Abstract
The effectiveness of activated carbon (AC) in reducing the bioavailability of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was examined from the context of using in-situ sorbent amendments to remediate soils/sediments contaminated with polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/Fs). This technology has gained rapid acceptance based on observations that AC amendments predictably lower PCDD/F concentrations in water and bioaccumulation by simple aquatic organisms and earthworms; it has been assumed that bioavailability to mammals is similarly reduced, although this has been disproven for other sorbent materials. In this study TCDD was absorbed to a microporous AC using the incipient wetness method (TCDD-AC). An aqueous suspension of TCDD-AC, and an equivalent dosage of TCDD in corn oil, were administered by oral gavage to B6C3F1 mice. The relative bioavailability of TCDD-AC was determined by quantifying and comparing the hepatic induction of cyp1A1 (mRNA) and suppression of the IgM antibody forming cell cell (AFC) immune response, by the two forms of TCDD. A concentration-dependent response was observed for both assays when TCDD in corn oil was administered to mice. However, when equivalent masses of TCDD were administered as TCDD-AC, no induction of cyp1A1 or suppression of the IgM AFC response was observed. The absence of these two sensitive AHR-mediated responses in mice provides the first direct evidence that AC can sequester TCDD in a form that eliminates its bioavailability to mammals. These results support the premise that AC can be used to reduce the bioeffective dose of TCDD delivered to mammals, and that AC amendments may provide a low cost alternative to traditional remediation technologies.
Keywords: TCDD, Dioxin, Activated Carbon, Remediation, Sorbent Amendment, Bioavailability
Introduction
Polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs) are a group of persistent organic pollutants widely distributed in soils and sediments, frequently found at Superfund sites, and regulated at very low levels due to their potency as toxicants. These compounds are formed as unintended byproducts of anthropogenic activities including waste incineration, industrial production of herbicides and wood preservatives, and historically in chlor-alkali processes. Natural formation occurs via microbial biosynthesis, volcanic activity, forest fires, and in some clay deposits [1–3]. Significant improvements in emission controls coupled with more stringent regulations have contributed to an estimated 90% decrease in the annual release of dioxins into the environment between 1987 and 2000 [4]. Despite these reductions, soils and sediments remain contaminated at levels of concern due to the persistence of PCDD/Fs in natural environments [5]. This persistence is due to many factors including their structural resistance to microbial metabolism or other environmentally significant transformation processes, as well as limited conditions where biodegradation is naturally feasible, and their very low water solubilities and vapor pressures [6–8].
Polychlorinated dibenzo-p-dioxins and furans are regulated in units of toxic equivalents (TEQs). These compounds are commonly found in the environment as mixtures composed of congeners having different numbers of Cl substituents located at different positions on the two phenyl rings. The toxicity of PCDD/Fs is mediated in part through the aryl hydrocarbon receptor (AhR) and has been associated with immune suppression, chloracne, abnormal endocrine and nervous system function in humans, as well as liver and other organ cancers in animals [9]. The AhR-mediated toxicity of PCDD/Fs is known to be congener specific. The most toxic, with the correspondingly highest affinity for the AhR, is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which has been assigned a toxic equivalency factor (TEF) of 1.0. Other congeners are given a TEF value (from zero to one) based on their potency relative to TCDD. The toxic equivalents (TEQ) of a particular PCDD/F mixture is the sum of each congener concentration multiplied by its specific TEF. This method is used for regulatory purposes and takes into account the cumulative toxicity and concentration of all dioxin congeners comprising the mixture based on the assumption of additive effects on AhR activity [10].
Measurable levels of PCDD/Fs are present in nearly all soils in the U.S. Background concentrations in urban/suburban soils are greater and more variable than those found in rural soil samples, viz. 7-186 ng/kg and 2-23 ng/kg TEQ, respectively [11]. The widespread PCDD/F contamination results from a variety of factors including their formation both naturally [12, 13] and during anthropogenic activities, and their ability to partition between gas and particulate phases subjecting them to long-range atmospheric transport [14]. Atmospheric deposition, direct discharge, erosion and flooding events transport PCDD/Fs to surface waters. Aquatic sediments, in particular, represent an important sink for hydrophobic organic contaminants like PCDD/Fs [15]. The contaminated sediments act as a continual PCDD/F source, creating a concentration gradient from sediment pore water to the overlying water column [16].
Traditional remediation methods for soils and sediments at sites contaminated with toxic recalcitrant contaminants such as PCDD/Fs typically involves either removal of the contaminated material via dredging or excavation followed by disposal in a hazardous waste landfill, or hydraulic isolation using clay slurry walls and caps. The high costs, adverse ancillary environmental impacts, and long-term ineffectiveness of these techniques have motivated the development of new remediation approaches [17]. One promising technology involves the use of in-situ sorbent amendments to sequester PCDD/Fs in forms with reduced bioavailability and hence less potential for exposure; activated carbon (AC), organoclay, and apatite have been identified as the most promising additives [18]. The use of AC has been widely investigated due to its traditional role in water and wastewater treatment, and because similarly high surface area carbonaceous materials are components, albeit at low levels, of soils and sediments comprising approximately 4% to 9%, respectively, of the total organic carbon content that is naturally present [19, 20]. Furthermore, the ecological effects of AC amendments are thought to be minimal and likely short-term [21, 22], and implementing this technology is minimally invasive which reduces habitat destruction.
