Abstract
Maintained gamma band activity is a key element of higher brain function, participating in perception, executive function, and memory. The pedunculopontine nucleus (PPN), as part of the reticular activating system (RAS), is a major source of the “bottom-up” flow of gamma activity to higher regions. However, interruption of gamma band activity is associated with a number of neurological and psychiatric disorders. This review will focus on the role of the PPN in activating higher regions to induce arousal and descending pathways to modulate posture and locomotion. As such, PPN deep brain stimulation (DBS) can not only help regulate arousal and stepping, but continuous application may help maintain necessary levels of gamma band activity for a host of other brain processes. We will explore the potential future applications of PPN DBS for a number of disorders that are characterized by disturbances in gamma band maintenance.
Keywords: Alzheimer’s disease, bipolar disorder, bottom-up gamma, coma, depression, mesencephalic locomotor region, neglect, Parkinson’s disease, pedunculopontine nucleus, reticular activating system, schizophrenia, top-down beta
Introduction
“Bottom-up” brain processing involves the activation of lower brain centers by sensory events such that the information moves to higher centers to promote perception. “Top-down” processing involves the influence imposed by higher centers on the perception of incoming stimuli. Recent findings suggest that bottom-up and top-down signaling use different frequency channels, specifically gamma and beta frequencies, respectively (Bastos et al. 2015). It has been long known that gamma band activity recorded over the cortex using the electroencephalogram (EEG) appears to participate in sensory perception, problem solving, and memory (Eckhorn et al. 1988; Gray and Singer, 1989; Philips and Takeda, 2009; Palva et al. 2009; Voss et al. 2009). Coherent activity across cortical regions is thought to contribute to the merging or “binding” of information from various regions into a united whole that contributes to perception (Llinas et al. 1991; Llinas and Pare, 1991; Singer, 1993). Coherent gamma band activity is also thought to give rise to consciousness, but only when maintained for prolonged periods. That is, consciousness is associated with maintained, but not interrupted, gamma band activity (Vanderwolf, 2000 a, b). The question then arises, how is “bottom-up” gamma activity generated, and how is it maintained? Given that repetitive sensory stimulation is not reflected by matching evoked cortical activity as the rates exceed 20–30 Hz, how are cortical synaptic circuits supposed to maintain firing rates of 40–60 Hz or higher? Auditory stimuli, for example, produce an evoked response in the primary auditory cortex to every stimulus, but when frequencies exceed ~20 Hz, the cortical evoked synapses begin to fail (e.g. Erwin and Buchwald, 1986). Likewise, visual evoked responses cannot follow rates faster that ~30 Hz before synapses begin to fail, that is, before there is “flicker fusion” or the perception that one is watching a movie rather than a sequence of static images. Thus, cortical synaptic circuits appear incapable of maintaining prolonged activity at high rates without failing. What mechanisms help maintain high frequency activity, and what are the consequences of interruptions in gamma band activity? If failure to maintain gamma band activity leads to abnormal function, can gamma band activity be induced and maintained artificially?
Mechanisms of Gamma Activity
The cortical mechanisms behind gamma activity include not only fast-spiking neurons (Cunningham et al. 2004), but also inhibitory interneurons with intrinsic membrane oscillations in the gamma range (Steriade and Llinas, 1988; Llinas et al. 1991; Steriade, 1999). In the thalamus, thalamocortical neurons have intrinsic properties needed to generate subthreshold gamma band membrane potential oscillations (Pedroarena and Llinas, 1997). Some cortical interneurons can generate intrinsic gamma oscillations through the activation of voltage-dependent, persistent sodium channels (Llinas et al. 1991), but, in thalamocortical neurons, the main mechanism responsible for gamma band activity involves high threshold P/Q-type voltage-gated calcium channels located in the dendrites (Pedroarena and Llinas, 1997). The same intrinsic properties mediating gamma oscillations are present in the thalamus of several vertebrate species, suggesting the mechanism is well conserved in evolution (Llinas and Steriade, 2006).
Calcium channels are known to play an important role in intrinsic properties and synaptic transmission throughout the central nervous system (Katz and Miledi, 1965; Llinas and Hess, 1976; Caterall, 1988; Llinas, 1988; Llinas et al. 2007). P/Q-type channels (Cav2.1) are present throughout the brain (Hillman et al. 1991; Uchitel et al. 1992; Llinas et al. 2007). N-type calcium channels are found in the brainstem, appear mainly during the early postnatal period, and some are replaced by P/Q-type channels later in development (Iwasaki and Takahashi, 1998; Westenbroek et al. 1992). Importantly, P/Q-type knockout mice have deficient gamma band activity in the EEG, abnormal sleep-wake states, ataxia, are prone to seizures, and die within 3 weeks, suggesting a lack of high frequency activity (Llinas et al. 2007). Therefore, both the cortex and thalamus appear to generate gamma activity, and they do so via sub-populations of cells with intrinsic sodium-dependent or calcium channel-dependent membrane oscillations.
In addition, the hippocampus and cerebellum have the intrinsic and synaptic properties necessary to generate gamma band oscillatory activity. Hippocampal oscillatory activity in the gamma range (30–90 Hz) has been extensively described to be functional associated with entorhinal cortex afferents (Charpak et al. 1995). Neurons in the entorhinal cortex can also oscillate at gamma frequencies, playing a key role in maintaining hippocampal gamma oscillations (Chrobak and Buzsaki, 1998). Gamma band activity in the CA1 area has been divided into high (>65 Hz) and low (~25–60 Hz) gamma components that differentially couple CA1 and CA3 subfields, respectively (Colgin et al. 2009). Entorhinal cortex in charge of providing information about object and place recognition appears to use high gamma oscillations to “bind” CA1 activity in rodents (Bussey et al. 1999), on the other hand, CA1 low gamma oscillations appear locked to the slower frequencies present in CA3, which is in charge of memory storage (Colgin et al. 2009; Colgin and Moser, 2010). This suggests the use of different frequency bands for separate hippocampal functions.
Gamma band activity has been described in the Purkinje cell layer in the cerebellum, and also in distal white matter (Lang et al. 2006; Middleton et al. 2008). Such cerebellar activity is coherent with that of the cortex and thalamus. Cortico-cerebellar coherence at gamma frequencies is manifested in monkeys during performance of a precision grip task (Soteropoulos and Baker, 2006), and cerebello-thalamic activity is synchronized with neocortical activity at gamma frequencies (Timofeev and Steriade, 1997). Also, it has been proposed that both cerebellar and thalamocortical networks might oscillate at the same frequencies to enable information exchange among these brain areas (Middleton et al. 2008), very much like distant cortical areas display coherent activity during “binding” of sensory events. However, gamma band activity in the motor cortex lags behind coherent activity in basal ganglia structures (Lalo et al. 2008; Trottenberg et al. 2006), suggesting that motor cortex gamma synchronization reflects a momentary arousal-related event for enabling the initiation of movement (Brucke et al. 2012; Jenkinson et al. 2013). That is, structures such as the reticular activating system (RAS) and thalamus may play an early permissive role in the control of movement (Garcia-Rill et al. 2016c). One of the most important recent breakthroughs in the wake-sleep field is the discovery of beta (20–30 Hz)/gamma (30–60 Hz) band activity in the RAS.
PPN Gamma Activity
The pedunculopontine nucleus (PPN) is active during waking and rapid eye movement (REM) sleep, and is the only RAS nucleus that fires selectively in relation to states involving high frequency beta/gamma activity (Garcia-Rill, 2015). The other nuclei, the locus coeruleus and raphe nuclei, both fire in relation to waking and slow wave sleep (SWS), but not REM sleep (Garcia-Rill, 2009). The PPN is made up of different populations of cholinergic, glutamatergic, and GABAergic neurons (Wang and Morales, 2009), receives sensory input in parallel to primary sensory afferents. The PPN sends ascending projections through the intralaminar thalamus to the cortex and thus modulating arousal, as well as descending projections through reticulospinal pathways of the pons and medulla thus modulating posture and locomotion (Garcia-Rill, 2015). Figure 1 shows how afferent input can simultaneously activate thalamocortical projections to elicit arousal and reticulospinal projections to set posture and trigger locomotion. The ascending projections of the PPN are especially relevant to cortical arousal. The intralaminar thalamic nuclei, including the parafascicular (Pf) and centre median (CM), represent the main thalamic target of the brainstem PPN nucleus (Capozzo et al. 2003; Hallanger and Wainer 1988; Scarnati et al. 1987; Steriade and Glenn, 1982). These two nuclei in turn project to the cerebral cortex and basal ganglia structures (Jones 1985, 2002; Otake and Nakamura 1998; Sadikot et al. 1990, 1992; Steriade and Glenn, 1982). As such, ascending PPN inputs to these regions are likely to play an important role in regulating arousal and in coordinating motor activity. In this regard, stimulation of the PPN elicits a prolonged response in cells in both of these regions (Kobayashi et al. 2004a). However, differences in the frequency dependence of firing induced by different trains of PPN stimuli (the highest responses are elicited by stimulation at gamma frequencies) in these two nuclei indicate that they differ in the manner in which they relay PPN information to the cortex and basal ganglia (Kobayashi et al. 2004). Briefly, PPN ascending projections have been proposed to generate “bottom-up” gamma, while its descending projections modulate posture and locomotion during waking and REM sleep (Garcia-Rill et al. 2016b, 2017).
