Abstract
Background
Adjuvant sunitinib significantly improved disease-free survival (DFS) versus placebo in patients with locoregional renal cell carcinoma (RCC) at high risk of recurrence after nephrectomy (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.59–0.98; p = 0.03).
Objective
To report the relationship between baseline factors and DFS, pattern of recurrence, and updated overall survival (OS).
Design, setting, and participants
Data for 615 patients randomized to sunitinib (n = 309) or placebo (n = 306) in the S-TRAC trial.
Outcome measurements and statistical analysis
Subgroup DFS analyses by baseline risk factors were conducted using a Cox proportional hazards model. Baseline risk factors included: modified University of California Los Angeles integrated staging system criteria, age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), weight, neutrophil-to-lymphocyte ratio (NLR), and Fuhrman grade.
Results and limitations
Of 615 patients, 97 and 122 in the sunitinib and placebo arms developed metastatic disease, with the most common sites of distant recurrence being lung (40 and 49), lymph node (21 and 26), and liver (11 and 14), respectively. A benefit of adjuvant sunitinib over placebo was observed across subgroups, including: higher risk (T3, no or undetermined nodal involvement, Fuhrman grade ≥2, ECOG PS ≥1, T4 and/or nodal involvement; hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.55–0.99; p = 0.04), NLR ≤3 (HR 0.72, 95% CI 0.54–0.95; p = 0.02), and Fuhrman grade 3/4 (HR 0.73, 95% CI 0.55–0.98; p = 0.04). All subgroup analyses were exploratory, and no adjustments for multiplicity were made. Median OS was not reached in either arm (HR 0.92, 95% CI 0.66–1.28; p = 0.6); 67 and 74 patients died in the sunitinib and placebo arms, respectively.
Conclusions
A benefit of adjuvant sunitinib over placebo was observed across subgroups. The results are consistent with the primary analysis, which showed a benefit for adjuvant sunitinib in patients at high risk of recurrent RCC after nephrectomy.
Patient summary
Most subgroups of patients at high risk of recurrent renal cell carcinoma after nephrectomy experienced a clinical benefit with adjuvant sunitinib.
Trial registration
Keywords: Adjuvant, Disease-free survival, Renal cell carcinoma, Sunitinib
1. Introduction
The prognosis for renal cell carcinoma (RCC) depends on stage and on additional tumor and patient-specific risk factors obtained at diagnosis. Nearly 20% of all patients with RCC are diagnosed with locoregional disease [1], and up to 40% of these patients experience relapse after nephrectomy and develop metastasis [2,3]. Adjuvant therapies to decrease relapse after nephrectomy are needed. A decision to adopt a new adjuvant therapy in standard clinical practice depends on consideration of a patient’s estimated risk of recurrence, the clinical benefit of the additional treatment, and the additive treatment related morbidity.
In the phase 3 S-TRAC study, sunitinib (50 mg once daily) was administered on a 4-wk on/2-wk off treatment schedule to patients with locoregional RCC at high risk of tumor recurrence after nephrectomy [4]. Adjuvant sunitinib significantly improved disease-free survival (DFS) versus placebo (median 6.8 vs 5.6 yr) according to blinded independent central review (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.59–0.98; p = 0.03) [4]. At 5 yr, the DFS rate gain was 8% in favor of sunitinib over placebo. Overall survival (OS) data were not mature at data cutoff [4]. The most common (>5% of patients) grade ≥3, all-causality adverse events (AEs) in the sunitinib group were palmar-plantar erythrodysesthesia (16%), neutropenia (8.5%), hypertension (7.8%), and thrombocytopenia (6.2%) [4].
In the present study, we examined treatment outcomes for subgroups of patients defined according to baseline characteristics, and report here the sites of tumor recurrence in the two treatment arms. Updated OS data, based on an additional 10 mo of follow-up, are also provided.
2. Patients and methods
2.1. Patients and treatment
As described previously [4], key inclusion criteria in the S-TRAC study included: nonmetastatic locoregional RCC defined as T3 or T4, no or undetermined nodal involvement, or any T stage with local nodal involvement; and for all patients, any Fuhrman grade and any Eastern Cooperative Oncology Group performance status (ECOG PS). In addition, patients had to have clear cell histology, no previous systemic therapy, ECOG PS ≤2 before nephrectomy, no evidence of macroscopic residual disease/metastasis (confirmed by blinded independent central review), and treatment initiation within 3–12 wk after nephrectomy. Patients were randomized to receive treatment with sunitinib or placebo for nine cycles (~1 yr) until recurrence, second cancer, significant toxicity, or consent withdrawal.
