Abstract
Objective:
Sleep changes substantially during adolescence; however, our understanding of age-related differences in specific electroencephalographic waveforms during this developmental period is limited.
Method:
Sigma power, spindle characteristics and cognitive data were calculated for fast (~13Hz) central and slow (~11Hz) frontal sleep spindles for a large cross-sectional sample of adolescents (N=134, aged 12-21 years, from the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study).
Results:
Older age (and advanced pubertal development) was associated with lower absolute sigma power and greater fast spindle density, with spindles having a shorter duration and smaller amplitude and occurring at a faster average frequency than at a younger age. Spindle characteristics were not directly associated with cognition. An indirect relationship (age*density) provided some evidence for an association between better episodic memory performance and greater spindle density only for younger adolescents.
Conclusion:
Spindle characteristics in adolescents differed according to age, possibly reflecting underlying differences in thalamo-cortical connectivity, and may play a role in episodic memory early in adolescence.
Significance:
Sleep spindles may serve as a marker of adolescent development, likely reflecting brain maturational status. Investigating specific spindle characteristics, in addition to sigma power, is necessary to fully characterize spindles during adolescence.
Keywords: adolescence, development, sigma, cognition, memory, EEG
1.1. Introduction
Adolescence is a period of development associated with widespread changes to brain networks (Power et al., 2010), likely driven by numerous brain maturational processes to support more efficient brain processing. Sleep also undergoes considerable change during adolescence, the most well-evidenced change being a dramatic decline in slow wave activity (SWA), or delta power (Feinberg et al., 2006, Tarokh et al., 2010, Baker et al., 2012, Baker et al., 2016). This decline is thought to reflect brain maturational processes (Feinberg et al., 2010, 2013), and evidence links the age-related decline in SWA, at least in part, with underlying brain structural differences particularly in frontal and parietal brain regions (Goldstone et al., 2018).
Sleep spindles are a defining feature of stage two (N2), non-rapid eye-movement (NREM) sleep (De Gennaro et al., 2003, Berry et al., 2012) and consist of short bursts (~0.5-3 seconds) of oscillatory EEG activity within the sigma frequency range (12-15Hz) of the EEG power spectrum. Some evidence suggests that sleep spindles can be further decomposed into slow (9-12 Hz) and fast (12-15 Hz) spindles, with the most commonly studied ‘fast’ spindles typically occurring at central sites, and ‘slow’ spindles predominantly occurring at frontal sites (Jankel et al., 1985, Jobert et al., 1992). The neurocircuitry supporting fast spindles is fairly well understood and a large body of research has identified the role of the thalamus in sleep spindle generation (for a review see: Steriade et al. (1988)). Briefly, spindle rhythms are generated in the reticular nucleus of the thalamus, which are then transmitted to thalamic relay nuclei and then expressed in the cortex, via a network of reciprocal thalamocortical pathways. Although generated in the thalamus, full spindle expression and synchronization is coordinated by thalamo-cortical networks (Destexhe et al., 1999) and recent evidence identifies that the cortex may initiate spindle expression by inducing down-states in the thalamus, thus triggering the generation of spindles which are then transmitted back to the cortex (Mak-McCully et al., 2017).
Given the substantial development of thalamo-cortical networks during childhood and adolescence (Fair et al., 2010), it is feasible that there may be concurrent changes in sleep spindles. Indeed, studies have identified that peak sigma frequency increases across childhood and adolescence (Shinomiya et al., 1999, Tarokh et al., 2010), spindle duration and amplitude increase across early childhood (McClain et al., 2016), and older adolescents have greater spindle density than younger adolescents (Bodizs et al., 2014). More recently, longitudinal changes to both peak sigma frequency and sigma power during adolescence have been observed (Campbell et al., 2016), and a study of sleep spindles across the lifespan reported a peak in spindle density in adolescence (Purcell et al., 2017). Furthermore, as the coupling of SWA and sleep spindles has been demonstrated, (Uchida et al., 1994, Ueda et al., 2001), the large decrease in slow-wave activity (SWA) which occurs during adolescence may be expected to be accompanied by changes to spindle density and peak frequency during adolescence. Taken together, these findings suggest that sleep spindles may be modified during this developmental period.
Although not completely understood, sleep spindles are thought to play a role in several functions related to sleep, memory, and cognition. During sleep, spindles are hypothesized to promote the stabilization of sleep by potentially protecting the cortex from incoming sensory stimuli (Dang-Vu et al., 2010, Schabus et al., 2012). Additionally, sleep spindles are implicated in sleep-dependent memory consolidation and general cognitive ability, or intelligence (see Fogel et al. (2011) for a review). Typically, sleep spindle density increases following training on a task and greater spindle density during the night, or even during a daytime nap (Nishida et al., 2007), is often associated with better performance on the same task the following day. However, given that intra-individual sleep spindle properties are consistent across nights (De Gennaro et al., 2000) and correlate with general ability, sleep spindles may be an electrophysiological ‘fingerprint’ representing intelligence or ‘learning aptitude.’ Although spindle-cognition associations have been less well studied in adolescents, greater spindle activity has been associated with better cognitive performance in adolescents, particularly for the fluid IQ domain (Reynolds et al., 2018c).
Here, we aimed to investigate how sleep spindle characteristics differ as a function of age across adolescence and how such spindle differences may relate to SWA. We took advantage of the baseline assessment of the large NCANDA sample to also investigate sex and race effects as well as potential relationships between pubertal development and sleep spindles. Furthermore, given the known role of sleep spindles in cognition, we also investigated how sleep spindle density may predict performance on episodic memory tasks and tasks of more general ability for this age group.
2.1. Method
2.1.1. Participants
134 participants (63 female) aged 12-21 (mean= 15.6 ± 2.21 years) were included from the sleep substudy of the larger National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study, which took place at SRI International and University of Pittsburgh (see Brown et al. (2015) for a full description of the NCANDA methodology and baseline sample characteristics and Baker et al. (2016) for baseline sleep characteristics). See Table 1 for final sample characteristics, including polysomnography (PSG) measures.