Recently, a number of studies have investigated the efficacy of in-situ AC sorbent amendments as a remediation alternative in soils and sediments contaminated with hydrophobic organic contaminants (HOCs). These studies have documented predictable reductions in pore water HOC concentrations and lowered bioaccumulation by simple aquatic organisms or earthworms. For instance, laboratory studies have shown that a 1-5% AC amendment to sediments manifested a 70-99% reduction of aqueous phase PCDDs as well as a corresponding 70-90% reduction in bioaccumulation by benthic organisms [15]. A limited number of field-scale studies have similarly shown reductions in HOC porewater concentrations and/or bioaccumulation in sediments amended with AC; increasing effectiveness over time was observed and attributed to progressive mass transfer [15, 23-26]. Thin-layer capping using a mixture of an inert material and AC resulted in a nearly 90% reduction of fluxes into the water column and the resulting bioaccumulation of PCDD/Fs by two aquatic invertebrates [27]. Similar studies reported that AC amendments to PCDD contaminated soils decreased porewater concentrations by as much as 99%, and TEQs in earthworms decreased concomitantly by 78-99% depending on soil characteristics [28]. Greater reduction efficiencies were achieved using sorbent particles of smaller size [29, 30]. These reductions in aqueous concentrations are largely predictable as ACs represent high surface area absorbents with a porous structure that is intrinsically suitable for sorption of nonpolar aromatic compounds [31]. Over 90% of porosity of the AC used in this study is from micropores, which are pores less than 2 nm in diameter (Table 1). The surfaces of these exceptionally small pores have a high affinity for planar hydrophobic compounds. Furthermore, the small diameter of the pores limits diffusion/release of the sorbed contaminants out of the material.
Table I.
Porosity and surface area of activated carbon
| Product Name | Supplier | Source | Surface area (m2/g) | Total Pore Volume (cc/g) |
Dominant pores (percentage of total pore volume) * |
|---|---|---|---|---|---|
| Darco G60 | Sigma Aldrich | Lignite Coal | 1076.2 | .671 | Mesopore (56.7%) |
| Darco FM-1 | Cabot Corp. | Lignite Coal | 650 | .505 | Mesopore (71.4%) |
| F400 | Calgon Carbon Corp. | Bituminous Coal | 948 | .521 | Micropore (69.1%) |
| TOG-LF 80×325 | Calgon Carbon Corp. | Bituminous Coal | 1220 | .450 | Micropore (67.2%) |
| WPC | Calgon Carbon Corp. | Coconut | 801 | .320 | Micropore (90.5%) |
Pore diameters: micropore: 0-2 nm and mesopore: 2-50 nm.
Although the studies described above suggest that AC amendments could reduce the potential for exposure of aquatic invertebrates and earthworms to HOCs, there is a paucity of direct evidence showing that sequestration of HOCs by AC reduces bioavailability to mammals [32], and none that measure an appropriate bioresponse. Studies documenting reduced bioavailability and exposure of higher order organisms to AC-sequestered HOCs are essential prior to the widespread adoption of this new remediation technology. In fact, the importance of evaluating the bioavailability of sorbed contaminants to an appropriate mammalian model was highlighted in two recent studies which clearly showed that even when bound to silica or intercalation between the individual sheets of montmorillonite clay, the bioavailability of sorbed TCDD to mice, as evidenced by suppression of immune function and induction of cyp1a1 mRNA, were not reduced compared to that observed when TCDD was administration in corn oil [33, 34]. Suppression of humoral immunity and induction of cyp1a1 mRNA in mice were specifically selected as these are widely established and arguably two of the most sensitive biomarkers of TCDD exposure [33, 34]. A study of PCDD/F-contaminated soils and sediments by Budinsky et al. [35] suggested the beneficial impacts of carbonaceous materials in soils and sediments on reduction of oral bioavailability of PCDD/Fs. Furthermore, oral ingestion of ball clays contaminated naturally with PCDDs and used as feed additives has been a major PCDD exposure route in livestock leading to their entry into the human food chain [36]. Whether PCDDs contained in ball clays were bound predominately to clay components or particulate organic constituents is unclear, but at least a fraction remained bioavailable. Also, young children are known to inadvertently ingest a significant amount (ca. 100-200 mg/day) of soil relative to their body mass [37, 38].
The use of sorbent amendments, mostly involving AC, has been rapidly accepted in remediation based on the premise that AC will sequester organic contaminants in a form which substantially reduces their bioavailability and hence exposure potential, especially for humans. The arguably premature acceptance of this new strategy in the management of contaminated sediments and soils rests almost entirely on studies showing that AC predictably reduces aqueous phase concentrations and hence (bio)accumulation by passive samplers, simple aquatic organisms and earthworms. One prior study indicated loss of TCDD accumulation in rat livers when TCDD was administered after sorption by AC [32]. Surprisingly, this single study used a mammalian model to provide evidence for the efficacy of AC in reducing PCDD/F bioavailability to mammals and hence human exposure potential, though this contention forms the basis of using AC amendments in remediation aimed at protecting human health. The goal of the present work is to address this critical knowledge gap. Worldwide remediation efforts for soils and sediments contaminated with PCDD/Fs have already begun incorporating AC amendments [20, 39]. Remarkably, the selection of this remedy has occurred with very limited data from one study [32] indicating that AC can reduce the bioavailability of TCDD in mammals and by inference humans. Our results show for the first time that AC can sequester TCDD in a form that eliminates its bioavailability to an appropriate mammalian (mouse) model using signature TCDD-induced biomarkers, namely suppression of immune function and induction of cyp1a1. These results provide the best direct evidence to support the feasibility of activated charcoal as a potential remediation technology for reducing human exposure from dioxin-contaminated soils and sediments and an alternative to capping and dredging.
Materials and Methods
Selection and Characterization of activated Carbon
Five activated carbons were characterized for possible use in this study (Table I). Surface area and pore size analyses were determined from measurements of nitrogen absorption at 77°K (LabQMC, Boynton Beach, FL, USA). Three criteria were used for the selection of an appropriate AC for the mice exposures: 1) Compatibility with water, i.e. the ability of AC to stay in suspension so that reproducible aliquots could be withdrawn and administered by oral gavage; 2) The physical ability of AC particles to pass through the oral gavage needle; 3) A preponderance of micropore volume to maximize sequestration of TCDD as indicated by thermogravimetric analysis (TGA) and pyrolysis mass spectrometry (described below).