Figure 1. PPN modulation of cortical arousal and posture and locomotion.
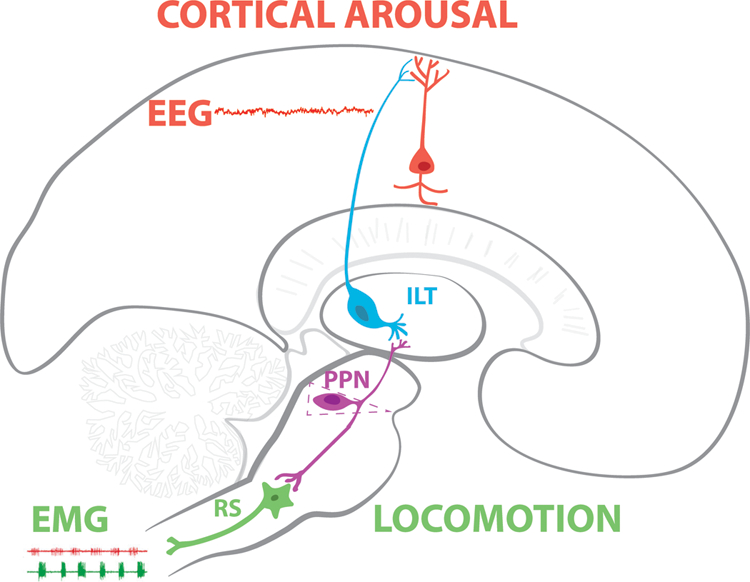
Afferent input arising in parallel to primary sensory pathways activates PPN neurons with intrinsic gamma oscillations. Output from PPN ascends to the intralaminar thalamus (ILT) where cells also manifest N- and P/Q-type calcium channel-mediated intrinsic gamma oscillations. In turn, the ILT projects to upper layers of the cortex to activate pyramidal neurons. Differences in coherence during waking vs REM sleep suggest that the PPN, as the source of both mechanisms, helps modulate cortical EEG in the gamma band range. Simultaneously, descending projections from the PPN modulate the firing of reticulospinal (RS) neurons to trigger locomotion during waking or changes in posture, such as atonia, during REM sleep. This general diagram is not intended to represent accurate anatomical locations and connectivity. The reader is referred to Garcia-Rill (2015) for much more detailed anatomical sections, 3D reconstructions, and connectivity.
Multiple in vivo studies have shown that PPN neurons manifest beta/gamma frequencies during waking and REM sleep, but not during SWS (Sakai et al. 1990; Steriade et al. 1990; Kayama et al. 1992; Datta and Siwek, 2002; Datta et al. 2009; Boucetta et al. 2014). Moreover, gamma band activity is present in the cortical EEG and the PPN of the cat in vivo when the animal is active (Steriade et al. 1990); and in the region of the PPN in humans during stepping, but not at rest (Fraix et al. 2013). A study in the primate showed that PPN neurons fired at low frequencies ~10 Hz at rest, but the same cells fired at gamma frequencies when the animal awakened, or when it walked on a treadmill (Goetz et al. 2016). That is, the same cells were involved in both arousal and motor control, and its role in various arousal-related behaviors, partly through the cerebellum, has been confirmed (Scarnati et al. 2016). That is, a wealth of evidence shows that PPN cells are active at beta/gamma frequencies in relation to both waking and movement across species (rodent, feline, primate, man) as well as in vitro and in vivo.
A number of novel studies described the presence of beta/gamma frequency intrinsic membrane oscillations as the maximal natural frequency of all PPN neurons, regardless of cell type or transmitter type (Garcia-Rill et al. 2013, 2014a, b, 2015, 2016a; Kezunovic et al. 2011; Urbano et al. 2014). Every PPN neuron expresses high threshold N- or P/Q-type calcium channels, and there are three cell types in the PPN, those that have only N-type calcium (modulated by cAMP/PK), those with both N- and P/Q-type, and those that have only P/Q-type calcium (modulated by CaMKII), channels (Luster et al. 2015, 2016). These and other results suggest that the gamma activity generated by the PPN is different during waking (through CaMKII and P/Q-type channels) than during REM sleep (through cAMP/PK and N-type channels). That is, the PPN provides at least in part, the driving for “bottom-up gamma” (Garcia-Rill et al. 2016b). The differential effects of gamma activity during waking and REM sleep are maintained at the level of the cortical EEG. The difference between gamma band activity during waking vs REM sleep appears to be a lack of coherence across distant cortical regions (Castro et al. 2013). These data demonstrate that gamma band activity generated in the PPN has effects on the cortical EEG, and they do so differentially depending on state. That is, brainstem driving of EEG gamma activity (i.e. bottom-up gamma) during waking carries with it coherence across cortical areas, but driving of EEG gamma activity during REM sleep does not exhibit coherence across distant cortical regions (Cavelli et al. 2015; Torterolo et al. 2015). In summary, ascending PPN gamma helps drive the cortical EEG but differs in promoting coherence during waking but not during REM sleep, while descending PPN projections modulate posture and locomotion during waking and REM sleep atonia during REM sleep (Garcia-Rill, 2015). Figure 2 depicts the intracellular pathways for the calcium channels involved in generating gamma oscillations in PPN neurons. P/Q-type calcium channels act through CaMKII intracellular mechanisms, while N-type calcium channels act through cAMP/PK mechanisms. The former is involved in waking, which modulates cortical EEG gamma activity with coherence across distant sites, while the latter is involved in REM sleep, which modulates cortical EEG gamma band activity without coherence.
Figure 2. Mechanisms of gamma band activity generated by the PPN.
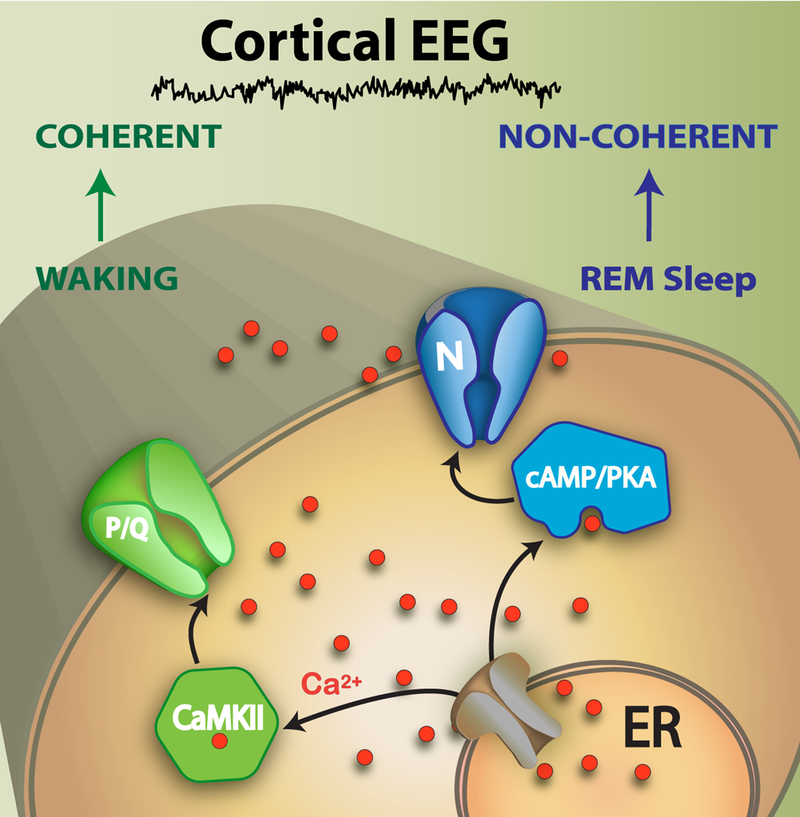
P/Q-type calcium channels are modulated by CaMKII intracellular mechanisms to produce coherent cortical EEG gamma band activity during waking. N-type calcium channels are modulated by cAMP/PKA intracellular mechanisms to produce non-coherent cortical EEG gamma band activity during REM sleep. At the cortical level, gamma band activity during waking carries with it coherence across distant sites, but during REM sleep, there is no coherence in gamma activity across distant sites. Waking and REM sleep, both of which are regulated by the PPN, both induce gamma band activity in the cortical EEG, but differ in the coherence between distant sites. Disorders in which gamma band activity is reduced, and those in which there is low coherence across distant cortical sites, may both be amenable to PPN DBS.