Table 1:
Demographic and polysomnographic characteristics for the sample included in this study, separated by site. PSG variables marked with a + denote variables calculated as a percentage of time in bed (+) and total sleep time (++).
| SRI | Pittsburgh | |||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Age | 15.2 (1.9) | 12.1-20.0 | 17.1 (2.5) | 12.8-21.9 |
| PDS | 3.0 (0.7) | 1.4-4.0 | 3.3 (0.7) | 1.2-4.0 |
| % | N | % | N | |
| Female | 46.3 | 50 | 50 | 13 |
| Male | 53.7 | 58 | 50 | 13 |
| White | 79.6 | 86 | 61.5 | 16 |
| Black | 3.7 | 4 | 38.5 | 10 |
| Asian | 16.7 | 18 | 0 | 0 |
| Mean (SD) | Range | Mean (SD) | Range | |
| Total sleep time (mins) | 441.8 (49.5) | 300.5-556.5 | 465.4 (67.8) | 256.5-577.5 |
| Sleep efficiency (%) | 92.3 (.05) | 65-99 | 89.8 (7.4) | 70-97 |
| Sleep onset latency (mins) | 13.5 (15.2) | 0.5-53 | 13.4 (11.2) | 1.5-49 |
| Number of awakenings | 22.0 (9.0) | 6-45 | 25.9 (9.9) | 12-48 |
| Wake after sleep onset (%)+ | 4.8 (3.8) | 1-26 | 7.6 (7.0) | 2-27 |
| NREM (N2 & N3) (%)++ | 74.0 (4.5) | 50-82 | 72.9 (5.6) | 56-76 |
| REM (%)++ | 20.0 (4.7) | 4-35 | 19 (5.8) | 5-30 |
2.1.2. Procedure
Full procedures are reported in Baker et al. (2016). All but seven participants had a clinical polysomnographic screening/adaptation night before the sleep architecture recording. Recordings were made at SRI International (n = 108) or the University of Pittsburgh (n = 26) NCANDA sites. Participants were requested to maintain a regular sleep-wake schedule for 5 nights before recordings but were not monitored to check compliance. All participants went to bed in the laboratory at their self-reported typical bed-times. Each night, a breath alcohol test (S75 Pro, BACtrack Breathalyzers, San Francisco, CA, USA) and urine drug test (10 Panel iCup drug test kit, Instant Technologies, Inc., Chicago, IL, USA) confirmed the absence of recent alcohol or drug use. Girls who were postmenarche (51 of 62 female participants) were studied irrespective of menstrual cycle phase. Within 30 days of their overnight recording, participants also underwent a battery of cognitive tasks (see Sullivan et al. 2016 for a full description of all tasks and baseline results for the entire NCANDA sample). Pubertal status was determined by self-assessment with the Pubertal Development Scale (PDS) (Petersen et al., 1988), a validated measure of pubertal stage that shows modest concordance with a physical exam and that correlates with basal gonadal hormone levels. Progesterone in saliva collected within the first hour of waking was analyzed in the majority of girls (n = 45, all at SRI) to determine menstrual phase. Only 4 girls had progesterone levels > 40 pg.ml-1, reflecting probable recordings in the post-ovulatory luteal phase. The limits of detection for progesterone were 5.1 pg.ml-1. The intra-assay coefficient of variation was 22.2-93.2 pg.ml-1.
2.1.3. Cognitive measures
We chose to limit our analyses to measures of memory ability and two measures of more ‘general’ cognitive ability, from the full NCANDA neuropsychological test battery (defined in Brown et al. (2015)). All tasks, other than WRAT-4, which is a pencil and paper test, were subtests of the WebCNP battery (Computerized Neuropsychological Testing System, University of Pennsylvania School of Medicine) and were completed on Apple laptops (13-inch MacBook Air, OS X 10.8). Cognitive measures assessed in these analyses were as follows:
Episodic memory
Participants completed three episodic memory tasks: Face (FMT), Verbal (VMT) and Short Visual Object Learning (SVOLT). Participants were shown a set of stimuli (either faces, words or 3D-euclidean objects), following which they were shown stimuli from the same category (20 targets for memory and 20 distractors) and asked to report whether they had seen each item or not (immediate recall). Participants were then re-tested approximately 25 minutes after the immediate recall trial (delayed recall), without prior warning this second recall trial will occur. For each task, both immediate and delayed recall was tested. The performance measure used for all tasks was an efficiency measure, calculated as: number of correctly remembered items/average correct trial reaction time, as defined in Sullivan et al. (2016).
General ability
WRAT-4 combined score (WRATc): The Wide Range Achievement Test-4 (WRAT4) is widely used to assess general ability in word reading and math calculation (Wilkinson et al., 2006, Dell et al., 2008); these scores were combined to create a ‘general ability’ composite score.
Matrix analysis test (PMAT): this is a multiple-choice task, designed to test mental abstraction and flexibility. Participants must conceptualize spatial, design, and numerical relations that range in difficulty from very easy to increasingly complex. Previous studies have used similar tasks to assess general ability or learning ‘aptitude’ in relation to sleep spindles (Bodizs et al., 2005, Schabus et al., 2006).
2.1.4. Assessment of PSG and sleep EEG
Standard PSG was performed at both sites using Compumedics Grael HD-PSG system (Compumedics, Abbotsford, Victoria, Australia) (see Baker et al. (2016) for details). EEG (Fp1, Fp2, F3, F4, FC3, FC4, C3, C4, CP3, CP4, P3, P4, O1, O2 referenced to the contralateral mastoids), submental electromyogram, and bipolar electrooculogram were recorded according to American Academy of Sleep Medicine (AASM) guidelines (Iber et al., 2007) and was sampled at 256 Hz. Sleep stages and arousals (< 15 s) were scored according to AASM criteria.
EEG data within NREM sleep (N2 and N3 combined) were analyzed on selected electrodes (C3 and F3), using the EEGLAB toolbox (Delorme et al., 2004) for MATLAB (MathWorks, Natick, MA, USA). The contralateral derivation was used in cases when poor signal quality precluded analysis of the left derivation (maximum of four cases for any electrode). EEG was re-referenced to the average mastoid and filtered at 0.3–36 Hz with half-amplitude cutoffs at 0.15 and 36.15 Hz. Fast Fourier transform analysis was conducted on each 30-s epoch using a 4-s sliding Hanning window to calculate power density values with 0.125 Hz resolution. Epochs containing arousals were excluded. In addition, an automated process was applied to reject outlier epochs from N2 and N3 sleep separately, in both the time and frequency domains. In the time domain, if the EEG was flat for greater than 5 s during the 30-s epoch, or if the maximum value exceeded the median by 10 times the median absolute deviation, that epoch was removed. In the frequency domain, if the power of any band exceeded the median by 15 times the median absolute deviation, that epoch was also removed. No more than 5% of epochs were rejected with this process. Absolute power density (μV2.Hz−1) values were then averaged across the sigma (12- 15 Hz) and delta (0.3-4Hz) frequency bands for F3 and C3 electrodes. For each electrode site, relative sigma and delta power were calculated as absolute sigma or delta power divided by the sum of the six typical frequency bands: delta (0.3-4 Hz), theta (4-8 Hz), sigma (12-15 Hz), alpha (8-12 Hz), beta1 (15-23 Hz) and beta2 (23-30 Hz).