Loading of AC with TCDD
The extremely low water solubility of 2,3,7,8-TCDD, 19.3 parts per trillion [35], made impractical the use of aqueous solutions to load AC with the quantities of TCDD necessary for oral administration to mice, our chosen mammalian model. Therefore, TCDD was loaded into the pore structure of WPC AC using the incipient wetness method as described in our previous work with silica [34] and montmorillonite clay [33]. The method involves the addition TCDD dissolved in a volume of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) equal to the specific pore volume of a known mass of AC. The DMSO is removed at 200°C under vacuum leaving TCDD within the pore structure of AC, referred to hereafter as TCDD-AC. Thermogravimetric analysis was used to quantify any mass loss during heating.
For this study, DMSO spiking solutions of TCDD were prepared at four concentrations (0, 1, 10, and 100 μg/mL) using a stock solution of TCDD (100 μg/mL) obtained from AccuStandard Inc (New Haven, CT). The WPC AC was weighed (500 mg) into screw-top Corex glass centrifuge tubes (30 mL) then 160 μL of the TCDD-DMSO solutions at each concentration were applied directly on the AC creating a slightly wetted powder. The centrifuge tubes were closed with polytetrafluoroethylene (PTFE) lined screw caps and the whole was vortex mixed for approximately 20 min. until the AC returned to a free-flowing powder. The mixtures were then allowed to equilibrate for 24 hours prior to isothermal removal of DMSO at 200°C under vacuum. This procedure resulted in final TCDD concentrations of ca. 0, 0.32, 3.2, and 32 μg/Kg of AC. Finally, 156.25 mg of each TCDD-AC dose were weighed into 20 mL glass scintillation vials then mixed with 5 mL deionized water to form aqueous suspensions. Four 5 mL aliquots of corn oil were prepared by spiking 50 μL of the 0, 1.0, 10, or 100 μg/mL TCDD in DMSO into 4.95 mL of corn oil. This resulted in 5 mL corn oil or aqueous AC suspensions with TCDD concentrations of 0, 0.01, 0.1, and 1.0 μg/mL. All mice received 200 μL of the solution corresponding to their respective treatment group.
Animals
Five to eight-week old pathogen-free B6C3F1 female mice were purchased from Charles River Breeding Laboratories (Portage, MI, USA). Upon arrival, 45 mice were randomized and transferred into 9 plastic cages (five animals per cage) containing sawdust bedding; each cage containing five mice comprised a different treatment group. Mice were acclimated for one week, during which food (Purina Certified Laboratory Chow) and water were provided ad libitum until body weights of 17-20 g were reached. Animal holding rooms were maintained on a 12:12 hour light:dark cycle at a temperature of 21 to 24 °C and a relative humidity of 40 to 60%. All procedures involving mice were in accordance with the Michigan State University Institutional Animal Care and Use Committee.
In-Vivo antibody-forming cell response
The experimental matrix consisted of nine different treatment groups as summarized in Table II. Six of the nine treatment groups (each comprised of five mice) were administered TCDD, either freely dissolved in corn oil or as TCDD-AC suspensions in water, at doses of 0.1, 1.0, and 10 μg TCDD/kg body weight/day (Table II) based on an average mouse weight of 20 g. One other group was kept naive (no oral exposures) and the two remaining treatment groups received only vehicle (i.e. corn oil or AC suspension) with no TCDD. Mice were dosed by oral gavage (200 μL) once per day for four consecutive days. The WPC AC and the TCDD-AC samples were delivered as aqueous suspensions by removing 200 μL from the 5 mL suspensions contained in glass scintillation vials. Care was taken by regularly mixing suspensions between deliveries to mice to eliminate settling of the suspension. After three consecutive days of administration by oral gavage, each treatment group (except the naïve control) received 5 × 108 sheep red blood cells (sRBC) (Colorado Serum Company, Denver, CO, USA) by intraperitoneal injection to initiate a humoral immune response. Four days post sRBC sensitization (day 7), mice were sacrificed via dislocation of the neck and total body, liver, and spleen weights were recorded. The timeline for the various treatments is summarized in Table II.
Table II.
Mice treatment groups and timeline for treatments
| Group* | Treatment |
|---|---|
| 1 | Corn Oil Vehicle |
| 2 | Corn Oil + TCDD (0.1 ug/kg) |
| 3 | Corn Oil + TCDD (1 ug/kg) |
| 4 | Corn Oil + TCDD (10 ug/kg) |
| 5 | Activated Carbon |
| 6 | Activated Carbon + TCDD (0.1 ug/kg) |
| 7 | Activated Carbon + TCDD (1 ug/kg) |
| 8 | Activated Carbon + TCDD (10 ug/kg) |
| 9 | Naive |
Each group consisted of n=5 mice
The Jerne plaque assay was performed for the enumeration of anti-sRBC IgM antibody-forming cells (AFCs) [41]. Briefly, 100 μL aliquots of the recovered splenocytes were combined with 0.5% melted agar (Difco/BD), guinea pig complement (Gibco/Invirtogen), and sRBC. The mixture was vortex mixed and poured on to a Petri dish, overlaid with a 24 × 50 mm glass microscope coverslip and incubated for 3 h at 37°C. The AFCs, each of which is an antibody secreting plasma cell, were enumerated using a Bellco (Vineland, NJ, USA) plaque viewer at 6.5× magnifications. Cells were enumerated with ZI Coulter particle counter (Beckman Coulter, Pasadena, CA, USA). Data are presented as anti-sRBC IgM AFC/1×106 spleen cells in culture.
Real time polymerase chain reaction
Mouse livers were placed in TRI Reagent (Sigma-Aldrich) and stored at −70°C. On the day of RNA extraction, homogenized livers were phase separated with bromochlorophenol, and RNA was precipitated from the aqueous phase by adding isopropanol. A Promega SV total RNA isolation system was employed for the remaining extraction, purification, and DNase treatment. Reverse transcription of total RNA using random primers was carried out with a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The cDNA was amplified with a Taqman primer/probe set for mouse cyp1al (Applied Biosystems) and analyzed using a 7900 HT fast real-time polymerase chain reaction (PCR) system (Applied Biosystems). Fold change values were calculated using the ΔΔCT method [42].