2.2. Analyses
Disease recurrence was determined via centrally confirmed imaging and/or histological findings. Prespecified subgroup analyses of DFS by baseline risk factors were conducted using a Cox proportional hazards model. The baseline risk factors were as follows: University of California Los Angeles integrated staging system (UISS) criteria [5]; age; gender; ECOG PS before first dose (as opposed to ECOG PS in risk groups before nephrectomy); weight; and neutrophil-to-lymphocyte ratio). Post hoc analyses of DFS by Fuhrman grade were also performed. Interaction terms (treatment × baseline factors) were analyzed to investigate possible interactions between treatment and the baseline factors in a univariate model. All subgroup analyses were exploratory, and no adjustments for multiplicity were made.
Patients were followed for survival status (regardless of treatment duration) every 12 wk until the time of the final analysis. OS was defined as the time from the date of randomization to the date of death due to any cause. In the absence of confirmation of death, survival time was censored at the last date on which the patient was known to be alive. OS was estimated using the Kaplan-Meier method, and data were compared using a two-sided log-rank test, stratified by UISS risk group.
3. Results
3.1. Patients and treatment
Overall, 615 patients (n = 309 sunitinib; n = 306 placebo) were enrolled from 97 sites, including 73 in Europe, 15 in Asia, and nine in the Americas. Of these patients, 306 were treated with sunitinib and 304 with placebo. Patient characteristics, summary of treatment, and treatment outcome are summarized in Table 1. Overall, 71% of patients received sunitinib for six or more cycles (8 mo) and 56% completed the full 1-yr treatment. It should be noted that the trial permitted a dose decrease to 37.5 mg/d, but not to 25 mg/d. The most common reasons for treatment discontinuation in the sunitinib group included AEs (28%), relapse (7.2%), and patient refusal to continue treatment for reason other than AEs (4.6%). In the placebo-treated patients, the most common reasons for treatment discontinuation included relapse (19%), AEs (5.9%), and patient refusal to continue treatment for a reason other than AEs (2.6%).
Table 1 –
S-TRAC selected baseline characteristics and summary of study outcome
| Sunitinib | Placebo | |
|---|---|---|
| Patients assigned for treatment/treated (n) | 309/306 | 306/304 |
| Median age, yr (interquartile range) | 57 (49–64) | 58 (51–66) |
| Male/female (%) | 72/28 | 75/25 |
| ECOG PS, n (%) | ||
| 0 | 228 (74) | 220 (72) |
| 1 | 79 (26) | 84 (28) |
| ≥2 | 1 (0.3) | 0 |
| UCLA integrated staging system, n (%) | ||
| T3 low a | 115 (37) | 112 (37) |
| T3 high b | 165 (53) | 166 (54) |
| T4 or any T/N+ c | 29 (9.4) | 28 (9.2) |
| Patients who completed full 1-yr treatment, n (%) | 170 (56) | 211 (69) |
| Median treatment duration, mo (interquartile range) | 12.4 (6.0–12.5) | 12.4 (9.2–12.5) |
| Median daily dose, mg (interquartile range) | 45.9 (38.4–50) | 50 (49–50.2) |
| Median DFS, yr (95% CI) d | 6.8 (5.8–NR) | 5.6 (3.8–6.6) |
| DFS hazard ratio (95% CI) for sunitinib vs placebo d | 0.76 (0.59–0.98) |
CI = confidence interval; DFS = disease-free survival; ECOG PS = Eastern Cooperative Oncology Group performance status; NR = not reached; UCLA = University of California Los Angeles.
T3, no or undetermined nodal involvement, no metastasis, any Fuhrman grade, ECOG PS 0 or Fuhrman grade 1, ECOG PS 1.
T3, no or undetermined nodal involvement, no metastasis, Fuhrman grade ≥2, ECOG PS ≥1.
T4 or any T with nodal involvement, no metastasis, any Fuhrman grade, any ECOG PS.