2.1.4.1. Spindle characteristics
For all spindle analyses, C3 was used to identify and characterize fast spindles, and F3 was used for slow spindles. While sleep spindle characteristics are stable across nights (De Gennaro et al., 2000), there is a large amount of variability across participants (Werth et al., 1997), therefore, we applied semi-automated spindle detection and quantification, as previously used (de Zambotti et al., 2015). This consisted of an adaptation of methods employed by Bodizs et al. (2009) and Andrillon et al. (2011). Spindle limits were decided on an individual basis, where zero crossing points on a spectral profile, computed for all artefact-free N2 and N3 sleep between 9 and 16 Hz, indicated the largest spectral peaks (computed at F3 for slow and C3 for fast spindles). For each participant, spindle limits were visually inspected to confirm they encompassed the highest spectral power within the fast and slow spindle ranges (Bodizs et al., 2009). Slow and fast spindle peaks were robustly identified using the zerocrossing method and manual correction spindle limits was required for fewer than 5% of participants for fast spindles; slow spindle limits were manually adjusted for 15% of participants. 1 participant was excluded from the slow spindle analysis due to failure to detect a reliable slow spindle peak in the spectral profile. Spindle analyses were computed on electrodes F3 (slow spindles) and C3 (fast spindles). Data were then bandpass filtered to a 1.5Hz band around the peak frequency (i.e. peak frequency ± 0.75Hz) for each participant. Thus, if the peak frequency was identified as 15 Hz, the band pass would have been set for 14.25–15.75 Hz. The Hilbert transform was then applied to this data to calculate the instantaneous amplitude envelope of the spindle frequency data. As in Andrillon et al. (2011), a threshold of mean + 3SD of the amplitude envelope was set for spindle detection and a threshold of mean + 1SD for the start and end of the spindle. For each participant the following spindle characteristics were then calculated: 1) spindle duration: calculated as the average duration (ms) of the segment of each spindle that met the individually-calculated peak amplitude criterion for 500-3000ms, 2) spindle amplitude: the average amplitude of the midpoint of all detected spindles and 3) spindle density: the total number of spindles per minute of N2 and N3 sleep.
2.1.5. Statistical analysis
All analyses were conducted using IBM SPSS Statistics Version 23 for Windows (IBM Corp., Armonk, NY). Multivariate regression models were used to investigate how well age and demographic factors predicted spindle properties and sigma power, with all variables entered together. As sex and race have been associated with spindle properties, especially for younger participants (Purcell et al., 2017), these variables were included in the model as covariables along with site (SRI=reference, female = reference). Race included three categories: White (reference), Black and Asian. All models contained the following variables: age (or PDS), sex, race and site. Variance inflation factor (VIF) was used to confirm absence of multicollinearity between these variables (VIF < 2).
Subsequent regression models investigating how well spindle density predicted cognitive ability included the above demographic variables and spindle density. As spindle density differed as a function of age and site, both age*density and site*density interaction terms were also included in these models assessing cognitive ability.
When variables were not normally distributed (e.g. power data), they were normalized with a natural log function before analysis. Continuous variables (age and density) were centered at the mean before creating any interaction terms.
2.1.5.1. Assessing pubertal status
To assess whether age or pubertal status better predicted spindle characteristics, age was replaced with pubertal status in all models and model outcomes were compared to those which included age. Model fit was assessed by Schwarz Bayesian Criterion (SBIC). R2 and SBIC for each spindle characteristic are reported in Table 2. Pubertal status data were missing for five participants, resulting in a final sample size of 129 for this additional analysis.
Table 2:
model outcomes comparing age vs. pubertal status (PDS) as predictors of spindle characteristics. These comparison models were calculated for the 126 participants with PDS measures, to allow direct comparison between model statistics. For each significant model, R2 and Schwarz Bayesian Criterion are reported. Models with the better fit are highlighted in bold. N/A indicates a non-significant model.
| Frequency | Amplitude | Density | Duration | Sigma power (absolute) |
Sigma power (relative) |
|
|---|---|---|---|---|---|---|
| Fast Spindles | ||||||
| Age | R2 .25 SBIC: −143.75 |
R2 .28 SBIC: 362.34 |
R2 .19 SBIC: −364.27 |
R2 .30 SBIC: 1119.47 |
R2 .25 SBIC: −5.80 |
R2 .12 SBIC: 10.43 |
| PDS | R2 .24 SBIC: −142.46 |
R2 .25 SBIC: 367.49 |
R2 .22 SBIC: −368.69 |
R2 .28 SBIC: 1123.38 |
R2 .23 SBIC: −2.54 |
R2 .11 SBIC: 12.71 |
| Slow Spindles | ||||||
| Age | N/A | R2 .29 SBIC: 474.87 |
N/A | R2 .13 SBIC: 1130.16 |
R2 .26 SBIC: −7.75 |
N/A |
| PDS | N/A | R2 .28 SBIC: 477.44 |
N/A | R2 .11 SBIC: 1133.05 |
R2 .22 SBIC: −6.45 |
N/A |
2.1.5.2. Assessing the relationship between spindle characteristics and SWA
To assess the potential associations between spindle characteristics and SWA (delta power) during adolescence, spindle characteristics were correlated with SWA. In addition, as the proportion of N3:N2 sleep decreases across adolescence, it is possible that age-related differences in spindle characteristics are associated with such proportional differences in N2 and N3 sleep. A proportional measure of N3 sleep was calculated by dividing %N3 by %N2 (total N2 or N3 duration/TST *100) and correlated with spindle characteristics, after controlling for age. Significant p-values are reported after Bonferroni correction for multiple comparisons.
3.1. Results
3.1.1. Differences between fast and slow spindle characteristics
Fast spindles had significantly longer durations (p<.001), greater density (p=.005) and smaller amplitude (p<.001), compared to slow spindles. In addition, relative sigma power was larger for central, compared to frontal, locations (p<.001). Spindle frequency was not tested for significance, because it was used to differentiate between spindle types. Table 3 details average characteristics for each spindle type.
