Abstract
Supplementation of probiotics to very low birth weight (VLBW) infants has been extensively studied, with multiple meta-analyses reporting probiotics decrease the risk of necrotizing enterocolitis (NEC) and death. Despite availability of this evidence, the decision to initiate routine probiotic supplementation to preterm infants continues to be a complex one. There are uncertainties regarding the use of probiotics, including selecting the appropriate product, dose and target population. Additionally, availability of specific probiotic products and regulatory oversight varies by country, raising concerns regarding the safety and efficacy of specific probiotic products. In this review, we summarize the latest evidence on probiotic use in preterm infants and discuss considerations that may help guide clinicians who are considering routine probiotic supplementation.
Keywords: Infant, neonate, preterm, microbiome, probiotics, necrotizing enterocolitis, sepsis
Introduction
In 1999, Dr. Angela Hoyos published the first cohort study of routine neonatal probiotic administration to prevent necrotizing enterocolitis (NEC)[1]. Dr. Hoyos proposed the hypothesis that “modifying the intestinal microflora colonization of all the newborns in the unit would decrease the incidence of NEC.” Over a 1 year period from 1994 to 1995, all infants admitted to a neonatal intensive care unit in Bogota, Colombia were supplemented with Lactobacillus acidophilus and Bifidobacterium infantis (Infloran) until the time of discharge. Compared to historical controls before routine supplementation, the probiotic treated infants had a lower incidence of NEC (3.0% compared to 6.6%) and fewer NEC-related deaths. Since this initial report, many randomized trials have been conducted evaluating the effect of probiotics on the risks of NEC, late-onset sepsis, and mortality in preterm infants. Four recent meta-analyses (Table 1) summarize the results of these trials, demonstrating favorable effects of probiotics in reducing the risk of NEC, late-onset sepsis, and death in preterm infants. Despite the substantial evidence available that supports the use of probiotics, the decision to initiate routine probiotic supplementation to preterm infants continues to be a complex one for many clinicians. In this review, we discuss some of the considerations that may help guide clinicians who are considering routine probiotic supplementation.
Table 1.
Summary of recent meta-analyses evaluating treatment effects of probiotics.
| Outcome | Year | Trials, n | Patients, n | Relative risk of outcome (95 % CI) |
|---|---|---|---|---|
| Necrotizing Enterocolitis (Bell stage II or III) | ||||
| Sawh et al.[18] | 2016 | 35 | 10520 | 0.53 (0.42–0.66) |
| Dermyshi et al.[48] | 2017 | 29 | 8535 | 0.57 (0.47–0.70) |
| Chang et al.[28] | 2017 | 25 | 7345 | 0.60 (0.48–0.74) |
| Thomas et al.[49] | 2017 | 23 | 7325 | 0.57 (0.43–0.74) |
| Late-onset sepsis | ||||
| Sawh et al.[18] | 2016 | 28 | 8707 | 0.88 (0.77–1.00) |
| Rao et al.[50] | 2016 | 37 | 9416 | 0.86 (0.78–0.94) |
| Dermyshi et al.[48] | 2017 | 28 | 7987 | 0.88 (0.80–0.97) |
| Death from any cause | ||||
| Sawh et al.[18] | 2016 | 27 | 9507 | 0.79 (0.68–0.93) |
| Dermyshi et al.[48] | 2017 | 27 | 8156 | 0.77 (0.65–0.92) |
| Chang et al.[28] | 2017 | 21 | 6291 | 0.75 (0.60–0.92) |
| Thomas et al.[49] | 2017 | 22 | 6954 | 0.72 (0.57–0.92) |
Abbreviations: RR, relative risk; CI, confidence interval; NEC, necrotizing enterocolitis.
Adapted from Patel RM and Underwood MA. Semin Ped Surg. 2018[3] [with permission]
Biologic Plausibility for Observed Effects of Probiotics in Preterm Infants
Biologic plausibility is one of several important considerations in determining the relationship between a therapy (or exposure) and disease[2]. There is both pre-clinical and human data that support the mechanisms by which probiotics may reduce the risk of NEC in preterm infants.
