Abstract
To evaluate the influence of sex and gender on clinical characteristics and survival in multiple system atrophy (MSA), we reviewed MSA patients with autonomic testing 1998–2012. Of 685 patients, 52% were male. Median survival overall was 7.3 years for males, 7.6 years for females. Survival from diagnosis was 2.9 years in males, 3.8 years in females. Females were more likely to initially manifest motor symptoms. Males were more likely to have orthostatic intolerance and early catheterization. In conclusion, our data show longer survival from diagnosis in females and slight overall survival benefit which may be related to initial motor manifestations.
Keywords: multiple system atrophy, sex, gender, parkinsonism, ataxia, autonomic
Introduction
Since the Institute of Medicine highlighted poorly understood sex differences in brain function, there have been further advances in understanding sex and gender influences in neurodegenerative disease1, 2. Multiple system atrophy (MSA) is a neurodegenerative disorder characterized by autonomic failure and motor involvement of predominantly parkinsonism (MSA-P) or cerebellar ataxia (MSA-C). Clinical manifestations of autonomic failure may include orthostatic hypotension, genitourinary dysfunction with sexual dysfunction and urinary incontinence, anhidrosis and constipation3, 4. Differences in autonomic and motor symptoms in males and females with MSA have been variably reported with many studies focusing on survival. Longer survival has been reported in males in some studies5–7 while others showed no difference8–14 or longer survival in females15. As results on sex and gender differences in MSA are conflicted, we sought to examine the influence of sex and gender on clinical features and survival in MSA.
Methods
All patients with the diagnosis of probable or possible MSA evaluated at Mayo Clinic, Rochester between January 1998 and December 2012 were retrospectively reviewed3. Inclusion criteria included standardized autonomic function testing. Patients were excluded if secondary causes of parkinsonism, ataxia or autonomic failure were considered more likely than MSA or clinical and laboratory features suggested an alternative diagnosis to MSA. Severity of autonomic dysfunction was graded using the composite autonomic severity score16. Initial symptoms were defined as motor (including parkinsonism, ataxia, tremor, and falls), autonomic (orthostatic intolerance, syncope, urinary dysfunction, bowel symptoms, sexual dysfunction, and anhidrosis), or combined if both autonomic and motor symptom onset occurred simultaneously or within 1 month. In males, erectile dysfunction was considered as the onset symptom if it occurred within 1 year from onset of urinary symptoms signifying genitourinary failure. Time of diagnosis refers to the date that patient received the diagnosis of possible or probable MSA. Clinical phenotype of MSA-P or MSA-C was assigned based on predominant symptom complex and examination findings at initial evaluation as recorded from clinical history, neurologic examination, and standardized patient-completed symptom questionnaire as previously described14. Living patients were called to assess for development of symptoms since last neurologic examination. Survival data was obtained from the clinical record, Social Security Death Index or telephone contact.
Statistical analyses were performed using SAS, version 9.3 (SAS Inc, N.C.), with statistical significance set at p < 0.05. Student’s t-test was used for normally distributed continuous variables with Mann-Whitney U-test for skewed data; Chi-square analysis was used for categorical variables. We have reported both unadjusted and false discovery rate corrected p-values in this study. Traditional approaches for controlling error rates in the presence of multiple comparisons include strong and weak control of familywise error rates, using techniques such as the Bonferroni correction17. The false discovery rate approach is shown to be more powerful than methods like the Bonferroni correction that control false positive rates18.
Survival analyses were performed using Kaplan-Meier estimates with survival curves. For patients without a death date or recent contact, the last contact date was used as the censor date. This study was approved by the Mayo Clinic Institutional Review Board.
Results
Of 685 patients with MSA, 356 (52%) were male (Table 1). There was no difference in age of onset or MSA subtype between males and females. A total of 568 patients were deceased with autopsy confirmation of MSA in 36 patients. Of the remaining 117 patients, 80 were considered alive based on phone call or encounter in the medical record within the past 6 month; there were 37 patients without recent contact and no known death date. Median overall survival from the time of onset to death was 7.3 years for males and 7.6 years for females (p = 0.0401; Figure). The median time from diagnosis to death was 2.9 years in males and 3.8 years in females (p = 0.0009; Figure).