Table 3:
Mean (SD) spindle characteristics for fast (12-15 Hz) spindles selected from C3 and slow spindles (9-12 Hz), selected from F3. Relative sigma power was calculated as (sigma power/total power)*100 at the two electrode sites.
| Fast (C3) |
Slow (F3) |
|
|---|---|---|
| Amplitude*** (μV) |
16.74 (4.82) |
22.38 (7.83) |
| Duration*** (ms) |
1062.63 (102.29) |
1008.60 (97.21) |
| Density** (spindles per minute) |
2.96 (.28) |
2.88 (.03) |
| Frequency (Hz) |
12.94 (.58) |
11.02 (.91) |
| Sigma power (absolute; μV2.Hz−1) |
4.39 (2.40) |
5.01 (3.14) |
| Sigma power*** (relative) |
1.15 (.68) |
0.81 (.49) |
p < .001,
p = .005.
3.1.2. Age, pubertal status, and sex associations with spindle properties
Across spindle characteristics, age and PDS performed similarly, with age resulting in the marginally better fit compared to PDS, except for spindle density where PDS performed marginally better than age. Models predicted between 13 and 31% of the variance in sleep spindle characteristics. See Table 2 for comparison of age and PDS model fit and Table 4 for full model statistics for each characteristic.
Table 4:
Full model statistics are displayed for fast and slow spindle characteristics that were significantly predicted by independent variables (age, sex, race and site). Standardized Beta (β) and associated p-values for each variable are listed below the model statistics.+ Denotes where statistics for pubertal development scale (PDS) are reported, as it provided a marginally better fit that age (fast density only).
| Fast spindles | ||||||
|---|---|---|---|---|---|---|
| Frequency: R2 = .24, F(5,127) = 7.98, p<.001 | ||||||
| Amplitude: R2 = .27, F(5, 127) = 9.40, p<.001 | ||||||
| Duration: R2 = .31, F(5, 127) = 11.01 p<.001 | ||||||
| Density: R2 = .22, F(5, 127) = 6.72, p<.001 | ||||||
| Central sigma power (absolute): R2 = .25, F(5, 127) = 8.33, p <.001 | ||||||
| Central sigma power (relative): R2 = .12, F(5, 127) = 3.45, p =.005 | ||||||
| Frequency | Amplitude | Duration | Density | Absolute sigma |
Relative sigma |
|
| Age/PDS+ |
β = .17, p=.03 |
β = −.39, p<.001 |
β = −.35, p<.001 |
β = .32, p=.001+ |
β = −.39, p<0.001 |
β = .26, p=0.004 |
| Sex |
β = .19 p=.02 |
NS | NS | NS | NS | NS |
| Black | NS |
β = −.23, p=.009 |
NS | NS |
β = −.28, p=0.002 |
NS |
| Asian | NS | NS |
β = −.24 p=.002 |
β = .20 p=.019 |
NS | NS |
| Site |
β = −.32, p<.001 |
NS |
β = −.30, p=.001 |
β = .29, p=.002 |
NS | NS |
| Slow spindles | ||||||
| Amplitude: R2 = .29, F(5, 126) = 10.25, p<.001 | ||||||
| Duration: R2 = .13, F(5, 126) = 3.80, p=.003 | ||||||
| Frontal sigma power (absolute): R2 = .25, F(5, 126) = 8.53, p <.001 | ||||||
| Amplitude | Duration | Abs sigma | ||||
| Age/PDS |
β = −.37, p<.001 |
β = −.27, p=.004 |
β = −.32, p<.001 |
|||
| Sex | NS | NS | NS | |||
| Black |
β = −.23, p=.01 |
β = −.23, p=.02 |
β = −.20 p=.02 |
|||
| Asian | NS | NS | NS | |||
| Site | NS | NS | NS | |||
3.1.2.1. Fast spindles
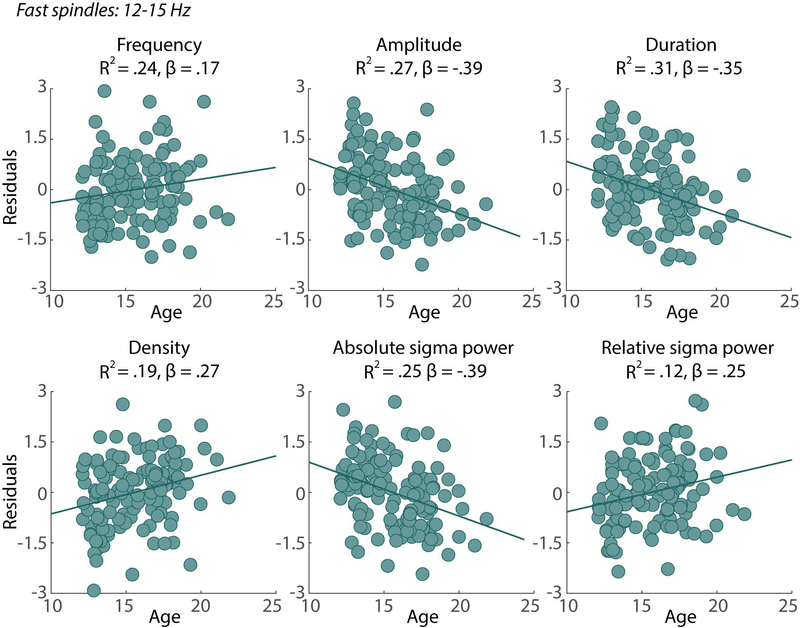
Older age was associated with faster spindle frequency (p=.03), smaller spindle amplitude (p<.001), shorter spindle duration (p=.002) and greater spindle density (p<.001). For every one-year increase in age, spindle frequency increased by 0.045 (Hz), amplitude decreased by 0.78 (μV), duration decreased by 14.96 (ms) and density increased by .03 (spindles). For spindle density, PDS provide a marginally better fit compared to age. More advanced PDS was associated with greater spindle density (p<.001) with a one-unit increase in PDS associated with an increase in spindle density of 0.10 (spindles). See Figure 1 for fast spindle characteristics by age.
Figure 1:
Significant relationships between age and fast spindle 1) frequency 2) amplitude, 3) duration and 4) central sigma power. To show relationships between spindle characteristics and age after controlling for additional factors, residuals are plotted after adjusting for the effect of site, sex and race. R2 = the R2 of the full model, which included age and demographic variables, β= the β of the main effect of age within each model.
In addition, sex was associated with faster spindle frequency (p=.02), with girls exhibiting faster spindle frequencies than boys. Race was also associated with spindle properties; compared to Caucasian participants, African American participants had significantly smaller spindle amplitude (p=.009) and Asian participants had significantly shorter spindle duration (p=.002) and greater spindle density (p=.019). Site was also associated with some spindle properties; compared to participants at SRI, Pittsburgh participants had faster spindle frequency (p<.001) and shorter spindle duration (p=.001).