Moreover, N- and P/Q-type calcium channels are arranged all along the dendrites of PPN cells, since fast calcium imaging studies showed that high threshold calcium channel signals are evident throughout the dendrites of these neurons (Hyde et al. 2013). This exquisite organization of rafts in the lipid environment (Brady et al. 2004), is likely disturbed in optogenetic studies in which over expression of a bacterial calcium channel is amplified by horse promoter to pepper the dendrites of PPN neurons, doubtlessly impairing the manifestation of threshold-dependent calcium channels. For this reason, optogenetic experiments on wake-sleep control will alter the function of those channels generating high frequency intrinsic membrane oscillations, making conclusions untenable. In addition, considering the latency of effects on wake-sleep cycles, their direct causation by light stimulation can easily be questioned. Finally, stimulation with light would induce a tonic low threshold calcium influx unlike the rapid oscillations normally induced by the switching of calcium and potassium channels driving intrinsic membrane oscillations (Garcia-Rill et al. 2013, 2014a,b). Stimulation at the appropriate frequencies (20–60 Hz) to drive beta/gamma intrinsic membrane oscillations, such as those used in PPN DBS, would induce activity closer to the normal physiological manifestation.
Revisiting the MLR
The mesencephalic locomotor region (MLR) was originally described in very specific terms. In the precollicular-postmamillary transected cat, stimulation using long duration (0.5–1.0 msec), low amplitude (10–100 uA) pulses delivered at specific frequencies (40–60 Hz) could elicit locomotion on a treadmill when the body was suspended on a sling (Shik et al. 1966). Moreover, increasing stimulus amplitude would change stepping from a walk to a trot to a gallop, otherwise known as “controlled” locomotion on a treadmill. This is markedly different from the “unspecific” progression elicited by multiple brain regions in an intact animal (Shik et al. 1966; Armstrong, 1986; Garcia-Rill, 1986). These studies identified a locus, the MLR, independent of higher structures such as the cortex and cerebellum that could specifically help drive behavioral approach and escape. We investigated the neuronal substrates mediating this very specific effect and found that low threshold sites in the rat and cat brains were located within the PPN when labeled for cholinergic neurons (Garcia-Rill et al. 1987). We further showed that the region could be chemically activated, eliminating the potential for electrical stimulation of fibers of passage (Garcia-Rill et al. 1985). However, we pointed out that there were other nearby sites that would lead to locomotion, especially when stimulated using different parameters (Skinner and Garcia-Rill, 1984; Garcia-Rill 1986, 1991). We also pointed out that locomotion had to be “recruited” rather than induced, since it did not immediately follow the onset of stimulation, and that the effective region overlapped with elements of the RAS, especially the PPN (Garcia-Rill and Skinner. 1988, 1991).
We have repeatedly cast doubt on the specificity of this region, concluding that the MLR was NOT a “locomotion-specific” area, but rather a region that could be stimulated to induce rhythmic activity, that is, a “rhythmogenic” area (Garcia-Rill and Skinner, 1988; Skinner and Garcia-Rill, 1990). In other words, stimulating at the natural frequency of this region would activate all of its efferent projections, including ascending ones projecting to the thalamus modulating arousal, and descending ones to reticulospinal systems modulating posture and gait (Garcia-Rill, 1991; Reese et al. 1995). It was not until years later that we discovered the reason for the need of using 40–60 Hz stimulation, the fact that all PPN cells, regardless of transmitter type, as described above, manifest intrinsic membrane oscillations in the beta/gamma range mediated by high threshold calcium channels (Simon et al. 2010; Kezunovic et al. 2011). This provides an explanation for the need to stimulate at specific frequencies, why the region is rhythmogenic, and why rhythms are “recruited” as current levels must depolarize cells sufficiently to activate high threshold calcium channels.
Amazingly enough, a multitude of authors have made assertions about the location of the MLR without labeling for cholinergic neurons in locomotion preparations (Noga et al. 2017; Takakusaki et al. 2003), or even carrying out actual “controlled” locomotion on a treadmill studies using PPN stimulation (Mena-Segovia and Bolam, 2017; Sherman et al. 2015). Moreover, using intact (non-transected) animals likely will only reveal the many ways in which the cortex and cerebellum can induce “unspecific” behavioral approach and escape in intact animals, but will not identify the site(s) essential for “controlled” locomotion (Noga et al. 2017). The issue of “controlled” vs “unspecific” locomotion requires some explanation. The original description of the MLR (Shik et al 1966) was designed to expose those regions that could specifically elicit locomotion on a treadmill. The precollicular-premammillary transection led to spontaneous locomotion when the treadmill was turned on and sometimes even in the absence of a moving treadmill. Presumably, even more anterior regions, including intact non-transected animals, would similarly elicit stepping spontaneously. Thus, the use of a precollicular-postmammillary transection was essential to eliminate all forms of spontaneous or sensory-driven (treadmill movement) induction of stepping. In the intact animal, far too many regions influence the initiation of stepping, making stimulation within any one area merely a contributor, not a “specific” locomotion-inducing area. Given the absence of spontaneous locomotion in the precollicular-postmammillary cat, Shik et al (1966) identified very specific conditions that qualified as “controlled” locomotion, namely, the ability of slowly increasing stimulus amplitude to elicit a walk, then a trot, then a gallop, i.e. controlling the step cycle. The lowest amplitudes of stimulation were found to require long duration pulses in the 0.5–1.0 msec range, and to need rates in the order of 40–60 Hz (Shik et al 1966). Given these rigid requirements, one can be assured that a stimulation site indeed drives stepping in the absence of sensory or central commands. As mentioned above, we found that other nearby sites (e.g. mesencephalic trigeminal nucleus at the edge of the central gray, ventral edge of the inferior colliculus, and posterior edge of the substantia nigra at the edge of the PPN), but these regions were considered too close to the PPN to warrant separate assignation. However, stimulation of only lateral, but not medial cuneiform nucleus, was effective, suggesting that it is the posterior PPN (which is embedded in the lateral cuneiform) that is responsible for the induced stepping, and not the cuneiform nucleus as a whole (Garcia-Rill 1986, 1991; Garcia-Rill and Skinner, 1988, 1991; Garcia-Rill et al. 1996; Reese et al. 1995).
Based on suggestions over the years that the PPN is involved in the locomotor deficits in Parkinson’s disease (Garcia-Rill 1986, 1991; Garcia-Rill and Skinner, 1988, 1991; Garcia-Rill et al. 1983, 1996; Reese et al. 1995), there are now over 200 patients implanted with PPN deep brain stimulation (DBS) electrodes. Early neuropathological studies showing PPN degeneration in PD (e.g. Hirsch et al. 1987, Jellinger, 1988) led to studies on the primate demonstrating lesions of the PPN induced akinesia (Munro-Davies et al. 1999), and the suggestion of using PPN DBS for treating akinesia (Jenkinson et al. 2005), along with a series of clinical studies in humans (Mazzone et al. 2005). We should note that histological verification of stimulation sites in most patients implanted for PPN DBS is not available. Given that caveat, Ferraye et al (2010) found that bilateral PPN stimulation at 15 Hz and 25 Hz improved freezing of gait and decreased falls. Moro et al (2010) used unilateral stimulation at 50 Hz and 70 Hz, and showed improvements in falls and motor scores. Stefani et al (2007, 2013) used PPN stimulation at 10 Hz and 25 Hz, with some improvements in motor scores but a significant improvement in sleep patterns and modest improvement in gait. Alessandro et al (2010) using 25 Hz stimulation showed no motor improvements but significant amelioration in sleep scores and executive function. Thevanasathan et al (2010, 2012) showed that stimulation at 20–35 Hz improved reaction time and fall scores, and that gait freezing was significantly improved, particularly with bilateral stimulation. The latter study is one of the few that used double-blind analysis and established that bilateral stimulation was more effective than unilateral. These results taken together suggest that stimulation, especially bilaterally, at mid-range frequencies (25–50 Hz or beta/gamma) improved gait scores and prevented falls. It is likely that the differences across the results of different groups are due to the selection of patients, of surgical approach, of method of verification of stimulation site, of parameters of stimulation, and of measures of end result. In addition, a number of specific issues remain to be clarified before this therapy can be used consistently across groups, namely, current practice in DBS is to stimulate using 50–100 μsec pulses, which may preferentially be activating intrinsic axons and fibers of passage rather than neurons; current practice in DBS is to use frequencies >60 Hz, which, when applied to PPN, may tend to inactivate these neurons by depolarization block. Only some studies have used stimulation at 40–60 Hz, to good effect (Mazzone et al. 2008; 2009; Moro et al. 2010).