According to blinded independent central review.
3.2. Subgroup analysis
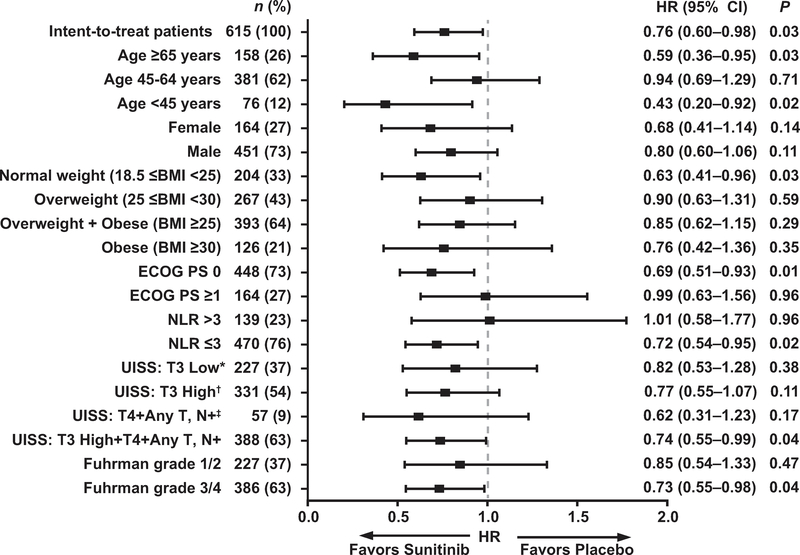
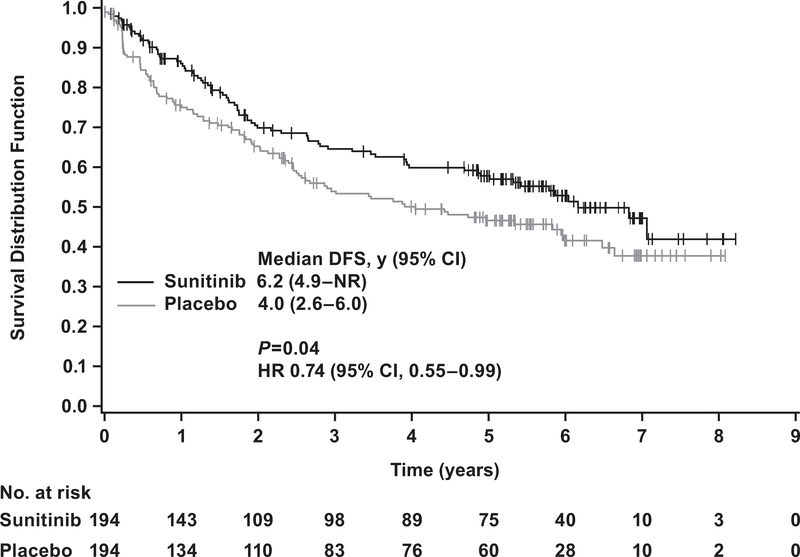
The majority of subgroups selected according to baseline characteristics experienced longer DFS on sunitinib compared to placebo (Fig. 1). Subgroups favoring sunitinib by HR with an upper 95% CI boundary <1.0 included age <45 yr (HR 0.43, 95% CI, 0.20–0.92) or ≥65 yr (HR 0.59, 95% CI 0.36–0.95); normal weight (HR 0.63, 95% CI 0.41–0.96); and ECOG PS 0 (HR 0.69, 95% CI 0.51–0.93). Other groups included Fuhrman grade 3 or 4 tumors (HR 0.73, 95% CI 0.55–0.98); higher risk (HR 0.74, 95% CI 0.55–0.99; Fig. 2); and neutrophil-to-lymphocyte ratio ≤3 (HR 0.72, 95% CI 0.54–0.95). The CIs were wide for the subgroups because of the small sample size. In addition, none of the interaction terms (treatment × baseline factors) were statistically significant.
