Abstract
Diabetes and metabolic disorders are leading causes of micro- and macrovascular complications. Furthermore, efforts to treat these complications are hampered by metabolic memory, a phenomenon in which prior exposure to hyperglycemia predisposes diabetic patients to the continued development of vascular diseases despite subsequent glycemic control. Persistently increased levels of oxidant stress and inflammatory genes are key features of these pathologies. Biochemical and molecular studies showed that hyperglycemia induced activation of NF-κB, signaling and actions of advanced glycation end products and other inflammatory mediators play key roles in the expression of pathological genes. In addition, epigenetic mechanisms such as posttranslational modification of histones and DNA methylation also play central roles in gene regulation by affecting chromatin structure and function. Recent studies have suggested that dysregulation of such epigenetic mechanisms may be involved in metabolic memory leading to persistent changes in the expression of genes associated with diabetic vascular complications. Further exploration of these mechanisms by also taking advantages of recent advances in high throughput epigenomics technologies will greatly increase our understanding of epigenetic variations in diabetes and its complications. This in turn can lead to the development of novel new therapies.
19.1. Introduction
Diabetes is the leading cause of macro- and microvascular complications such as atherosclerosis, hypertension, nephropathy, retinopathy and neuropathy (Beckman et al. 2002; He and King 2004; Sharma and Ziyadeh 1995). These vascular complications affect all major organs leading to heart failure, kidney failure, blindness and limb amputation and can significantly enhance mortality rates in patients with diabetes relative to the normal population. Current projections predict a significant surge in obesity, diabetes and related metabolic disorders worldwide and in particular among the younger population, likely due to changes in lifestyle. This can greatly increase the economic burden associated with diabetes and its complications. Intense efforts are therefore needed to find more effective therapeutic approaches to curb the progression of diabetic complications. Diabetes increases the blood glucose levels(hyperglycemia) as a result of lack of insulin production (type 1 diabetes, T1D) or increased insulin resistance(type 2 diabetes, T2D), with the latter often being associated with hyperlipidemia. Hyperglycemia has been implicated in chronic inflammation and increased oxidant stress, the major risk factors for the development of vascular complications (Giacco and Brownlee 2010; Sheetz and King 2002; Devaraj et al. 2010). Clinical trials have demonstrated that strict glycemic control is critical to reduce the incidence of diabetic complications. They also showed that diabetic patients are at continued risk for increased vascular complications even long after achieving normal blood glucose levels, suggesting a ‘metabolic memory’ or ‘legacy’ effect of prior hyperglycemic exposure (Writing Team DCCT/EDIC Research Group 2002; Colagiuri et al. 2002). These clinical studies were further supported by experimental evidence showing that vascular cells exposed to diabetic milieu retain their pro-inflammatory diabetic phenotype for extended periods even after glucose normalization (Ihnat et al. 2007a; Villeneuve and Natarajan 2010; Pirola et al. 2010). Metabolic memory is a major challenge in the prevention of diabetic vascular complications and it is imperative to examine the molecular mechanisms involved and develop novel therapies.
Transcription regulation plays a central role in the expression of inflammatory and other pathologic genes. In general, the recruitment of transcription factors (TFs) to the cis-elements located in the promoters and enhancers plays a key role in gene regulation. However, it is now clear that epigenetic mechanisms in chromatin, i.e., changes that occur without alterations in the DNA sequence, also play important roles in gene transcription. These mechanisms control chromatin access to transcription regulators in mammalian cells and dictate active or repressed states of genes (Li et al. 2007; Murr 2010; Kouzarides 2007; Bannister and Kouzarides 2011). Furthermore, environmental factors and nutrients can affect epigenetic states and regulate the expression of genes associated with various diseases including cancer and diabetes (Sharma et al. 2010; Liu et al. 2008; Ling and Groop 2009). Recent studies have also implicated epigenetic mechanisms in the phenomenon of metabolic memory. An increased understanding of these changes in chromatin events can yield critical new information about the metabolic memory of vascular complications and aberrant expression of inflammatory genes under diabetic conditions that can lead to the identification of novel new therapeutic targets. The current review discusses the role of epigenetics in diabetic vascular complications and some of the recent developments in this area.
19.2. Inflammatory Gene Expression and Vascular Complications
Diabetes is associated with significantly accelerated rates of vascular diseases like atherosclerosis which is multi-cellular in nature involving interactions between endothelial cells (EC), vascular smooth muscle cells (VSMC) and monocytes in the vessel wall (Ross 1999). Endothelial dysfunction induced by oxidant stress, oxidized lipids and other inflammatory mediators increases monocyte adhesion to EC, which then migrate into subendothelial space where interaction with VSMC promotes their differentiation into macrophages. The uptake of oxidized lipids by macrophages leads to foam cell formation. VSMC proliferation and migration to the sites of lesion also contribute to the formation of the atherosclerotic plaque. In advanced stages of the disease plaque rupture releases pro-inflammatory and pro-thrombotic mediators resulting in stroke (Ross 1999). EC dysfunction and macrophage infiltration also play key roles in diabetic nephropathy and retinopathy (King 2008; Giacco and Brownlee 2010; Wang and Harris 2011). All cell types involved in vascular complications produce inflammatory cytokines and chemokines, and regulate the functions of each other through autocrine and paracrine actions. Pro-inflammatory chemokines such as monocyte chemoattractant protein-1(MCP-1), cytokines including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), and growth factors such as Angiotensin II(Ang II) and macrophage colony stimulating factor (M-CSF) play key roles in the initiation and progression of vascular complications (Charo and Taubman 2004; Libby et al. 2002; Weiss et al. 2001). Several studies have demonstrated that diabetes exacerbates the production of inflammatory mediators in the vessel wall leading to further acceleration of vascular dysfunction. Enhanced activity of the pro-inflammatory TF NF-κB plays a key role in the increased inflammatory gene expression in VSMC, EC and monocytes under diabetic conditions. These cells exhibit enhanced oxidant stress and inflammatory genes such as TNF-α, MCP-1, Fractalkine (CX3CL1), IL-6, M-CSF and arachidonic acid metabolizing enzymes such as cyclooxygenese-2 (COX-2) and lipoxygenases, and the receptor for advanced glycation end products (RAGE) under diabetic conditions (Devaraj et al. 2006; De Martin et al. 2000; Barnes and Karin 1997; Brownlee 2001; Natarajan and Nadler 2004; Shanmugam et al. 2003a, b; Guha et al. 2000; Li et al. 2006; Reddy et al. 2009; Hatley et al. 2003; Min et al. 2010; Meng et al. 2010; Villeneuve et al. 2008; Yan et al. 2009). While the transcription of inflammatory genes is most often regulated by NF-κB TF (Barnes and Karin 1997; Glass and Witztum 2001), other TFs such as CREB and STATs have also been implicated (Reddy et al. 2006, 2009; Sahar et al. 2005, 2007; Chava et al. 2009). Biochemical studies have also established the role of multiple upstream signal transduction mechanisms involving increased oxidant stress, polyol pathway, AGEs and RAGE, Protein kinase C (PKC), AT1R, oxidized lipids, tyrosine kinases and mitogen activated protein kinases (MAPKs) that can lead to the activation of TFs regulating genes involved in diabetic complications (Brownlee 2005; Natarajan and Nadler 2004; Yan et al. 2008; Marrero et al. 2005; Reddy et al. 2006). However, therapies based on these mechanisms have not been fully adequate to effectively prevent the progression of various diabetic vascular complications suggesting the need to explore additional mediators and drug targets.