Overall, most studies have shown salutary effects on gait, posture, sleep, and even cognitive measures (for review, see Garcia-Rill et al. 2014a). The most effective frequencies are, not surprisingly, in the 20–70 Hz (beta/gamma) range (see review in Garcia-Rill et al. 2014a), but few below 20 Hz as recently purported (Noga et al. 2017). One study (Nosko et al. 2015) found that 10–25 Hz stimulation was effective for akinesia, gait disturbances, and daytime sleepiness, but symptoms were aggravated at these frequencies in one patient. Such low frequencies produce few beneficial effects for PPN DBS, most workers choosing >20 Hz (i.e. beta frequencies), most use higher frequencies in the beta/gamma range if they wish to obtain ameliorative effects. In those studies using lower frequencies, it is not clear if they need to use higher amplitude stimuli to elicit the desired effects. The higher, more effective frequencies (beta/gamma) are in keeping with the natural frequencies of firing of PPN neurons when they are activated (Garcia-Rill et al. 2014b; Goetz et al. 2016; Kezunovic et al. 2011; Luster et al. 2015; Simon et al. 2010; Urbano et al. 2012). Moreover, from a philosophical viewpoint, given the existence of spinal pattern generators for walking and brainstem oscillators for respiration and mastication, why would there be a region high in the brainstem dedicated to locomotor control? We reiterate our position that the term MLR is not “locomotion-specific”, is an outdated term not based on current findings, and should be retired.
Clinical Implications
Before implementing the directions envisioned below, considerable more preclinical and clinical research is needed on the role of disturbed gamma band activity and of the role of the PPN in the pathophysiology of these disorders. From the foregoing, we can expect, in general, that any condition characterized by wake-sleep disturbances will involve the PPN/RAS. We can also expect the manifestation of the following symptoms from disturbances in RAS “bottom-up” gamma band function; a) interruption of gamma band activity will manifest itself in problems with “binding” or perceiving appropriately, and in failure to attend to a stimulus or task for prolonged periods; b) increased REM sleep drive basically suggests that waking drive with cortical coherence is decreased, while REM sleep drive with lack of cortical coherence is increased, thereby leading to localized dream-like perceptions while awake (hallucinations) without an organized consciousness; c) daytime sleepiness, on the one hand suggests a decrease in P/Q-type channel activity, while on the other hand insomnia suggests an increase in P/Q-type channel activity, at least in some cases; and d) maintained consciousness would be impaired, among other possibilities. In the future, the use of PPN DBS at appropriate frequencies, therefore, could serve to stabilize gamma band output, preventing interruptions, and permitting more stable conscious goal-directed behavior. Presumably, an intervention such as DBS would be undertaken only in cases intractable to current medications. In addition, there may be a way of identifying those who might most benefit. Given findings showing a lack of gamma coherence in the EEG during REM sleep, and given the fact that most disorders manifest an increase in REM sleep drive, those patients with decreased cortical gamma coherence may respond to PPN DBS by regaining more coherence across distant sites in the cortex. That is, post-treatment EEGs should show increased gamma coherence across distant cortical sites. Briefly, PPN DBS may provide the maintained background of “bottom-up” gamma frequency activity necessary for generating proper consciousness, perception, and other higher functions.
Neurological Disorders
Parkinson’s disease (PD) patients show sleep disturbances that include disturbed REM sleep drive (specifically the presence of REM sleep during both night and day, along with hallucinations (Arnulf et al. 2000)), decreased SWS, frequent awakenings leading to daytime sleepiness, and insomnia (Jankovic, 2007; Ondo, 2014; Schrempf et al. 2014). The most frequent sleep disorder in PD patients was insomnia, present in over 80% of those tested (Alatriste-Booth et al. 2014). The use of PPN DBS in Parkinson’s disease (PD) targeted patients with axial symptoms and gait disturbances (Mazzone et al. 2005), and later studies used improved techniques for localization and visualization (Mazzone et al. 2008, 2009; Zrinzo et al. 2008). In general, PD patients showed improvements in gait when stimulation frequency was ~25 Hz (Ferraye et al. 2010), while stimulation at 50–70 Hz showed improvements in falls (Moro et al. 2010). In fact, decreasing PPN cell number was found to be related to an increase in falls (Kucinski and Sarter, 2015). Sleep scores and executive function also were improved by stimulation at ~25 Hz (Stefani et al. 2007, 2013; Alessandro et al. 2010). Double-blind studies established that bilateral stimulation was better than unilateral stimulation, and frequencies of 20–35 Hz improved reaction time, fall scores, and gait (Thevanasathan et al. 2010, 2012). A number of contributions to the present volume are made on this topic so no additional comments will be made except that the P50 potential, which is generated by the PPN (Garcia-Rill and Skinner, 2001), is a measure of level of arousal and sensory gating. The P50 potential is abnormal in PD patients (Teo et al. 1997), normalizes after bilateral pallidotomy (Teo et al. 1998), and could be used to monitor the effects of PPN DBS to determine if such treatment corrects any increases in amplitude or decreases in habituation, along with cognitive and motor symptoms. In addition, changes in the cortical EEG from non-coherent to coherent would be expected after PPN DBS in at least those patients initially manifesting lack of coherence in the waking EEG.
Alzheimer’s disease (AD) is characterized by sleep disturbances such as daytime sleepiness (Ferman et al. 2014), but no major changes in REM sleep drive, unlike that seen in a number of other disorders. Moreover, AD and dementia are marked by increased gamma band EEG activity with increased gamma coherence (Basar et al. 2017; Wang et al. 2017). Therefore, it would not be advisable to use PPN DBS at gamma frequencies lest symptoms be amplified by the added activation. However, it would be interesting to determine if very high frequency (>100 Hz) PPN DBS could be used to depolarize block PPN activity and thereby decrease gamma coherence along with, presumably, deleterious symptomatology. Interestingly, increased gamma band power suggests more powerful “bottom-up gamma” driving the cortex, the question remains, is “top-down beta” changed in this disease, suggesting that cortical influence is diminished.
Epilepsy represents the epitome of low frequency states. The origin of epilepsy cannot be traced to the PPN, rather, stimulation of the PPN may be used to counteract the tendency in epilepsy of synchronizing brain activity at low frequencies. The 10 Hz frequency of the EEG spectrum is at the fulcrum between higher frequencies (beta, gamma) that can tilt the brain to higher functions such as perception, consciousness, and cognition, or tilt it towards lower frequencies (theta, delta) that represent states of increasing coherence that drive the brain towards sleep (Garcia-Rill et al. 2016a, b). The state with the greatest coherence at low frequencies is epilepsy, therefore, interventions that drive the brain towards higher frequencies may prevent the effects of seizures. One possibility behind the beneficial uses of vagal stimulators is that the high frequency stimulation retrogradely affects brain activity towards higher frequencies. On the one hand, the PPN is part of a circuit that promotes seizures. Kindling reduces the firing of PPN neurons (Nolte et al 2006), suggesting that lower activity in PPN is associated with triggered seizure activity. On the other hand, stimulation of cholinergic PPN neurons reduced focal limbic seizures (Furman et al. 2015), suggesting that PPN stimulation may be a viable therapeutic avenue in epilepsy. PPN DBS could provide the necessary background of high frequency activity to drive the brain away from low frequency states and seizures.
Psychiatric Disorders
Aside from delusions and hallucinations, attentional impairment and withdrawal, apathy and cognitive impairment (Andreasen and Flaum, 1991), schizophrenia manifests severe wake-sleep abnormalities. These include hypervigilance, decreased SWS, especially deep sleep stages, increased REM sleep drive, and fragmented sleep (Caldwell and Domino, 1997; Feinberg et al. 1969; Itil et al. 1972; Jus et al. 1973; Zarcone et al. 1975), suggesting involvement of the RAS in schizophrenia. We showed that intractable inpatients, but not outpatients, had increased cholinergic cell number in the PPN, suggesting increased PPN output and hypervigilance (Garcia-Rill et al. 1995). There are also postural and motor disturbances (King, 1974; Manschrek, 1986), as well as eye movement dysregulation (Holzman and Proctor, 1973; Karson et al. 1990). The P50 potential shows decreased habituation in patients with schizophrenia and in some clinically unaffected first-degree relatives (Olincy et al. 2010). Moreover, aberrant gamma band activity and coherence during cognitive tasks or attentional load have been reported in schizophrenic patients (Ulhass and Singer, 2010; Hirano et al. 2015). These results suggest that the generation and maintenance of gamma band activity is abnormal in schizophrenia.