It is important to begin by considering NEC as a multifactorial disease. Risk factors include prematurity, microbial dysbiosis, and enteral feeding[3, 4]. Prematurity is perhaps the strongest risk factor as the risk of NEC is inversely related to gestational age [5], premature infants have a propensity towards gut inflammation mediated by Toll-like receptor-4 [6], and are at higher risk for microbial dysbiosis[4]. Other factors that may play a role include abnormal gut vascular regulation, red blood cell transfusion and anemia[3, 7]. Colonization during the weeks after birth is influenced by antibiotic exposure, mode of delivery and diet (e.g. breastfeeding).[8] Epidemiologic studies support the role of microbial dysbiosis in NEC, as exposure to medications that alter intestinal microbiota (systemic antibiotics[9] and acid-blockers [10]) have been associated with an increased risk of NEC. Breastfeeding has important influences on the development of the gut microbiome in infancy[11] and supports increased microbial diversity[12] and, compared to formula, decreases the risk of NEC[13, 14]. In a meta-analysis of 14 studies, infants who developed NEC, compared to control infants, had decreased diversity of gut bacteria and an increased relative abundance of Proteobacteria and a decreased relative abundance of Firmicutes and Bacteroidetes[15].
There are several mechanisms by which probiotics may protect the immature gut against inflammation and injury[3, 16, 17]. These mechanisms include: 1.) Blunting the inflammatory response; 2.) Decreasing intestinal permeability; 3.) Producing butyrate and other short-chain fatty acids (which act as an energy source for colonocytes, decrease the intestinal pH and oxygen tension, and suppress the growth of pathogenic Enterobacteriacea); 4.) Competitively excluding other microbes; 5.) Regulating cellular immunity; 6.) Upregulating cytoprotective genes. It is important to note that the mechanisms of probiotic action may vary based on the specific strain.
Data from Randomized Trials of Probiotic Supplementation to Preterm Infants
As mentioned previously, there have been several recent meta-analyses that summarize the treatment effects of probiotics (Table 1). However, trials have varied in probiotic strains and doses used. Most trials have initiated supplementation within several days of birth or at first feeding and continued for at least 4 weeks or until discharge[18]. Most commonly, strains used contain either Lactobacillus and Bifidobacterium, individually or in combination[3, 18]. Despite the clinical heterogeneity, there has been low statistical heterogeneity in findings in recent meta-analyses for NEC (Table 1), suggesting consistent relative risk effects of probiotic supplementation across studies.
However, the largest trial of probiotic supplementation to date, the Probiotics in Preterm Infants (PiPS) trial did not find a difference in the risk of NEC between the probiotic group and placebo group[19]. This study treated 1315 infants with Bifidobacterium breve BBG-001 or placebo and found no difference in the risk of NEC between probiotics vs. placebo treatment arms (adjusted risk ratio = 0.93; 95% CI: 0.68–1.27)[14]. There was no harm reported with the use of probiotics in this trial. The authors of this study also reported that 20% of infants in the placebo group were colonized with the probiotic organism by 2 weeks of age and 49% were colonized by 36 weeks post-menstrual age, with possible cross-contamination noted at every study site. This could have diminished the results of the trial toward the null; however, the incidence of NEC was not significantly different among infants colonized with the probiotic compared to those not colonized (7% vs. 13%, adjusted risk ratio = 0.68; 99% CI: 0.43–1.09). Importantly, inclusion of this trial results in a cumulative meta-analysis has a small effect on changing the average treatment effect of probiotics on NEC from a pooled relative risk of 0.47 to 0.53[3]. The results of the PiPS trial highlights the importance of considering each clinical trial individually, including the product used, alongside the results from meta-analyses. Because the meta-analyses to date show a strong treatment effect on NEC and death, it is unlikely that any additional studies will change the conclusion that probiotics decrease the risk of NEC and death.
Additional trials or observational cohort studies comparing different probiotic strains, products or approaches to administration could be informative to clinicians.