Statistical Analysis
Differences between means of treatment groups were determined with a parametric analysis of variance. Statistically significant differences between treatment groups and their controls were determined using Dunnett’s two tailed t test. Statistical analyses for real-time PCR were performed on the ΔCT values using Prism version 4.0a (Graphpad, La Jolla, CA, USA).
Results and Discussion
TCDD adsorption to WPC-activated carbon by incipient wetness
The black carbonaceous materials used as sorbents in this study strongly absorb incident radiation in both visible (Raman) and mid-infrared (FTIR) regions, hence these spectroscopic methods could not be used to assess TCDD-sorbent interactions. Additionally, established reference extraction methods used in the analysis of highly hydrophobic organic contaminants by high-resolution gas chromatography mass spectrometry (GC/MS) have been shown to be ineffective (i.e. low and variable recoveries) in quantifying PCDD/Fs and PCBs bound to porous graphitic carbonaceous materials like ACs [43–46]. Alternative methods were developed to help evaluate the physicochemical state of TCDD in the TCDD-AC samples and to validate dosage. Thermogravimetric analysis (TGA) of WPC AC, which had been subjected to the incipient wetness method utilizing dibenzo-p-dioxin (DD), showed that >90% of DMSO was removed by isothermal heating at 200°C for 2 h. Significant tailing observed in the TGA scan at 200°C indicating the continued gradual loss of small amounts of DMSO consistent with its presence in the micropores; similar tailing was not observed for a predominantly mesoporous AC (results not shown). Some minor additional DMSO loss occurred when the temperature was raised from 200 to 400°C Pyrolysis gas chromatography coupled mass spectrometry (GC/MS) was also used to characterize the WPC AC loaded with DD in DMSO via incipient wetness. Pyrolysis at 250°C and 350°C showed only the loss of DMSO (79 m/z) but not DD. Loss of DD (184 m/z) was only observed directly at temperatures of 550°C and above as shown in Figure 1. The pyrolysis GC/MS results using DD as a conservative surrogate for PCDD/Fs strongly support the conclusion that addition of TCDD dissolved in DMSO directly to the AC powder via the incipient wetness method, and the subsequent removal of DMSO at temperatures up to 250°C with isothermal treatment, would not result in the loss of TCDD. Furthermore, the high temperature required to desorb DD (melting point = 122°C, boiling point = 283.5°C) from the AC sample suggests that DD, and by inference the even more thermally stable TCDD (melting point = 305°C, boiling point = 500°C), is unaffected by the 200°C used to remove the DMSO and therefore left to reside within the micropores of WPC. This method of dosing was used in two prior studies with no loss of TCDD [33, 34].
Figure 1.
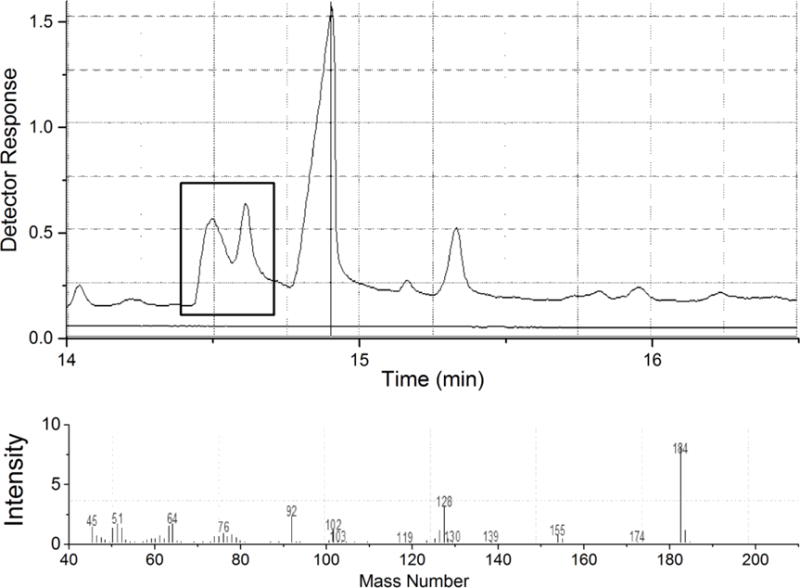
Chromatogram and mass spectrum obtained from pyrolysis GC/MS analysis of WPC activated carbon loaded with dibenzo-p-dioxin (DD) dissolved in dimethyl sulfoxide (DMSO) at 550°C. Direct observation of the loss of DD was observed for the two peaks shown in rectangular box.
Effect of WPC AC on humoral immune function
Prior to TCDD animal exposures, the effects of orally administered vehicle (WPC AC in water) on the IgM antibody response was examined in a preliminary mouse study since toxic effects of AC exposure have been observed in benthic invertebrates [21, 22]. Mice (four per treatment group) received a 200 μL dose of either water or a suspension of WPC AC in water for four consecutive days, with sensitization by injecting sRBC (i.e., antigen) on day 3. A third treatment group received the 200 μL water, but were not sensitized with sRBC to assess the background anti-sRBC response. The mice were sacrificed on day 7 and enumeration of IgM AFCs was performed using the Jerne plaque assay (Figure 2) as described above. The non-sensitized control group showed a minimal number of B cells secreting IgM antibodies directed against sRBC. The sRBC sensitized group receiving water showed a strong IgM response at approximately 700 IgM secreting B cells/million spleen cells. Likewise, the group that received the AC suspension showed a similar IgM response. These results demonstrate that WPC AC alone, at the 20 g/L suspension dosage, does not interfere with the IgM antibody response in mice. These results allowed determination of whether TCDD sequestered by the AC would illicit suppression of the humoral immune response as widely established when TCDD is administered in a bioavailable form such as dissolved in corn oil [33, 34].
Figure 2.