In schizophrenia, as well as the other disorders mentioned, patient selection is paramount. There are major differences in the symptom clusters of equally diagnosed patients, pointing to the heterogeneity in patients with the disease. One indicator that could be used is the measure of gamma band activity, so that if it is intermittent or not maintained, that could represent an inclusion criterion. Likewise, symptoms of sleep dysregulation, especially increased REM sleep drive and hypervigilence, may also point to the involvement of the PPN. The same measures could be applied to the neurological disorders outlined here.
The wake-sleep patterns manifested in the EEG of bipolar disorder patients include fragmented sleep, decreased SWS, increased vigilance, and increased REM sleep drive (Harvey et al. 2009; Kadrmas and Winokur, 1979; Kupfer et al. 1978). Adults with bipolar disorder manifest reduced sleep time and longer sleep onset during manic episodes, which have decreased REM sleep latency and increased REM sleep duration, while during depression they experience insomnia or hypersomnia, also with decreased REM sleep latency and increased REM sleep density (Kaplan and Williams, 2017; Hegerl and Hensch, 2014). Recent meta-analyses described fragmented sleep (including increased sleep latency, waking after sleep onset, and overall sleep efficiency) in bipolar disorder (Ng et al. 2015; Geoffroy et al. 2015). These patients also show decreased habituation of the arousal-related P50 potential (Olincy and Martin, 2005; Schulze et al. 2007), indicative of hypervigilance as a hallmark of the disease. Reduced maintained gamma band activity has been reported in bipolar disorder patients (Ozerdem et al. 2011).
Postmortem studies in humans found increased expression of neuronal calcium sensor protein (NCS-1) in the brains of some schizophrenic and bipolar disorder patients compared to controls and major depression patients (Bergson et al. 2003; Koh et al. 2003). Some patients had a 50% increase in expression, while others were within the normal range. We found that high concentrations of NCS-1, as would be evident in overexpression, decreased gamma oscillations in PPN neurons, but low concentrations promoted gamma oscillations (D’Onofrio et al. 2015). We also discovered that lithium, a front line treatment for bipolar disorder, normalized the effects of excessive NCS-1 on PPN gamma oscillation (D’Onofrio et al. 2016). Interestingly, NCS-1 down regulates N-type calcium channels in some cell lines (Gambino et al. 2007). This may mean that NCS-1 may normally inhibit N-type channel function (REM sleep drive), while promoting P/Q-type channel function (waking drive), but when overexpressed the effects may be reversed.
These findings are all suggestive of a deleterious effect on gamma oscillations in schizophrenia and bipolar disorder. We also know that existing forms of treatment are only partially effective in alleviating symptoms in only some patients. No therapy is effective in all patients, speaking to the heterogeneity of these diseases. This requires that careful screening for potential salutary effects is essential, along with monitoring of effectiveness over time. Nevertheless, in some cases, perhaps those with clear lack of gamma coherence across distant cortical sites, PPN DBS may be an effective therapy in schizophrenia and bipolar disorder.
Neglect, Coma, Vegetative States, Minimally Conscious States
Neglect can result from strokes or trauma to the contralateral hemisphere, but can also be caused by thalamic or RAS lesions. Lesions to the left hemisphere result in only transient neglect, while right side lesions lead to permanent contralateral deficits. Unilateral lesions of the cortex or thalamus produce unilateral neglect (Orem et al. 1973; Watson et al. 1981), but bilateral lesions of the intralaminar thalamus produce akinetic mutism (Mills and Swanson, 1978), while bilateral RAS lesions produce coma (Watson et al, 1974, 1981). Decreased arousal exacerbates symptoms of neglect (Coslett et al. 1987; Demeurisse et al. 1998; Storrie-Baker et al. 1997; Watson et al. 1977). Therefore, measures that increase arousal should relieve the symptoms of neglect. For example, the cold pressor test can be used to transiently reduce neglect (Storrie-Baker et al. 1997). We recorded the P50 potential, which is generated by the PPN, from left spatial neglect patients who showed a decrease in amplitude before, along with an increase after, cold pressor testing. In addition, the stimulant modafinil eliminated the neglect and restored P50 potential amplitude and habituation to normal levels (Woods et al. 2006). These findings suggest that neglect represents a decrement in arousal levels, and restoration of arousal levels will decrease or eliminate the symptom.
Recently, we proposed a mechanism by which full consciousness is achieved in a stepwise fashion (Garcia-Rill, 2017). A study on recovery from anesthesia found that recovery passes through several discreet activity states with an orderly progression through intermediate states rather than a continuous recovery of consciousness from anesthesia (Hudson et al. 2014). The EEG of the comatose patient is similar to that during general anesthesia, which is referred to as a “drug-induced coma”, and both states are characterized by burst suppression (Brown et al. 2010). In coma, it may be that either the ability to generate significant stepwise levels of gamma activity, and/or the mechanism for maintaining gamma oscillations, are disrupted. Given the multiple potential causes of coma, it is difficult to determine how each insult could affect stepwise gamma band generation or maintenance mechanisms specifically. Nevertheless, the insult or damage to the brain induces an inability to step through all the levels needed to reach full consciousness. Based on the amount or degree of damage, partial “levels” of consciousness may be achieved in different patients. For example, in the chronic “vegetative state”, the EEG changes may reflect stepwise shifts that do not reach higher levels of consciousness, whereas in the “minimally conscious state”, some degree of consciousness is attained, but the next higher level cannot be fully manifested. That is, gamma band activity is not maintained. PPN DBS may provide a stable level of gamma band activity that could overcome some of the deficits described, especially in cases in which current clinical treatments are ineffective. Moreover, EEG changes can profile the various states of “minimally conscious”, “vegetative”, and “locked-in” (Malinowska et al. 2013), representing a potential monitoring tool for judging effectiveness.
A table listing the disorders described above has been included. Certain caveats are needed, however, given that patients with these conditions may be mentally or cognitively impaired. Appropriate ethical considerations and oversight by institutional review boards is essential for patient selection, consent, and inclusion/exclusion criteria. Obviously, PPN DBS may be used only in cases that have proven intractable to standard treatment and the continued impairment represents major disability and/or danger to self and others.
Conclusions
A number of disorders may be amenable to PPN DBS in addition to PD, given disturbances in gamma band activity that parallel those seen in PD that may be ameliorated by such treatment. Targeting those patients who show decreased waking cortical EEG gamma band coherence across distant sites may increase the potential salutary effects of this now well-established therapy. By normalizing gamma band coherence across distant sites during waking, a number of serious symptoms may be alleviated, including disturbances in perception, erroneous executive function, and lapsed memory, as well as achieving more normal wake-sleep cycles and vigilance, in addition to regulation of motor disturbances.
Table 1.
Potential Effects of PPN DBS in Various Disorders
| Disorder | Symptom | Effect of PPN DBS |
|---|---|---|
| Neurological | ||
| Parkinson’s Disease | Increased REM sleep | Decrease REM sleep |
| Fragmented sleep | Normalize sleep | |
| Insomnia | Normalize sleep | |
| Cognition | Improve attention | |
| Gait and posture | Improve | |
| Alzheimer’s Disease | Increased gamma | 40–60 Hz negative effect >100 Hz positive effect? |
| Epilepsy | Low frequency EEG | Increase EEG frequency |
| Psychiatric | ||
| Schizophrenia | Fragmented sleep | Normalize sleep |
| Interrupted gamma | Increase gamma | |
| Increased REM sleep | Decrease REM sleep | |
| Bipolar Disorder | Fragmented sleep | Normalize sleep |
| Interrupted gamma | Increase gamma | |
| Increased REM sleep | Decrease REM sleep | |
| Trauma/Other | ||
| Neglect | Contralateral neglect | Increase gamma |
| Decreased arousal | Increase arousal | |
| Coma | Vegetative | Increase gamma |
| Minimally conscious | Increase gamma | |
| Locked-in | Increase gamma | |
Highlights.
The salutary use of PPN DBS in Parkinson’s disease suggests improvement in a number of symptoms other than motor. The PPN, as a major source of gamma band activity, can be considered a target for regulating the maintenance of gamma band activity using DBS. Disturbances in gamma band activity are present in a number of disorders that may be amenable to such therapy in intractable cases.
Acknowledgments
Supported by NIH award P30 GM110702 from the IDeA program at NIGMS to the CTN, allowing the center to generate over $100 million in grant support for its members over the last 15 years. EGR would also like to express profound gratitude to all of the Federal funding agencies, especially NIH and NSF that have continuously funded his lab for the last 40 years.