19.3. Metabolic Memory of Diabetic Vascular Complications
Landmark clinical trials such as the Diabetes Control and Complications Trial (DCCT) with T1D patients showed that intensive glycemic control is critical for the prevention or reduction of long term vascular complications. In the follow up Epidemiology of Diabetes Interventions and Complications study (EDIC), the DCCT enrollees were subsequently monitored after placing both the conventional and intensive treatment groups on the same intensive glycemic control. Results from EDIC showed that patients previously receiving conventional therapy during DCCT continued to develop micro-and macrovascular complications at a much greater rate relative to those in the intensive treatment group throughout (Writing Team DCCT/EDIC Research Group 2002; Nathan et al. 2005). In other clinical trials involving T2D patients, levels of hyperglycemia at the time of diagnosis correlated with risk for developing complications in T2D patients (Colagiuri et al. 2002). Furthermore, fluctuations in post-prandial glycemic levels have also been implicated in the increased risk for vascular complications (Ceriello et al. 2008). Overall, these clinical studies have suggested that the persisting effects of prior exposure to hyperglycemia termed ‘metabolic memory’ or “legacy effect” can have long lasting deleterious effects in diabetes patients.
Studies using cell culture and animal models further established the role of metabolic memory in vascular complications. These included demonstration of increased oxidative stress, fibrotic and inflammatory gene expression in high glucose (HG) treated EC even several days after return to normal glucose (Ihnat et al. 2007b; Roy et al. 1990; El-Osta et al. 2008). VSMC derived from leptin receptor deficient db/db mice, a model of T2D, exhibited enhanced pro-inflammatory gene expression, monocyte binding and migration even after culturing in vitro for a few passages compared with non-diabetic db/+ controls (Li et al. 2006; Villeneuve et al. 2008). VSMC from db/db mice also showed persistently increased activation of signal transduction pathways such as oxidant stress, tyrosine kinase, MAPK and downstream pro-inflammatory TFs NF-κB and CREB (Li et al. 2006). EC and macrophages from db/db mice also exhibited enhanced inflammatory genes and activation of NF-κB, suggesting that diabetes induces a pre-activated phenotype in target cells involved in vascular complications (Hatley et al. 2003; Li et al. 2006; Wen et al. 2006). In retinal EC, HG inhibited the expression of the antioxidant gene superoxide dismutase (sod2) and this persisted even 4 days after reversal to normal glucose (Zhong and Kowluru 2011). In streptozotocin injected dogs with T1D, retinal complications persisted even after achieving normoglycemia with islet transplantation (Engerman and Kern 1987). Furthermore, in T1D rats, poor glycemic control for short periods followed by intensive glycemic control prevented the development of retinopathy. However, intensive glycemic control could not prevent retinopathy and related biochemical parameters in animals that had poor glycemic control for much longer durations (Chan et al. 2010; Kowluru 2003). Together these studies demonstrated the role of metabolic memory in diabetic complications and recent studies now suggest that epigenetic mechanisms may be involved in these events.
19.4. Epigenetic Mechanisms of Gene Regulation in Chromatin: DNA Methylation and Histone Post Translational Modifications (PTMs)
In mammalian cells, chromosomal DNA is tightly packaged into chromatin by histone proteins along with other chromatin assembly factors. Chromatin is a highly organized structure consisting of numerous nucleosome particles. Each nucleosome is composed of about 147 bp DNA wrapped around an octamer histone protein complex made up of dimers of core histone proteins H2A, H2B, H3 and H4 (Li et al. 2007; Workman and Kingston 1998). The histone proteins were initially proposed to be passive components supporting chromatin structure. However, it is now clear that they actively participate in transcriptional regulation. Epigenetic mechanisms regulate dynamic switching of chromatin between active (euchromatin) and inactive (heterochromatin) states that determines the transcription status of target genes and the biological outcomes (Li et al. 2007; Kouzarides 2007; Murr 2010). These mechanisms include DNA cytosine methylation, covalent post translational modifications (PTMs) of nucleosomal histones, small non-coding RNAs or microRNAs (miRNAs) and large intergenic noncoding RNAs (lincRNAs) (Murr 2010). While epigenetics usually refers to heritable changes, including those conferred mitotically or meiotically, that occur without changes in DNA sequence, more recently the definition has been modified to include the structural adaptation of chromosomal regions (Bird 2007). Thus, both DNA methylation and histone PTMs can work together to control epigenetic transmission (Bird 2007; Berger et al. 2009; Portela and Esteller 2010). Epigenetic mechanisms genomewide are now termed as the ‘Epigenome’ and major efforts are underway to understand how alterations in epigenome status can modulate diverse patho-physiolological conditions (Bernstein et al. 2007; Maunakea et al. 2010), especially since epigenetic changes can be induced by environmental factors.
DNA methylation which occurs at cytosine residues in CpG dinucleotides is one of the most stable epigenetic marks (Miranda and Jones 2007). CpGs can occur in groups called ‘CpG islands’ near promoters and methylation of promoter CpG islands often leads to gene repression. DNA methylation is regulated by DNA methyl transferases that mediate the transfer of methyl groups from S-adenosyl methionine (SAM). Recent studies have also implicated a role for DNA de-methylation in cellular processes but the identity of the enzymes that mediate DNA demethylation is not fully clear (Wu and Zhang 2010). Abnormal DNA methylation or demethylation at the promoters of oncogenes and tumor suppressors is a major mechanism involved in the development of cancer (Miranda and Jones 2007; Sharma et al. 2010). However, only limited information is available on the role of DNA methylation in diabetes, metabolism and vascular complications. Some of these include the demonstration of DNA methylation in Agouti gene expression (Morgan et al. 1999), DNA hypomethylation in aortic tissues from atherosclerosis animal models (Turunen et al. 2009) and differential DNA methylation in monocytes of T1D patients with diabetic nephropathy compared with patients who did not develop nephropathy (Bell et al. 2010). Further information on DNA methylation related to vascular complications can be obtained from recent reviews (Dong et al. 2002; Turunen et al. 2009; Reddy and Natarajan 2011).