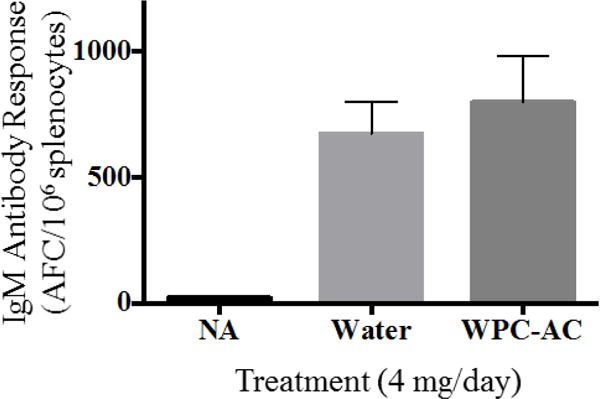
Preliminary mice feeding experiment with three different treatments: NA - no antigenic sensitization with sRBC, Water – 200 μL of water per day for 4 consecutive days then sensitized with sRBC, and Char – activated carbon (20 mg/mL) in 200 μL of water per day for 4 consecutive days then sensitized with sRBC. * Denotes p < 0.05 compared to water control group.
Effect of TCDD adsorbed on WPC AC on humoral immune function
The effect of TCDD on immune function in mice was evaluated by quantifying the elicitation of antigen-specific (sRBC) IgM AFCs. In agreement with previous work, TCDD in corn oil suppressed the anti-sRBC IgM AFC response in a dose-responsive manner. However, in direct contrast with previous work using silica and smectite clay sorbents, exposure of TCDD sequestered (sorbed) by WPC AC did not produce suppression of the AFC response, which was observed when TCDD was administered in corn oil (Figure 3). In addition, a dose-dependent correlation was observed for both spleen and liver weights with decreasing spleen mass and increasing liver mass associated with increasing exposure to TCDD in corn oil (Figure 4). Spleen and liver masses of mice exposed to the same levels of TCDD in the form of TCDD-AC showed no significant change compared to those of the AC vehicle control. There was a modest increase in liver mass induced by both the corn oil and AC vehicles compared to the naive treatment group.
Figure 3.
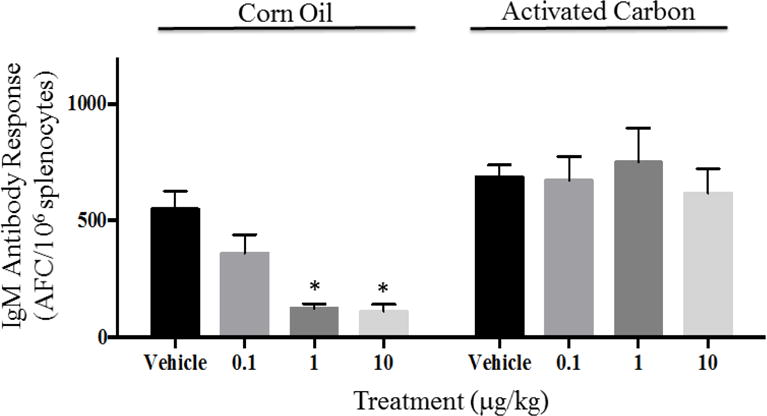
Tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of humoral activity observed using corn oil vehicle and not observed in activated carbon. *Denotes p < 0.05 compared to respective vehicle control group. Results are representative of two separate experiments.
Figure 4.
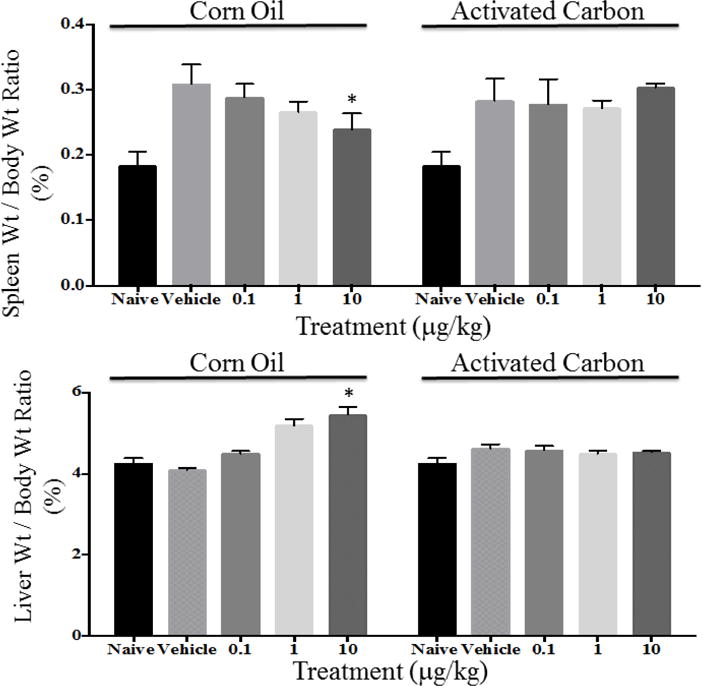
Spleen (A) and liver (B) weight to total body weight ratio. *Denotes p < 0.05 compared to the respective vehicle control.
To confirm the results described above, biodistribution of TCDD administered in corn oil or in the WPC AC suspension was verified using induction of cyp1al gene expression in liver, a gene readily induced in response to TCDD treatment (Figure 5). The TCDD dissolved in corn oil showed a dose-dependent increase in cyp1a1 fold induction at the mRNA level. In contrast, exposure to TCDD sequestered by AC did not induce cyp1a1 mRNA levels, when compared to the control. In fact, a slight reduction was observed for TCDD-AC at all three sorbed TCDD concentrations, as compared to the control (Figure 5). These results demonstrate that the bioavailability of TCDD when administered orally in corn oil was blocked when TCDD was sequestered by AC. The bioactivity of TCDD when administered in corn oil vehicle are in agreement with our previous studies [33, 34]. However, unlike silica and saponite clay sorbents loaded with TCDD using the same incipient wetness method, the use of WPC AC as a sorbent for TCDD was completely effective in eliminating TCDD bioavailability in the mouse model [33, 34]. This is likely due to differences in the mechanisms of sorption between the different sorbent materials. Unlike the formation of complexes involving exchangeable cations shown to be key to sorption in clay materials [31], affinity for AC is due to hydrophobic pore-filling processes stabilized by van der Waals attractions [47, 48].