Footnotes
Conflict of Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Alatriste-Booth V, Rodriguez-Violante M, Camacho-Ordonez A, Cervantes-Arriaga A, 2014. Prevalence and correlates of sleep disorders in Parkinson’s disease: a polysomnographic study. Arq. Neuropsiquiatr 73, 241–245. [DOI] [PubMed] [Google Scholar]
- Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, Placidi F, Romigi A, Iani C, Marzetti F, Peppe A, 2010. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive problems. J. Neurol. Sci 289, 44–48. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, 1991. Schizophrenia: the characteristic symptoms. Schiz. Bull 17, 27–49. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, 1986. Supraspinal contributions to the initiation and control of locomotion. Prog. Neurobiol 26, 273–361. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Bonnet AM, Damier P, Bejjani BP, Seilhean D, Derenne JP, Agid Y, 2000. Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurol 55, 281–288. [DOI] [PubMed] [Google Scholar]
- Basar E, Femir B, Emek-Savas DD, Guntekin B, Yener GG, 2017. Increased long distance event-related gamma band connectivity in Alzheimer;s disease. Neuroimage Clin 14, 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, et al. , 2015. Visual areas exert feedforward and feedback through distinct frequency channels. Neuron 85, 390–40. [DOI] [PubMed] [Google Scholar]
- Bergson C, Levenson R, Goldman-Rakic P, Lidow MS, 2003Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling, Trends Pharmacol. Sci 24, 486–492. [DOI] [PubMed] [Google Scholar]
- Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE, 2014. Discharge profiles across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci 34, 4708–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND, 2010. General anesthesia, sleep, and coma. New Engl. J. Med 363, 2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucke C, Huebl J, Kempf F, Krauss JK, Yarrow, et al. , 2008. Pallidal gamma activity is correlated to movement amplitude in patients with dystonia. Clin. Neurophysiol 119, (S1)49. [Google Scholar]
- Brady JD, Rich TC, Le X, Stafford K, Fowler CJ, et al. , 2004. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol 65, 503–511. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, and Aggleton JP, 1999. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J. Neurosci 19, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell DF, Domino EF, 1997. Electroencephalographic and eye movement patterns during sleep in chronic schizophrenic patients. Electroenceph. Clin. Neurophysiol 22, 414–420. [DOI] [PubMed] [Google Scholar]
- Capozzo A, Florio T, Cellini R, Moriconi U, Scarnati E, 2003. The pedunculopontine nucleus projection to the parafascicular nucleus of the thalamus: an electrophysiological investigation in the rat. J. Neural Transm 110, 733–47. [DOI] [PubMed] [Google Scholar]
- Castro S, Falconi A, Chase M, Torterolo P, 2013. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur. J. Neurosci 37, 1330–1339. [DOI] [PubMed] [Google Scholar]
- Caterall WA, 1988. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium 24, 307–323. [DOI] [PubMed] [Google Scholar]
- Cavelli M, Castro S, Schwartzkopf N, Chase M, Falconi A, Torterolo P, 2015. Coherent cortical oscillations decrease during REM sleep in the rat. Behav. Brain Res 281, 318–325. [DOI] [PubMed] [Google Scholar]
- Charpak S, Paré D, Llinás RR, 1995. The entorhinal cortex entrains fast CA1 hippocampal oscillations in the anaesthetized guinea-pig: role of the monosynaptic component of the perforant path. Eur. J. Neurosci 7, 1548–1557. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G, 1998. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J. Neurosci 18, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, et al. , 2009. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, 2010. Gamma oscillations in the hippocampus. Physiology (Bethesda) 25, 319–329. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Bowers D, Heilman KM, 1987. Reduction of cerebral activation after right hemisphere stroke. Neurol, 37, 957–962. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, et al. , 2004. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc. Nat. Acad. Sci. USA 101, 7152–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF, 2002. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res 70, 79–82. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF Stack EC, 2009. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci 163, 397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeurisse G, Hublet C, Paternot J, 1988. Quantitative EEG in subcortical neglect. Neurophysiol. Clin 28, 259–265. [DOI] [PubMed] [Google Scholar]
- D’Onofrio S, Kezunovic N, Hyde JR, Luster B, Messias E, Urbano FJ, Garcia-Rill E, 2015. Modulation of gamma oscillations in the pedunculopontine nucleus (PPN) by neuronal calcium sensor protein-1 (NCS-1): relevance to schizophrenia and bipolar disorder. J. Neurophysiol 113, 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio S, Urbano FJ, Mesias E, Garcia-Rill E, 2016. Lithium decreases the effects of neuronal calcium sensor protein 1 in pedunculopontine neurons. Physiol. Rep 4, e12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, et al. , 1988. Coherent oscillations: a mechanism of feature linking in the visual system? Biol. Cybern 60, 121–130. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS, 1986b. Midlatency auditory evoked responses: Differential recovery cycle characteristics. Electroenceph Clin Neurophysiol, 64, 417–423. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Braun M, Koresko R.l., Gottleib F, 1969. Stage 4 sleep in schizophrenia. Arch. Gen. Psychiat 21, 262–266. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Smith GF, Dickson DW, Graff-Radford NR, Lin SC, et al. , 2014. Abnormal daytime sleepiness in dementia with Lewy bodies compred to Alzheimer’s disease using the Multiple Sleep Latency Test. Alz. Res. Ther 16, 76, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, et al. 2010. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 133, 205–14. [DOI] [PubMed] [Google Scholar]
- Fraix V, Bastin J, David O, Goetz L, Ferraye M, et al. , 2013. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE 8, e83919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman M, Zhan Q, McCaffery C, Lerner BA, Motelow JE, et al. , 2015. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: effects on cortical physiology. Epilepsia 56; e198–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino F, Pavlowsky A, Begle A, Dupont JL, Bahi N, et al. , 2007. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+ -channel and neurite elongation. Proc. Nat. Acad. Sci 104, 9063–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, 1986. The basal ganglia and the locomotor regions. Brain Res. Rev 11, 47–63. [PubMed] [Google Scholar]
- Garcia-Rill E, 1991. The pedunculopontine nucleus. Prog. Neurobiol 36, 363–389. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, 2009. Sleep and arousal states: reticular activating system. In: New Encyclopedia of Neuroscience, Squire LR, Bloom F, Spitzer N, Gage F, Albright T, Eds., Elsevier, Oxford, England: Vol. 8, pp.137–143. [Google Scholar]
- Garcia-Rill E, 2015. Waking and the Reticular Activating System in Health and Disease, Elsevier, New York, pp 313. [Google Scholar]
- Garcia-Rill E, 2017. Bottom-up gamma and stages of waking. Med. Hypoth 104, 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, et al. , 1995. Mesopontine neurons in schizophrenia. Neurosci 66, 321–335. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, D’Onofrio S, Luster B, Mahaffey S, Urbano FJ, et al. , 2016a. The 10 Hz Frequency: a fulcrum for transitional brain states. Translat. Brain Rhyth, 1, 7–13. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, D’Onofrio S, Mahaffey S, 2016b. Bottom-up Gamma: the Pedunculopontine Nucleus and Reticular Activating System. Transl. Brain Rhythm 1, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ, 1987. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res. Bull 18, 731–738. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ, 2013. Coherence and frequency in the reticular activating system (RAS). Sleep Med. Rev 17, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E, 2014a. The physiology of the pedunculopontine nucleus- implications for deep brain stimulation. J. Neural Transm 122, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, et al. , 2014b. Gamma band activity in the RAS- intracellular mechanisms. Exptl. Brain Res 232, 1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, et al. , 2015. Implications of gamma band activity in the pedunculopontine nucleus. J. Neural Transm 123, 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Reese NB, Skinner RD, 1996. Arousal and locomotion: from schizophrenia to narcolepsy. In The Emotional Motor System, Holstege G and Saper C, (Eds.). Prog. Brain Res 107, 417–434. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, 1988. Modulation of rhythmic function in the posterior midbrain. Neurosci 17, 639–654. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, 1991. Modulation of rhythmic functions by the brainstem, In Neurobiology of Human Locomotion, Shimamura M, Grillner S and Edgerton VR, (Eds.) Japan Sci. Soc. Press, Tokyo, pp. 137–158. [Google Scholar]
- Garcia-Rill E, Skinner RD, 2001. The sleep state-dependent P50 midlatency auditory evoked potential. In: Sleep Medicine, Lee-Chiong TL, Carskadon MA and Sateia MJ (Eds.), Hanley & Belfus, Philadelphia, pp. 697–704. [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA, 1983. Activity in the mesencephalic locomotor region during locomotion. Exptl Neurol 82, 606–622. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA, 1985. Chemical activation of the mesencephalic locomotor region. Brain Res 330, 43–54. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Virmani T, Hyde JR, D’Onofrio S, Mahaffey S, 2016c. Arousal and the control of perception and movement. Curr. Tends Neurol 10, 53–64. [PMC free article] [PubMed] [Google Scholar]
