Abstract
Objectives
To test the hypothesis that higher blood levels of neurotrophic proteins (proteins that support neuronal survival and function) in the first 2 weeks of life are associated with a lower risk of cognitive impairment at 10 years.
Study design
We evaluated 812 10-year-old children with neonatal blood specimens enrolled in the multicenter prospective Extremely Low Gestational Age Newborn Study, assessing 22 blood proteins collected on 3 days over the first 2 weeks of life. Using latent profile analysis, we derived a cognitive function level based on standardized cognitive and executive function tests. We defined high exposure as the top quartile neurotrophic protein blood level on ≥2 days either for ≥4 proteins or for a specific cluster of neurotrophic proteins (defined by latent class analysis). Multinomial logistic regression analyzed associations between high exposures and cognitive impairment.
Results
Controlling for the effects of inflammatory proteins, persistently elevated blood levels of ≥4 neurotrophic proteins were associated with reduced risk of moderate (OR, 0.35; 95% CI, 0.18–0.67) and severe cognitive impairment (OR, 0.22; 95% CI, 0.09–0.53). Children with a cluster of elevated proteins including angiopoietin 1, brain-derived neurotrophic factor, and regulated upon activation, normal T-cell expressed, and secreted had a reduced risk of adverse cognitive outcomes (OR range, 0.31–0.6). The risk for moderate to severe cognitive impairment was least with 0–1 inflammatory and >4 neurotrophic proteins.
Conclusions
Persisting elevations of circulating neurotrophic proteins during the first 2 weeks of life are associated with lowered risk of impaired cognition at 10 years of age, controlling for increases in inflammatory proteins.
Advances in neonatal intensive care have increased the survival of extremely preterm children born at <28 weeks of gestation.1 Increased survival rates have not been accompanied by similar improvements in neurodevelopmental outcomes, and one-quarter of survivors have cognitive impairment.2,3 Reduction of the risk of cognitive impairment depends on an improved understanding of its etiology.
The Extremely Low Gestational Age Newborn (ELGAN) Study was designed to test the hypothesis that perinatal inflammation is associated with persisting brain structural and functional disorders. In the ELGAN cohort of about 1000 children born at <28 weeks of gestation, neonatal elevations of specific inflammation-associated protein biomarkers in blood robustly predicted cognitive impairment at 2 years of age.4,5 These indicators of neonatal systemic inflammation also were associated with impaired cognition at 10 years of age.6
In the ELGAN Study, blood samples were taken in the first 2 weeks of life and we measured levels of neurotrophic proteins, including growth factors, neurotrophins, and angiotrophins that might influence developmental outcomes.7,8 These proteins support the growth, survival, and differentiation of developing neurons. Lower levels of these proteins may signal less resilience against inflammation-associated injury and higher levels may prevent damage.9 Inflammation-related proteins are associated with cognitive impairment, and neurotrophic proteins may be associated with better cognitive outcomes, but elevations within these 2 protein families that operate in opposite directions frequently occur simultaneously. We, therefore, tested the hypothesis that higher blood levels of neurotrophic proteins measured in the first 2 weeks of life would be associated with a lesser risk of cognitive impairment at 10 years of age, controlling for the presence of inflammation-associated proteins.
Methods
The ELGAN Study is a multicenter, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants. From 2002 to 2004, women delivering at <28 weeks of gestation were asked for consent to enroll their child into the study. This analysis includes 812 children (a subset of the surviving 1200 study participants) who had ≥2 sets of neonatal blood samples for proteins and were evaluated at 10 years of age (Figure 1; available at www.jpeds.com). This study was approved by the institutional review boards of all participating institutions and informed consent was obtained from all participants.
Figure 1.
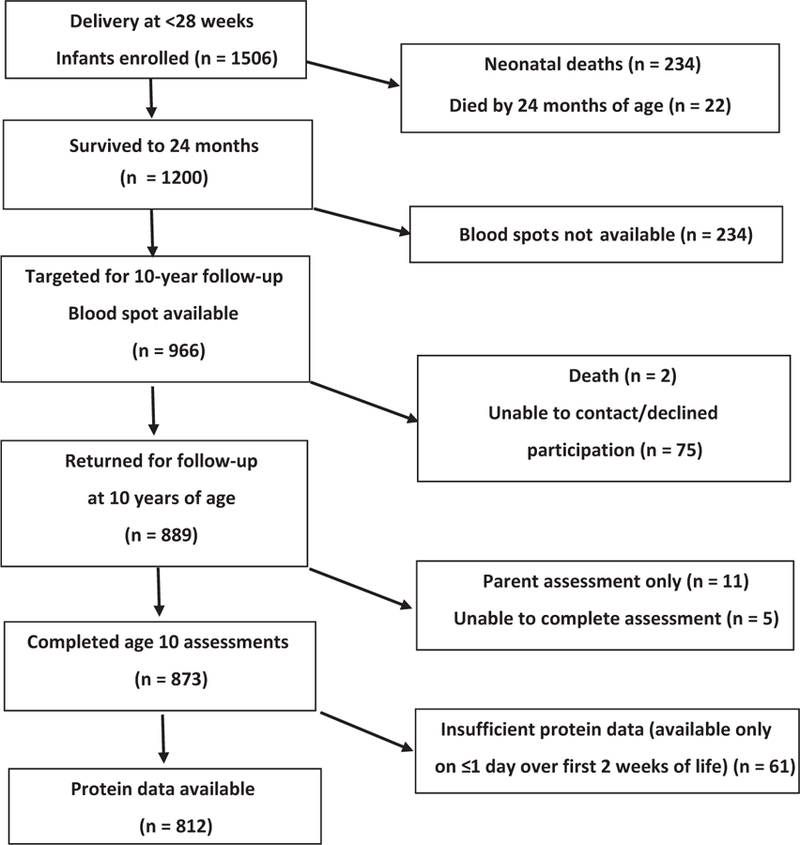
Study enrollment flow chart.
Cognitive Assessment and Derivation of Levels of Function
Child assessments included the full-scale IQ from the School-Age Differential Ability Scales–II Verbal and Nonverbal Reasoning scales10 and executive function (EF) (from the School-Age Differential Ability Scales–II Verbal and Nonverbal Reasoning scales and the Developmental NEeuroPSYchological Assessment, 2nd Edition11).