Fig. 1 –
Disease-free survival by subgroup. * T3 tumor, no or undetermined nodal involvement, no metastasis, any Fuhrman grade, ECOG PS 0; or Fuhrman grade 1, ECOG PS 1. † T3 tumor, no or undetermined nodal involvement, no metastasis, Fuhrman grade ≥2, ECOG PS ≥1. ‡ T4 tumor or any T stage with nodal involvement, no metastasis, any Fuhrman grade, any ECOG PS. BMI = body mass index; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; NLR = neutrophil-to-lymphocyte ratio; UISS = University of California Los Angeles integrated staging system.
Fig. 2 –
Disease-Free survival in patients at higher risk according to blinded independent central review. Higher risk was defined as T3, no or undetermined nodal involvement, no metastasis, Fuhrman grade ≥2, ECOG PS ≥1; or T4 and/or nodal involvement. CI = confidence interval; HR = hazard ratio; DFS = disease-free survival; NR = not reached.
3.3. Site of relapse
According to blinded independent review, 97 patients in the sunitinib arm and 122 patients in the placebo arm developed distant disease recurrence. The most common (≥5%) sites of distant recurrence were lung and lymph node (Table 2). There was no difference in sites of recurrence between the treatment arms. In 219 recurrences observed across both arms, 89, 47, 36, and 25 occurred in lung, lymph node, retroperitoneum, and liver, respectively. Only ten and seven recurrences were observed in bone and brain, respectively.
Table 2 –
Most common (≥1% of patients in any group) sites of distant recurrence
| Site of relapse | Patients, n (%) |
|
|---|---|---|
| Sunitinib (n = 309) | Placebo (n = 306) | |
| Lung | 40 (13) | 49 (16) |
| Lymph node | 21 (6.8) | 26 (8.5) |
| Retroperitoneum | 16 (5.2) | 20 (6.5) |
| Liver | 11 (3.6) | 14 (4.6) |
| Adrenal gland | 10 (3.2) | 6 (2) |
| Bone | 3 (1) | 7 (2.3) |
| Pancreas | 4 (1.3) | 5 (1.6) |
| Brain | 3 (1) | 4 (1.3) |
| Peritoneum/omentum | 3 (1) | 4 (1.3) |
| Mediastinum | 1 (0.3) | 4 (1.3) |
3.4. Overall survival
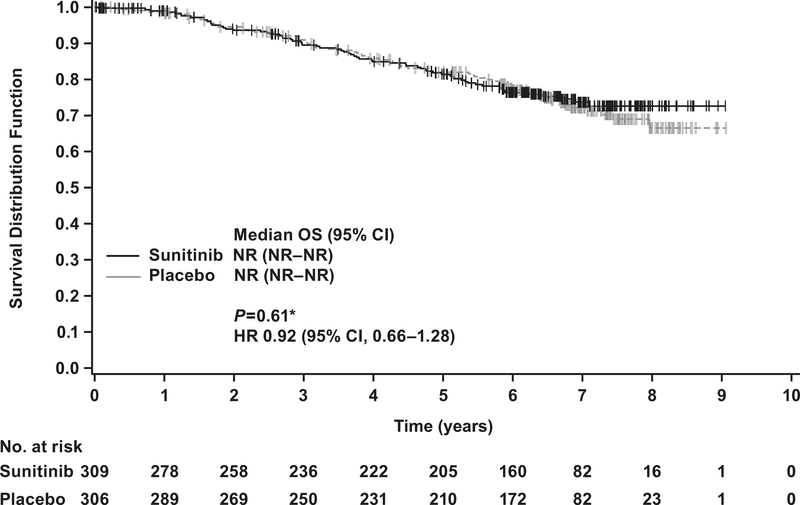
At the cutoff date for the updated OS analysis (January 31, 2017), 67 patients in the sunitinib arm and 74 patients in the placebo arm had died. The median follow-up time was 6.6 yr in the sunitinib arm and 6.7 yr in the placebo arm. The median OS was not reached in either arm. The HR for sunitinib versus placebo was 0.92 (95% CI 0.66–1.28; p = 0.6; Fig. 3).
Fig. 3 –
Kaplan-Meier estimates of overall survival (OS) in the intent-to-treat population. * Two-sided p value from log-rank test stratified by University of California, Los Angeles integrated staging system high-risk group: T3 or T4, no or undetermined nodal involvement, no metastasis, or any T stage with local nodal involvement, and for all patients, any Fuhrman grade and any Eastern Cooperative Oncology Group performance status. CI = confidence interval; ECOG PS =; HR = hazard ratio; NR = not reached.