Table 1.
Demographics and clinical characteristics of MSA patients
| All MSA Patients 685 (100) |
Male MSA Patients 356 (52) |
Female MSA Patients 329 (48) |
p-valuea | False Discovery Rate adjusted p-values | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at Onset | 60.9 (9.6) | 60.6 (9.8) | 61.3 (9.3) | 0.8323 | 0.9047 |
| Probable MSA | 594 (87) | 300 (84) | 294 (89) | 0.0498 | 0.0845 |
| Subtype | 0.3019 | 0.4193 | |||
| MSA-P | 430 (63) | 230 (65) | 200 (61) | ||
| MSA-C | 255 (37) | 126 (35) | 129 (39) | ||
| Clinical Features | |||||
| Parkinsonism | 526 (77) | 280 (79) | 246 (70) | 0.2295 | 0.3404 |
| Ataxia | 338 (50) | 176 (50) | 162 (49) | 0.8737 | 0.9101 |
| Falls at any time | 458 (67) | 226 (63) | 232 (71) | 0.0507 | 0.0845 |
| Falls within 3 years | 358 (52) | 170 (48) | 188 (57) | 0.0140 | 0.0318 |
| Bladder Symptoms | 572 (84) | 297 (83) | 275 (84) | 0.9551 | 0.9551 |
| Urinary Catheterization | 111 (16) | 79 (22) | 32 (10) | <0.0001 | 0.0004 |
| Urinary Catheterization within 3 years | 58 (8) | 39 (11) | 19 (6) | 0.0190 | 0.0396 |
| Early Orthostatic Intoleranceb | 240 (35) | 142 (40) | 98 (30) | 0.0056 | 0.0156 |
| Stridor | 176 (26) | 93 (26) | 83 (25) | 0.7887 | 0.9047 |
| Dream enactment behavior | 304 (44) | 161 (45) | 143 (43) | 0.6433 | 0.8041 |
| Autonomic Testing | |||||
| Supine systolic blood pressure | 148 (27.2) | 149 (26.3) | 147 (28.3) | 0.2315 | 0.3404 |
| Supine diastolic blood pressure | 83 (14.9) | 84 (13.1) | 81.1 (16.5) | 0.0091 | 0.0228 |
| Systolic blood pressure at 1 minute | 120 (26.5) | 118 (26.3) | 122 (26.6) | 0.0448 | 0.0845 |
| Diastolic blood pressure at 1 minute | 74 (18.9) | 74 (16.5) | 74.4 (21.3) | 0.7993 | 0.9047 |
| Systolic blood pressure at 5 minutes | 112 (26.5) | 109 (26.3) | 116 (26.2) | 0.0001 | 0.0004 |
| Diastolic blood pressure at 5 minutes | 68 (27.1) | 66 (31.5) | 72 (21) | 0.0036 | 0.0113 |
| Systolic blood pressure drop at 1 minute | 28.4 (22.4) | 31.6 (22.0) | 25.0 (22.5) | <0.0001 | 0.0004 |
| Diastolic blood pressure drop at 1 minute | 7.7 (12.4) | 9.4 (11.1) | 5.8 (13.5) | <0.0001 | 0.0004 |
| Systolic blood pressure drop at 5 minute | 36.1 (30.7) | 41.1 (30.4) | 30.7 (30.1) | <0.0001 | 0.0004 |
| Diastolic blood pressure drop at 5 minute | 10.5 (14.5) | 13.2 (14.5) | 7.7 (14.0) | <0.0001 | 0.0004 |
| Systolic blood pressure <90 mmHg at 5 minutes | 171 (25) | 106 (30) | 65 (20) | 0.0027 | 0.0096 |
| CASS Totalc | 5.50 (2.39) | 5.52 (2.16) | 5.47 (2.62) | 0.3979 | 0.5236 |
Values displayed are mean (standard deviation) for continuous variables and frequency (%) for categorical variables.