Even though the development and maturation of neural circuits underlying aspects of IQ and EF differ from each other,12 both can be reliably measured by 10 years of age.13 As previously reported, we evaluated cognitive outcomes using latent class analysis (LCA) classifications, which represented both IQ and EF abilities, to provide a better predictor of adaptive outcomes, such as academic success, than IQ alone.2,14 Children were classified into their most likely latent class for analysis. With LCA, we identified 4 subgroups of children in our cohort corresponding with overall cognitive functioning that was normal (34% of cohort; normal mean IQ and EF scores), low-normal (41%; mean IQ and EF scores ranging from 0.5 to 1.5 SDs below norm), moderately impaired (17%; mean IQ and EF measures from 1.5 to 2.5 SDs below norm), and severely impaired (8%; mean IQ and EF measures from 2.5 to 4.0 SDs below norm).2
Assessment of Inflammation and Neutrophic Proteins
Blood Protein Measurements.
Drops of whole blood were collected on postnatal days 1 (range, 1–3 days), 7 (range, 5–8 days), and 14 (range, 12–15 days).15,16 Protein concentration quartiles were normalized for gestational age and day of collection.17 Because single day elevations of proteins are not as strongly associated with cognitive outcomes as are persistent elevations,6 we defined protein concentration elevation as being in the highest quartile on ≥2 of 3 measures obtained.
Identification of Clusters of Neurotrophic and Inflammation-Associated Proteins with LCA.
The specific neurotrophic proteins evaluated are listed in Table I (available at www.jpeds.com). Given that the blood levels of neurotrophic proteins under study might correlate with one another, we conducted separate LCA analyses on each postnatal day, fitting models with 2–5 classes and choosing an appropriate model based on fit statistics, entropy, interpretability, and consistency of results across days (Table II; available at www.jpeds.com). Analyses consistently identified 3 subgroups of children with similar patterns of neurotrophin elevations. Based on these analyses, we categorized children into 3 distinct subgroups (Table III; available at www.jpeds.com). The neurotrophic-related protein group (NRG) 1 had ≤2 elevated proteins; NRG2 had elevations of ≥3 proteins including ≥2 of the following 3 neurotrophic proteins: regulated upon activation, normal T-cell expressed, and secreted (RANTES), brain-derived neurotrophic factor (BDNF), and angiopoietin 1 (Ang-1); and NRG3 had low levels on ≥2 of 3 NRG2 proteins, RANTES, BDNF, and Ang-1, but elevated levels of ≥3 of the other neurotrophic proteins (Table III).
Table I.
Inflammation-related and neurotrophic proteins considered in analyses
| Inflammation-related proteins | Proteins with neurotrophic properties |
|---|---|
| Interleukin-1b (IL-1β) | Interleukin-6 receptor (IL-6R) |
| Interleukin-6 (IL-6) | Regulated upon activation, and normal T-cell expressed, and (presumably) secreted (RANTES) |
| Tumor necrosis factor-alpha (TNF-α) | Brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (BFGF) |
| Intercellular adhesion molecule-1 (ICAM-1) | Insulin-like growth factor-1 (IGF-1) |
| Interleukin-8 (IL-8) | Vascular endothelial growth factor (VEGF) |
| Matrix metallopeptidase 9 (MMP-9) | Vascular endothelial growth factor receptor-1 (VEGF-R1) |
| C-reactive protein (CRP) | Vascular endothelial growth factor receptor-2 (VEGF-R2) |
| Serum amyloid A (SAA) | Placental growth factor (PIGF) |
| Angiopoietin 1 (ANG-1) | |
| Angiopoietin 2 (ANG-2) | |
| Thyroid-stimulating hormone (TSH) |
Table II.
Fit indices for LCA on neurotrophic and inflammatory proteins*
| Day of life | No. of classes | AIC | BIC | SSABIC | Lo-Mendell-Rubin P value† | Entropy |
|---|---|---|---|---|---|---|
| Fit indices for LCA on neurotrophic proteins | ||||||
| 1 | 2 | 9491.2 | 9608.6 | 9529.2 | <.001 | 0.85 |
| 3 | 9301.1 | 9479.5 | 9358.8 | <.001 | 0.75 | |
| 4 | 9248.2 | 9487.6 | 9325.6 | .327 | 0.72 | |
| 5 | 9197.2 | 9497.6 | 9294.4 | .073 | 0.73 | |
| 7 | 2 | 9626.6 | 9744.3 | 9664.9 | <.001 | 0.79 |
| 3 | 9443.8 | 9622.7 | 9502.1 | .001 | 0.77 | |
| 4 | 9378.9 | 9619.0 | 9457.1 | .135 | 0.78 | |
| 5 | 9312.4 | 9613.7 | 9410.5 | .089 | 0.77 | |
| 14 | 2 | 8947.0 | 9062.2 | 8982.8 | <.001 | 0.83 |
| 3 | 8780.0 | 8955.2 | 8834.6 | <.001 | 0.71 | |
| 4 | 8679.8 | 8914.9 | 8753.0 | .005 | 0.78 | |
| 5 | 8589.6 | 8884.7 | 8681.5 | .142 | 0.78 | |
| Fit indices for LCA on inflammatory proteins | ||||||
| 1 | 2 | 6217.5 | 6297.2 | 6243.3 | <.001 | 0.83 |
| 3 | 6034.2 | 6156.2 | 6073.7 | <.001 | 0.86 | |
| 4 | 5896.0 | 6060.3 | 5949.2 | <.001 | 0.85 | |
| 5 | 5878.5 | 6085.0 | 5945.3 | .074 | 0.86 | |
| 7 | 2 | 6596.8 | 6676.9 | 6622.9 | <.001 | 0.77 |
| 3 | 6379.6 | 6502.0 | 6419.4 | <.001 | 0.82 | |
| 4 | 6269.6 | 6434.4 | 6323.2 | <.001 | 0.84 | |
| 5 | 6252.0 | 6459.2 | 6319.5 | .013 | 0.85 | |
| 14 | 2 | 5810.8 | 5889.2 | 5835.2 | <.001 | 0.82 |
| 3 | 5680.0 | 5799.9 | 5717.3 | <.001 | 0.88 | |
| 4 | 5575.9 | 5737.3 | 5626.2 | <.001 | 0.84 | |
| 5 | 5572.3 | 5775.1 | 5635.4 | .152 | 0.84 | |
AIC, Akaike information criteria; BIC, Bayesian information criteria; SSABIC, sample size adjusted Bayesian information criteria.
Separate analyses were conducted for proteins measured at days 1, 7, and 14.
Lo-Mendell-Rubin adjusted likelihood ratio test of k vs k-1 classes.
Table III.