Histone PTMs can play major roles in the regulation of gene expression by modifying chromatin structure and by providing anchoring sites for co-activators, co-repressors and other chromatin proteins. Histone PTMs in association with other epigenetic mechanisms such as DNA methylation can modulate the euchromatin (accessible) or heterochromatin (inaccessible) status of chromatin (Murr 2010; Kouzarides 2007; Bannister and Kouzarides 2011). Several histone PTMs have been identified including phosphorylation (serine and threonine), acetylation (lysine), methylation (lysine and arginine), ubiquitination (lysine) and sumoylation (lysine) (Kouzarides 2007). Histone PTMs usually act in concert with each other to form a ‘histone code’ that dictates the transcriptional states of genes leading to biological phenotypes (Jenuwein and Allis 2001). The function of histone acetylation and methylation has been widely studied in the pathogenesis of cancer (Portela and Esteller 2010). Recent studies have examined their role in diabetes and vascular complications.
19.5. Histone Lysine Acetylation and Methylation in Gene Regulation
In general histone lysine acetylation (HKAc) promotes formation of open chromatin and is associated with active promoters. Acetylation of amino-terminal residues in histone tails neutralizes the positive charge weakening DNA-histone and nucleosome-nucleosome interactions (Kouzarides 2007; Roth et al. 2001). Furthermore, acetylated lysines can provide binding sites for chromatin remodeling proteins. These events can relax chromatin structure and make it more accessible to transcription factors and other regulators to promote gene transcription. HKAc is mediated by histone acetyl transferases (HATs). Several HATs including CBP, p300, pCAF, Tip60, SRC-1, SRC-2 and SRC-3 also act as co-activators. HATs can acetylate multiple lysine residues of histone and non-histone proteins (Roth et al. 2001; Kouzarides 2007). Genomewide studies using chromatin immunoprecipitation linked to Next Generation DNA Sequencing (ChIP-Seq) revealed differential regulation of specific promoters by individual HATs (Ramos et al. 2010). Interestingly, ChIP-Seq and ChIP coupled with microarray analysis (ChIP-on-chip) studies also showed that p300 is recruited to enhancers and its location along with other histone PTMs such as H3K4me1 could be used to predict enhancers genomewide in mammalian cells (Visel et al. 2009; Hon et al. 2009; Jin et al. 2011).
Histone acetylation is removed by histone deacetylases (HDACs), which are sub-divided into four groups (Class I, Class II, Class III and clas IV) depending on structure, function and mode of action. Class III proteins also known as Sirtuins are unique in that they require NAD+ as co-factor, and respond to changes in metabolic status (Kouzarides 2007; Yang and Seto 2007). Removal of acetyl groups by HDACs restores the positive charge of histone lysines and promotes chromatin condensation, leading to reduced TF accessibility and inhibition of transcription. The functions of HDACs are quite complex mainly due to their low substrate specificity. However, studies in knockout mice showed they could be involved in specific function such as embryonic stem cell differentiation and EC proliferation (Dovey et al. 2010; Margariti et al. 2010). Overall, histone lysine acetylation can play significant roles in gene regulation related to biological and pathophysiological states.
Histone lysine methylation (HKme) on the other hand is associated with both active and inactive promoters depending on the specific lysine residue methylated. Furthermore, these lysines can be mono, di or tri methylated adding another level of complexity (Shilatifard 2006; Kouzarides 2007; Bannister and Kouzarides 2011). Histone H3 lysine 9 methylation (H3K9me2/3), H3K27me2/3, and H4K20me3 are generally associated with gene repression, while histone H3K4me1/2/3 and H3K36me3 are usually associated with active genes and gene bodies. Histone methyl transferases (HMTs) catalyze HKme while histone demethylases (HDMs) mediate removal of methyl groups. HMTs and HDMs are quite specific, e.g., SUV39H1 HMT mediates H3K9me2 and H3K9me3, and the MLL family members mediate H3K4me1-me3. Conversely, lysine specific demethylase 1(LSD1) removes H3K4 -me1 and -me2, and Jhdm2a demethylates H3K9me2 (Kouzarides 2007; Shi and Whetstine 2007; Trojer and Reinberg 2006). Most HMTs contain the SET domain which mediates lysin methyltransferase activity (Kouzarides 2007). The discovery of several HDMs demonstrated that HKme is indeed reversible and established the dynamic regulation of HKme in gene expression. Lysine methylation marks are recognized by chromo, tudor, MBT and nonrelated PHD domain containing proteins, which regulate the function of HKme (Kouzarides 2007).
Increasing evidence supports the critical function of HKme in diverse pathophysiological conditions including cancer (Bhaumik et al. 2007; Portela and Esteller 2010) and recently in diabetes and its vascular complications (Villeneuve and Natarajan 2010; Pirola et al. 2010; Giacco and Brownlee 2010; Reddy and Natarajan 2011). Because HKme is relatively more stable than other modifications, it is likely to be involved in transcriptional memory. Interestingly, studies showed that the polycomb group of proteins that mediate H3K27me3 remained bound to chromatin during DNA replication implicating a potential epigenetic role for such histone marks in the maintenance of transcription memory (Bantignies and Cavalli 2006; Francis et al. 2009). Thus, HKme could be a mediator of metabolic memory resulting from exposure to environment and dietary factors and diabetic stimuli such as HG, AGEs and oxidized lipids.
Arginine methylation of histones (HRme) can be associated with gene activation or repression. Arginine can be mono or dimethylated, and the dimethylation can be asymmetric or symmetric depending on the methyltransferase involved (Kouzarides 2007). At least 9 protein arginine methyltransferases (PRMTs) have been identified including coactivator-associated arginine methyltransferase 1 (CARM1). HRme is removed by arginine demethylases such as JMJD6. HRme might play key role in inflammation, cell proliferation and differentiation (Wysocka et al. 2006; Miao et al. 2006).
Role of epigenetic mechanisms including histone PTMs and DNA methylation in gene transcription and interaction with environment is summarized in Fig. 19.1. Recent advances in next generation sequencing (NGS) have significantly enhanced our technical capabilities to analyze genomewide changes in histone PTMs and DNA methylation (Metzker 2010; Hawkins et al. 2010; Maunakea et al. 2010). This area is poised to make tremendous progress towards understanding the function of epigenetic mechanisms under normal and disease states.
Fig. 19.1.