4. Discussion
Results from this report on the S-TRAC study show that 71% of patients completed 8 mo of adjuvant sunitinib treatment and 56% completed the full year of treatment. Of the treatment discontinuations reported for the sunitinib arm, 28% were related to AEs, which is similar to the discontinuation rate reported for sunitinib in the metastatic RCC setting [6]. Furthermore, the 28% discontinuation rate reported in S-TRAC was over a median treatment duration of 12 mo, whereas in the metastatic setting, discontinuation rates of 24% and 20% due to AEs were reported for pazopanib and sunitinib, respectively, over a median treatment duration of 8 mo [4,6]. For most patients, toxicities related to adjuvant sunitinib were managed via supportive care and a dose reduction or interruption. Nevertheless, there is still a need to improve the management of some side effects in the adjuvant setting. For example, hand-foot syndrome occurred more frequently in the adjuvant than in the metastatic setting [6–8]. Whether this is because of a more physically active population receiving treatment in the adjuvant setting or a more specific toxicity is not yet clear. In addition, an alternative schedule (2 wk on treatment followed by 1 wk off treatment) should be explored with the aim of decreasing the frequency of side effects and severity, as reported in the metastatic setting [9].
In addition to the positive outcome in the overall population of the S-TRAC study, the majority of subgroups defined according to baseline characteristics experienced longer DFS on sunitinib compared to placebo, including the prespecified subgroup of patients with higher risk of recurrence (defined as T3, no or undetermined nodal involvement, Fuhrman grade ≥2, and ECOG PS ≥1; or T4 and/or nodal involvement) compared to the overall population, as well as the subgroup of patients with Fuhrman grade 3/4.
This analysis has limitations in that all the subgroup analyses were exploratory, and no adjustments for multiplicity were made. However, the results are consistent with the primary analysis, showing a benefit for adjuvant sunitinib treatment in patients at high risk of recurrent RCC after nephrectomy.
Overall, 31% and 40% of patients in the sunitinib and placebo arms, respectively, developed distant disease recurrence. The most common sites of relapse included lung, lymph node, and retroperitoneum. Bone and brain metastases (1% each) were less common after adjuvant sunitinib compared to the higher percentages documented in metastatic RCC (30% for bone and 8% for brain) [8,10,11]. Knowing the patterns of recurrence in these patients may help physicians to monitor patients who are deemed at high risk of recurrence for metastases following nephrectomy.
The updated OS data were not mature after an additional 10 mo of follow-up, but the results show that OS was not compromised. With a 40% survival rate at 10 yr after nephrectomy in patients at high risk (UISS criteria) of recurrent RCC [12,13], and given the number of patients and timeframe required, this study was not powered to show an improvement in OS.
Specifically, a trial designed to demonstrate a 25% improvement in OS (HR 0.8) with a two-sided α value of 0.05 and 80% power would require approximately 1650 patients and 18.5 yr of follow-up. This is a conservative estimate that does not account for the dropouts that are likely in a study that is ongoing over this time period. Although improving patient OS is a goal of adjuvant treatment, it is widely understood that for many indications for which survival is long and/or many subsequent therapies are available, it is challenging to measure. Therefore, adjuvant treatments in various tumor types, including colon cancer, breast cancer, melanoma, and gastrointestinal stromal tumors, have been approved by regulatory authorities and implemented as a standard of care on the basis of relative risk reductions in DFS or recurrence-free survival with limited or no OS data [14–19]. Approval was based on, among other reasons, the strength/magnitude of the benefit, positive risk-benefit assessment, and unmet medical needs. Indeed, for colon cancer and gastrointestinal stromal tumors, the benefit in DFS later translated to a benefit in OS [14–16,19,20].
The phase 3 studies ASSURE and S-TRAC assessed the efficacy of adjuvant sunitinib versus placebo in patients with RCC, but had different outcomes for the primary endpoint analysis [4,21]. In ASSURE, there was no improvement in investigator-assessed DFS [21], whereas independently assessed DFS improved significantly in S-TRAC [4]. Differences between the ASSURE and S-TRAC studies are summarized in Table 3. In addition to the differences described therein, the difference in DFS according to investigator assessment for the placebo arms (median 6.6 yr in ASSURE and 4.5 yr in S-TRAC) illustrates the differences in the patient populations [4,21].