Figure 5.
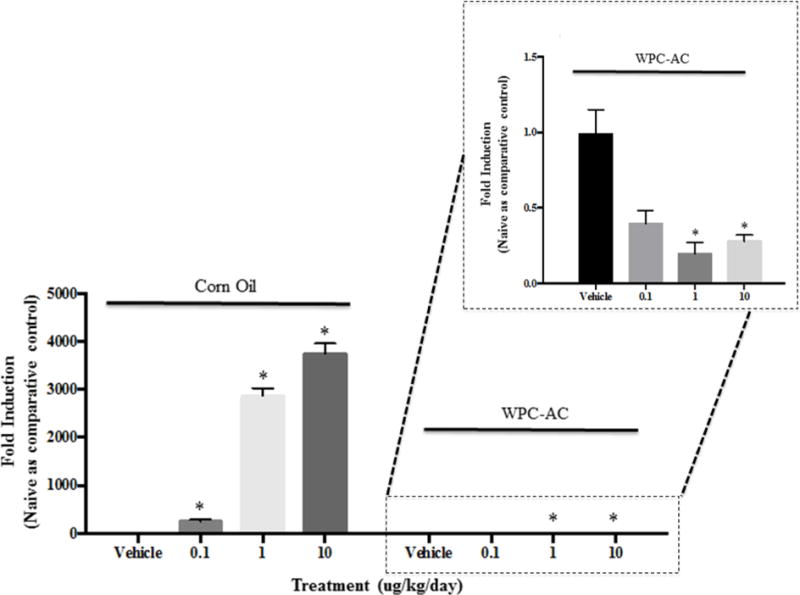
Effects on cyp1a1 mRNA induction and scaled response of the fold induction in mice exposed to TCDD-AC. *Denotes p < 0.05 compared to their respective vehicle (TCDD = 0) control.
Implications of the bioavailability of TCDD sequestered in AC
This research is meant to contribute to a better understanding and evaluation of the impact of sorbent amendments, specifically AC, on the bioavailability of TCDD to mammals, and by inference protection of human health. Remediation strategies for PCDD/F contaminated soils and sediments utilizing AC amendments have garnered rapid acceptance; they are already being used at major sites of PCDD/F contamination throughout the world despite limited peer-reviewed studies employing mammalian models to demonstrate reduced bioavailability and exposure potential to humans [20,39]. In the present study, the humoral immune response and induction of liver cyp1a1 mRNA levels, measures of relative bioavailability [49], were studied using mice exposed to TCDD either freely available in corn oil or sequestered by WPC AC. Whereas prior studies have documented that AC amendments can manifest reduction in freely available HOC concentrations and bioaccumulation by simple aquatic invertebrates and earthworms, this study demonstrates that when such a contaminant, i.e. TCDD, is strongly sequestered by AC, its oral bioavailability to a mammalian (mouse) model is eliminated. This is an important new finding, but it was not a forgone conclusion simply related to reduction in aqueous phase concentrations. In fact, our two prior studies showed that TCDD sequestered by either silica or by smectite clay produced an identical bioresponse (i.e. immune system suppression) in mice as that observed when TCDD was administered, freely available, in corn oil [33, 34]. Thus reductions in aqueous phase TCDD concentrations resulting from sorbent amendments are alone not good predictors of reduced bioavailability in mammals. The present results, demonstrating that AC can be used to reduce or eliminate TCDD mammalian bioavailability, supports the notion that soil and sediment amendments with carbonaceous sorbents may provide a low-cost, ecosystem friendly alternative to traditional remediation techniques such as dredging/excavation and landfilling. These results represent the first step in the evaluation of AC amendments as a remediation alternative. This should serve as a proof of concept showing the potential for AC as a material capable of eliminating mammalian bioavailability of sequestered TCDD. In this context, regulatory criteria may need to be adjusted to reflect this diminution of bioavailability. However, additional work is needed to characterize different types of carbonaceous materials, with different physical properties including pore structures and surface areas and their effectiveness as sorbent amendments in more complex environments and with prior PCDD/F contamination.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Ashwini Phadnis-Moghe, Jingeng Li, Natalia Kovalova, Mike Rizzo, Joseph Henriquez, and Jiajun Zhoufor for their assistance with mouse handling as well as Premachandra Gnanasiri for assistance with activated carbon preparations. Research reported in this publication was supported by AgBio Research at Michigan State University and the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES004911. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Kulkarni PS, Crespo JG, Afonso CAM. Dioxins sources and current remediation technologies - A review. Environ Int. 2008;34(1):139–153. doi: 10.1016/j.envint.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Alcock RE, Jones KC. Dioxins in the environment: A review of trend data. Environ Sci Technol. 1996;30(11):3133–3143. [Google Scholar]
- 3.Silk PJ, Lonergan GC, Arsenault TL, Boyle CD. Evidence of natural organochlorine formation in peat bogs. Chemosphere. 1997;35(12):2865–2880. [Google Scholar]
- 4.US E.P.A. An inventory of sources and environmental releases of dioxin-like compounds in the U.S. for the years 1987, 1995, and 2000 2006 [Google Scholar]
- 5.Yamashita N, Kannan K, Imagawa T, Villeneuve DL, Hashimoto S, Miyazaki A, Giesy JP. Vertical profile of polychlorinated dibenzo-p-dioxins, dibenzofurans, naphthalenes, biphenyls, polycyclic aromatic hydrocarbons, and alkylphenols in a sediment core from Tokyo Bay, Japan. Environ Sci Technol. 2000;34(17):3560–3567. [Google Scholar]
- 6.Orazio CE, Kapila S, Puri RK, Yanders AF. Persistence of chlrinated dioxins and furans in the soil environment. Chemosphere. 1992;25(7-10):1469–1474. [Google Scholar]
- 7.Tysklind M, Fangmark I, Marklund S, Lindskog A, Thaning L, Rappe C. Atmospheric transport and transformation of polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ Sci Technol. 1993;27(10):2190–2197. [Google Scholar]
- 8.Kao CM, Chen SC, Liu JK, Wu MJ. Evaluation of TCDD biodegradability under different redox conditions. Chemosphere. 2001;44(6):1447–1454. doi: 10.1016/s0045-6535(00)00464-1. [DOI] [PubMed] [Google Scholar]