Data from Cohort Studies of Routine Probiotic Supplementation to Preterm Infants
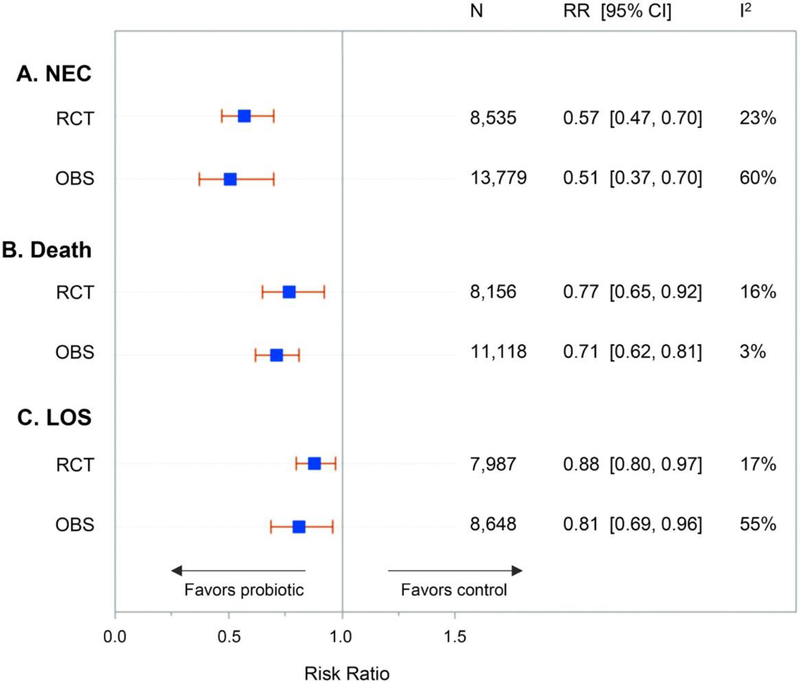
When evaluating the efficacy of a treatment in a controlled trial, it is also important to evaluate the effects of this treatment in routine practice, as the efficacy seen in trials may not be reproducible in routine practice. There are many implementation cohort studies for use of probiotics in preterm infants to evaluate the effects of supplementation in routine practice. The largest implementation cohort study used Infloran, a probiotic preparation of Lactobacillus acidophilus and Bifidobacterium bifidum[20]. The study, which took place in Germany, included over 5000 infants and found infants supplemented with Infloran, compared to those not supplemented, had a lower risk of surgical NEC (adjusted OR = 0.58, 95% CI: 0.37–0.91) [15]. Another large cohort study of 3093 infants < 29 weeks’ gestation in the Canadian Neonatal Network found prophylactic probiotic supplementation was associated with a lower risk of NEC (adjusted OR = 0.64; 95% CI: 0.41–0.99) and death (adjusted OR = 0.41; 95% CI 0.26–0.63), with 21% of infants in the cohort receiving probiotic supplementation[21]. Infants in this study were supplemented with one of two products available in Canada with oversight under the Natural Health Products Regulation in Canada. These included Florababy® (B. breve, B. bifidum, B. infantis, and B. longum) or Biogaia® (Lactobacillus reuteri). Prior to this multicenter cohort study, both of these products were reported in single-center cohort studies in the United States (US) and Canada to be associated with significant reductions in the risk of NEC[22, 23]. Other implementation cohort studies from France, Australia, and Switzerland have reported significant decreases in the incidence of NEC after routine use of probiotics[3]. Additionally, the pooled treatment effects of probiotics on NEC, death, and late-onset sepsis in observational studies are similar to those in clinical trials (Figure 1). These findings support the external validity of the pooled estimates of probiotic treatment effects from randomized trials, and increase confidence in the findings from meta-analyses of randomized trials.
Figure 1.
Treatment effects of probiotics in randomized trials (RCT) and observational studies (OBS) on necrotizing enterocolitis (NEC), death and late-onset sepsis (LOS). From Patel RM and Underwood MA. Semin Ped Surg. 2018[3] [with permission]
Concerns and Uncertainties Regarding Probiotic Supplementation
There is an abundance of data supporting the use of probiotics. Research supports both the biologic plausibility and consistent benefit with minimal risk. Despite this abundant evidence in support of probiotics, there continues to be justifiable reasons to carefully consider its implementation (Table 2). First, in the US, there is no FDA-approved probiotic drug formulation (live-bio therapeutic product) and all currently available products are considered to be regulated as dietary supplements. This has led to concerns regarding the quality and safety of these products[24]. A recent study evaluated 16 probiotic products to determine whether the contents by DNA and culture were consistent with their label. In this study, only 1 of 16 products contained the product that matched the label[25]. Additionally, the fact that there has been one case of probiotic related death from mucormycosis associated with a contaminated probiotic product confirms that concerns regarding quality and safety are valid[26]. Second, there continues to be uncertainty as to which strain and dose is optimal in our patients due to the heterogeneity in both clinical trials and implementation cohort studies. However, in the most recent Cochrane review on probiotics and meta-analysis by Sawh et al.[18], there was no statistical evidence of heterogeneity in treatment effects by probiotic strains[27]. This contrasts another meta-analysis with conflicting findings, suggesting multiple-strain products are superior to single-strain products[28]. However, potential differences between strains need to be balanced with selection of appropriate products, as discussed below. In addition, many studies have shown reductions in the risk of NEC associated with routine supplementation, as discussed previously, despite the use of a variety of products. Finally, there is the potential for probiotic-associated sepsis[29], although reports have been limited in frequency to occasional case reports and probiotics have had a beneficial effect on the risk of late-onset sepsis.