Inflammatory and neurotrophic proteins through LCA*
| IRG 1 Low elevation (n = 634) | IRG 2† ≥3 Elevated proteins including CRP or SAA (n = 130) | IRG 3‡ ≥3 Elevated proteins Not CRP or SAA (n = 48) | |
|---|---|---|---|
| IRG | |||
| CRP | 6.8 | 86.9 | .0 |
| SAA | 6.9 | 68.5 | .0 |
| IL-1β | 7.1 | 43.8 | 62.5 |
| IL-6 | 6.1 | 51.5 | 52.1 |
| TNF-alpha | 7.4 | 54.6 | 75.0 |
| IL-8 | 4.3 | 58.5 | 68.7 |
| ICAM-1 | 8.4 | 62.3 | 39.6 |
| MMP-9 | 8.5 | 33.1 | 56.2 |
| NRG 1 Low Elevation (n = 552) | NRG 2§ 2 of RANTES, BDNF, Ang-1 Elevated (n = 133) | NRG 3¶ 3 + Other Proteins Elevated (n = 127) | |
| NRG | |||
| RANTES | 5.8 | 77.4 | 12.6 |
| BDNF | 5.8 | 85.7 | 5.7 |
| Ang-1 | 2.1 | 87.2 | 12.3 |
| IL-6R | 7.2 | 43.6 | 51.2 |
| BFGF | 6.2 | 28.6 | 48.4 |
| IGF-1 | 13.6 | 17.3 | 41.0 |
| VEGF | 10.1 | 42.9 | 40.9 |
| VEGF-R1 | 10.2 | 30.1 | 40.9 |
| VEGF-R2 | 7.4 | 51.1 | 40.9 |
| PIGF | 8.2 | 19.5 | 41.0 |
| Ang-2 | 5.6 | 42.9 | 36.9 |
| TSH | 14.1 | 26.3 | 40.9 |
BFGF, Basic fibroblast growth factor; ICAM-1, intercellular adhesion molecule-1; IGF, insulin-like growth factor; IL, interleukin; MMP, matrix metallopeptidase; PIGF, placental growth factor; TNF, tumor necrosis factor; TSH, thyroid-stimulating hormone; VEGF, vascular endothelial growth factor.
The columns represent the percentage for each protein listed on the left that have sustained highest quartile value in each of the 3 inflammatory or neurotrophic risk groups of children.
IRG2 has a preponderance of high values for CRP and SAA and lesser elevations of the other proteins.
IRG3 has a reduced likelihood of having high values for CRP or SAA while having elevated values of the other inflammatory proteins.
NRG2 has a preponderance of high values for RANTES, BDNF, and Ang-1 and lesser elevations of the other proteins.
NRG3 has reduced likelihood of having high values for RANTES, BDNF, and Ang-1, while having elevated values of the other neurotrophic proteins.
The analyses also included values for 8 inflammation-related proteins obtained at the same 3 time points as the neurotrophic proteins. These inflammatory-related proteins have been associated with structural and functional neurological outcomes in previous ELGAN Study analyses Table I.4,18,19 As with the neurotrophic proteins, we conducted LCA on postnatal days 1, 7, and 14 and found that a 3-class solution was most consistent across all 3 days (Table II). Based on these analyses, we identified 3 distinct subgroups of children: inflammatory group (IRG) 2 had ≥3 elevated proteins that included elevation of either C-reactive protein (CRP) or serum amyloid A (SAA). IRG3 had normal CRP and SAA but had elevations of ≥3 other inflammatory proteins (Table III).
We a priori operationally defined neurotrophic protein exposure in 2 ways. First, we considered the number of sustained elevated inflammatory and sustained elevated neurotrophic proteins as measures of the breadth of inflammatory or neurotrophic exposure (0–1 proteins [referent group], 2–3 proteins, >4 proteins). Second, we considered the at-risk subgroups of children based on a pattern of elevated proteins derived from LCA.
Statistical Analyses
We tested the hypothesis that elevation of neurotrophic proteins in the first 2 weeks of life is associated with a decreased risk of cognitive impairment. Because high concentrations of inflammation-related proteins can prompt high concentrations of putative neurotrophic proteins,20–29 in our analyses we controlled for elevations of inflammatory proteins when evaluating risks associated with neurotrophic proteins.
Maternal and infant characteristics of the study sample were compared across neurotrophin risk groups with a χ2 test; characteristics associated with risk groups at P < .05 were considered potential confounders. Multivariable multinomial logistic regression models were used to examine adjusted associations of elevated inflammatory and neurotrophic proteins with cognitive impairment at 10 years of age. All analyses controlled child sex and maternal education.3,30 Secondary analyses also controlled for additional potential confounding by variables found to be associated with either neurotrophic or inflammatory protein exposure in preliminary analyses. Adjusted associations based on these logistic regression models were described with ORs and 95% CIs. In secondary analyses, variables found to be associated with either neurotrophic or inflammatory protein exposure were examined for confounding and controlled for if the adjusted ORs for neurotrophic or inflammatory proteins changed by ≥10%. Secondary analyses also examined the association between number of neurotrophic and inflammatory proteins and IQ alone, categorized as <70, 70–85, and >85.
Results
Demographic Characteristics and Protein Elevations
Maternal and child characteristics, including maternal body mass index and maternal smoking status, did not significantly differ across neurotrophic protein risk groups of children (P > .10), with the exception of birth weight z-score (P < .05) (Table IV). Children with a greater number of elevated neurotrophic proteins were more likely to have elevated inflammatory proteins (P < .001). For example, 32% of children with >4 elevated neurotrophic proteins had >4 elevated inflammatory proteins, but only 4% of children with 0–1 elevated neurotrophic proteins had >4 elevated inflammatory proteins. Elevated inflammatory proteins were associated with maternal race, marital status, smoking during pregnancy, and birth weight z-score category (data not shown). Among these risk factors, only maternal race was found to confound the association between elevated proteins and cognitive impairment in multivariable analyses described elsewhere in this article.
Table IV.