Between male and female
Within 1 year of onset
Score ranges from 0 (no abnormalities) to 10 (maximal autonomic failure)
Abbreviations: CASS, composite autonomic severity score; MSA, multiple system atrophy; MSA-C, multiple system atrophy-cerebellar; MSA-P, multiple system atrophy parkinsonism
Figure. Survival curves for female and male MSA patients.
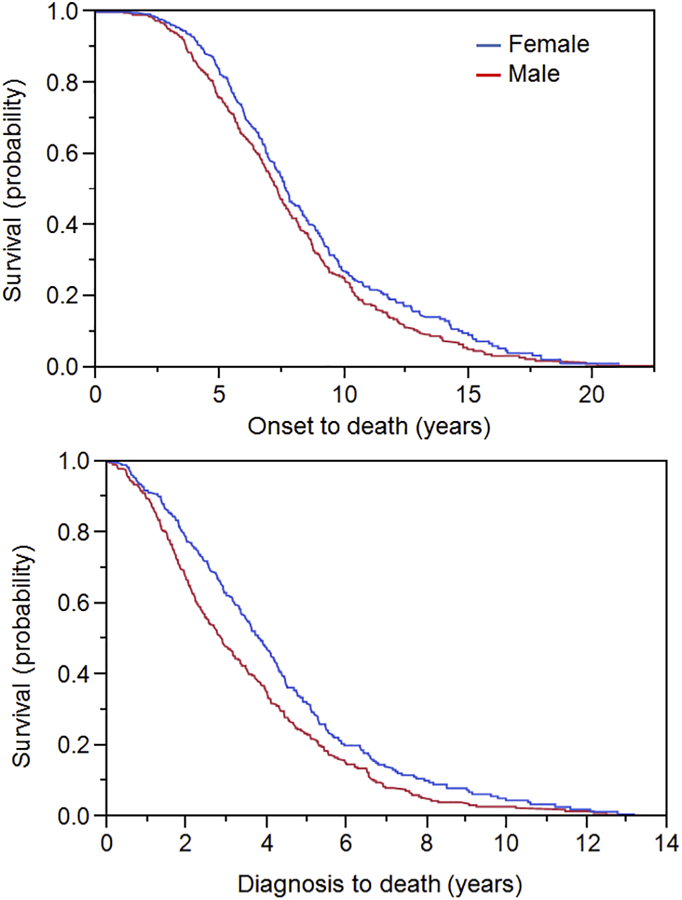
Censored Kaplan-Meier survival curves for female (blue) and male (red) MSA patients showing probability of death from symptom onset (top) and probability of death from time of diagnosis (bottom).
There was a significant difference between males and females in the type of onset symptoms reported when categorized as motor-only symptoms, autonomic-only symptoms, or combined (motor and autonomic symptoms occurring within 1 month) (p = 0.0026). In all patients, initial motor symptoms were most common; however, motor-only symptoms at onset were more common in females (236 patients, 72%) compared to males (213 patients, 60%) while autonomic-only symptoms were more common in males (116 patients, 33%) compared to females (70 patients, 21%). Combined symptoms occurred a minority of the time (50 patients, 7%). There was no difference in sexes regarding symptoms which led patients to neurology presentation (p = 0.0531). In both sexes, motor symptoms were most likely to lead to presentation to a neurologist (375 patients, 55%). While solely autonomic symptoms rarely brought patients to presentation (74 patients, 11%), the combination of autonomic and motor symptoms led to presentation in approximately a third of patients (236 patients, 34%).
Clinical features present at any point in the disease course and autonomic testing results at presentation are shown in Table 1. Falls early in the disease course were more common in females. Regarding autonomic symptoms, bladder symptoms were similar between sexes however males were more likely to undergo urinary catheterization, especially early in the disease course. Orthostatic intolerance within the first year of the disease course was also more common in males. Objectively, males had a larger and more severe drop in blood pressure during head-up tilt testing.