3.1.2.2. Slow spindles
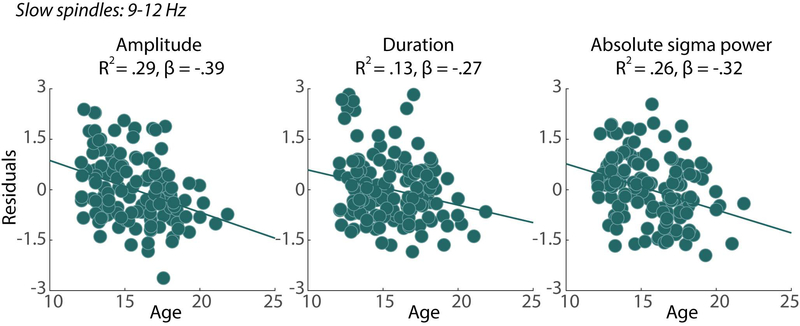
Older age was associated with smaller spindle amplitude (p<.001) and shorter spindle duration (p=.004). For every one-year increase in age, spindle amplitude decreased by 1.13 (μV) and duration decreased by 10.67 (ms). Compared to Caucasian participants, African American participants had significantly smaller spindle amplitude (p=.01) and shorter spindle duration (p=.02). See Figure 2 for slow spindle characteristics by age.
Figure 2:
Significant relationships between age and slow spindle 1) amplitude, 2) duration and 4) frontal sigma power. To show relationships between spindle characteristics and age after controlling for additional factors, residuals are plotted after adjusting for the effect of site, sex and race. R2 = the R2 of the full model, which included age and demographic variables, β= the β of the main effect of age within each model.
3.1.2.3. Sigma power
Older age was associated with weaker absolute frontal and central sigma power (p<.001), and greater relative central sigma power (p=.004). For every one-year increase in age frontal sigma power decreased by 0.39 (μV2.Hz−1) and central sigma power decreased by 0.38 (μV2.Hz−1). Compared to Caucasian participants, African American participants exhibited significantly weaker absolute frontal (p=.02) and central (p=0.002) sigma power. See Figures 1 and 2 for age-sigma power relationships.
Associations between spindle characteristics SWA and slow wave sleep
After controlling for age and site, absolute SWA was significantly correlated with spindle amplitude (fast: r=.43, p<.001, slow: r=.49, p<.001) and frequency (fast: r=−.36, p<.001, slow: NS). SWA was also significantly associated with relative (C3: r=−.49, p<.001, F3: r=−.45, p<.001) and absolute (C3: r=.38, p<.001, F3: r=.41, p<.001) sigma power. Greater SWA was associated with greater spindle amplitude, slower spindle frequency, less relative and greater absolute sigma power.
After controlling for age and site, proportion of N3 sleep (N3:N2) significantly correlated with spindle frequency (fast: r=−.36, p<.001, slow: r= −.32, p<.001) and relative sigma power (C3: r=−.36, p<.001, F3: r=−.33, p<.001). Greater proportion of N3 sleep was associated with slower spindle frequency and less relative sigma power. No other N3:N2-spindle correlations were significant.
3.1.3. Associations between spindle density and cognitive ability
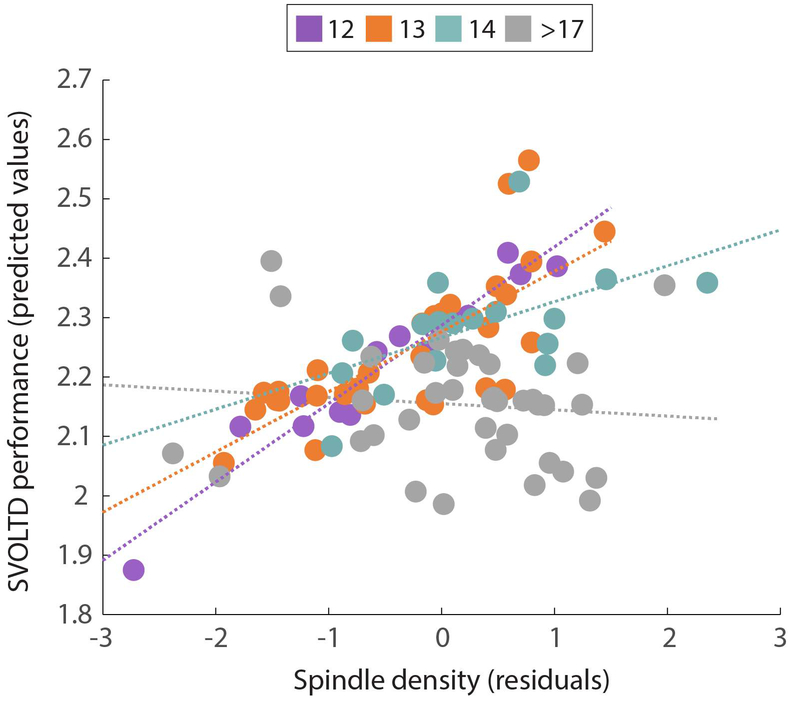
Spindle density did not predict performance on the tasks assessing more general ability (PMAT and WRATav). Similarly, spindle density did not directly predict performance on any of the episodic memory tasks for either immediate or delayed recall. Spindle density*age (β= −.31, p=.003) and density*site (β= .31, p=.02) interactions significantly predicted delayed recall performance on the short visual object (SVOLT-D) task, with the model explaining 12.5% of the variance in SVOLT-D (R2 = .125, F(7, 124) = 2.47, p=.01). Greater spindle density was associated with better SVOLT-D performance, which differed as a function of age, with the strongest relationship for younger adolescents. See Figure 3.
Figure 3:
A subset of participants (those aged 12-14 and older than 17 years) are plotted for illustrative purposes, to depict the significant age*density interaction that predicted episodic memory performance as assessed by delayed recall on the short visual object learning task (SVOLT-D). Spindle density predicted memory performance for younger participants (seen for ages 12-14 in the plot) but not for participants aged over 17 years.
Age was the only significant predictor of the site*density interaction term, suggesting that differences in participant’s ages between sites may contribute to the site*density effect on SVOLT-D.
3.1.4. Socio-economic status
In order to investigate whether site or race effects on spindle characteristics could be explained by socio-economic status (SES) a proxy measure (parent’s highest level of education) was included into models. However, SES was not found to be a significant predictor of any spindle characteristics, and any site or race effects remained significant when SES was also included in the model.