Description of the sample of children with measured proteins (n = 812)
| Overall n | Neurotrophic risk group* (proteins included in top quartile) |
No. of elevated neurotrophic proteins† |
|||||
|---|---|---|---|---|---|---|---|
| 1: None (n = 552) | 2: RANTES, BDNF, ANG-1 (n = 133) | 3: >3—not RANTES, BDNF, ANG-1 (n = 127) | 0–1 (n = 395) | 2–3 (n = 232) | ≥4 (n = 185) | ||
| Maternal characteristics | |||||||
| Racial identity | |||||||
| White | 505 | 62 | 68 | 62 | 63 | 59 | 68 |
| Black | 208 | 27 | 19 | 27 | 26 | 31 | 20 |
| Other | 90 | 11 | 13 | 10 | 11 | 10 | 12 |
| Hispanic | |||||||
| Yes | 80 | 10 | 14 | 7 | 9 | 9 | 11 |
| No | 731 | 90 | 86 | 93 | 91 | 91 | 89 |
| Body mass index | |||||||
| Under | 64 | 8 | 7 | 11 | 9 | 6 | 10 |
| Normal | 390 | 49 | 52 | 50 | 49 | 50 | 51 |
| Over | 152 | 20 | 22 | 13 | 22 | 16 | 19 |
| Obese | 179 | 23 | 20 | 26 | 21 | 29 | 20 |
| Education, years | |||||||
| ≤12 | 322 | 40 | 43 | 40 | 41 | 38 | 45 |
| >12, <16 | 185 | 24 | 22 | 24 | 22 | 27 | 22 |
| ≥16 | 283 | 36 | 35 | 36 | 38 | 35 | 33 |
| Marital status, single | |||||||
| Yes | 329 | 40 | 37 | 47 | 38 | 43 | 42 |
| No | 483 | 60 | 63 | 53 | 62 | 57 | 58 |
| Public insurance | |||||||
| Yes | 286 | 36 | 31 | 37 | 37 | 33 | 34 |
| No | 526 | 64 | 69 | 63 | 63 | 67 | 66 |
| Cigarette exposure in pregnancy | |||||||
| None | 567 | 73 | 68 | 68 | 74 | 70 | 67 |
| Passive | 120 | 14 | 16 | 20 | 14 | 15 | 18 |
| Smoker | 108 | 13 | 16 | 12 | 12 | 15 | 14 |
| Newborn characteristics | |||||||
| Sex | |||||||
| Male | 413 | 51 | 47 | 55 | 51 | 53 | 48 |
| Female | 399 | 49 | 53 | 45 | 49 | 47 | 52 |
| Gestational age, weeks | |||||||
| 23–24 | 173 | 21 | 20 | 24 | 21 | 24 | 20 |
| 25–26 | 366 | 44 | 46 | 48 | 45 | 44 | 46 |
| 27 | 273 | 35 | 35 | 28 | 35 | 32 | 34 |
| Birth weight z-score | |||||||
| <−2 | 49 | 7 | 2 | 8 | 6 | 6 | 6 |
| ≥−2, <−1 | 106 | 12 | 11 | 19 | 11 | 16 | 14 |
| ≥−1 | 657 | 81 | 87 | 73 | 82 | 79 | 81 |
| Inflammatory proteins | |||||||
| IRG1 | 634 | 87 | 65 | 51 | |||
| IRG2 | 130 | 11 | 23 | 32 | |||
| IRG3 | 48 | 2 | 12 | 17 | |||
| Inflammatory proteins | |||||||
| 0–1 | 540 | 85 | 59 | 38 | |||
| 2–3 | 164 | 11 | 27 | 30 | |||
| ≥4 | 108 | 4 | 14 | 32 | |||
No significant differences in characteristics by neurotrophic risk group except for birth weight z-score (P = .043) and inflammatory proteins (P < .001).
No significant differences in characteristics by number of neurotrophic proteins except for inflammatory proteins (P < .001).
Patterns of Inflammatory and Neurotrophic Protein Elevations
In each of 3 strata defined in terms of the number of elevations of inflammatory proteins (columns), the risk of cognitive impairment decreased as the number of elevations of neurotrophic proteins increased (Figure 2). For example, for children with >4 elevated inflammatory proteins, the percent with cognitive impairment decreased from 56.3% to 22.0% as the number of neurotrophic proteins increased from 0 to 1 to >4 (OR, 0.22; 95% CI, 0.07–0.70). Similarly, for children with 0–1 elevated inflammatory proteins, the percent with cognitive impairment decreased from 22.2% to 10.0% (OR, 0.39; 95% CI, 0.17–0.89). In the 3 strata defined by the number of elevations of neurotrophic proteins, the risk of cognitive impairment increased with elevated number of inflammatory proteins. Multiple logistic regression analysis of these data (Table IV) show significant effects of both neurotrophic (P = .004) and inflammatory proteins (P < .001), with no significant multiplicative interaction (P = .670). The risk for moderate or severe cognitive impairment was greatest in the presence of >4 inflammatory and 0–1 neurotrophic proteins (56%) and least when there were 0–1 inflammatory and >4 neurotrophic proteins (10%).
Figure 2.

Percent of children with moderate or severe cognitive impairment as a function of the number of elevated neurotrophic and inflammatory proteins.
Associations between Protein Elevations and Cognitive Impairment
Adjusting for the number of elevations of inflammatory proteins, elevation of >4 neurotrophic proteins was associated with a reduced risk of moderate and severe cognitive impairment (Table V). Elevation of 2–3 neurotrophic proteins also was associated with decreased risk of cognitive impairment in the severely impaired and low normal groups of children. Secondary analyses examining confounding by other maternal and infant characteristics identified race as a confounder; associations of >4 elevated inflammatory and neurotrophic proteins with severe and moderate cognitive impairment were somewhat attenuated, but remained significant after additionally controlling for race. Secondary analyses using IQ alone as the outcome showed similar associations with elevated proteins. For IQ categories, both 2–3 and >4 elevated inflammatory proteins significantly increased the odds of an IQ of <70, but not an IQ of 70–85, whereas both 2–3 and >4 elevated neurotrophic proteins were associated with lower odds of an IQ of <70, but not an IQ of 70–85 (data not shown).
Table V.
Impairment on LCA measure*
| LCA-based cognitive impairment level (n = 812) |
||||
|---|---|---|---|---|
| Severe n = 64 | Moderate n = 135 | Low Normal n = 333 | Normal n = 280 | |
| Elevated inflammatory proteins | ||||
| 0–1 | Ref | Ref | Ref | Ref |
| 2–3 | 1.67 (0.75–3.73) | 3.47 (1.98–6.09) | 1.34 (0.84–2.14) | Ref |
| ≥4 | 5.68 (2.30–14.00) | 5.89 (2.83–12.24) | 2.72 (1.49–4.99) | Ref |
| Elevated neurotrophic proteins | ||||
| 0–1 | Ref | Ref | Ref | Ref |
| 2–3 | 0.48 (0.24–0.97) | 0.70 (0.41–1.19) | 0.66 (0.44–0.99) | Ref |
| ≥4 | 0.22 (0.09–0.53) | 0.35 (0.18–0.67) | 0.63 (0.39–1.00) | Ref |
Values are adjusted OR (95% CI).
Bold values represent ORs for which the 95% confidence limits do not include 1; ie, significantly different from 1 at P < .05.
At 10 years of age for those with elevated inflammatory and neurotrophic proteins on 2 of the first 3 postnatal measurements (controlling for infant sex, maternal education, and the other set of proteins).
The latent class category resulting from the analysis of neurotrophic proteins that was characterized by elevated proteins, including 2 of 3 proteins (RANTES, BDNF, or Ang-1), was associated with a lesser risk of cognitive impairment for the severely impaired, moderately impaired, and low-normal categories (Table VI). The latent class with elevated levels of other neurotrophic proteins, but not RANTES, BDNF, and Ang-1, was not associated with cognitive outcome (Table VI). In LCA-defined inflammation subgroups of children, IRG2 (pre-dominantly SAA and CRP elevations) was significantly associated with all 3 adverse cognitive outcome groups relative to the reference group, but IRG3 (≥3 proteins other than SAA and CRP) was significantly associated with increased risk in the moderately impaired children only.