Sexual dysfunction was differentially addressed between females and males: 246 (69%) male patients had sexual dysfunction addressed and documented in the clinical record compared to 17 (5%) female patients (p = 0.0001). When sexual dysfunction was addressed and documented, it was present in 237 (96%) males and 5 (29%) females (p = 0.0001).
Discussion
This study shows sex and gender differences in MSA regarding symptom onset and presentation, and in survival. In addition to a slightly longer overall disease course, females were more likely to have motor onset of symptoms and receive the diagnosis of MSA earlier than males. Males were more likely to have autonomic symptoms at onset, which tended to be more severe throughout the disease course. While the overall survival benefit was 3.6 months in females, the difference in time from diagnosis to death was almost 1 year between sexes. These findings are likely due to both biological (sex) differences and perhaps societal (gender) issues which influence presentation and time to diagnosis.
Our findings build on previous large studies in MSA which suggested a gender difference in survival15. Our earlier work with this cohort did not find a survival difference between males and females but noted a significant difference in the time from diagnosis to death14. We hypothesized that females came to presentation earlier than males based on a differing symptom complex which led us to further evaluate all patients in the cohort with regard to symptoms at onset and presentation and in doing so, we found that an additional 27 patients (4%) in the cohort had died over the extended study period. Using updated death data, a small survival difference between males and females became evident. This highlights the importance of having death data on a significant number of subjects which may contribute to the lack of survival differences found in prospective studies in MSA10, 13.
The symptom complex at onset may relate to the earlier diagnosis of MSA in females, specifically that motor presentation likely prompts earlier referral to a neurologist rather than autonomic symptom onset. Males, who tend to have more autonomic symptoms at onset, may seek care at other specialists prior to motor symptoms becoming evident, leading to a delay in diagnosis. Our findings of less severe urinary dysfunction in females with MSA are consistent with previous reports19 however an alternative possibility is that autonomic symptoms are less emphasized in females, specifically that females may attribute bladder dysfunction to other causes or minimize symptoms. Additionally, our study showed that sexual dysfunction is rarely addressed in females. Another explanation for the shortened survival in males is that by not including longstanding sexual dysfunction, the disease course is artificially shortened. However, sexual dysfunction in males may be due to a myriad of causes and only sexual dysfunction occurring in the present of urinary dysfunction is likely to be neurologic.
In keeping with earlier studies, the severity of autonomic dysfunction, as evidence by early need for catheterization and orthostatic hypotension, may be contributing to worse survival in males 20–22. However, early falls are a strong predictor for shortened survival in MSA and even though females were more likely to manifest early falls, they maintained a slight survival advantage14. One explanation may be that autonomic symptoms may be additive as a negative prognosticator in males. Another possibility is that earlier diagnosis in females expedites involvement in subspecialty care which may improve survival.
Both biologic issues and gender may be influencing the differences seen in the study. The autonomic nervous system is sexually dimorphic beyond the genital system with differences in blood pressure regulation23, thermoregulation24, and urinary tract25. Anatomical and physiological differences are likely contributing to sex differences in symptom manifestation and neurodegeneration in MSA. Gender issues related to assessment of urogenital symptoms in men and women are also evident. These differences gain importance in the context of the current consensus criteria which uses autonomic findings of orthostatic hypotension and urinary incontinence to diagnose probable MSA3. Less severe autonomic failure in females may lead to diagnostic uncertainty or impact inclusion in clinical trials that require patients fulfill criteria for probable but not possible MSA.
Gender issues may be influencing time to diagnosis in MSA. There is some data in gender-specific studies to suggest that men may delay seeking care when they become ill26, 27. However, many of these studies focus on differences between men and women rather than a particular group and data on gender differences in neurodegenerative disease is lacking.
Weaknesses of this study include its retrospective nature with different providers evaluating patients over an extended period; for instance, patient reporting of bladder symptoms could include frequency, urgency, retention and/or incontinence. These symptoms may be related to confounding sex-specific factors such as childbirth or benign prostatic hypertrophy. However, the use of standardized patient-completed questionnaires which included autonomic symptoms and motor symptoms helped reduce bias from differing approaches and documentation.