- Geoffroy PA, Scott J, Boudebesse C, Lajnef M, Henry C, et al. , 2015. Sleep in patients with remitted bipolar disorders; a meta-analysis of actigraphy studies. Acta Psychiat. Scand 131, 89–99. [DOI] [PubMed] [Google Scholar]
- Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, et al. , 2016. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J. Neural Transm 123, 667–678. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W, 1989. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Nat. Acad. Sci. USA 86, 1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, et al. , 2016. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J. Neural Transm 123, 667–678. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH,1988. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J. Comp. Neurol 274, 483–515. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Talbot LS, Gershon A, 2009. Sleep disturbances in bipolar disorder across the lifespan. Clin. Psychol 16, 256–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Hensch T, 2014. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev 44, 45–57. [DOI] [PubMed] [Google Scholar]
- Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, et al. , 1991. Localization of P-type calcium channels in the central nervous system. Proc. Natl. Acad. Sci. USA 88, 7076–7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, et al. , 2015. Spontaneous gamma activity in schizophrenia, JAMA Psychiat 72, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel A, Duyckaerts C, Javoy-Agid F Neuronla loss in the pedudunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. USA, 84, 5976–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Hughes DW, 1973. Eye tracking patterns in schizophrenia, Science 18, 179–181. [DOI] [PubMed] [Google Scholar]
- Hudson AE, Calderon DP, Pfaff DW, Proekt A, 2014. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc. Nat. Acad. Sci. USA 111, 9283–9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JR, Kezunovic N, Urbano FJ, Garcia-Rill E, 2013. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J. Appl. Physiol 115, 1402–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itil T.m., Hsu W, Klingenberg W, Saletu B, Gannon P, 1972. Digital computer-analyzed all-night sleep EEG patterns (sleep prints) in schizophrenics. Biol. Psychiat 4, 3–16. [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T, 1998. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J. Physiol. (Lond) 509, 419–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, 2007. Parkinson’s disease: clinical features and diagnosis, J. Neurol. Neurosurg. Psychiat 79, 368–376. [DOI] [PubMed] [Google Scholar]
- Jellinger K, 1988. The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiat 51, 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Kuhn AA, Brown P, 2013. Gamma oscillations in the human basal ganglia. Exp. Neurol 245, 72–76. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Azziz T, Stein JF, 2005. Pedunculopontine nucleus: a new target for deep brain stimulation for akinesia. Neurorep 16, 1875–1876. [DOI] [PubMed] [Google Scholar]
- Jones EG, 1985. The Thalamus, Plenum Press, New York. [Google Scholar]
- Jones EG, 2002. Thalamic organization and function after Cajal. Brain Res 136, 333–357. [DOI] [PubMed] [Google Scholar]
- Jus K, Bouchard M, Jus A, Villeneuve A, Lachance R, 1973. Sleep EEG studies in untreated long-term schizophrenic patients, Arch. Gen. Psychiat 29, 286–290. [DOI] [PubMed] [Google Scholar]
- Kadrmas A, Winokur G, 1979. Manic depressive illness and EEG abnormalities. J. Clin. Psychiat 40, 306–307. [PubMed] [Google Scholar]
- Kaplan KA, Williams R, 2017. Hypersomnia: an overlooked, but not overestimated, sleep disturbance in bipolar disorder. Evid. based Mental Health 20, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson CN, Dykman RA, Paige SR, 1990. Blink rates in schizophrenia. Schiz. Bull 16, 345 354. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R, 1965. The effect of calcium on acetylcholine release from motor nerve terminals. Proc. R. Soc. Lond. B Biol. Sci 161, 483–495. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E, 1992. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res 569, 210–220. [DOI] [PubMed] [Google Scholar]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. , 2011. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN). Eur. J. Neurosci 34, 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.l., 1974. A sensory-integrative approach to schizophrenia. Amer, J, Occup, Ther 28, 529 536. [PubMed] [Google Scholar]
- Kobayashi T, Good C, Biedermann J, Barnes C, Skinner RD, Garcia-Rill E, 2004. Developmental changes in pedunculopontine nucleus (PPN) neurons. J. Neurophysiol 91, 1470–81. [DOI] [PubMed] [Google Scholar]
- Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic P, et al. , 2003. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients, Proc. Natl. Acad. Sci 100, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, Sarter M, 2015. Modeling Parkinson’s disease falls associated with brainstem cholinergic systems decline. Behav. Neurosci 129, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG, Coble P, McPartland RJ, Ulrich RF, 1978. The application of EEG sleep for the differential diagnosis of affective disorders. Amer. J. Psychiat 135, 69–74. [DOI] [PubMed] [Google Scholar]
- Lalo E, Thobois S, Sharott A, Polo G, Mertens P, et al. , 2008. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J. Neurosci 28, 3008–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás RR, 2006. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J. Physiol. (Lond) 571, 101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, 1988. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242, 1654–1664. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Grace AA, Yarom Y, 1991. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc. Nat. Acad. Sci. USA 88, 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Hess R, 1976. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc. Nat. Acad. Sci. USA 73, 2520–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Paré D, 1991. Of dreaming and wakefulness. Neurosci 44, 521–535. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Soonwook C, Urbano FJ, Hee-Sup S, 2007. γ-Band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc. Nat. Acad. Sci. USA 104, 17819–17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M, 2006. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol 95, 3297–3308. [DOI] [PubMed] [Google Scholar]
- Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E, 2015. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol. Rep 3, e12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster B, Urbano FJ, Garcia-Rill E, 2016. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN). Physiol. Rep e12787. [DOI] [PMC free article] [PubMed]
- Malinowska U, Chatelle C, Bruno MA, Noirhomme Q, Laureys S, et al. , 2013. Electroencephalographic profiles for differentiation of disorders of consciousness. BioMedical. Eng. Online 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschrek TC, 1986. Motor abnormalities in schizophrenia, In: Nasrallah HA, Weinberger DR (Eds.), Elsevier, Amsterdam, pp. 65–96. [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A, 2005. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neurorep 16, 1877–1891. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Sposato S, Scarnati E, Stefani A, 2005. Brain stimulation and movement disorders: where we going? Proc. 14th Meet. World Soc. Stereotac. Funct. Neurosurg. (WSSFN), Monduzzi, Bologna. [Google Scholar]
- Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E, 2008. Stereotactic surgery of nucleus tegmenti pedunculopontine [corrected], Brit. J. Neurosurg 22, S33–S40. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Insola A, Sposato S, Scarnati E, 2009. The deep brain stimulation of the pedunculopontine tegmental nucleus, Neuromod 12, 191–204. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam P, 2017. Rethinking the epdunculopontine nucleus: from cellular organization to function. Neuron 94, 7–18. [DOI] [PubMed] [Google Scholar]
- Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, et al. , 2008. High-frequency network oscillations in cerebellar cortex. Neuron 58, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RP, Swanson PD, 1978. Vertical oculomotor apraxia and memory loss. Arch. Neurol 4, 149–153. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, et al. 2010. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain 133, 215–24. [DOI] [PubMed] [Google Scholar]
- Munro-Davies LE, Winter J, Azziz T, Steain JF, 1999. The role of the pedunculopontine region in basal-ganglia mechanisms of akinesia. Expt. Brain Res 128, 511–517. [DOI] [PubMed] [Google Scholar]
- Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, et al. , 2015. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med. Rev 20, 46–58. [DOI] [PubMed] [Google Scholar]
- Noga BR, Sanchez FJ, Villamil LM, O’Toole C, Kasicki S, et al. , 2017. LFP oscillations in the mesencephalic locomotor region during voluntary locomotion. Frontiers Neural Circuits 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte MW, Loscher W, Gernert M, 2006. Pedunculopontine neurons are involved in network changes in the kindling model of temporal lobe epilepsy. Neurobiol. Dis 23, 206–218. [DOI] [PubMed] [Google Scholar]
- Nosko D, Ferraye MU, Fraix V, Goetz L, Chabardes S, Pollak P, Debu B, 2015. Low-frequency vesus high-frequency stimulation of the pedunculopontine nucleus area in Parkinson’s disease: a randomized controlled trial. J. Neurol. Neurosurg. Psychiat 86, 674–679. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L, 2005. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Amer. J. Psychiat 162, 43–49. [DOI] [PubMed] [Google Scholar]