Table 2.
Factors to be considered prior to implementing routine use of probiotics
| Support Starting Routine Probiotic Supplementation | Do Not Support Starting Routine Probiotic Supplementation |
|---|---|
| Pre-clinical and human data support biologic plausibility. | No regulator-approved drug formulation (in the United States or United Kingdom). |
| Numerous RCTs enrolling >10,000 infants show consistent benefit (low heterogeneity) in decreasing the risk of NEC. | Concerns regarding product quality and contamination. |
| Large magnitude of effect on NEC in meta-analysis (decreases relative risk by approximately one-half), as well as potential benefits in reducing the risk of late-onset sepsis and all-cause mortality. | Well conducted, multicenter trial (PiPS) showed no benefit on NEC (of note, relatively high rate of cross-colonization with probiotic strain in control arm). |
| Multiple implementation cohort studies support effectiveness of probiotic supplementation in routine practice. | Uncertainty regarding optimal product/strain, including dose and duration of supplementation. |
| Meta-analysis for subgroup of infants with birth weight <1000 g infants show no increased risks of sepsis. | Limited studies with long-term follow-up data (although the two studies to date show no evidence of harm and one shows a lower risk of deafness). |
| Low relative cost of supplementation. | High number needed to treat for centers with low NEC incidence. |
| NEC remains a major cause of death in preterm infants. | Other opportunities (e.g. increasing human milk feeding) to decrease the risk of NEC. |
Two long-term follow-up studies have not found any evidence of adverse effects (or benefits) in long-term neurodevelopmental outcomes. Akar et al. published a randomized trial of 400 VLBW infants with follow-up of 249 infants at 18–24 months’ corrected age. In this trial the use of Lactobacillus reuteri had no effect on the risk of adverse neurocognitive outcome assessed using the Bayley Scales of Infant and Toddler Development II[30]. Follow-up of the ProPrems trial found no differences in neurodevelopmental impairment at a mean age of 30 months, but did find a lower incidence of deafness among probiotic-treated children[31]. In addition, some centers have observed increases in the risk of NEC after routine supplementation of probiotics[32, 33]. While it is unlikely that probiotics cause NEC, given the abundance of trial data showing benefit, the lack of a beneficial effect does raise questions regarding the effects in certain populations or potential co-treatments (e.g. antibiotics) that may influence the treatment effects of probiotics. These uncertainties and risks need to be carefully considered with a multidisciplinary team in order to determine whether the benefits of starting probiotic supplementation outweigh the risks.
Deciding Whether or Not to Start Routine Supplementation
There are a number of factors to consider regarding whether or not to start routine supplementation of probiotics in your NICU (Table 2). In addition to these factors, we recommend considering the following four key questions:
Have other efforts to reduce NEC been applied in your unit?
What is the baseline incidence of NEC within your unit?
Is the target population in your unit similar to the population studied in trials and implementation cohort studies?
Which probiotic products are available to your unit?
Have other efforts to reduce NEC been applied in your unit?
One of the most important interventions to reduce the risk of NEC is human milk feeding, especially mother’s own milk. Many centers have demonstrated clinically significant declines in their incidence of NEC following quality improvement efforts to improve the use of human milk for infants at their center[34]. This includes efforts to increase feeding mother’s own milk as well as providing donor human milk when mother’s milk is not available. Therefore, improvements in human milk feeding should be the major focus of efforts to decrease NEC. Additionally, human milk oligosaccharides present in breastmilk can promote the growth of certain probiotic bacteria, such as B. infantis[35]. While some randomized trials have combined the use of prebiotics with probiotics[36], this strategy cannot replace the additional beneficial components of breastmilk that may protect against NEC including lowering gastric pH, enhancing intestinal motility, and decreasing epithelial permeability [37, 38].
What is the baseline incidence of NEC within your unit?
Centers should consider their baseline NEC incidence and number of infants needed to treat (NNT) to prevent one case of NEC, as one of several factors used when deciding whether or not to start probiotic supplementation. As baseline risks decrease, the number of infants who receive probiotic supplementation (NNT) increases in order to prevent one case of NEC (Table 3). We derived estimates of NNT, with accompanying confidence intervals[39], based on ranges of the incidence of NEC reported in the literature and estimates of treatment effect on NEC from Sawh et al.[18]. Although NNT informs decisions at the unit or population level about the tradeoffs for prophylactic therapies among at-risk populations between benefit and risk, for an individual infant the risk is binary – either they do or do not develop NEC. Therefore, the NNT should be considered alongside the severity of the disease that is being prevented, including the associated case-fatality and morbidity. In the case for NEC, this is substantial, with NEC accounting for 10% of deaths in US intensive care units [40] and the most common single cause of death between 2 weeks and 2 months of age in extremely preterm infants[41].