4.1. Discussion
In this large group of adolescents, we found that older age was associated with faster spindle frequency and greater fast spindle density, although spindles were of shorter duration and smaller amplitude. Absolute sigma power, which encompassed the fast spindle frequency band, was weaker in older, compared to younger, adolescents, suggesting that sigma power more closely mirrors differences in spindle amplitude and duration rather than density, at least in adolescents. There were fewer age-related differences in slow spindle characteristics, although slow spindle duration was shorter and amplitude was weaker in older adolescents. These results show that sleep spindles differ across the adolescent developmental period, possibly reflecting underlying developmental differences in thalamocortical networks. Furthermore, proportion of N3:N2 sleep was only associated with spindle frequency, suggesting that changes to sleep architecture during this period of development do not fully account for differences in spindle characteristics. Finally, spindle density was not directly associated with episodic memory or measures of ‘general intelligence’.
4.1.1. Spindle characteristics, age, and pubertal development
Here we identified significant spindle-age associations, using cross-sectional data. Our findings are consistent with a previous longitudinal study that identified a decline in absolute sigma power across adolescence (Campbell et al., 2016) and with the life-span age trajectories identified by Purcell et al. (2017). The latter study used data from multiple studies (n=11,630) and identified a u-shaped trajectory for spindle density across the lifespan; showing an increase with age across childhood, a peak during adolescence and then a decline across adulthood, and that spindle duration declined with age from childhood to late adulthood (Purcell et al., 2017). Here, we identified that greater spindle density was associated with both older age and advanced pubertal stage. While absolute sigma power was lower, relative sigma power was higher in older adolescents in our study, probably reflecting the greater reduction in SWA than for sigma power with advancing age (Baker et al., 2016).
We also identified that older age was associated with a faster peak frequency, as have others (Shinomiya et al., 1999, Tarokh et al., 2010, Campbell et al., 2016, Purcell et al., 2017). However, independent of the age effect, spindle frequency was also related to proportion of N3:N2 sleep (N3:N2) and SWA. Across adolescence, the amount of N3 sleep decreases and the amount of N2 sleep subsequently increases, thus N3:N2 could be thought of as a measure of ‘sleep maturation.’ Thus, here we identified that faster peak spindle frequency and greater relative C3 sigma power were associated with more advanced ‘sleep maturity’ (i.e. less N3:N2). The relationships that we found between spindle frequency and N3:N2 as well as with SWA support a previous suggestion by Campbell and Feinberg (2016) that an increase in spindle frequency across adolescence may reflect a decreased level of thalamocortical hyperpolarization as sleep becomes less deep, since hyperpolarization is negatively associated with spindle frequency (Steriade et al., 1988). Following this line of thought, decreased thalamo-cortical hyperpolarization would increase the number of available neurons able to produce spindles, which corresponds to our results; older adolescence (and thus potentially reduced hyperpolarization) was associated with greater spindle density. However, we did not find a direct association between SWA and spindle density, although this could be because SWA is not a sensitive enough measure of thalamo-cortical hyperpolarization.
For this sample, the age-peak spindle frequency association was weaker compared to relationships found for other spindle characteristics we investigated. This may be due to the fact that our youngest participant was 12 years old, as other studies that have found strong associations between age and spindle frequency have also included children younger than 10 (Shinomiya et al., 1999, Tarokh et al., 2010, Campbell et al., 2016). Indeed, Shinomiya et al. (1999) identified that while frontal spindles abruptly increased in frequency during early adolescence, they had stabilized by the age of 13 and the peak frequency of centroparietal spindles exhibited a gradual linear increase with age, which may not have been captured in this cross-sectional analysis.
Overall, our results highlight the relevance of considering specific spindle characteristics alongside sigma power, as relationships with age diverge depending on the characteristic investigated (e.g. greater spindle density but shorter spindle duration with older age). Although the relationship between sigma power and age corresponds with the relationship between spindle duration and amplitude, it does not match spindle density, meaning that developmental differences to spindle activity may not be entirely accurately estimated when using sigma power as a proxy of spindle activity. This divergence may be particularly important for adolescent samples given that adolescence is a unique developmental period during which dramatic developmental changes occur to both brain structure and function – therefore, age may have unique associations with specific spindle characteristics. The functional consequences of these disassociations remain to be seen. It may be that frequent, shorter, lower-amplitude spindles are characteristic of more efficient brain networks, due to processes such as synaptic pruning and myelination which result in ‘smaller’ spindles in late, compared to early, adolescence. Alternatively, these differences may be simultaneous consequences of multiple developmental brain changes that occur during adolescence.
4.1.2. Spindle characteristics as a measure of brain maturation
The consistent associations between age and spindle properties during adolescence supports the idea that spindle characteristics may reflect the maturational status of brain, likely reflecting a combination of synaptic pruning, myelination and changes to brain structure, processes which are also known to occur during this period. Previously, we identified that age-related differences in SWA were mediated by brain maturational differences, (i.e. cortical thickness and gray matter volume (Goldstone et al., 2018) while others have also suggested that sleep EEG characteristics are a proxy of brain maturation (Feinberg et al., 2006, Feinberg et al., 2013). Given that the well-established spindle neurocircuitry comprises of reciprocal thalamo-cortical connections, it seems feasible to suggest that differences in spindle activity during adolescence may be a marker of thalamo-cortical connectivity strength or efficiency. As synaptic pruning occurs, and thalamo-cortical connectivity becomes more refined and efficient, spindle density may increase. If processing speed can be assumed to reflect neural efficiency, the specific association between faster processing speed and greater spindle density during adolescence (Nader et al., 2015) may support this theory. Furthermore, results from a study of white matter also suggest that spindle characteristics are a consequence of thalamic and temporal connectivity, potentially reflecting myelination processes (Piantoni et al., 2013). Similarly, in older adults, the magnitude of spindle density loss and the extent to which spindles enhance memory has been shown to be predicted by white matter integrity, further suggesting that spindle characteristics reflect brain maturation or integrity (Mander et al., 2017). Alternatively, a bi-directional relationship may exist, whereby sleep spindles help shape thalamo-cortical pathways during adolescence, promoting more efficient, or stronger, connectivity between the thalamus and the cortex. However, it is difficult to disassociate this relationship in human studies. Animal research may allow us to discern the directionality of the relationship between sleep spindles and thalamo-cortical connectivity during adolescence.