Future work to further elucidate sex and gender differences in MSA will be helpful to understand the prognostic differences between males and females and encompass sex and gender issues in biomarker development and clinical trial design.
Highlights.
Sex and gender may influence clinical characteristics and survival in patients with MSA.
The median duration from diagnosis to death is almost 1 year shorter in males than females with MSA.
Males are more likely to report only autonomic symptoms at onset compared to females and were more likely to report orthostatic intolerance and undergo early urinary catheterization compared to females.
Females were more likely to report onset of motor symptoms and have falls within the first 3 years of disease compared to males.
Acknowledgments
Funding Sources: Supported in part by NIH (P01NS44233, U54NS065736, K23NS075141, R01 FD004789, R01 NS092625) and Mayo CCaTS (UL1TR000135), and Cure MSA Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure/Conflict of Interest: There is no support or financial issues from all authors relative to the research covered in the submitted manuscript.
Financial disclosures:
Elizabeth A. Coon reports no disclosures.
Renee M. Nelson reports no disclosures.
David M. Sletten reports no disclosures.
Mariana D. Suarez reports no disclosures.
J. Eric Ahlskog reports no disclosures.
Eduardo E. Benarroch reports no disclosures.
Paola Sandroni reports no disclosures.
Phillip A. Low reports no disclosures.
Wolfgang Singer reports no disclosures.
References
- 1.Medicine Io. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, D.C.: National Academy Press, 2001. [PubMed] [Google Scholar]
- 2.Zagni E, Simoni L, Colombo D. Sex and Gender Differences in Central Nervous System-Related Disorders. Neurosci J 2016;2016:2827090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med 2015;372:249–263. [DOI] [PubMed] [Google Scholar]
- 5.Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain 1994;117 ( Pt 4):835–845. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Mov Disord 2008;23:294–296. [DOI] [PubMed] [Google Scholar]
- 7.O’Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 2008;131:1362–1372. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 2002;125:1070–1083. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology 1997;48:384–393. [DOI] [PubMed] [Google Scholar]
- 10.Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 2013;12:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm (Vienna) 2014;121:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa JJ, Singer W, Parsaik A, et al. Multiple system atrophy: prognostic indicators of survival. Mov Disord 2014;29:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low PA, Reich SG, Jankovic J, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol 2015;14:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coon EA, Sletten DM, Suarez MD, et al. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain 2015;138:3623–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Jeon BS, Lee JY, Yun JY. Survival of Korean patients with multiple system atrophy. Mov Disord 2011;26:909–912. [DOI] [PubMed] [Google Scholar]
- 16.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748–752. [DOI] [PubMed] [Google Scholar]
- 17.Osborne J Estimating the false discovery rate using SAS. SAS Users Group International Proceedings 2006;190:1–10. [Google Scholar]
- 18.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014;67:850–857. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Sakakibara R, Uchiyama T, et al. Time-dependent changes and gender differences in urinary dysfunction in patients with multiple system atrophy. Neurourol Urodyn 2014;33:516–523. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain : a journal of neurology 2002;125:1070–1083. [DOI] [PubMed] [Google Scholar]
- 21.Tada M, Onodera O, Ozawa T, et al. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Archives of neurology 2007;64:256–260. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa JJ, Singer W, Parsaik A, et al. Multiple system atrophy: prognostic indicators of survival. Movement disorders : official journal of the Movement Disorder Society 2014;29:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol 2016;101:349–355. [DOI] [PubMed] [Google Scholar]
- 24.Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci 2016;196:75–80. [DOI] [PubMed] [Google Scholar]
- 25.Patra PB, Patra S. Sex differences in the physiology and pharmacology of the lower urinary tract. Curr Urol 2013;6:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs 2005;49:616–623. [DOI] [PubMed] [Google Scholar]
- 27.Wyke S, Hunt K, Ford G. Gender differences in consulting a general practitioner for common symptoms of minor illness. Soc Sci Med 1998;46:901–906. [DOI] [PubMed] [Google Scholar]