Table 3.
Estimates of Number of Infants Needed to Treat to Prevent One Outcome of NEC
| Baseline NEC incidence | Absolute risk reduction | Number needed to treat (95% CI) |
|---|---|---|
| 1.0% | 0.5% | 213 (172–294) |
| 2.0% | 0.9% | 106 (86–147) |
| 3.0% | 1.4% | 71 (57–98) |
| 4.0% | 1.9% | 53 (43–74) |
| 5.0% | 2.4% | 43 (34–59) |
| 7.5% | 3.5% | 28 (23–39) |
| 10.0% | 4.7% | 21 (17–29) |
| 12.5% | 5.9% | 17 (14–24) |
| 15.0% | 7.1% | 14 (11–20) |
| 20.0% | 9.4% | 11 (9–15) |
Estimates for number needed to treat derived using data from meta-analysis by Sawh et al. PeerJ. 2016 (RR of NEC of 0.53; 95% CI 0.42–0.66; n=10,520 infants; 35 trials) and assumes similar treatment spopulation. Abbreviations: NEC, necrotizing enterocolitis; CI, confidence interval.
Is the target population in your unit similar to the population studied in trials and implementation cohort studies?
Most trials of probiotic supplementation have enrolled very low birth weight (VLBW) infants weighing <1500 g, although infants <1000 g birth weight may have been underrepresented in some of these trials [42]. However, a large German cohort study demonstrated beneficial effects of probiotics in the ELBW population [20] and subgroup analyses of the ProPrems trial did not find any significant differences (based on tests for heterogeneity) in effects of probiotics on NEC between ELBW infants <1000g and infants weighing ≥ 1000 g in the trial[43]. Clinicians should also consider specific inclusion and exclusion criteria prior to implementing routine probiotic use. For example, in our center’s previously published experience with probiotic supplementation, we studied infants <1500 g. Use of probiotics in more mature populations to prevent NEC may substantially increase the NNT to prevent NEC, as the baseline incidence would be lower in this population (Table 3).
Which probiotic products are available to your unit?
There is a wide variety of commercially available probiotic products (Table 4). We identified these products by performing a Google search using the following search phrases: ““probiotics for infants”, “infant probiotics”, “commercially available infant probiotics”, “commercially available probiotics infant”. Given the availability of specific probiotic products may differ by geographic region and the variety used in trials and observational studies to date[3], no specific product can be broadly recommended. In the US, there are currently no probiotic products approved under the medicinal live biotherapeutic pathway, although one product has completed phase 2 study with phase 3 trial planning underway (https://clinicaltrials.gov/ct2/show/NCT02472769 ; http://ibtherapeutics.com/the-phase-3-study-protocol-is-modified-after-ibts-meeting-with-the-fda/). Therefore, clinicians and centers should research specific products and evaluate the evidence of efficacy and safety reported with the specific use of the product and the manufacturing practices used (e.g. GMP). In Canada, there are two products, as previously mentioned, that have oversight under the Natural Health Products Regulation. In the United Kingdom, 17% of units that responded to a 2018 survey reported using probiotic products with each site using multi-strain formulations as either Labinic Drops ® or Infloran® [44]. This was similar to a prior survey in 2014. In a survey of 500 NICUs in the US, a similar minority (14.0%) reported routine probiotic supplementation, although a larger variety of probiotic products was used, with single strain Lactobacillus-containing products being the most common [45]. A summary of specific products studied in randomized trials and observational studies was reported in a recent review[3].
Table 4.