4.1.3. Spindle characteristics and demographic factors
We identified smaller spindle amplitudes, shorter slow spindle durations and weaker absolute sigma power in African American, compared to Caucasian, participants, a finding that has been consistently reported across the lifespan (Purcell et al., 2017). However, no race differences were identified for relative sigma power, which suggests that race differences are not specific to the sigma frequency band and are likely driven by race differences in total power. Similarly, greater fast spindle density and shorter fast spindle durations were identified in Asian, compared to Caucasian, participants, a finding, which to our knowledge has not been reported previously. However, given the small proportion of Asian and African American participants within this sample, these results should be interpreted with caution and require further investigation. Furthermore, despite identical equipment and sleep protocol (with a minor difference in wake-up times) at both sites, site was found to significantly predict spindle density, frequency and duration. Pittsburgh participants were generally older than SRI participants, but age did not fully account for the site effect. Similarly, socio-economic status did not account for the site effect, indicating that additional factors, not assessed here, may account for the site effects. As has been previously stated (Pfefferbaum et al., 2016), these results indicate the importance of accounting for site differences in multi-site studies in establishing normal developmental changes. The longitudinal nature of the ongoing NCANDA sample will allow us to investigate the relationship between age and spindle characteristics more robustly, whilst continuing to account for site differences.
4.1.4. Spindle density and cognitive ability
In addition to the age-related associations with spindle characteristics, we also investigated the relationship between spindle density and episodic memory. We did not identify any significant main effects of spindle density and cognitive performance. However, for the visual object learning task, a density*age interaction significantly predicted memory performance, suggesting a positive association between spindle density and episodic memory performance, which was greatest for younger adolescents. This suggests that there may be something specific about spindle density and visual-object episodic memory ability during early adolescence that changes across development. A previous study identified moderate correlations between fast spindle density and narrative memory in children (Chatburn et al., 2013), providing some evidence that spindles may be more closely related to cognition in children compared to adolescents. However, further studies are required to identify how spindle-cognition relations may change across the period of childhood-adolescence. Spindle density did not predict face or verbal episodic memory, however superior performance on these two tasks, compared to SVOLT, may have masked any association between spindle density and performance. Future studies should utilize different tasks to investigate the relationship between spindle characteristics and specific forms of memory.
We failed to find any significant relationships with spindle density and more general tasks. By contrast, others have identified associations in adults between spindle density and performance on the Raven’s Advanced Progressive Matrices Task (Bodizs et al., 2005, Schabus et al., 2006), which is similar in procedure to the task we employed. While some studies have identified associations in children between spindle frequency and IQ (Geiger et al., 2011, Gruber et al., 2013), associations between IQ and spindle amplitude, duration, or density have not been found (Gruber et al., 2013). Although a metaanalysis identified relationships between spindle activity and specific cognitive domains, such as fluid IQ and working memory, in adolescents, a consistent relationship with full IQ was not identified (Reynolds et al., 2018c). Similarly, others have identified that only a composite measure of ‘spindle activity’ (density*amplitude) predicted full-scale IQ in adolescents (Nader et al., 2015) and children (Hoedlmoser et al., 2014). Possibly, the relationship between spindle density and IQ is different for adolescents and children, compared to adults. It is also possible that the age, or developmental, effects on sleep spindles may be greater than the predictive power of spindles of general ability during childhood and adolescence, which is why we fail to find an association between spindles and cognition here. Future work may look to explore whether stronger spindle-cognition relationships are identified when specific memory consolidation processes are investigated, as has been reported in adults (Fogel et al., 2011).
4.1.5. Study limitations
There are several potential limitations to this study. First, we present data from a cross-sectional study of adolescent development, which may have resulted in an oversimplification of the relationship between age and spindle characteristics. However, others have reported similar linear increases in spindle frequency and declines in sigma power across adolescence in both cross-sectional (Shinomiya et al., 1999) and longitudinal (Campbell et al., 2016). In addition, our age group ranged from 12-21 years, thus precluding characteristics of spindles earlier in development. It is possible that major changes in sleep spindle characterizes may have already occurred because participants had already entered into puberty at the time of the study. However, the PDS data (available for 131 of 136 participants), indicates that only 25 participants were rated as post-pubertal, while 106 spanned from pre- to late-pubertal, suggesting considerable variability in PDS for the sample. Future longitudinal analyses on this data set will be able to better answer how sleep spindles change across the remainder of this adolescent period. Furthermore, the lack of younger adolescents in our sample may have limited our ability to fully identify sex differences in spindle characteristics because of sex differences in pubertal development, with girls developing earlier than boys. Further, girls were studied irrespective of menstrual phase, which may have influenced spindle properties, as previous research has identified that reproductive hormones affect sleep spindles (Baker et al., 2007). However, progesterone levels identified that the majority of girls tested were not in the post-ovulatory luteal phase at the time of their sleep recording, suggesting that any influence of menstrual phase on spindles was minimal. Finally, here we used a semi-automated method for detecting sleep spindles, as has been validated previously (Bodizs et al., 2005, Bodizs et al., 2009) and used in both adults (Bodizs et al., 2005, Bodizs et al., 2009, de Zambotti et al., 2015) and adolescents (Bodizs et al., 2014, Reynolds et al., 2018a, Reynolds et al., 2018b). However, as with any automated/semi-automated method, it is possible that this method may have introduced some unknown bias, despite its previous use and validation. Nevertheless, this semi-automated method affords the investigation of spindle characteristics in a large sample, which would otherwise be infeasible using visual scoring methods. Given that the patterns of results were similar to previous adolescent studies and are in line with what may be expected developmentally, we do not believe that the spindle detection method resulted in unreliable results. Future work would benefit from validation of such semi-automated methods in adolescent, as well as adult, samples.
4.1.6. Conclusion
We identified consistent age-related differences in spindle characteristics, notably faster peak spindle frequency and greater fast spindle density but with spindles being shorter and of smaller amplitude, during adolescence, likely reflecting maturation in thalamo-cortical networks. We found some evidence for relationships between SWA and spindles that require further investigation. Furthermore, we provided preliminary evidence to suggest that spindle density was associated with episodic memory performance on an object-based task, in younger adolescents (<17 years old) only, suggesting functional links between sleep spindles and aspects of memory beyond daily memory consolidation in early adolescence that warrant further investigation.
Highlights.
Younger adolescents exhibit fewer fast spindles compared to older adolescents.
Sleep spindles may serve as a marker of adolescent brain development.
Sleep spindle changes during adolescence are not fully characterized by sigma power.
Acknowledgements
We thank our lab manager, Stephanie Claudatos, and research assistants, David Dresser, David Sugarbaker, Justin Greco, Sarah Inkelis, Rebecca Carr, Lena Kardos, Devika Nair, Leonardo Rosas and Sarah Gratzmiller for their effort in collecting data for this project, and all research participants.