- 9.Mukerjee D. Health impact of polychlorinated dibenzo-p-dioxins: A critical review. J Air Waste Manag Assoc. 1998;48(2):157–165. doi: 10.1080/10473289.1998.10463655. [DOI] [PubMed] [Google Scholar]
- 10.Safe SH. Development validation and problems with the toxic equivalency factor approach for risk assessment of dioxins and related compounds. J Anim Sci. 1998;76(1):134–141. doi: 10.2527/1998.761134x. [DOI] [PubMed] [Google Scholar]
- 11.Urban JD, Wikoff DS, Bunch ATG, Harris MA, Haws LC. A review of background dioxin concentrations in urban/suburban and rural soils across the United States: Implications for site assessments and the establishment of soil cleanup levels. Sci Total Environ. 2014;466:586–597. doi: 10.1016/j.scitotenv.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 12.Gadomski D, Tysklind M, Irvine RL, Burns PC, Andersson R. Investigations into vertical distribution of PCDDs and mineralogy in three ball clay cores from the United States exhibitinh the natural formation pattern. Environ Sci Technol. 2004;38(19):4956–4963. doi: 10.1021/es049579h. [DOI] [PubMed] [Google Scholar]
- 13.Ferrario JB, Byrne CJ, Cleverly DH. 2,3,7,8-dibenzo-p-dioxins in mined clay products from the United States: Evidence for possible natural origin. Environ Sci Technol. 2000;34(21):4524–4532. [Google Scholar]
- 14.Lohmann R, Jones KC. Dioxins and furans in air and deposition: A review of levels, behaviour and processes. Sci Total Environ. 1998;219(1):53–81. doi: 10.1016/s0048-9697(98)00237-x. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh U, Luthy RG, Cornelissen G, Werner D, Menzie CA. In-situ Sorbent Amendments: A New Direction in Contaminated Sediment Management. Environ Sci Technol. 2011;45(4):1163–1168. doi: 10.1021/es102694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen G, Broman D, Naes K. Freely dissolved PCDD/F concentrations in the Frierfjord, Norway: comparing equilibrium passive sampling with “active” water sampling. J Soils Sediments. 2010;10(2):162–171. [Google Scholar]
- 17.Bridges TS, Gustavson KE, Schroeder P, Ells SJ, Hayes D, Nadeau SC, Palermo MR, Patmont C. Dredging processes and remedy effectiveness: Relationship to the 4 Rs of environmental dredging. Integr Enviro Assess Manage. 2010;6(4):619–630. doi: 10.1002/ieam.71. [DOI] [PubMed] [Google Scholar]
- 18.US E.P.A. Use of amendments for in situ remediation at superfund sediment sites 2013 [Google Scholar]
- 19.Cornelissen G, Gustafsson O, Bucheli TD, Jonker MTO, Koelmans AA, Van Noort PCM. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol. 2005;39(18):6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- 20.Patmont CR, Ghosh U, LaRosa P, Menzie CA, Luthy RG, Greenberg MS, Cornelissen G, Eek E, Collins J, Hull J, Hjartland T, Glaza E, Bleiler J, Quadrini J. In Situ Sediment Treatment Using Activated Carbon: A Demonstrated Sediment Cleanup Technology. Integr Enviro Assess Manage. 2015;11(2):195–207. doi: 10.1002/ieam.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckingham B, Buys D, Vandewalker H, Ghosh U. Observations of limited secondary effects to benthic invertebrates and macrophytes with activated carbon amendment in river sediments. Environ Toxicol Chem. 2013;32(7):1504–1515. doi: 10.1002/etc.2231. [DOI] [PubMed] [Google Scholar]
- 22.Janssen EML, Beckingham BA. Biological responses to activated carbon amendments in sediment remediation. Environ Sci Technol. 2013;47(14):7595–7607. doi: 10.1021/es401142e. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman JR, Werner D, Ghosh U, Millward RN, Bridges TS, Luthy RG. Effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environ Toxicol Chem. 2005;24(7):1594–1601. doi: 10.1897/04-368r.1. [DOI] [PubMed] [Google Scholar]
- 24.Cho YM, Werner D, Choi YJ, Luthy RG. Long-term monitoring and modeling of the mass transfer of polychlorinated biphenyls in sediment following pilot-scale in-situ amendment with activated carbon. J Contam Hydrol. 2012;129:25–37. doi: 10.1016/j.jconhyd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Choi YJ, Cho YM, Gala WR, Luthy RG. Measurement and modeling of activated carbon performance for the sequestration of parent- and alkylated-polycyclic aromatic hydrocarbons in petroleum-impacted sediments. Environ Sci Technol. 2013;47(2):1024–1032. doi: 10.1021/es303770c. [DOI] [PubMed] [Google Scholar]
- 26.Choi YJ, Cho YM, Werner D, Luthy RG. In situ sequestration of hydrophobic organic contaminants in sediments under stagnant contact with activated carbon. 2. Mass transfer modeling. Environ Sci Technol. 2014;48(3):1843–1850. doi: 10.1021/es404209v. [DOI] [PubMed] [Google Scholar]
- 27.Josefsson S, Schaanning M, Samuelsson GS, Gunnarsson JS, Olofsson I, Eek E, Wiberg K. Capping efficiency of various carbonaceous and mineral materials for in situ remediation of polychlorinated dibenzo-p-dioxin and dibenzofuran contaminated marine sediments: Sediment-to-water fluxes and bioaccumulation in boxcosm tests. Environ Sci Technol. 2012;46(6):3343–3351. doi: 10.1021/es203528v. [DOI] [PubMed] [Google Scholar]