Characteristics of Some Commercially Available Infant Probiotic Products
| Product Name | Manufacturer, Country | Probiotic contents on packaging | Dose in CFU on packaging |
|---|---|---|---|
| Culturelle Baby Grow + Thrive | Culturelle, USA | Lactobacillus rhamnosus GG | 10 billion |
| Evivo | Evolve Biosystems, USA | B. infantis | 8 billion |
| GutPro Infant Probiotic Powder | Organic 3, Inc., USA | B. infantis, B. bifidum, B. breve, B. longum, B. lactis, L. gasseri, L. salivarius | 6 billion |
| BioGaia Protectis Baby | BioGaia, Sweden | L. reuteri | 100 million |
| HLC Neonate Powder | Seroyal (Pharmax), USA | L. acidophilus, L. paracasei, B. animalis subsp. lactis | 3 billion |
| Nexabiotic Probiotic Powder | Dr Formulas, USA | S. boulardii, L. acidophilus, S. thermophilus, B. animalis lactis, L. delbrueckii LE, L. rhamnosus LB3, B. coagulans, B. subtilis, L. plantarum LM, E. faecium, L. casei, L. helveticus, L. plantarum, L. rhamnosis, L. salivarius, L. lactis, L. paracasei, L. brevis, L. gasseri, B. bifidum, B. breve, B. lactis, B. longum | 17.25 billion |
| GNC Milestones Baby Probiotic Drops | General Nutrition Centers, Inc., USA | L. fermentum | 200 million |
| Gerber Gentle Everyday Probiotic Drops | Gerber, USA | B. lactis | 1 billion |
| Toddler’s Blend Probiotic | Flora Health, USA | L. casei, L. rhamnosus, L. acidophilus, B. infantis, B. bifidum, B. breve | 1 billion |
| Mommy’s Bliss | Mommy’s Bliss Inc., USA | L. rhamnosus GG | 1 billion |
| Raw Probiotics Kids | Garden of Life, USA | L. gasseri, L. plantarum, B. lactis, L. casei, L. acidophilus | 5 billion |
| Baby’s Jarro-Dophilus + GOS | Jarrow Formulas, USA | B. breve, B. longum, B. lactis, B. bifidum, L. casei, L. rhamnosus | 3 billion |
| Baby Probiotic Supplement | Zarbee’s Naturals, USA | L. casei var. rhamnosus | 1 billion |
| Ther-biotic For Infants | Klaire Labs, USA | L. rhamnosus, L. casei, L. paracasei, L. gasseri, L. salivarius, B. infantis, B. bifidum, B. longum, B. breve, B. lactis | 5 billion |
| Ultimate Flora Baby Probiotic | Renew Life Formulas, USA | B. breve, L. rhamnosus, B. bifidum, B. infantis, B. longum | 4 billion |
| ProBiota Infant | Seeking Health, USA | L. rhamnosus, L. casei, L. paracasei, L. gasseri, L. salivarius, B. infantis, B. bifidum, B. longum, B. breve, B. lactis | 5 billion |
| Tiny Tummies | LoveBug Probiotics, USA | B. infantis, B. lactis, L. rhamnosus GG | 1 billion |
| HMF Natogen | Seroyal (Pharmax), USA | B. animalis subsp. Lactis, L. acidophilus (CUL-21), L. acidophilus (CUL-60), L. paracasei | 3 billion |
| UpSpring Baby Probiotic | UpSpring, USA | L. rhamnosus | 2 billion |
| MetaKids Baby Probiotic | Metagenics, USA | B. animalis ssp. Lactis, L. rhamnosus GG | 1 billion |
| Life Start Vegan | Natren, USA | B. infantis | 1 billion |
| Morinaga M-16V | Morinaga Milk Industry Co., Japan | B. breve | 1 billion |
| Probactiol Infantis | Metagenics, USA | B. bifidum, L. acidophilus, B. infantis | 4.5 billion |
| Optibac Live Cultures for Babies and Children | Optibac, UK | L. acidophilus, B. infantis, B. bifidum | 3 billion |
| Infloran | Multiple manufacturers and countries, including Switzerland | L. acidophilus, B. bifidum | 2 billion |
| Labinic Drops | Biofloratech Ltd, UK | L. acidophilus, B. infantis, B. bifidum | 2 billion |
| ProPrems | NeoBiomics, Sweden | B. infantis, B.lactis and Streptococcus thermophilus | Under development |
Factors to consider prior to implementing routine probiotic supplementation
Once the decision has been made to implement probiotic supplementation at a given center, several practical issues need to be addressed. A team-based approach engaging relevant expertise is important, including pharmacy, infectious disease/microbiology and nursing leadership. A pharmacist can assist in adding a product to the hospital formulary if one is not available at the hospital and engage local drug and therapeutics committees. Infectious disease colleagues may provide input on the suitability of specific blood culture medium for detecting the bacterial species in the probiotic product should an infant require evaluation for possible probiotic-associated bloodstream infection. Infectious disease can also guide appropriate antimicrobial coverage for suspected probiotic-associated infections. Whether or not to obtain consent and how best to inform parents is likely important to individualize to local practice, although one report suggests most parents embrace probiotic supplementation[46]. Some authors have suggested local evaluation of bacterial species of probiotics as a quality-control measure [47], although such an approach for single-dose preparations may not always be practical or useful. Engaging nursing leadership can help formulate appropriate procedures for probiotic preparation and supplementation. Avoiding probiotic supplementation with other nursing activities, such as intravenous medication administration or accessing of catheters, is suggested to reduce the potential for probiotic-associated bloodstream infection. Good hand-hygiene processes, including handwashing, should be monitored and encouraged. The rationale for and approach to implementation should be documented.