Table 3 –
Differences between the S-TRAC and ASSURE studies
| Category and variable | ASSURE [21] | S-TRAC [4] | ||||
| Study conduct | ||||||
| Study sites (n) | 226 | 97 | ||||
| Patients treated with sunitinib/placebo (n) | 647/647 | 309/306 | ||||
| Regions | USA, Canada | Americas, Europe, Asia, Australia, Middle East | ||||
| Treatment arms (n) | 3 | 2 | ||||
| Blinded independent central review of scans | ||||||
| At baseline | No | Yes | ||||
| At recurrence | No | Yes | ||||
| Stratification | Histology (CC vs NCC) | 1. ECOG PS (<2 vs 2) | ||||
| 2. Surgery (laparoscopic vs open) | 2. Risk category | |||||
| 3. ECOG PS (0 vs 1) | 3. Country | |||||
| 4. Risk category | ||||||
| Patient characteristics | 79 | >99 | ||||
| CC RCC (%) | ||||||
| NCC RCC (%) | 21 a | <1 | ||||
| Risk groups included | ≥T1b G3–4 and/or N+ b | ≥T3 and/or N+ c | ||||
| RCC stage I–II (%) | 33 a | 0 | ||||
| Treatment | ||||||
| Completed the full 1-yr treatment (%) | 49 | 56 | ||||
| Dose administered | ||||||
| Starting dose levels | 2 (50 mg and 37.5 mg) | 1 (50 mg) | ||||
| Sunitinib starting dose of 50 mg/d (%) | 70 d | 100 | ||||
| Minimum dose reduction allowed (mg) | 25.0 | 37.5 | ||||
| Median number of cycles (n) | 8 | 9 | ||||
| Medial actual cumulative sunitinib exposure, mg (IQR) | 6800 (2600–9900) | 9638(5550–12200) | ||||
| Safety | ||||||
| Discontinuations due to AEs/refusal/other (%) | 41 | 32 | ||||
| AEs (%) | G3 | G4 | G5 | G3 | G4 | G5 |
| Hypertension | 17 | <1 | 0 | 8 | 0 | 0 |
| Fatigue | 17 | 1 | 0 | 4 | <1 | 0 |
| Hand-foot skin reaction | 15 | 0 | 0 | 15 | 1 | 0 |
| Diarrhea | 10 | 0 | 0 | 4 | 0 | 0 |
| All AEs | 57 | 5 | 1 | 48 | 12 | 0 |
AE = adverse events; CC = clear cell; ECOG PS = Eastern Cooperative Oncology Group performance status; G = grade; IQR = interquartile range; NCC = non–clear cell; RCC = renal cell carcinoma.
In the sunitinib arm.
pT1b, G3–4, no or undetermined nodal involvement, no metastasis, or any T, any G, with local nodal involvement (fully resected), and no metastasis.
T3 or T4, no or undetermined nodal involvement, no metastasis, or any T stage with local nodal involvement; and for all patients, any Fuhrman grade and any ECOG PS.
The remaining patients started at a reduced dose of 37.5 mg.
One subgroup analysis for ASSURE did not elucidate a group that benefited from treatment with sunitinib [22]. However, the differences between ASSURE and S-TRAC in trial design, patient population, and dosing should be considered in conglomerate rather than in isolation [4,21]. For example, owing to the differences in dosing observed across the two trials, subgroup analyses based on exposure in ASSURE may not explain the benefit observed in S-TRAC [22]. Cross-study comparison between subsets of patients selected retrospectively from the ASSURE trial and the primary analysis of S-TRAC must be interpreted with caution given the many differences between the two trials and the limitations of such comparisons.
5. Conclusions
The DFS benefit with adjuvant sunitinib for in patients with locoregional RCC at high risk of tumor recurrence after nephrectomy as demonstrated in the primary analysis for S-TRAC was supported by subgroup analyses. The majority of subgroups experienced longer DFS with adjuvant sunitinib compared to placebo, including patients at higher risk of recurrence (T3, no or undetermined nodal involvement, Fuhrman grade ≥2, and ECOG PS ≥1; or T4 and/or nodal involvement) and those with Fuhrman grade 3/4. The updated OS data are not mature enough to draw reliable conclusions regarding the impact of adjuvant sunitinib treatment on OS; however, no detrimental effect on OS was observed for sunitinib treatment.