Table VI.
Cognitive impairment*
| LCA-based cognitive impairment level (n = 812) |
||||
|---|---|---|---|---|
| Severe n = 64 | Moderate n = 135 | Low Normal n = 333 | Normal n = 280 | |
| Elevated inflammatory proteins | ||||
| IRG1 | Ref | Ref | Ref | Ref |
| IRG2 | 3.79 (1.72–8.36) | 3.93 (2.08–7.41) | 2.54 (1.48–4.34) | Ref |
| IRG3 | 2.18 (0.61–7.78) | 3.18 (1.29–7.83) | 1.53 (0.69–3.38) | Ref |
| Elevated neurotrophic proteins | ||||
| NRG1 | Ref | Ref | Ref | Ref |
| NRG2 | 0.31 (0.12–0.79) | 0.46 (0.24–0.88) | 0.62 (0.39–0.98) | Ref |
| NRG3 | 0.85 (0.37–1.95) | 1.03 (0.55–1.95) | 1.00 (0.60–1.67) | Ref |
IRG2 defined by elevation of CRP or SAA with other proteins having little or modest elevations.
IRG3 defined by ≥3 other protein elevations without CRP and SAA elevations.
NRG2 defined by elevation of 2 of RANTES, BDNF, or ANG-1 with other proteins having little or modest elevations.
NRG3 defined by ≥3 other proteins elevated protein elevations without CRP and SAA elevations.
Values are adjusted OR (95% CI).
Bold values represent ORs for which the 95% confidence limits do not include 1; ie, significantly different from 1 at P < .05.
At 10 years of age for those with sustained elevated inflammatory and neurotrophic proteins (controlling for infant sex and maternal education).
There were no significant interactions (P > .65) between neurotrophic and inflammatory proteins related to cognitive outcomes. Adjustments for birth weight z-score, the only demographic or birth characteristic associated with the neurotrophic protein LCA groups of children, did not substantively change the association between neurotrophic protein elevations and cognitive outcome.
Discussion
Whereas the sustained presence of inflammation-related proteins in the blood during the first 2 weeks after birth was associated with adverse cognitive outcomes at 10 years of age, increased levels of circulating neurotrophic proteins were associated with a lesser risk of cognitive impairment. The lower risk for cognitive impairment associated with elevation of neurotrophic proteins was evident whether indexed by the number of elevated proteins or by a cluster of neurotrophic proteins derived from LCA that included RANTES, BDNF, and Ang-1. With both approaches, the association was stronger with severe compared with moderate cognitive impairment.
We examined the association between elevated neurotrophic proteins in the first weeks of life with cognitive abilities, adjusting for inflammation. This approach was undertaken based on the notion that inflammation-related and neurotrophic proteins correlate with each other but seem to influence the risk of cognitive impairment in opposite directions. That inflammation-related and neurotrophic proteins are both elevated more often than expected by chance, suggests ≥2 possible underlying biological models. In 1 model, inflammatory and neurotrophic protein elevations have a common initiator, prompting an inflammatory cascade as well as enhancing production of neurotrophic proteins. A number of in vitro neonatal models suggest that BDNF and other neurotrophins are decreased rather than increased when exposed to a lipopolysaccharide stimulus, suggesting that this mechanism is less likely.24 A second, more likely model invokes neurotrophic protein elevations as a consequence of inflammation, possibly as a nonspecific upregulation of many proteins or an attempt of the body to”downregulate” inflammation.
As part of our evaluation of 12 proteins thought to have neurotrophic properties, we sought to understand whether these neurotrophic proteins fluctuated independently or in concert. LCA identified 3 patterns of elevated neurotrophic protein values. One group of children, NRG2, with elevations of RANTES, BDNF, and Ang-1, were least at risk for adverse cognitive outcomes. Support for a neuroprotective role for RANTES, BDNF, and Ang-1 includes the association of RANTES with a decreased risk of attention deficit hyperactivity disorder in our cohort.31 Also, in preterm infants, higher BDNF values are associated with lower odds of failing developmental milestones32 and developing retinopathy of prematurity.33 In rodent brain ischemia and head trauma models, BDNF also is associated with neuroprotective effects.34,35 Ang-1 in experimental models seems to ameliorate the impact of cerebral ischemia and stroke in rats.36,37
We found no interaction between elevated neurotrophic and inflammatory proteins with respect to cognitive risk. This finding implies that elevated neurotrophic proteins are protective against cognitive impairment regardless of the level of inflammatory proteins, rather than only providing protection in the presence of elevated inflammatory proteins. However, statistical tests for interaction have lower power, and this null result should be interpreted cautiously.
We conceptualized our outcome as cognitive impairment and chose to summarize IQ and EF variables using LCA, which identified subgroups of children by degree of cognitive impairment. Other approaches to identifying impairment categories, such as categorizing impairment based on IQ and EF z-scores or a factor analysis approach, would also be valid. Similarly, because severity and breadth of inflammatory exposure seems to be a critical predictor of outcome,6 we conceptualized inflammatory and neurotrophic protein exposure as representing exposure categories and used LCA to define the exposure categories.