Role of epigenetic mechanisms in gene transcription. Epigenetic mechanisms play central roles in gene regulation mediated by transcription factors (TF) by maintaining active (euchromatin) or repressed (heterochromatin) states of chromatin. These include DNA methylation (DNAMe) and histone post translational modifications (PTMs). In the repressed state, chromatin at repressed genes can be enriched with DNAMe [mediated by DNA methyl transferases (DNMTs)] and repressive histone PTMs such H3K9 methylation (H3K9me), H3K27me and H4K20me mediated by histone methyltransferases (HMTs) SUV39H1, Ezh2 and SUV420H2 respectively. In active states, chromatin is enriched by H3K acetylation (H3KAc) and H4KAc, and H3K4me and H3K36me. Histone acetyltransferases (HAT) such as CBP/p300 and SRC-1 mediate acetylation, while H3K4me is catalyzed by HMTs such as SET7 and MLL family members. Actions of HATs are opposed by histone deacetylases (HDAC). Actions of HMTs are countered by histone demethylases (HDM) such as LSD1 and JARID, which erase H3K4me marks and the JMJD family members, which erase H3K9me and H3K27me marks. This results in gene repression or activation depending on the modification that has been erased. Environmental factors and extracellular signals can affect epigenetic states to modulate the expression of genes associated with various diseases including cancer and diabetes. Ac-lysine acetylation; Me: lysine methylation
19.6. Histone Modifications in Diabetes
Histone modifications were shown to play key role in pancreatic islet specific expression of insulin and related genes in response to changing glucose levels. Under HG conditions, the islet specific transcription factor Pdx1 was shown to recruit a co-activator HAT p300 and a H3K4 methyltransferase SET7/9 at the insulin promoter. This was associated with increased promoter levels of the activation marks H3K9/14Ac and H3K4Me and insulin expression. Conversely, under low glucose conditions, Pdx1could recruit HDAC1 and HDAC2 to the insulin promoter, leading to the inhibition of insulin expression. Interestingly, Pdx1 also induced SET7/9 expression, which in turn increased the expression of genes involved in glucose induced insulin secretion (Deering et al. 2009; Chakrabarti et al. 2003). Recent studies showed that the Polycomb group of proteins including Ezh2 that mediates H3K27me3, JMJD3 that demethylates H3K27me3, and accessory proteins such as Bmi-1 play key roles in pancreatic β-cell proliferation and regeneration through regulation of the tumor suppressor protein p16INK4a (Dhawan et al. 2009; Chen et al. 2009). Histone modifications were also reported in adipocyte differentiation since a regulatory role of H3KAc was identified in the expression of C/EBP-delta, a key transcription factor involved in adipocyte differentiation (Nakade et al. 2007). LSD1(demethylase) and SETDB1(methyltransferase) functions were required for the regulation of H3K4me2 and H3K9me2 respectively during adipogenesis (Musri et al. 2010). Furthermore, knockdown of the H3K9me2 demethylase Jhdm2a (Jmjd1a) resulted in obesity and hyperlipidemia in mice, providing direct evidence for H3Kme in the development of diabetes (Tateishi et al. 2009). The class III HDAC, SIRT1 was found to play an important role in energy metabolism, and SIRT1 activators such as resveratrol can inhibit insulin resistance. Several SIRT1 modulators are being evaluated for the treatment of insulin resistance (Haigis and Sinclair 2010; Blum et al. 2011).
19.7. Histone Modifications in Inflammatory Gene Expression
Evidence shows that NF-κB mediated inflammatory gene expression induced by pro-inflammatory signals was associated with changes in HKAc and HKme in vascular cells and monocytes. Ang II induced IL-6 expression was associated with increased NF-κB activation and promoter H3K9/14Ac in VSMC. Ang II enhanced the recruitment of TFs (NF-κB and CREB) along with co-activator HATs steroid receptor coactivator-1 (SRC-1) and p300/CBP to the IL-6 promoter. Furthermore, Ang II induced IL-6 expression was inhibited by a p300 mutant lacking HAT activity and a SRC-1 mutant lacking ERK phosphorylation site (Sahar et al. 2007). Oxidized lipids also increased H3KAc at the IL-6 and MCP-1 promoters in VSMC. This increased acetylation and gene expression was dependent on Src kinase activity (Reddy et al. 2009). Role of p300 and p/CAF was also reported in NF-κB mediated chemokine(C-C motif) ligand 11 (CCL11) expression in TNF-α stimulated human airway smooth muscle cells (Clarke et al. 2008).
TNF-α increased H3KAc at the IL-6 promoter, and recruitment of CBP/p300 was required for the optimal induction of NF-κB mediated IL-6 and IL-8 expression in EC (Vanden Berghe et al. 1999). TNF-α induced EC specific E-selectin expression was associated with increased H3K4me2, H3K9/14 Ac and H4K12Ac along with p300/CBP recruitment, which promoted nucleosome remodeling to increase chromatin access to NF-κB (Edelstein et al. 2005). Furthermore, these studies also showed the role of HDAC2 in association with Sin3A in the post-induction repression of these inflammatory genes. EC specific expression of endothelial nitric oxide(eNOS) was associated with H3K4me, H3K9/14Ac and Ser10-phosphorylation at the eNOS core promoter (Fish et al. 2005). Oxidized LDL could induce H3KAc, and recruitment of HATs along with NF-κB at inflammatory gene promoters in EC and these events could be reversed by statin treatment (Dje N’Guessan et al. 2009). Together, these reports demonstrate the role of histone PTMs in regulating the expression of inflammatory genes in vascular cells.
Lipopolysaccharide (LPS) induced the expression of several HDACs along with inflammatory genes in macrophages (Aung et al. 2006). Interestingly, HDAC inhibitors attenuated the expression of some inflammatory genes but also increased the expression of pro-atherogenic genes (Halili et al. 2010). In dendritic cells derived from human monocytes, LPS induced inflammatory gene expression was associated with a rapid decrease in the levels of the repressive mark H3K9me3 followed by an increase to basal levels at later time points. This suggested a regulatory role of H3K9me3 in negative feedback mechanisms associated with post induction repression of inducible inflammatory genes (Saccani and Natoli 2002). Similar decrease in H3K9me3 at early time points and restoration to control levels at later time points was also noted in VSMC stimulated with TNF-α (Villeneuve et al. 2008). LPS mediated inflammatory gene expression was associated with decreases in repressive epigenetic mark H3K27me3 in macrophages. Further studies revealed that LPS also increased the expression of the H3K27me3 demethylase JMJD3 which was shown to regulate inflammatory genes in macrophages (De Santa et al. 2007, 2009). In THP-1 monocytes, TNF-α stimulation increased H3K4me at inflammatory gene promoters and promoted the recruitment of SET7/9, a H3K4 methyl transferase, along with NF-κB at inflammatory gene promoters. SET7/9 gene silencing revealed that a subset of NF-κB regulated inflammatory genes required SET7/9 and its mehyltransferase activity in TNF-α stimulated THP-1 cells (Li et al. 2008). TNF-α induced inflammatory gene expression was also associated with increased H3R17me and recruitment of the arginine methyltransferase CARM1 (Miao et al. 2006). Thus, NF-κB mediated inflammatory gene expression can be fine tuned by histone modifications in both vascular cells and monocytes, key players in the pathogenesis of vascular complications.