Conclusion
After reviewing the evidence and considering the questions above, clinicians may use their own experience and expertise to determine if there is sufficient evidence for routine clinical use of probiotics in their NICU. If after reviewing all the factors discussed above, clinicians decide to begin routine supplementation, they may find it helpful to consult with other centers with experience in probiotic implementation. Organizations such as the NEC Society (www.NECsociety.org) can help clinicians connect with these centers. For those clinicians who decide not to begin routine supplementation, we recommend using quality improvement principles [34] to address other ways to prevent NEC.
ACKNOWLEDGEMENTS
Portions of this review were previously published online as part of a joint blog post for SIGNEC.org and NECsociety.org.
The review was supported, in part, by the National Institutes of Health under award K23 HL128942 (R.M.P.), which had no role in the content of this review.
Conflicts of interest: Dr. Patel consults for Shipman and Goodwin, LLC and received partial travel support from Danone Nutricia to attend the 2018 SIGNEC UK meeting.
REFERENCES
- [1].Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. [DOI] [PubMed] [Google Scholar]
- [2].Lucas RM, McMichael AJ. Association or causation: evaluating links between “environment and disease”. Bull World Health Organ. 2005;83:792–5. [PMC free article] [PubMed] [Google Scholar]
- [3].Lama DJ, Owyong M, Parkhomenko E, Patel RM, Landman J, Clayman RV. Fluid Dynamic Analysis of Hand-Pump Infuser and UROMAT Endoscopic Automatic System for Irrigation Through a Flexible Ureteroscope. J Endourol. 2018;32:431–6. [DOI] [PubMed] [Google Scholar]
- [4].Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. 2015;78:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Battersby C, Longford N, Mandalia S, Costeloe K, Modi N, group UKNCNEs. Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012–13: a whole-population surveillance study. Lancet Gastroenterol Hepatol. 2017;2:43–51. [DOI] [PubMed] [Google Scholar]
- [6].Hackam DJ, Sodhi CP. Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis. Cell Mol Gastroenterol Hepatol. 2018;6:229–38 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saroha V, Josephson CD, Patel RM. Epidemiology of Necrotizing Enterocolitis: New Considerations Regarding the Influence of Red Blood Cell Transfusions and Anemia. Clinics in Perinatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A. 2014;111:12522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–42. [DOI] [PubMed] [Google Scholar]
- [11].Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, et al. Gut Microbiome Developmental Patterns in Early Life of Preterm Infants: Impacts of Feeding and Gender. PLoS One. 2016;11:e0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6:CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol. 2009;29:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Underwood MA. Probiotics and the prevention of necrotizing enterocolitis. J Pediatr Surg. 2018. [DOI] [PubMed] [Google Scholar]
- [17].Jakaitis BM, Denning PW. Commensal and probiotic bacteria may prevent NEC by maturing intestinal host defenses. Pathophysiology. 2014;21:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sawh SC, Deshpande S, Jansen S, Reynaert CJ, Jones PM. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ. 2016;4:e2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR, Probiotics in Preterm Infants Study Collaborative G. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. 2016;387:649–60. [DOI] [PubMed] [Google Scholar]
- [20].Hartel C, Pagel J, Rupp J, Bendiks M, Guthmann F, Rieger-Fackeldey E, et al. Prophylactic use of Lactobacillus acidophilus/Bifidobacterium infantis probiotics and outcome in very low birth weight infants. J Pediatr. 2014;165:285–9 e1. [DOI] [PubMed] [Google Scholar]
- [21].Singh B, Shah PS, Afifi J, Simpson CD, Mitra S, Dow K, et al. Probiotics for preterm infants: A National Retrospective Cohort Study. J Perinatol. 2019. [DOI] [PubMed] [Google Scholar]
- [22].Hunter C, Dimaguila MA, Gal P, Wimmer JE Jr., , Ransom JL, Carlos RQ, et al. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight < 1000 grams: a sequential analysis. BMC Pediatr. 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a North American neonatal intensive care unit. J Pediatr. 2014;164:980–5. [DOI] [PubMed] [Google Scholar]