Brain development in the extremely preterm infant involves dynamic and critical processes that are distinguishable from those occurring in the more mature brain, and many of these developmental processes seem to be adversely affected in several ways. First, to a greater extent than in the term-born infant’s brain, the immature brain demonstrates vigorous dendritic and axonal growth (particularly growth cone proliferation), as well as myelinogenesis and angiogenesis.38 Second, developing preoligodendrocytes and subplate neurons at 24–32 weeks of gestation are extremely vulnerable to physiological perturbations.39 Third, most programmed cell death (apoptosis) in neuronal populations occurs prenatally, whereas cell death in glia populations as well as production and pruning of connections are largely postnatal events.40 Fourth, to a greater extent than infants born at term, preterm infants encounter a host of potentially prenatal, perinatal, and postnatal harmful exposures, including inflammatory processes that frequently precipitate early delivery or occur in the context of postnatal illnesses, such as chronic lung disease, necrotizing enterocolitis, and sepsis.38,41 Fifth, preterm infants may have reduced capacities to synthesize proteins that promote cell growth or survival in the amounts needed for normal development,42 particularly when exposed to adverse stresses, such as inflammation and infection, which occur commonly in extremely preterm infants.9,43
Our finding that the presence of proteins with neurotrophic properties reduces risks for adverse cognitive impairment support the notion that such proteins can stimulate oligodendrocyte progenitor cell proliferation,44 induce oligodendrocyte progenitors “to undergo continuous self-renewal,”45 minimize oligodendrocyte progenitor and neuronal apoptosis,46,47 and rescue motor neurons from axotomy.48–50
These results suggest that there are complex determinants of adverse outcomes involving an interplay between imposed risks and host resistance and resilience. Both risk and protective factors likely are under the control of genetic and local environmental or epigenetic influences, which may either enhance or dampen inflammatory or neurotrophic and neuroprotective mechanisms.51 For example, inflammatory-promoting clinical conditions associated with low gestational age (such as chorioamnionitis, chronic lung disease [or protracted intubation],52 necrotizing enterocolitis,53 and sepsis52) may contribute to heightened adverse outcome risk. In contrast, antenatal exposure to magnesium,54 socioeconomic advantage,30 or exposures that enhance neurotrophic concentrations may positively influence the balance between risk and neuroprotection. Calabrese et al and Dhobale reviewed the effects of neurotrophic proteins in the perinatal period and noted that elevation of certain neurotrophins, including BDNF, are associated with maternal preeclampsia and fetal growth restriction, whereas neurotrophin-3 is diminished in the presence of placental inflammation.24,55 Further complicating our understanding of determinants of outcome is the observation that many of the proteins we characterize as either inflammatory or having neurotrophic properties may be pleiotrophic, functioning to enhance risk in some contexts and to protect brain function in others. For example, Ang-1 seems to have neurotrophic properties in these analyses, yet under other circumstances has proinflammatory characteristics.56,57 Similar data exist for BDNF,58–60 basic fibroblast growth factor,61 insulin-like growth factor-1,62 and erythropoietin.63,64
Our findings complement the notion that exogenously administered neurotrophic proteins might be therapeutic,65–69 lending support to growth factor clinical trials aimed at benefitting cognitive outcome,70–72 including one involving extremely preterm infants.73 The next steps should include analyses that evaluate the role that antecedent prepregnancy, prenatal, and postnatal characteristics have in modulating inflammation-associated and neurotrophic proteins, and to explore epigenetic mechanisms that likely play a role in such modulations.
Our study has several strengths. We included a large number of infants, collected our data prospectively, and had only modest attrition across 10 years of follow-up. Examiners at 2 and 10 years were not aware of the medical histories of the children they examined, and our analyses of protein data are of high quality, with high content validity.16,74,75
Although we sampled a wide range of inflammation-associated proteins known to be associated with neurologic damage, and a number of neurotrophic proteins, we did not evaluate all known inflammation-associated or neurotrophic proteins. We selected proteins on the basis of likely involvement in the fetal or neonatal inflammatory response or brain-protective properties, and the accuracy with which they could be measured reliably in whole blood spots using the Meso Scale Discovery and the Luminex multiplex platforms. Finally, rather than reporting absolute protein concentration values, we used a distribution-based definition of protein elevation based on gestational age, postnatal day, and the interval between processing blood samples, because normal values are not known, and values appear to be influenced by these factors.
We conclude that elevated levels of circulating neurotrophic proteins in the first 2 weeks after birth seem to decrease the risk of cognitive impairment at 10 of age years in children born extremely preterm. ■
Acknowledgments
J.F. received research support from Fulcrum Therapeutics, F. Hoffman-LaRoche, Ltd, Janssen Research and Development, SyneuRex International Corp, and Neuren Pharmaceuticals. The other authors declare no conflicts of interest.
Supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069–05; 2R01NS040069–09), the Office of the Director of NIH (1UG3OD023348–01), the National Institute of Child Health and Human Development (5P30HD018655–28), and the National Eye Institute (R01EY021820–01A1).
We are grateful to ELGAN Study participants and their families for their willingness to be engaged in the study for these many years and for the commitment and extra efforts that have made this work possible. We also acknowledge the inspiration, guidance and collaboration of Alan Levition and Elizabeth Allred in conducting the ELGAN Study.
Glossary
- ANG
Angiopoietin
- BDNF
Brain-derived neurotrophic factor
- CRP
C-reactive protein
- EF
Executive function
- ELGAN
Extremely Low Gestational Age Newborn
- IRG
Inflammatory risk group
- LCA
Latent class analysis
- NRG
Neurotrophin group
- RANTES
Regulated upon activation, normal T-cell expressed, and secreted
- SAA
Serum amyloid A
Appendix
Additional Members of the ELGAN Study
Boston Children’s Hospital, Boston, Massachusetts
Janice Ware, PhD
Taryn Coster, BA
Brandi Hanson, PsyD
Rachel Wilson, PhD
Kirsten McGhee, PhD
Patricia Lee, PhD
Aimee Asgarian, PhD
Anjali Sadhwani, PhD
Tufts Medical Center, Boston, Massachusetts
Ellen Perrin, MD
Emily Neger, MA
Kathryn Mattern, BA
Jenifer Walkowiak, PhD
Susan Barron, PhD
Baystate Medical Center, Springfield, Massachusetts
Bhavesh Shah, MD
Rachana Singh, MD, MS
Anne Smith, PhD
Deborah Klein, BSN, RN
Susan McQuiston, PhD
University of Massachusetts Medical School, Worcester, Massachusetts
Lauren Venuti, BA
Beth Powers, RN
Ann Foley, Ed M
Brian Dessureau, PhD
Molly Wood, PhD
Jill Damon-Minow, PsyD
Yale University School of Medicine, New Haven, Connecticut
Richard Ehrenkranz, MD
Jennifer Benjamin, MD
Elaine Romano, APRN
Kathy Tsatsanis, PhD
Katarzyna Chawarska, PhD
Sophy Kim, PhD
Susan Dieterich, PhD
Karen Bearrs, PhD
Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina
Nancy Peters, RN
Patricia Brown, BSN
Emily Ansusinha, BA
Ellen Waldrep, PhD
Jackie Friedman, PhD
Gail Hounshell. PhD
Debbie Allred, PhD
University Health Systems of Eastern Carolina, Greenville, North Carolina
Stephen C. Engelke, MD
Nancy Darden-Saad, BS, RN, CCRC
Gary Stainback, PhD
North Carolina Children’s Hospital, Chapel Hill, North Carolina
Diane Warner, MD, MPH
Janice Wereszczak, MSN, PNP
Janice Bernhardt, MS, RN
Joni McKeeman, PhD
Echo Meyer, PhD
Helen DeVos Children’s Hospital, Grand Rapids, Michigan
Steve Pastyrnak, PHD
Julie Rathbun, BSW, BSN, RN
Sarah Nota, BS
Teri Crumb, BSN, RN, CCRC
Sparrow Hospital, Lansing, Michigan
Madeleine Lenski, MPH
Deborah Weiland, MSN
Megan Lloyd, MA, EdS
University of Chicago Medical Center, Chicago, Illinois
Scott Hunter, PhD
Michael Msall, MD
Rugile Ramoskaite, BA
Suzanne Wiggins, MA
Krissy Washington, MA
Ryan Martin, MA
Barbara Prendergast, BSN, RN
Megan Scott, PhD
William Beaumont Hospital, Royal Oak, Michigan
Judith Klarr, MD
Beth Kring, RN
Jennifer DeRidder, RN
Kelly Vogt, PhD.