- Olincy DL, Braff LE, Adler KS, Cadenhead ME, Calkins M.e., et al. , 2014. Inhibition of the P50 cerebral evoked response to repeated auditory stimuli: results from the consortium on genetics of schizophrenia. Schizophr. Res 119, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondo WG, 2014. Sleep/wake problems in Parkinson’s disease: pathophysiology and clinicopathologic correlations. J. Neural Transm 121, S3–S13. [DOI] [PubMed] [Google Scholar]
- Orem J, Schlag-Rey M, Schlag J, 1973. Unilateral visual neglect and thalamic intralaminar lesions in the cat. Exp. Neurol 40, 784–797. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura T, 1998. Single midline thalamic neurons projecting to both the ventral striatum and the prefrontal cortex in the rat. Neurosci 86, 635–649. [DOI] [PubMed] [Google Scholar]
- Ozerdem A, Guntenkin B, Atagun I, Turp B, Başar E, 2011. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J. Affect. Disord 132, 325–332. [DOI] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM, 2009. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage 49, 3257–3268. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Llinás RR, 1997. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc. Nat. Acad. Sci. USA 94, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips S, Takeda Y, 2009. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int. J. Psychophysiol 73, 350–354. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD, 1995. The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog. Neurobiol 47, 105–133. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Francois C, 1992. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey:a PHA-L study of subcortical projections. J. Comp. Neurol 315, 137–159. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Francois C, 1990. The centre median and parafascicular thalamic nuclei project respectively to the sensorimotor and associative-limbic striatal territories in the squirrel monkey. Brain Res 510, 161–165. [DOI] [PubMed] [Google Scholar]
- Sakai K, El Mansari M, Jouvet M, 1990. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res 527, 213–223. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Gasbarri A, Campana E, Pacitti C, 1987. The organization of nucleus tegmenti pedunculopontinus neurons projecting to basal ganglia and thalamus:a retrograde fluorescent double labeling study in the rat. Neurosci. Lett 79, 11–6. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Vitale F, Capozzo A, Mazzone P, 2016. Cholinergic input from the pedunculopontine nucleus to the cerebellum: implications for deep brain stimulation in Parkinson’s disease. Neural Regen. Res 11, 729–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf W, Brandt MD, Storch A, Reichmann H, 2014Sleep disorders in Parkinson’s disease, J. Parkinsons Dis 4, 211–221. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall M, McDonald C, Marshall N, Walshe M, et al. , 2007. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol. Psychiat 62, 121–128. [DOI] [PubMed] [Google Scholar]
- Sherman D, Fuller PM, Marcus J, Yu J, Zhang P, et al. , 2015. Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and Parkinsonism. Frontiers Neurol 6, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN, 1966. Control of walking and running by means of electric stimulation of the midbrain. Biofizika 11, 659–666. [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, et al. , 2010. Gamma band unit activity and population responses in the pedunculopontine nucleus (PPN). J. Neurophysiol 104, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, 1993. Synchronization of cortical activity and its putative role in informtion processing and learning. Annu. Rev. Physiol 55, 349–374. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E, 1990. Brainstem modulation of rhythmic functions and behaviors, In: Brainstem Mechanisms of Behavior, Klemm WR and Vertes RP, (Eds.). John Wiley & Sons, New York, pp. 419–445. [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, et al. 2007. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease, Brain 130, 1596–1607. [DOI] [PubMed] [Google Scholar]
- Stefani A, Peppe A, Galati S, Stampanoni A, Bassi M, et al. 2013. The serendipity case of the pedunculopontine nucleus low-frequency brain stimulation: chasing a gait response, finding sleep, and cognitive improvement, Frontiers Neurol 4, 68(1–12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, 1999. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Handbook of Behavioral State Control. Cellular and molecular mechanisms, ed. Lydic R & Baghdoyan HA (CRC Press, New York: ), 327–347. [Google Scholar]
- Steriade M, Glenn LL, 1982. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J. Neurophysiol 48, 352–371. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR, 1988. The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev 68, 649–742. [DOI] [PubMed] [Google Scholar]
- Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R, 1990. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci 10, 2560–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie-Baker HJ, Segalowitz SJ, Black SE, McLean JA, Sullivan N, 1997. Improvement of spatial neglect with cold-water calorics: an electrophysiological test of the arousal hypothesis of neglect. J. Int. Neuropsychol. Soc 3, 394–402. [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E, 1990. Brainstem modulation of rhythmic functions and behaviors, In: Brainstem Mechanisms of Behavior, Klemm WR and Vertes RP, (Eds.), John Wiley & Sons, New York, pp. 419–445. [Google Scholar]
- Soteropoulos DS, Baker SN, 2006. Cortico-cerebellar coherence during a precision grip task in the monkey. J. Neurophysiol 95, 1194–1206. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T, 2003. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neurosci 119, 293–308. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco L, Al-Mefty K, Skinner R.d., Garcia-Rill E, 1997. Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease. Movement Dis 12, 655–664. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco l., Skinner RD, Garcia-Rill E, 1998. Disinhibition of the sleep-state dependent P1 potential in Parkinson’s disease-improvement after pallidotomy. Sleep Res Online 1, 62–70. [PubMed] [Google Scholar]
- Thevanasathan W, Silburn PA, Brooker H, Coyne TJ, Kahn S, et al. , 2010. The impact of low-frequency stimulation of the pedunculopontine nucleus region on reaction time in Parkinsonism, J. Neurol. Neurosurg. Psychiat 81, 1099–1104. [DOI] [PubMed] [Google Scholar]
- Thevanasathan W, Cole MH, Grapel C.l., Hyam JA, Jenkinson N, et al. , 2012. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation, Brain 135, 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M, 1997. Fast (mainly 30–100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J. Physiol 504, 153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Castro-Zaballa S, Cavelli M, Chase M, Falconi A, 2015. Neocortical 40 Hz oscillations during carbachol-induced rapid eye movement sleep and cataplexy. Eur. J. Neurosci 281, 318–325. [DOI] [PubMed] [Google Scholar]
- Trottenberg T, Fogelson N, Kuhn AA, Kivi A, Kupsch A, et al. , 2006. Subthalamic gamma activity in patients with Parkinson’s disease. Exp. Neurol 200, 56–65. [DOI] [PubMed] [Google Scholar]
- Uchitel OD, Protti DA, Sanchez V, Cherkesey BD, Sugimori M, et al. , 1992. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and trnsmitter release in mammalian synapses. Proc. Nat. Acad. Sci. USA 89, 3330–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P, et al. , 2012. Gamma band activity in the reticular activating system (RAS). Frontiers in Neurology; Sleep and Chronobiology 3, 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, D’Onofrio SM, Luster BR, J.R, Bosagno V, et al. , 2014. Pedunculopontine nucleus gamma band activity- preconscious awareness, waking, and REM sleep. Frontiers in Sleep and Chronobiol 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH, 2000. What is the significance of gamma wave activity in the pyriform cortex? Brain Res 877, 125–133. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, 2000. Are neocortical gamma waves related to consciousness? Brain Res 855, 217–224. [DOI] [PubMed] [Google Scholar]
- Voss U, Holzmann R, Tuin I, and Hobson JA, 2009. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep 32, 1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Y, Wang X, Yang H, Yu X, et al. , 2017. Enhanced gamma activity abd cross-frequency inyeraction of resting-state electroencephalographic oscillations in patienst with Alzheimer’s disease. Frontiers Aging Neuro 9, 243, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M, 2009. Pedunculopontine and laterodorsal tegmental nuclei contan distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci 29, 340–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Andriola M, Heilman KM, 1977. The electroencephalogram in neglect. J. Neurol. Sci 34, 343–348. [DOI] [PubMed] [Google Scholar]
- Watson RT, Heilman KM, Miller BD, King FA, 1974. Neglect after mesencephalic reticular formation lesions. Neurol, 24, 294–298. [DOI] [PubMed] [Google Scholar]
- Watson RT, Valenstein E, Heilman KM, 1981. Thalamic neglect. Possible role of the medial thalamus and reticularis in behavior. Arch. Neurol 38, 501–506. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, et al. , 1992. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron 9, 1099–1115. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Mennemeier M, Garcia-Rill E, Meythaler J, Mrak VW, et al. , 2006. Bias in magnitude estimation following left hemisphere injury. Neuropsychol, 44, 1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone V, Azumi K, Dement WC, Gulevich G, Kraimer H, et al. , 1975. REM phase deprivation and schizophrenia II, Arch. Gen. Psychiat 32, 1431–1436. [DOI] [PubMed] [Google Scholar]