- [24].Kolacek S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2017;65:117–24. [DOI] [PubMed] [Google Scholar]
- [25].Lewis ZT, Shani G, Masarweh CF, Popovic M, Frese SA, Sela DA, et al. Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr Res. 2016;79:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vallabhaneni S, Walker TA, Lockhart SR, Ng D, Chiller T, Melchreit R, et al. Notes from the field: Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement--Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155–6. [PMC free article] [PubMed] [Google Scholar]
- [27].AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014:CD005496. [DOI] [PubMed] [Google Scholar]
- [28].Chang HY, Chen JH, Chang JH, Lin HC, Lin CY, Peng CC. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS One. 2017;12:e0171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Esaiassen E, Cavanagh P, Hjerde E, Simonsen GS, Stoen R, Klingenberg C. Bifidobacterium longum Subspecies infantis Bacteremia in 3 Extremely Preterm Infants Receiving Probiotics. Emerg Infect Dis. 2016;22:1664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Akar M, Eras Z, Oncel MY, Arayici S, Guzoglu N, Canpolat FE, et al. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. J Matern Fetal Neonatal Med. 2017;30:411–5. [DOI] [PubMed] [Google Scholar]
- [31].Jacobs SE, Hickey L, Donath S, Opie GF, Anderson PJ, Garland SM, et al. Probiotics, prematurity and neurodevelopment: follow-up of a randomised trial. BMJ Paediatr Open. 2017;1:e000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Escribano E, Zozaya C, Madero R, Sanchez L, van Goudoever J, Rodriguez JM, et al. Increased incidence of necrotizing enterocolitis associated with routine administration of Infloran in extremely preterm infants. Benef Microbes. 2018;9:683–90. [DOI] [PubMed] [Google Scholar]
- [33].Kane AF, Bhatia AD, Denning PW, Shane AL, Patel RM. Routine Supplementation of Lactobacillus rhamnosus GG and Risk of Necrotizing Enterocolitis in Very Low Birth Weight Infants. J Pediatr. 2018;195:73–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patel AL, Panagos PG, Silvestri JM. Reducing Incidence of Necrotizing Enterocolitis. Clin Perinatol. 2017;44:683–700. [DOI] [PubMed] [Google Scholar]
- [35].Garrido D, Barile D, Mills DA. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr. 2012;3:415S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dilli D, Aydin B, Fettah ND, Ozyazici E, Beken S, Zenciroglu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr. 2015;166:545–51 e1. [DOI] [PubMed] [Google Scholar]
- [37].Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis! Semin Perinatol. 2017;41:36–40. [DOI] [PubMed] [Google Scholar]
- [38].Jakaitis BM, Denning PW. Human breast milk and the gastrointestinal innate immune system. Clin Perinatol. 2014;41:423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135:e59–65. [DOI] [PubMed] [Google Scholar]
- [41].Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patel RM, Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol. 2013;40:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics. 2013;132:1055–62. [DOI] [PubMed] [Google Scholar]
- [44].Duffield SD, Clarke P. Current use of probiotics to prevent necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2018. [DOI] [PubMed] [Google Scholar]
- [45].Viswanathan S, Lau C, Akbari H, Hoyen C, Walsh MC. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J Perinatol. 2016;36:1106–11. [DOI] [PubMed] [Google Scholar]
- [46].Sesham R, Oddie S, Embleton ND, Clarke P. Probiotics for preterm neonates: parents’ perspectives and present prevalence. Arch Dis Child Fetal Neonatal Ed. 2014;99:F345. [DOI] [PubMed] [Google Scholar]
- [47].Tarnow-Mordi W, Soll RF. Probiotic supplementation in preterm infants: it is time to change practice. J Pediatr. 2014;164:959–60. [DOI] [PubMed] [Google Scholar]
- [48].Dermyshi E, Wang Y, Yan C, Hong W, Qiu G, Gong X, et al. The “Golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology. 2017;112:9–23. [DOI] [PubMed] [Google Scholar]
- [49].Thomas JP, Raine T, Reddy S, Belteki G. Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: a meta-analysis and systematic review. Acta Paediatr. 2017. [DOI] [PubMed] [Google Scholar]
- [50].Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics. 2016;137:e20153684. [DOI] [PubMed] [Google Scholar]