19.8. Histone Lysine Modifications in Diabetic Vascular Complications
Several lines of evidence suggest that diabetes and diabetogenic agents promote changes in histone modifications in vascular cells and monocytes to regulate the expression of genes associated with vascular complications. HG induced increases in TNF-α and COX-2 genes in THP-1 monocytes was associated with increased H3KAc at their promoters along with increased promoter recruitment of NF-κB and co-activators CBP, p/CAF and SRC-1, and reduced occupancy of HDAC1 (Miao et al. 2004). Furthermore, increased levels of H3KAc was observed at inflammatory gene promoters in peripheral blood monocytes obtained from T1D and T2D patients, thus establishing in vivo relevance and demonstrating that inflammatory cells from diabetic patients may have a more open chromatin at pathologic genes (Miao et al. 2004). The RAGE ligand S100b also increased H3K9Ac, H3K14Ac and H3R17me as well as recruitment of p300 and CARM1 at the TNF-α promoter in THP-1 monocytes demonstrating that RAGE signaling can alter histone PTMs in chromatin (Miao et al. 2006). Recent studies reported that the anti-inflammatory agent curcumin blocked HG induced cytokine gene expression in THP-1 cells via inhibition of H3KAc and activity of p300 (Yun et al. 2011). These results further confirm the role of HKAc in inflammatory gene expression under diabetic conditions.
TNF-α induced inflammatory gene expression was enhanced in macrophages from T1D mice and this was associated with increased H3K4me and recruitment of SET7/9 at inflammatory gene promoters (Li et al. 2008). Genome-wide location approaches such as ChIP-on-chip showed that HG induced significant changes in H3K9me2 and H3K4me2 patterns at key genes in THP-1 monocytes (Miao et al. 2007). ChIP-on-chip studies using lymphocytes from T1D patients revealed significantly increased H3K9me2 levels at a subset of genes involved in inflammatory and autoimmune regulatory pathways relevant to the pathogenesis T1D and its complications (Miao et al. 2008).
Human VSMC cultured in HG displayed increases in inflammatory genes and this was associated with reduced H3K9me3 at their promoters (Villeneuve et al. 2008). In retinal EC, HG decreased global H3KAc, inhibited HAT activity and increased HDAC expression (Zhong and Kowluru 2010). Recent studies in retinal EC showed that HG mediated inhibition of the antioxidant superoxide dismutase (sod2) gene was associated with increased H4K20me3 at its promoter through increased expression of the corresponding HMT SUV420h2 (Zhong and Kowluru 2011). Another study reported decreases in H3K9me and increases in H3K4me along with increased recruitment of SET7/9 at fibrotic gene promoters by HG and the profibrotic growth factor TGF-β in renal mesangial cells which are involved in diabetic nephropathy (Sun et al. 2010). Furthermore, HG induced gene expression and changes in these histone modifications were blocked by a TGF-β antibody (Sun et al. 2010). These studies show that diabetic conditions can alter the levels of histone PTMs in target cells that play key roles in diabetes and its vascular complications.
19.9. Histone Modifications in Metabolic Memory
Metabolic memory has been implicated in the persistence of vascular complications even long after achieving normal glycemic levels in diabetes patients. This was attributed to the sustained increases in the levels of oxidant stress and inflammatory genes in the vasculature (Villeneuve and Natarajan 2010; Giacco and Brownlee 2010; Pirola et al. 2010). Recent studies using cell culture and animal models have suggested that HKme may be involved in metabolic memory of diabetic vascular complications.
EC treated with HG continued to express elevated levels of inflammatory genes even long after restoration to normal glucose medium mimicking metabolic memory. There was a sustained increase in p65 (NF-κB) expression and this was associated with increased levels of the activation mark H3K4me1 and the corresponding HMT Set7/9 at the p65 promoter (El-Osta et al. 2008). In addition, levels of repressive marks H3K9me2 and H3K9me3 were also reduced while the occupancy of the demethylase LSD1 was enhanced in HG treated EC. These changes persisted even after removal of HG suggesting a key role of H3Kme and its regulators in metabolic memory in EC (Brasacchio et al. 2009). Oxidant stress and reactive dicarbonyls such as methylglyoxal were implicated in these changes (El-Osta et al. 2008).
In another model of metabolic memory, aortic VSMC isolated from db/db mice were used. These cells displayed enhanced expression of IL-6, MCP-1 and MCSF-1 genes and increased monocyte binding relative to db/+ control cells, even after culturing for few passages in vitro, mimicking metabolic memory (Li et al. 2006; Villeneuve et al. 2008; Meng et al. 2010). ChIP assays showed that the repressive H3K9me3 mark was significantly reduced at the promoters of these inflammatory genes in VSMC of diabetic db/db mice relative to those from genetic control db/+ mice. Furthermore, TNF-α induced inflammatory gene expression was also significantly enhanced in these db/db cells (Villeneuve et al. 2008) and this was associated with persistently reduced levels of repressive H3K9me3 and occupancy of the H3K9me3 methyltransferase Suv39h1 at inflammatory gene promoters suggesting dysregulation of repressive mechanisms in diabetes. Suv39h1 levels were also significantly reduced in db/db VSMC and reconstitution by overexpression of Suv39h1 reversed the pro-inflammatory phenotype of db/db VSMC (Villeneuve et al. 2008). Thus, H3K9me3 and the corresponding HMT appear to play key roles in this model of metabolic memory related to vascular complications. In addition, recent studies showed that levels of the microRNA-125b were increased in db/db VSMC and that Suv39h1 was a direct target of miR-125b in mouse VSMC (Villeneuve et al. 2010). Furthermore, miR-125b could inhibit Suv39h1 expression, induce inflammatory genes, and reduce H3K9me3 at their promoters and also promote monocyte-VSMC binding in non-diabetic db/+ cells. In contrast, miR-125b inhibition reversed key pro-inflammatory phenotypes of diabetic db/db VSMC (Villeneuve et al. 2010). These results identified a novel role for microRNA mediated mechanisms in inflammatory gene expression in VMSC and possibly metabolic memory through downregulation of key repressive chromatin factors. Interestingly, SIRT1 can activate the methyltransferase activity of Suv39h1 (Vaquero et al. 2007) raising the intriguing question of whether SIRT1 activators could be used for treating diabetic complications or metabolic memory.
Studies with a T1D rat model of diabetic retinopathy have suggested a role for H4K20me3 in metabolic memory (Zhong and Kowluru 2011). There was persistently reduced expression of the key antioxidant gene sod2 in retinas from diabetic rats with poor glycemic control that developed retinopathy compared with non-diabetic rats, or diabetic rats with good glucose control that did not develop retinopathy. This was associated with increased levels of H4K20me3, H3K9Ac as well as NF-κB recruitment at the sod2 promoter and increases in the expression of the H4K20me3 methyl transferase Suv420h2 in diabetic retinas. Interestingly, these changes were sustained in rats displaying memory of retinopathy. Furthermore, in vitro studies with EC cultured in HG demonstrated the role of Suv420h2 in the persistent histone PTMs at the sod promoter. Thus, H4K20me3 and Suv420h2 were implicated in this model of metabolic memory associated with diabetic retinopathy.
