, Scholl FA, Pasmooij AM, Cassarino D, Chang HY, and Khavari PA (2009). Modeling inducible human tissue neoplasia identifies an extracellular matrix interaction network involved in cancer progression. Cancer Cell 15, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond N, Im JH, Garg R, Vega FM, Borda d’Agua B, Riou P, Cox S, Valderrama F, Muschel RJ, and Ridley AJ (2012). Cdc42 promotes transendothelial migration of cancer cells through beta1 integrin. J. Cell Biol 199, 653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, et al. (2009). Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med 15, 392–400. [DOI] [PubMed] [Google Scholar]
- Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. (2015). Integrative clinical genomics of advanced prostate cancer. Cell 162, 454. [DOI] [PubMed] [Google Scholar]
- Robinson SD, and Hodivala-Dilke KM (2011). The role of beta3-integrins in tumor angiogenesis: context is everything. Curr. Opin. Cell Biol 23, 630–637. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Iskratsch T, and Sheetz MP (2012). Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell Sci 125, 3025–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Widmaier M, Jakobson M, Ruppert R, Ussar S, Katsougkri D, Bottcher RT, Lai-Cheong JE, Rifkin DB, McGrath JA, and Fassler R (2014). Kindlin-1 controls Wnt and TGF-beta availability to regulate cutaneous stem cell proliferation. Nat. Med 20, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JB, Huh D, Noble LB, and Tavazoie SF (2015). Identification of molecular determinants of primary and metastatic tumour re-initiation in breast cancer. Nat. Cell Biol 17, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rys JP, DuFort CC, Monteiro DA, Baird MA, Oses-Prieto JA, Chand S, Burlingame AL, Davidson MW, and Alliston TN (2015). Discrete spatial organization of TGFbeta receptors couples receptor multimerization and signaling to cellular tension. Elife 4, e09300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Ellison D, Thomas GJ, Vallath S, Mather SJ, Hart IR, and Marshall JF (2010). High-resolution in vivo imaging of breast cancer by targeting the pro-invasive integrin alphavbeta6. J. Pathol 222, 52–63. [DOI] [PubMed] [Google Scholar]
- Sanz-Moreno V, and Marshall CJ (2010). The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr. Opin. Cell Biol 22, 690–696. [DOI] [PubMed] [Google Scholar]
- Schedin P, and Keely PJ (2011). Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol 3, a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X, Wrasidlo W, et al. (2011). Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell 19, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, and Ruoslahti E (1997). Alphavbeta3 Integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 16, 5600–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, and Fuchs E (2011). Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. U S A 108, 10544–10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, Benten D, Forbes SJ, Wells RG, and Iredale JP (2011). Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 53, 1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA (1997). Integrins, oncogenes, and anchorage independence. J. Cell Biol 139, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, Yebra M, Mielgo A, Lowy AM, Husain H, et al. (2014). An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol 16, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A, Gomez-Cuadrado L, Canel M, Muir M, Ring JE, et al. (2015). Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell 163, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro IM, Kolev VN, Vidal CM, Kadariya Y, Ring JE, Wright Q, Weaver DT, Menges C, Padval M, McClatchey AI, et al. (2014). Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci. Transl. Med 6, 237ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Kallur KG, Ryu JS, Parameswaran RV, Lindman H, Avril N, Gleeson FV, Lee JD, Lee KH, O’Doherty MJ, et al. (2015). Multicenter reproducibility of 18F-fluciclatide PET imaging in subjects with solid tumors. J. Nucl. Med 56, 1855–1861. [DOI] [PubMed] [Google Scholar]
- Sherman-Baust CA, Weeraratna AT, Rangel LB, Pizer ES, Cho KR, Schwartz DR, Shock T, and Morin PJ (2003). Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 3, 377–386. [DOI] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Inan MF, Reinhardt F, and Weinberg RA (2012). The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2, 706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, and Weinberg RA (2013). An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24, 481–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, and Weinberg RA (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U S A 106, 10290–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Ji X, Cao X, Dai X, Xu L, Zhao H, Guo X, Yan H, Zhang H, Zhu C, et al. (2017). Src inhibits the Hippo tumor suppressor pathway through tyrosine phosphorylation of Lats1. Cancer Res. 77, 4868–4880. [DOI] [PubMed] [Google Scholar]
- Slack-Davis JK, Hershey ED, Theodorescu D, Frierson HF, and Parsons JT (2009). Differential requirement for focal adhesion kinase signaling in cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol. Cancer Ther 8, 2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soikkeli J, Podlasz P, Yin M, Nummela P, Jahkola T, Virolainen S, Krogerus L, Heikkila P, von Smitten K, Saksela O, and Holtta E (2010). Metastatic outgrowth encompasses COL-I, FN1, and POSTN up-regulation and assembly to fibrillar networks regulating cell adhesion, migration, and growth. Am. J. Pathol 177, 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, et al. (2014). Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108. [DOI] [PubMed] [Google Scholar]
- Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, et al. (2014). Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer 14, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Guo SS, and Fassler R (2016). Integrin-mediated mechanotransduction. J. Cell Biol. 215, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, and Glukhova MA (2008). Beta1 Integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol 10, 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WL, and Weinberg RA (2013). The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 19, 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, and Yamada KM (1998). Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617. [DOI] [PubMed] [Google Scholar]
- Tavora B, Batista S, Reynolds LE, Jadeja S, Robinson S, Kostourou V, Hart I, Fruttiger M, Parsons M, and Hodivala-Dilke KM (2010). Endothelial FAK is required for tumour angiogenesis. EMBO Mol. Med 2, 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavora B, Reynolds LE, Batista S, Demircioglu F, Fernandez I, Lechertier T, Lees DM, Wong PP, Alexopoulou A, Elia G, et al. (2014). Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 514, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting DT, Wittner BS, Ligorio M, Vincent Jordan N, Shah AM, Miyamoto DT, Aceto N, Bersani F, Brannigan BW, Xega K, et al. (2014). Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 8, 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tod J, Hanley CJ, Morgan MR, Rucka M, Mellows T, Lopez MA, Kiely P, Moutasim KA, Frampton SJ, Sabnis D, et al. (2017). Pro-migratory and TGF-beta-activating functions of alphavbeta6 integrin in pancreatic cancer are differentially regulated via an Eps8-dependent GTPase switch. J. Pathol 243, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong HH, Xiong J, Ghotra VP, Nirmala E, Haazen L, Le Devedec SE, Balcioglu HE, He S, Snaar-Jagalska BE, Vreugdenhil E, et al. (2014). beta1 Integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci. Signal 7, ra15. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, and Comoglio PM (2001). A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 107, 643–654. [DOI] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, and Massague J (2014). Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, and Stingl J (2014). Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 28, 1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fu W, Im JH, Zhou Z, Santoro SA, Iyer V, DiPersio CM, Yu QC, Quaranta V, Al-Mehdi A, and Muschel RJ (2004). Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell Biol 164, 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Hung Y, Su CH, Peng ST, Guo YJ, Lai MC, Liu CY, and Hsu JW (2005). CD44 cross-linking induces integrin-mediated adhesion and transendothelial migration in breast cancer cell line by up-regulation of LFA-1 (alpha L beta2) and VLA-4 (alpha4beta1). Exp. Cell Res 304, 116–126. [DOI] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, and Muller WJ (2004). Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Lavelin I, and Geiger B (2013). Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell 24, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL, Cereser B, Crnogorac-Jurcevic T, McDonald S, Elia G, et al. (2015). Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell 27, 123–137. [DOI] [PubMed] [Google Scholar]
- Woods D, Cherwinski H, Venetsanakos E, Bhat A, Gysin S, Humbert M, Bray PF, Saylor VL, and McMahon M (2001). Induction of beta3-integrin gene expression by sustained activation of the Ras-regulated RafMEK-extracellular signal-regulated kinase signaling pathway. Mol. Cell. Biol 21, 3192–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Krasnitz A, Lucito R, Sordella R, Vanaelst L, Cordon-Cardo C, Singer S, Kuehnel F, Wigler M, Powers S, et al. (2008). DLC1 is a chromosome 8p tumor suppressor whose loss promotes hepatocellular carcinoma. Genes Dev. 22, 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Flores LM, Li Q, Zhou P, Xu F, Krop IE, and Hemler ME (2010). Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res. 70, 2256–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, Ridge SM, Jablonski EM, Therrien J, Tannheimer S, et al. (2018). Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 560, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Snider AK, and Ginsberg MH (2014). Talin and kindlin: the one-two punch in integrin activation. Front. Med. 8, 6–16. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Otero J, Chen Y, Kim YM, Koutcher JA, Satagopan J, Reuter V, Carver B, de Stanchina E, Enomoto K, et al. (2013). beta4 Integrin signaling induces expansion of prostate tumor progenitors. J. Clin. Invest 123, 682–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You D, Xin J, Volk A, Wei W, Schmidt R, Scurti G, Nand S, Breuer EK, Kuo PC, Breslin P, et al. (2015). FAK mediates a compensatory survival signal parallel to PI3K-AKT in PTEN-null T-ALL cells. Cell Rep. 10, 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, and Piccolo S (2015). Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol 17, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu G, Lang L, Li F, Fan X, Yan X, Yao S, Yan W, Huo L, Chen L, et al. (2017). Clinical translation of a dual integrin alphavbeta3- and gastrin-releasing peptide receptor-targeting PET radiotracer, 68Ga-BBNRGD. J. Nucl. Med 58, 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, de la Cruz CC, Sayles LC, Alleyne-Chin C, Vaka D, Knaak TD, Bigos M, Xu Y, Hoang CD, Shrager JB, et al. (2013). A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal. Cancer Cell 24, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al. (2015). Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol 17, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CQ, Popova SN, Brown ER, Barsyte-Lovejoy D, Navab R, Shih W, Li M, Lu M, Jurisica I, Penn LZ, et al. (2007). Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human nonsmall-cell lung cancer cells. Proc. Natl. Acad. Sci. U S A 104, 11754–11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni E, van der Horst G, van de Merbel AF, Chen L, Rane JK, Pelger RC, Collins AT, Visakorpi T, Snaar-Jagalska BE, Maitland NJ, and van der Pluijm G (2015). miR-25 modulates invasiveness and dissemination of human prostate cancer cells via regulation of alphav- and alpha6-integrin expression. Cancer Res. 75, 2326–2336. [DOI] [PubMed] [Google Scholar]