Acknowledgments
Patients treated at the Memorial Sloan Kettering Cancer Center were supported in part by a Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Funding/Support and role of the sponsor: These analyses were designed, funded, and conducted by Pfizer. The S-TRAC study from which data were collected for these analyses was sponsored by Pfizer. Medical writing support was provided by Vardit Dror of Engage Scientific Solutions and was funded by Pfizer Inc. The sponsor played a role in the design and conduct of the study; data collection, management, and analysis; and review and approval of the manuscript.
Financial disclosures: Robert J. Motzer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Robert J. Motzer has received research funding and consultant fees from Pfizer. Alain Ravaud is a member of advisory boards on renal cell carcinoma for Pfizer, Novartis, GSK, Roche, and BMS; has received institutional support grants from Pfizer and Novartis, and meetings expenses from Pfizer, Novartis, BMS, AstraZeneca, and MSD. Jean-Jacques Patard has received consulting fees from Pfizer and GSK. Daniel J. George has received honoraria and consulting fees from Dendreon, Sanofi, Novartis, and Bayer; consulting fees from Medivation, Merck, and Genentech; grants from Genentech/Roche, Novartis, Janssen, Astellas, Celldex, and Acerta; and grants and consulting fees from Exelixis, Pfizer, Sanofi, Innocrin Pharma, and BMS. Bernard Escudier has received consulting fees from Bayer, Pfizer, and Novartis; and honoraria from Bayer, Roche, Pfizer, Genentech, Novartis, and AVEO. Frede Donskov has received research funding from Pfizer, Novartis, and GSK. Ahmed Magheli has received financial compensation for speeches from Janssen, Bayer, Astellas, and Pfizer. Brigitte Laguerre has received honoraria from Pfizer. Michelle Casey, Paola Gerletti, Mariajose Lechuga, Xun Lin, and Lucile Serfass are employees of and own stock in Pfizer. Allan J. Pantuck has received consulting fees from Pfizer. Michael Staehler has received honoraria, consulting fees, and research grants from Pfizer, Bayer, GSK, Roche, BMS, Novartis, Exelixis, and AVEO. Hardev S. Pandha has received honoraria for advisory work from Ipsen and Esai. Piotr Tomczak, Giacomo Carteni, Jan Breza, Anup Patel, and Yen-Hwa Chang have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cancer Institute. SEER cancer statistics factsheets: kidney and renal pelvis cancer 2016. http://seer.cancer.gov/statfacts/html/kidrp.html
- 2.Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol 2013;40:482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843–52. [DOI] [PubMed] [Google Scholar]
- 4.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246–54. [DOI] [PubMed] [Google Scholar]
- 5.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 2001;19:1649–57. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31. [DOI] [PubMed] [Google Scholar]
- 7.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 2009;10:757–63. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 9.Bracarda S, Negrier S, Casper J, et al. How clinical practice is changing the rules: the sunitinib 2/1 schedule in metastatic renal cell carcinoma. Expert Rev Anticancer Ther 2017;17:227–33. [DOI] [PubMed] [Google Scholar]
- 10.Gore ME, Szczylik C, Porta C, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer 2015;113:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol 2012;23:973–80. [DOI] [PubMed] [Google Scholar]
- 12.Galligioni E, Quaia M, Merlo A, et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guerin: five-year results of a prospective randomized study. Cancer 1996;77:2560–6. [DOI] [PubMed] [Google Scholar]
- 13.Giberti C, Oneto F, Martorana G, Rovida S, Carmignani G. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur Urol 1997;31:40–8. [DOI] [PubMed] [Google Scholar]
- 14.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51. [DOI] [PubMed] [Google Scholar]
- 15.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. [DOI] [PubMed] [Google Scholar]
- 16.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage iii melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522–30. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265–72. [DOI] [PubMed] [Google Scholar]
- 20.Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J Clin Oncol 2016;34:244–50. [DOI] [PubMed] [Google Scholar]
- 21.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial JAMA Oncol. In press; 10.1001/jamaoncol.2017.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]