19.10. Summary
Studies in cultured cells and experimental models have identified the role of HG as well as downstream biochemical, signaling and chromatin mechanisms in vascular complications and metabolic memory (Fig. 19.2). However, diabetes is a multifactorial disease and other factors such as AGEs, oxidized lipids, proinflammatory growth factors, nutrients and lifestyles could also be involved. Recent advances in genomewide approaches such as ChIP-Seq and DNA methylation profiling will greatly accelerate our understanding of histone PTMs and epigenetic mechanisms regulating the expression of genes associated with diabetic complications and metabolic memory (Wang et al. 2009; Metzker 2010; Hawkins et al. 2010; Maunakea et al. 2010). These studies could potentially lead to the identification of several other histone PTMs, HMTs, HDMs and DNA methyl transferases in diabetic complications. Since epigenetic mechanisms and histone PTMs are reversible, there is ample potential for the development of novel therapies. Several candidates including inhibitors of HDACs, HATs and DNA methyltransferases are being tested in epigenetic therapy for cancer (Kelly et al. 2010; Selvi et al. 2010). Some of these are also being considered for treating vascular complications like restenosis (Pons et al. 2009; Natarajan 2011). Recent reports showing that histone modifications and inflammatory genes can be reversed by curcumin in monocytes treated with HG (Yun et al. 2011), and also renal dysfunction in diabetic rats (Chiu et al. 2009) by a TGF-b antibody in renal mesangial cells (Sun et al. 2010) and by statins in EC (Dje N’Guessan et al. 2009) reveal the potential of currently used drugs to directly or indirectly reverse the epigenetic mechanisms involved in diabetic vascular complications. Given the exponential increase in epigenetics research in recent years, epigenetic therapy for the treatment of diabetic complications could become a reality in the near future.
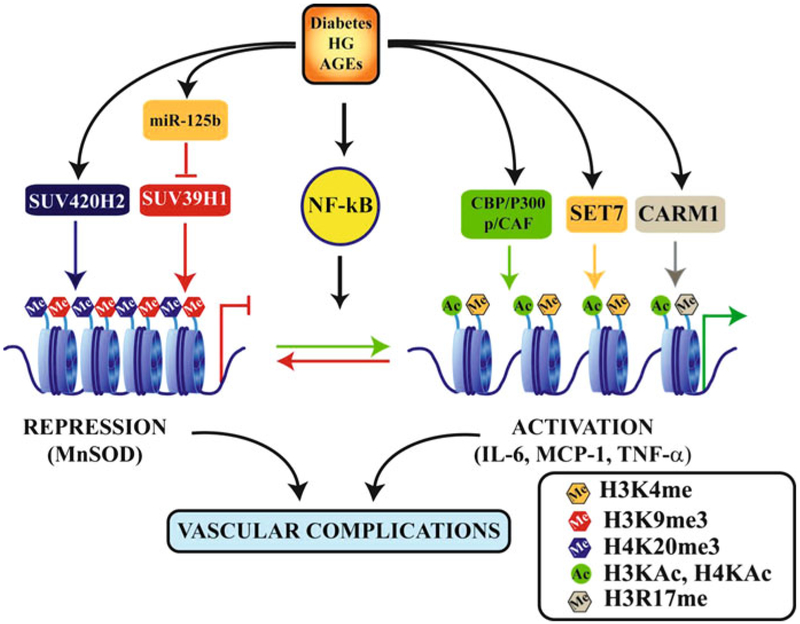
Fig. 19.2.
Epigenetic mechanisms of gene regulation and metabolic memory associated with vascular complications of diabetes. Diabetes and diabetogenic agents such as high glucose (HG) and advanced glycation end products (AGEs) activate transcription factors such as NF-κB which regulate gene expression. This is further regulated by epigenetic mechanisms in chromatin activated under these conditions. These include increases in the levels of active marks such as histone H3 or H4KAc and H3K4me or H3R17me through recruitment of co-activator HATs (CBP/p300 and p/CAF) and HMTs (SET7 and CARM1) respectively. Other as yet unidentified HATs, HMTs and HDMs may also be involved. Diabetes can also lead to loss or reduction in repressive marks like H3K9me3 through reduced recruitment of the HMT SUV39H1 as well as inhibition of its expression by miR-125b, which is increased in diabetes. This leads to increased expression of inflammatory genes. In addition, diabetes can also inhibit the expression of protective anti-oxidant genes such as manganese superoxide dismutase (MnSOD) by increasing promoter levels of the repressive mark H4K20me3 and its HMT, SUV420H2. Alterations in gene expression and histone PTMs at their promoters, such as reduced H3K9me3 or increased H3K4me1 and H4K20me3, can persist even after glucose normalization demonstrating the key role of such epigenetic mechanisms in metabolic memory which leads to sustained long term diabetic complications. Ac-lysine acetylation; Me: lysine or arginine methylation
Acknowledgements
The authors gratefully acknowledge grant support from the National Institutes of Health (NIDDK and NHLBI), the Juvenile Diabetes Research Foundation, and the American Diabetes Association.
References
- Aung HT, Schroder K, Himes SR, Brion K, van Zuylen W, Trieu A, Suzuki H, Hayashizaki Y, Hume DA, Sweet MJ, Ravasi T (2006) LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J 20:1315–1327 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21:381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantignies F, Cavalli G (2006) Cellular memory and dynamic regulation of polycomb group proteins. Curr Opin Cell Biol 18:275–283 [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071 [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P (2002) Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287:2570–2581 [DOI] [PubMed] [Google Scholar]
- Bell CG, Teschendorff AE, Rakyan VK, Maxwell AP, Beck S, Savage DA (2010) Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med Genomics 3:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A (2009) An operational definition of epigenetics. Genes Dev 23:781–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES (2007) The mammalian epigenome. Cell 128:669–681 [DOI] [PubMed] [Google Scholar]
- Bhaumik SR, Smith E, Shilatifard A (2007) Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 14:1008–1016 [DOI] [PubMed] [Google Scholar]
- Bird A (2007) Perceptions of epigenetics. Nature 447:396–398 [DOI] [PubMed] [Google Scholar]
- Blum CA, Ellis JL, Loh C, Ng PY, Perni RB, Stein RL (2011) SIRT1 modulation as a novel approach to the treatment of diseases of aging. J Med Chem 54:417–432 [DOI] [PubMed] [Google Scholar]
- Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A (2009) Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625 [DOI] [PubMed] [Google Scholar]
- Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57:1349–1354 [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, Francis J, Ziesmann SM, Garmey JC, Mirmira RG (2003) Covalent histone modifications underlie the developmental regulation of insulin gene transcription in pancreatic beta cells. J Biol Chem 278:23617–23623 [DOI] [PubMed] [Google Scholar]
- Chan PS, Kanwar M, Kowluru RA (2010) Resistance of retinal inflammatory mediators to suppress after reinstitution of good glycemic control: novel mechanism for metabolic memory. J Diabetes Complications 24:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Taubman MB (2004) Chemokines in the pathogenesis of vascular disease. Circ Res 95:858–866 [DOI] [PubMed] [Google Scholar]
- Chava KR, Karpurapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, Ichiki T, Glasgow WC, Rao GN (2009) CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol 29:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK (2009) Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 23:975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S (2009) Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 25:964–972 [DOI] [PubMed] [Google Scholar]
- Clarke DL, Sutcliffe A, Deacon K, Bradbury D, Corbett L, Knox AJ (2008) PKCbetaII augments NF-kappaB-dependent transcription at the CCL11 promoter via p300/CBP-associated factor recruitment and histone H4 acetylation. J Immunol 181:3503–3514 [DOI] [PubMed] [Google Scholar]
- Colagiuri S, Cull CA, Holman RR (2002) Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: U.K. prospective diabetes study 61. Diabetes Care 25:1410–1417 [DOI] [PubMed] [Google Scholar]
- De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA (2000) The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol 20:E83–E88 [DOI] [PubMed] [Google Scholar]
- De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G (2007) The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130:1083–1094 [DOI] [PubMed] [Google Scholar]