- 28.Fagervold SK, Chai YZ, Davis JW, Wilken M, Cornelissen G, Ghosh U. Bioaccumulation of polychlorinated dibenzo-p-dioxins/dibenzofurans in E. fetida from floodplain soils and the effect of activated carbon amendment. Environ Sci Technol. 2010;44(14):5546–5552. doi: 10.1021/es9027138. [DOI] [PubMed] [Google Scholar]
- 29.Chai YZ, Davis JW, Wilken M, Martin GD, Mowery DM, Ghosh U. Role of black carbon in the distribution of polychlorinated dibenzo-p-dioxins/dibenzofurans in aged field-contaminated soils. Chemosphere. 2011;82(5):639–647. doi: 10.1016/j.chemosphere.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Chai YZ, Currie RJ, Davis JW, Wilken M, Martin GD, Fishman VN, Ghosh U. Effectiveness of activated carbon and biochar in reducing the availability of polychlorinated dibenzo-p-dioxins/dibenzofurans in soils. Environ Sci Technol. 2012;46(2):1035–1043. doi: 10.1021/es2029697. [DOI] [PubMed] [Google Scholar]
- 31.Johnston CT, Khan B, Barth EF, Chattopadhyay S, Boyd SA. Nature of the interlayer environment in an organoclay optimized for the sequestration of dibenzo-p-dioxin. Environ Sci Technol. 2012;46(17):9584–9591. doi: 10.1021/es300699y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poiger H, Schlatter C. Influence of solvents and adsorbents on dermal and intestinal absorption of TCDD. Food Cos Toxicol. 1980;18(5):477–481. doi: 10.1016/0015-6264(80)90160-1. [DOI] [PubMed] [Google Scholar]
- 33.Boyd SA, Johnston CT, Pinnavaia TJ, Kaminski NE, Teppen BJ, Li H, Khan B, Crawford RB, Kovalova N, Kim SS, Shao H, Gu C, Kaplan BLF. Suppression of humoral immune responses by 2,3,7,8-tetrachlorodibenzo-p-dioxin intercalated in smectite clay. Environ Toxicol Chem. 2011;30(12):2748–2755. doi: 10.1002/etc.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan BLF, Crawford RB, Kovalova N, Arencibia A, Kim SS, Pinnavaia TJ, Boyd SA, Teppen BJ, Kaminski NE. TCDD adsorbed on silica as a model for TCDD contaminated soils: Evidence for suppression of humoral immunity in mice. Toxicology. 2011;282(3):82–87. doi: 10.1016/j.tox.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budinsky RA, Rowlands JC, Casteel S, Fent G, Cushing CA, Newsted J, Giesy JP, Ruby MV, Aylward LL. A pilot study of oral bioavailability of dioxins and furans from contaminated soils: Impact of differential hepatic enzyme activity and species differences. Chemosphere. 2008;70(10):1774–1786. doi: 10.1016/j.chemosphere.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Huwe JK. Dioxins in food: A modern agricultural perspective. J Agric Food Chem. 2002;50(7):1739–1750. doi: 10.1021/jf011265f. [DOI] [PubMed] [Google Scholar]
- 37.Vanwijnen JH, Clausing P, Brunekreef B. Estimated soil ingestion by children. Environ Res. 1990;51(2):147–162. doi: 10.1016/s0013-9351(05)80085-4. [DOI] [PubMed] [Google Scholar]
- 38.Stanek EJ, III, Calabrese EJ, Xu B. Meta-analysis of mass-balance studies of soil ingestion in children. Risk Analysis. 2012;32(3):433–447. doi: 10.1111/j.1539-6924.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 39.Lofrano G, Libralato G, Minetto D, De Gisi S, Todaro F, Conte B, Calabro D, Quatraro L, Notarnicola M. In situ remediation of contaminated marine sediment: an overview. Environ Sci Pollut Res. 2016 doi: 10.1007/s11356-016-8281-x. [DOI] [PubMed] [Google Scholar]
- 40.Marple L, Brunck R, Throop L. Water solubility of 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Environ Sci Technol. 1986;20:180–182. doi: 10.1021/es00144a012. [DOI] [PubMed] [Google Scholar]
- 41.Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140(356):405–&. [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Windal I, Miller DJ, De Pauw E, Hawthorne SB. Supercritical fluid extraction and accelerated solvent extraction of dioxins from high- and low carbon fly ash. Anal Chem. 2000;72(16):3916–3921. doi: 10.1021/ac9914972. [DOI] [PubMed] [Google Scholar]
- 44.Langenfeld JJ, Hawthorne SB, Miller DJ, Pawliszyn J. Kinetic study of supercritical fluid extraction of organic contaminants from heterogeneous environmental samples with carbon dioxide and elevated temperatures. Anal Chem. 1995;67(10):1727–1736. [Google Scholar]
- 45.Beckingham B, Ghosh U. Field-scale reduction of PCB bioavailability with activated carbon amendment to river sediments. Environ Sci Technol. 2011;45(24):10567–10574. doi: 10.1021/es202218p. [DOI] [PubMed] [Google Scholar]
- 46.Alexandrou N, Lawrence MJ, Pawliszyn J. Cleanup of complex organic mixtures using supercritical fluids and selective absorbents. Anal Chem. 1992;64(3):301–311. [Google Scholar]
- 47.Lesage G, Sperandio M, Tiruta-Barna L. Analysis and modelling of non-equilibrium sorption of aromatic micro-pollutants on GAC with a multi-compartment dynamic model. Chem Eng J. 2010;160(2):457–465. [Google Scholar]
- 48.Lui C, Li H, Johnston CT, Boyd SA, Teppen BJ. Relating clay structural factors to dioxin adsorption by smectites: Molecular dynamics simulations. Soil Sci Soc Amer J. 2012;76:110–120. [Google Scholar]
- 49.Council NR. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications. The National Academies Press; Washington, DC: 2003. p. 432. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