Brigham and Women’s Hospital, Boston, Massachusetts
Hidemi Yamamoto, BA
Stanthia Ryan, MD Candidate
Damilola Junaid, BS
Hassan Dawood, BS
Noah Beatty, BS
Ngan Luu, BA Candidate
Vanessa Tang, MD
Rosaria Rita Sassi, MD
Jenna-Malia Pasicznyk, RN
Footnotes
List of additional members of the ELGAN Study is available at www.jpeds.com(Appendix).
References
- 1.Washburn LK, Dillard RG, Goldstein DJ, Klinepeter KL, deRegnier RA, O’Shea TM. Survival and major neurodevelopmental impairment in extremely low gestational age newborns born 1990–2000: a retrospective cohort study. BMC Pediatr 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Cognitive functioning at age 10 years among children born extremely preterm: a latent profile approach. Pediatr Res 2017;82:614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuban KC, Joseph RM, O’Shea TM, Allred EN, Heeren T, Douglass L, et al. Girls and boys born before 28 weeks gestation: risks of cognitive, behavioral, and neurologic outcomes at age 10 years. J Pediatr 2016;173:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in post-natal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr 2012;160:395–401, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun 2013;29:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuban KC, Joseph RM, O’Shea TM, Heeren T, Fichorova RN, Douglass L, et al. Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. J Pediatr 2017;180:116–23, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leviton A, Blair E, Dammann O, Allred EN. The wealth of information conveyed by gestational age. J Pediatr 2005;146:123–7. [DOI] [PubMed] [Google Scholar]
- 8.Dammann O, Bueter W, Leviton A, Gressens P, Dammann CE. Neuregulin-1: a potential endogenous protector in perinatal brain white matter damage. Neonatology 2008;93:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dammann O, Leviton A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics 1999;104:541–50. [DOI] [PubMed] [Google Scholar]
- 10.Elliott CD. Differential ability scales San Antonio (TX): The Psychological Corporation; 2007. [Google Scholar]
- 11.Korkman M, Kirk U, Kemp S. NEPSY-II: clinical and interpretive manual 2nd ed. 2007. [Google Scholar]
- 12.Taylor HG, Filipek PA, Juranek J, Bangert B, Minich N, Hack M. Brain volumes in adolescents with very low birth weight: effects on brain structure and associations with neuropsychological outcomes. Dev Neuropsychol 2011;36:96–117. [DOI] [PubMed] [Google Scholar]
- 13.Harms MB, Zayas V, Meltzoff AN, Carlson SM. Stability of executive function and predictions to adaptive behavior from middle childhood to preadolescence. Front Psychol 2014;5:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung PJ, Opipari VP, Koolwijk I. Executive function and extremely preterm children. Pediatr Res 2017;82:565–6. [DOI] [PubMed] [Google Scholar]
- 15.Fichorova RN, Beatty N, Sassi RR, Yamamoto HS, Allred EN, Leviton A. Systemic inflammation in the extremely low gestational age newborn following maternal genitourinary infections. Am J Reprod Immunol 2015;73:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fichorova RN, Onderdonk AB, Yamamoto H, Delaney ML, DuBois AM, Allred E, et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio 2011;2:e00280–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leviton A, Allred EN, Yamamoto H, Fichorova RN, Investigators ES. Relationships among the concentrations of 25 inflammation-associated proteins during the first postnatal weeks in the blood of infants born before the 28th week of gestation. Cytokine 2012;57:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuban KCK, O’Shea TM, Alled EN, Paneth N, Hirtz D, Fichorova RN, et al. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J Child Neurol 2014;29:1692–8.24646503 [Google Scholar]
- 19.Leviton A, Kuban KC, Allred EN, Fichorova RN, O’Shea TM, Paneth N, et al. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum Dev 2011;87:325–30. [DOI] [PubMed] [Google Scholar]
- 20.Leviton A, Allred EN, Yamamoto H, Fichorova RN, Kuban K, O’Shea TM, et al. Antecedents and correlates of blood concentrations of neurotrophic growth factors in very preterm newborns. Cytokine 2017;94:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leviton A, Ryan S, Allred EN, Fichorova RN, O’Shea TM, Kuban K, et al. Antecedents and early correlates of high and low concentrations of angiogenic proteins in extremely preterm newborns. Clin Chim Acta 2017;471:1–5. [DOI] [PubMed] [Google Scholar]
- 22.Korzeniewski SJ, Allred E, Logan JW, Fichorova RN, Engelke S, Kuban KC, et al. Elevated endogenous erythropoietin concentrations are associated with increased risk of brain damage in extremely preterm neonates. PLoS ONE 2015;10:e0115083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto-Rivera CL, Fichorova RN, Allred EN, Van Marter LJ, Shah B, Martin CR, et al. The relationship between TSH and systemic inflammation in extremely preterm newborns. Endocrine 2015;48:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci 2014;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuno T, Nakanishi M. Neurotrophic factors increase tumor necrosis factor-alpha-induced nuclear translocation of NF-kappaB in rat PC12 cells. Neurosci Lett 2006;392:240–4. [DOI] [PubMed] [Google Scholar]
- 26.Patas K, Penninx BW, Bus BA, Vogelzangs N, Molendijk ML, Elzinga BM, et al. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain Behav Immun 2014;36:71–9. [DOI] [PubMed] [Google Scholar]
- 27.Barouch R, Appel E, Kazimirsky G, Brodie C. Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J Neuroimmunol 2001;112:72–7. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. J Neurosci Res 2001;65:322–31. [DOI] [PubMed] [Google Scholar]
- 29.Chien CC, Fu WM, Huang HI, Lai YH, Tsai YF, Guo SL, et al. Expression of neurotrophic factors in neonatal rats after peripheral inflammation. J Pain 2007;8:161–7. [DOI] [PubMed] [Google Scholar]
- 30.Joseph RM, O’Shea TM, Allred EN, Heeren T, Kuban KCK. for the ELGAN Study Investigators. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatr Res 2018;83(4):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allred EN, Dammann O, Fichorova RN, Hooper SR, Hunter SJ, Joseph RM, et al. Systemic inflammation during the first postnatal month and the risk of attention deficit hyperactivity disorder characteristics among 10 year-old children born extremely preterm. J Neuroimmune Pharmacol 2017;12:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghassabian A, Sundaram R, Chahal N, McLain AC, Bell E, Lawrence DA, et al. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev Psychopathol 2017;29:1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao R, Mashburn CB, Mao J, Wadhwa N, Smith GM, Desai NS. Brain-derived neurotrophic factor in infants <32 weeks gestational age: correlation with antenatal factors and postnatal outcomes. Pediatr Res 2009;65:548–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Lin Q, Wang P, Yao L, Leong K, Tan Z, et al. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co-overexpressing BDNF and VEGF in a rat model of cardiac arrest-induced global cerebral ischemia. Cell Death Dis 2017;8:e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalin I, Alyautdin R, Wong TW, Gnanou J, Kocherga G, Kreuter J. Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug Deliv 2016;23:3520–8. [DOI] [PubMed] [Google Scholar]