- De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, Testa G, Sung WK, Wei CL, Natoli G (2009) Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J 28:3341–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering TG, Ogihara T, Trace AP, Maier B, Mirmira RG (2009) Methyltransferase Set7/9 maintains transcription and euchromatin structure at islet-enriched genes. Diabetes 58:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I (2006) Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55:774–779 [DOI] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Jialal I (2010) Diabetes is a proinflammatory state: a translational perspective. Expert Rev Endocrinol Metab 5:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Tschen SI, Bhushan A (2009) Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev 23:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dje N’Guessan P, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B, Slevogt H, Weichert W, Hocke AC, Schmeck B, Suttorp N, Hippenstiel S (2009) Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol 29:380–386 [DOI] [PubMed] [Google Scholar]
- Dong C, Yoon W, Goldschmidt-Clermont PJ (2002) DNA methylation and atherosclerosis. J Nutr 132:2406S–2409S [DOI] [PubMed] [Google Scholar]
- Dovey OM, Foster CT, Cowley SM (2010) Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc Natl Acad Sci U S A 107:8242–8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein LC, Pan A, Collins T (2005) Chromatin modification and the endothelial-specific activation of the E-selectin gene. J Biol Chem 280:11192–11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman RL, Kern TS (1987) Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 36:808–812 [DOI] [PubMed] [Google Scholar]
- Fish JE, Matouk CC, Rachlis A, Lin S, Tai SC, D’Abreo C, Marsden PA (2005) The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem 280:24824–24838 [DOI] [PubMed] [Google Scholar]
- Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD (2009) Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell 137:110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Witztum JL (2001) Atherosclerosis: the road ahead. Cell 104:503–516 [DOI] [PubMed] [Google Scholar]
- Guha M, Bai W, Nadler JL, Natarajan R (2000) Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem 275:17728–17739 [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halili MA, Andrews MR, Labzin LI, Schroder K, Matthias G, Cao C, Lovelace E, Reid RC, Le GT, Hume DA, Irvine KM, Matthias P, Fairlie DP, Sweet MJ (2010) Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. J Leukoc Biol 87:1103–1114 [DOI] [PubMed] [Google Scholar]
- Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC (2003) Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem 278:25369–25375 [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Hon GC, Ren B (2010) Next-generation genomics: an integrative approach. Nat Rev Genet 11:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, King GL (2004) Microvascular complications of diabetes. Endocrinol Metab Clin North Am 33:215–238 [DOI] [PubMed] [Google Scholar]
- Hon GC, Hawkins RD, Ren B (2009) Predictive chromatin signatures in the mammalian genome. Hum Mol Genet 18:R195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihnat MA, Thorpe JE, Ceriello A (2007a) Hypothesis: the ‘metabolic memory’, the new challenge of diabetes. Diabet Med 24:582–586 [DOI] [PubMed] [Google Scholar]
- Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, Lacza Z, Cselenyak A, Ross K, Shakir S, Piconi L, Kaltreider RC, Ceriello A (2007b) Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia 50:1523–1531 [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1080 [DOI] [PubMed] [Google Scholar]
- Jin F, Li Y, Ren B, Natarajan R (2011) PU.1 and C/EBP(alpha) synergistically program distinct response to NF-kappaB activation through establishing monocyte specific enhancers. Proc Natl Acad Sci U S A 108:5290–5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TK, De Carvalho DD, Jones PA (2010) Epigenetic modifications as therapeutic targets. Nat Biotechnol 28:1069–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GL (2008) The role of inflammatory cytokines in diabetes and its complications. J Periodontol 79:1527–1534 [DOI] [PubMed] [Google Scholar]
- Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- Kowluru RA (2003) Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes 52:818–823 [DOI] [PubMed] [Google Scholar]
- Li SL, Reddy MA, Cai Q, Meng L, Yuan H, Lanting L, Natarajan R (2006) Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes 55:2611–2619 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128:707–719 [DOI] [PubMed] [Google Scholar]
- Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R (2008) Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem 283:26771–26781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105:1135–1143 [DOI] [PubMed] [Google Scholar]
- Ling C, Groop L (2009) Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 58:2718–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Y, Tollefsbol TO (2008) Gene-environment interactions and epigenetic basis of human diseases. Curr Issues Mol Biol 10:25–36 [PMC free article] [PubMed] [Google Scholar]
- Margariti A, Zampetaki A, Xiao Q, Zhou B, Karamariti E, Martin D, Yin X, Mayr M, Li H, Zhang Z, De Falco E, Hu Y, Cockerill G, Xu Q, Zeng L (2010) Histone deacetylase 7 controls endothelial cell growth through modulation of beta-catenin. Circ Res 106:1202–1211 [DOI] [PubMed] [Google Scholar]
- Marrero MB, Fulton D, Stepp D, Stern DM (2005) Angiotensin II-induced signaling pathways in diabetes. Curr Diabetes Rev 1:197–202 [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Chepelev I, Zhao K (2010) Epigenome mapping in normal and disease States. Circ Res 107:327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Park J, Cai Q, Lanting L, Reddy MA, Natarajan R (2010) Diabetic conditions promote binding of monocytes to vascular smooth muscle cells and their subsequent differentiation. Am J Physiol Heart Circ Physiol 298:H736–H745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker ML (2010) Sequencing technologies – the next generation. Nat Rev Genet 11:31–46 [DOI] [PubMed] [Google Scholar]
- Miao F, Gonzalo IG, Lanting L, Natarajan R (2004) In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem 279:18091–18097 [DOI] [PubMed] [Google Scholar]
- Miao F, Li S, Chavez V, Lanting L, Natarajan R (2006) Coactivator-associated arginine methyltransferase-1 enhances nuclear factor-kappaB-mediated gene transcription through methylation of histone H3 at arginine 17. Mol Endocrinol 20:1562–1573 [DOI] [PubMed] [Google Scholar]
- Miao F, Wu X, Zhang L, Yuan YC, Riggs AD, Natarajan R (2007) Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J Biol Chem 282:13854–13863 [DOI] [PubMed] [Google Scholar]
- Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R (2008) Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes 57:3189–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Q, Bai YT, Jia G, Wu J, Xiang JZ (2010) High glucose enhances angiotensin-II-mediated peroxisome proliferation-activated receptor-gamma inactivation in human coronary artery endothelial cells. Exp Mol Pathol 88:133–137 [DOI] [PubMed] [Google Scholar]
- Miranda TB, Jones PA (2007) DNA methylation: the nuts and bolts of repression. J Cell Physiol 213:384–390 [DOI] [PubMed] [Google Scholar]
- Morgan HD, Sutherland HG, Martin DI, Whitelaw E (1999) Epigenetic inheritance at the agouti locus in the mouse. Nat Genet 23:314–318 [DOI] [PubMed] [Google Scholar]