Funding
This study was supported by the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA); grants: AA021690 (DBC), AA021697 (EVS), and AA021696 (FCB & IMC). MdZ, FB, and IC have received research funding unrelated to this work from Ebb Therapeutics Inc., Fitbit Inc., and International Flavors & Fragrances Inc.
Footnotes
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci 2011;31:17821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med 2007;8:613–22. [DOI] [PubMed] [Google Scholar]
- Baker FC, Turlington SR, Colrain I. Developmental changes in the sleep electroencephalogram of adolescent boys and girls. J Sleep Res 2012;21:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Willoughby AR, de Zambotti M, Franzen PL, Prouty D, Javitz H, et al. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and Neurodevelopment in Adolescence Sample. Sleep. 2016;39:1429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. The AASM manual for the scoring of sleep and associated events Rules, Terminology and Technical Specifications. Darien, Illinois: American Academy of Sleep Medicine; 2012. [Google Scholar]
- Bodizs R, Gombos F, Ujma PP, Kovacs I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Front Hum Neurosci. 2014;8:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodizs R, Kis T, Lazar AS, Havran L, Rigo P, Clemens Z, et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res 2005;14:285–92. [DOI] [PubMed] [Google Scholar]
- Bodizs R, Kormendi J, Rigo P, Lazar AS. The individual adjustment method of sleep spindle analysis: methodological improvements and roots in the fingerprint paradigm. J Neurosci Methods. 2009;178:205–13. [DOI] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Maturational Patterns of Sigma Frequency Power Across Childhood and Adolescence: A Longitudinal Study. Sleep. 2016;39:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatburn A, Coussens S, Lushington K, Kennedy D, Baumert M, Kohler M. Sleep spindle activity and cognitive performance in healthy children. Sleep. 2013;36:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20:R626–7. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev 2003;7:423–40. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Bertini M. Topographical distribution of spindles: variations between and within nrem sleep cycles. Sleep Res Online. 2000;3:155–60. [PubMed] [Google Scholar]
- de Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC. Menstrual Cycle-Related Variation in Physiological Sleep in Women in the Early Menopausal Transition. J Clin Endocrinol Metab. 2015;100:2918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell CA, Harrold B, Dell T. Test Review: Wilkinson GS, & Robertson GJ (2006). Wide Range Achievement Test—Fourth Edition. Lutz, FL: Psychological Assessment Resources. WRAT4 Introductory Kit (includes manual, 25 test/response forms [blue and green], and accompanying test materials): $243.00. Rehabilitation Counseling Bulletin; 2008;52:57–60. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Cortically-induced coherence of a thalamic-generated oscillation. Neuroscience. 1999;92:427–43. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn 2010;72:56–65. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol 2013;304:R296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol 2006;291:R1724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev 2011;35:1154–65. [DOI] [PubMed] [Google Scholar]
- Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P. The Sleep EEG as a Marker of Intellectual Ability in School Age Children. Sleep. 2011;34:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A, Willoughby AR, de Zambotti M, Franzen PL, Kwon D, Pohl KM, et al. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct Funct 2018;223:669–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Wise MS, Frenette S, Knaauper B, Boom A, Fontil L, et al. The association between sleep spindles and IQ in healthy school-age children. Int J Psychophysiol 2013;89:229–40. [DOI] [PubMed] [Google Scholar]
- Hoedlmoser K, Heib DPJ, Roell J, Peigneux P, Sadeh A, Gruber G, et al. Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. Sleep. 2014;37:1501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SftAAoSM. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st Ed. Westchester Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jankel WR, Niedermeyer E. Sleep spindles. J Clin Neurophysiol 1985;2:1–35. [DOI] [PubMed] [Google Scholar]
- Jobert M, Poiseau E, Jahnig P, Schulz H, Kubicki S. Topographical analysis of sleep spindle activity. Neuropsychobiology. 1992;26:210–7. [DOI] [PubMed] [Google Scholar]
- Mak-McCully RA, Rolland M, Sargsyan A, Gonzalez C, Magnin M, Chauvel P, et al. Coordination of cortical and thalamic activity during non-REM sleep in humans. Nat Commun 2017;8:15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Zhu AH, Lindquist JR, Villeneuve S, Rao V, Lu B, et al. White Matter Structure in Older Adults Moderates the Benefit of Sleep Spindles on Motor Memory Consolidation. J Neurosci 2017;37:11675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain IJ, Lustenberger C, Achermann P, Lassonde JM, Kurth S, LeBourgeois MK. Developmental Changes in Sleep Spindle Characteristics and Sigma Power across Early Childhood. Neural Plast 2016;2016:3670951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader RS, Smith CT. Correlations between adolescent processing speed and specific spindle frequencies. Front Hum Neurosci 2015;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 1988;17:117–33. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, et al. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2016;26:4101–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantoni G, Poil SS, Linkenkaer-Hansen K, Verweij IM, Ramautar JR, Van Someren EJ, et al. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci 2013;33:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The Development of Human Functional Brain Networks. Neuron. 2010;67:735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun 2017;8:15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Gradisar M, Coussens S, Short MA. Sleep spindles in adolescence: a comparison across sleep restriction and sleep extension. Sleep Med 2018a;50:166–74. [DOI] [PubMed] [Google Scholar]
- Reynolds CM, Gradisar M, Short MA. Reliability of sleep spindle measurements in adolescents: How many nights are necessary? J Sleep Res 2018b:e12698. [DOI] [PubMed] [Google Scholar]
- Reynolds CM, Short MA, Gradisar M. Sleep spindles and cognitive performance across adolescence: A meta-analytic review. J Adolesc 2018c;66:55–70. [DOI] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Heib DP, Boly M, Desseilles M, Vandewalle G, et al. The Fate of Incoming Stimuli during NREM Sleep is Determined by Spindles and the Phase of the Slow Oscillation. Front Neurol 2012;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci 2006;23:1738–46. [DOI] [PubMed] [Google Scholar]
- Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clin Electroencephalogr 1999;30:39–43. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 1988;68:649–742. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, et al. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30:449–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Atsumi Y, Kojima T. Dynamic relationships between sleep spindles and delta waves during a NREM period. Brain Res Bull. 1994;33:351–5. [DOI] [PubMed] [Google Scholar]
- Ueda K, Nittono H, Hayashi M, Hori T. Spatiotemporal changes of slow wave activities before and after 14 Hz/12 Hz sleep spindles during stage 2 sleep. Psychiatry Clin Neurosci 2001;55:183–4. [DOI] [PubMed] [Google Scholar]
- Werth E, Achermann P, Dijk DJ, Borbely AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol 1997;103:535–42. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide range achievement test: Psychological Assessment Resources; 2006. [Google Scholar]