- 36.Meng Z, Li M, He Q, Jiang S, Zhang X, Xiao J, et al. Ectopic expression of human angiopoietin-1 promotes functional recovery and neurogenesis after focal cerebral ischemia. Neuroscience 2014;267:135–46. [DOI] [PubMed] [Google Scholar]
- 37.Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R, et al. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther 2014;20:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci 2011;29:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol 2005;15:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev 2010;20:327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 2014;75:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders EJ, Harvey S. Peptide hormones as developmental growth and differentiation factors. Dev Dyn 2008;237:1537–52. [DOI] [PubMed] [Google Scholar]
- 43.Larpthaveesarp A, Ferriero DM, Gonzalez FF. Growth factors for the treatment of ischemic brain injury (growth factor treatment). Brain Sci 2015;5:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raff MC, Lillien LE. Differentiation of a bipotential glial progenitor cell: what controls the timing and the choice of developmental pathway? J Cell Sci Suppl 1988;10:77–83. [DOI] [PubMed] [Google Scholar]
- 45.Bögler O, Wren D, Barnett S, Land H, Noble M. Cooperation between two growth factors promotes extended self renewal and inhibits differentiation of oligodendrocyte type-2 astrocyte progenitor cells. Proc Natl Acad Sci USA 1990;87:6368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 1992;70:31–64. [DOI] [PubMed] [Google Scholar]
- 47.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oppenheim RW, Yin QW, Prevette D, Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature 1992;360:755–7. [DOI] [PubMed] [Google Scholar]
- 49.Yan Q, Elliott J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature 1992;360:753–5. [DOI] [PubMed] [Google Scholar]
- 50.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 1992;360:757–9. [DOI] [PubMed] [Google Scholar]
- 51.Hodyl NA, Aboustate N, Bianco-Miotto T, Roberts CT, Clifton VL, Stark MJ. Child neurodevelopmental outcomes following preterm and term birth: what can the placenta tell us? Placenta 2017;57:79–86. [DOI] [PubMed] [Google Scholar]
- 52.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002;140:171–6. [DOI] [PubMed] [Google Scholar]
- 53.Martin CR, Dammann O, Allred EN, Patel S, O’Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr 2010;157:751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: an individual participant data meta-analysis. PLoS Med 2017;14:e1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhobale M Neurotrophins: role in adverse pregnancy outcome. Int J Dev Neurosci 2014;37:8–14. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Wang Y, Li D, Liu Z, Zhao Z, Han D, et al. VEGF inhibits the inflammation in spinal cord injury through activation of autophagy. Biochem Biophys Res Commun 2015;464:453–8. [DOI] [PubMed] [Google Scholar]
- 57.Mussap M, Cibecchini F, Noto A, Fanos V. In search of biomarkers for diagnosing and managing neonatal sepsis: the role of angiopoietins. J Matern Fetal Neonatal Med 2013;26(Suppl 2):24–6. [DOI] [PubMed] [Google Scholar]
- 58.Prakash YS, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacol Ther 2014;143:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong CJ, Liou YJ, Tsai SJ. Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Res Bull 2011;86:287–97. [DOI] [PubMed] [Google Scholar]
- 60.Shamovsky IL, Ross GM, Riopelle RJ, Weaver DF. The interaction of neurotrophins with the p75NTR common neurotrophin receptor: a comprehensive molecular modeling study. Protein Sci 1999;8:2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodbury ME, Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J Neuroimmune Pharmacol 2014;9:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nieto-Estevez V, Defterali C, Vicario-Abejon C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci 2016;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartnicki P, Kowalczyk M, Rysz J. The influence of the pleiotropic action of erythropoietin and its derivatives on nephroprotection. Med Sci Monit 2013;19:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci 2014;10:921–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Q, Miller JA. The use of trophic factors in degenerative motoneuron diseases. Exp Neurol 1993;124:60–3. [DOI] [PubMed] [Google Scholar]
- 66.Seeburger JL, Springer JE. Experimental rationale for the therapeutic use of neurotrophins in amyotrophic lateral sclerosis. Exp Neurol 1993;124:64–72. [DOI] [PubMed] [Google Scholar]
- 67.Thoenen H, Hughes RA, Sendtner M. Trophic support of motoneurons: physiological, pathophysiological, and therapeutic implications. Exp Neurol 1993;124:47–55. [DOI] [PubMed] [Google Scholar]
- 68.Ishii DN, Marsh DJ. On the therapeutic potential for insulin-like growth factor use in motor neuron disease. Exp Neurol 1993;124:96–9. [DOI] [PubMed] [Google Scholar]
- 69.Appel SH, Smith RG. Can neurotrophic factors prevent or reverse motoneuron injury in amyotrophic lateral sclerosis? Exp Neurol 1993;124:100–2. [DOI] [PubMed] [Google Scholar]
- 70.Lioutas VA, Alfaro-Martinez F, Bedoya F, Chung CC, Pimentel DA, Novak V. Intranasal insulin and insulin-like growth factor 1 as neuroprotectants in acute ischemic stroke. Transl Stroke Res 2015;6:264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maiese K Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen Res 2015;10:518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwamoto T, Ouchi Y. Emerging evidence of insulin-like growth factor 2 as a memory enhancer: a unique animal model of cognitive dysfunction with impaired adult neurogenesis. Rev Neurosci 2014;25:559–74. [DOI] [PubMed] [Google Scholar]
- 73.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol 2015;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leviton A, Fichorova R, Yamamoto Y, Allred EN, Dammann O, Hecht J, et al. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine 2011;53:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McElrath TF, Fichorova RN, Allred EN, Hecht JL, Ismail MA, Yuan H, et al. Blood protein profiles of infants born before 28 weeks differ by pregnancy complication. Am J Obstet Gynecol 2011;204:e1–418, e12. [DOI] [PubMed] [Google Scholar]