- Murr R (2010) Interplay between different epigenetic modifications and mechanisms. Adv Genet 70:101–141 [DOI] [PubMed] [Google Scholar]
- Musri MM, Carmona MC, Hanzu FA, Kaliman P, Gomis R, Parrizas M (2010) Histone demethylase LSD1 regulates adipogenesis. J Biol Chem 285:30034–30041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade K, Pan J, Yoshiki A, Ugai H, Kimura M, Liu B, Li H, Obata Y, Iwama M, Itohara S, Murata T, Yokoyama KK (2007) JDP2 suppresses adipocyte differentiation by regulating histone acetylation. Cell Death Differ 14:1398–1405 [DOI] [PubMed] [Google Scholar]
- Natarajan R (2011) Drugs targeting epigenetic histone acetylation in vascular smooth muscle cells for restenosis and atherosclerosis. Arterioscler Thromb Vasc Biol 31:725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Nadler JL (2004) Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol 24:1542–1548 [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirola L, Balcerczyk A, Okabe J, El-Osta A (2010) Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol 6:665–675 [DOI] [PubMed] [Google Scholar]
- Pons D, de Vries FR, van den Elsen PJ, Heijmans BT, Quax PH, Jukema JW (2009) Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur Heart J 30:266–277 [DOI] [PubMed] [Google Scholar]
- Portela A, Esteller M (2010) Epigenetic modifications and human disease. Nat Biotechnol 28:1057–1068 [DOI] [PubMed] [Google Scholar]
- Ramos YF, Hestand MS, Verlaan M, Krabbendam E, Ariyurek Y, van Galen M, van Dam H, van Ommen GJ, den Dunnen JT, Zantema A, t Hoen PA (2010) Genome-wide assessment of differential roles for p300 and CBP in transcription regulation. Nucleic Acids Res 38:5396–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Natarajan R (2011) Epigenetic mechanisms in diabetic vascular complications. Cardiovasc Res 90:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, Natarajan R (2006) Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem 281:13685–13693 [DOI] [PubMed] [Google Scholar]
- Reddy MA, Sahar S, Villeneuve LM, Lanting L, Natarajan R (2009) Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 29:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD (2001) Histone acetyltransferases. Annu Rev Biochem 70:81–120 [DOI] [PubMed] [Google Scholar]
- Roy S, Sala R, Cagliero E, Lorenzi M (1990) Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A 87:404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani S, Natoli G (2002) Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev 16:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Dwarakanath RS, Reddy MA, Lanting L, Todorov I, Natarajan R (2005) Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: a novel cross-talk in the pathogenesis of atherosclerosis. Circ Res 96:1064–1071 [DOI] [PubMed] [Google Scholar]
- Sahar S, Reddy MA, Wong C, Meng L, Wang M, Natarajan R (2007) Cooperation of SRC-1 and p300 with NF-kappaB and CREB in angiotensin II-induced IL-6 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27:1528–1534 [DOI] [PubMed] [Google Scholar]
- Selvi BR, Mohankrishna DV, Ostwal YB, Kundu TK (2010) Small molecule modulators of histone acetylation and methylation: a disease perspective. Biochim Biophys Acta 1799: 810–828 [DOI] [PubMed] [Google Scholar]
- Shanmugam N, Kim YS, Lanting L, Natarajan R (2003a) Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem 278:34834–34844 [DOI] [PubMed] [Google Scholar]
- Shanmugam N, Reddy MA, Guha M, Natarajan R (2003b) High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52:1256–1264 [DOI] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN (1995) Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44:1139–1146 [DOI] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA (2010) Epigenetics in cancer. Carcinogenesis 31:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MJ, King GL (2002) Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288:2579–2588 [DOI] [PubMed] [Google Scholar]
- Shi Y, Whetstine JR (2007) Dynamic regulation of histone lysine methylation by demethylases. Mol Cell 25:1–14 [DOI] [PubMed] [Google Scholar]
- Shilatifard A (2006) Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75:243–269 [DOI] [PubMed] [Google Scholar]
- Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R (2010) Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol 21:2069–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Okada Y, Kallin EM, Zhang Y (2009) Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 458:757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Reinberg D (2006) Histone lysine demethylases and their impact on epigenetics. Cell 125:213–217 [DOI] [PubMed] [Google Scholar]
- Turunen MP, Aavik E, Yla-Herttuala S (2009) Epigenetics and atherosclerosis. Biochim Biophys Acta 1790:886–891 [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G (1999) The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem 274:32091–32098 [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450:440–444 [DOI] [PubMed] [Google Scholar]
- Villeneuve LM, Natarajan R (2010) The role of epigenetics in the pathology of diabetic complications. Am J Physiol Renal Physiol 299:F14–F25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R (2008) Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A 105:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve LM, Kato M, Reddy MA, Wang M, Lanting L, Natarajan R (2010) Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes 59:2904–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, Afzal V, Ren B, Rubin EM, Pennacchio LA (2009) ChIP-seq accurately predicts tissue specific activity of enhancers. Nature 457:854–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Harris DC (2011) Macrophages in renal disease. J Am Soc Nephrol 22:21–27 [DOI] [PubMed] [Google Scholar]
- Wang Z, Schones DE, Zhao K (2009) Characterization of human epigenomes. Curr Opin Genet Dev 19:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Sorescu D, Taylor WR (2001) Angiotensin II and atherosclerosis. Am J Cardiol 87:25C–32C [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL (2006) Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 147:2518–2525 [DOI] [PubMed] [Google Scholar]
- Workman JL, Kingston RE (1998) Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem 67:545–579 [DOI] [PubMed] [Google Scholar]
- Writing Team DCCT/EDIC Research Group (2002) Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Zhang Y (2010) Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Allis CD, Coonrod S (2006) Histone arginine methylation and its dynamic regulation. Front Biosci 11:344–355 [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM (2008) Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab 4:285–293 [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM (2009) The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med 11:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26:5310–5318 [DOI] [PubMed] [Google Scholar]
- Yun JM, Jialal I, Devaraj S (2011) Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J Nutr Biochem 22:450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Kowluru RA (2010) Role of histone acetylation in the development of diabetic retinopathy and the metabolic memory phenomenon. J Cell Biochem 110:1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Kowluru RA (2011) Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes 60:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]