Abstract
An excess incidence of prostate cancer (PCa) has been identified among World Trade Center (WTC) responders. In this study, we hypothesized that WTC dust, which contained carcinogens and tumor-promoting agents, could facilitate PCa development by inducing DNA damage, promoting cell proliferation and causing chronic inflammation. We compared expression of immunological and inflammatory genes using a NanoString assay on archived prostate tumors from WTC Health Program (WTCHP) patients and non-WTC PCa patients. Further, to assess immediate and delayed responses of prostate tissue to acute WTC dust exposure via intratracheal inhalation, we performed RNA-seq on the prostate of normal rats that were exposed to moderate to high doses of WTC dust. WTC PCa cases showed significant upregulation of genes involved in DNA damage and G2/M arrest. Cell type enrichment analysis showed that Th17 cells, a subset of pro-inflammatory T helper cells, were specifically upregulated in WTC patients. In rats exposed to WTC dust, we observed upregulation of gene transcripts of cell types involved in both adaptive immune response (dendritic cells and B cells) and inflammatory response (Th17 cells) in the prostate. Unexpectedly, genes in the cholesterol biosynthesis pathway were also significantly upregulated 30 days after acute dust exposure. Our results suggest that respiratory exposure to WTC dust can induce inflammatory and immune responses in prostate tissue.
Keywords: World Trade Center, Prostate Cancer, Inflammation, NanoString, RNA-seq
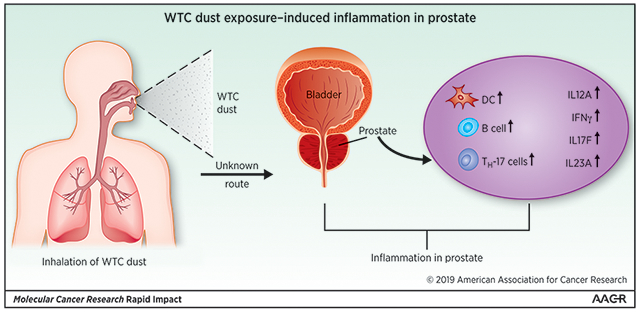
Graphical Abstract
INTRODUCTION
The attacks on the World Trade Center (WTC) in New York City on 9/11/2001 resulted in human exposure to multiple known and suspected human carcinogens, including soot, benzene and other volatile organic compounds (VOCs) from jet fuels. In addition, WTC dust and smoke contained asbestos, silica, cement dust, glass fibers, heavy metals, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and polychlorinated dibenzofurans (PCDFs) and dioxins from the burning and collapse of the planes and the towers [1]. Inhalation and ingestion of WTC dust and fumes caused respiratory illnesses caused by irritants and subsequent chronic inflammation [2–4]. An excess incidence of PCa has been identified among WTC rescue and recovery workers included in the WTC Health Program (WTCHP) at Mount Sinai Medical Center in New York, and in parallel among WTC-exposed firefighters in a separate program [4]. It is unclear whether the excess PCa is related to WTC-related exposures or represents an artifact due to surveillance bias. While the carcinogenic mechanisms of most of these agents on the prostate are not known, especially when inhaled in a complex mixture that changed in concentrations over time, they are likely to include both early (DNA damage, mutation, reduced DNA repair) as well as late events (cell proliferation, chronic inflammation) relevant to prostate carcinogenesis.
Chronically unresolved inflammation is associated with an increased risk of malignant disease. Approximately 20% of all human cancers are thought to be caused by chronic infection or chronic inflammatory states [5, 6]. In vitro and in vivo studies suggest that macrophages exposed to WTC dust can induce the secretion of cytokines and other inflammatory factors [7]. WTC dust exposure in mice is associated with inflammation, oxidative stress and epigenetic changes in the lung [8]. In an effort to understand the underlying causes of the excess incidence of PCa in WTC responders, we hypothesized that inhalation of WTC dust not only exposed prostate cells to genotoxic substances, but also might have facilitated PCa progression through longer-term mechanisms such as fostering chronic inflammation.
We studied the immunological and inflammatory cascade in archived PCa tissues of WTCHP patients using a NanoString assay, in comparison to non-WTC exposed PCa cases. In addition, we performed RNA-seq in rats to understand the immediate and delayed responses of healthy prostate tissue to acute WTC dust inhalation.
METHODS
Patient Recruitment Criteria
Archived paraffin-embedded tumor samples of WTC and non-WTC PCa cases were obtained from the Mount Sinai tumor bank. WTC PCa patients were consented to be part of the study; non-WTC patients were consented to an ongoing Genitourinary Cancer Biorepository at Mount Sinai. In total, 15 WTC patients and 15 non-WTC patients were included in this study, frequency matched for age, race/ethnicity and Gleason scores. WTC-related environmental exposure information was obtained from questionnaires administered to cohort members at the first visit, and was categorized into four mutually exclusive groups to reflect the intensity and duration of exposure to the dust, smoke, and debris. The group at very high exposure encompasses those who worked more than 90 days, were exposed to the dust cloud, and worked on the pile; high exposure was assigned to those who were exposed to the dust cloud, but either worked less than 90 days or did not work on the pile; intermediate exposure comprised those who were not exposed to the dust cloud and either worked between 40 and 90 days or worked on the pile [9].
NanoString Assay
All archived specimens were evaluated by a pathologist after H&E staining prior to RNA extraction. RNA was isolated from tissue scrolls or punch using the Promega Maxwell 16 LEV RNA FFPE Purification Kit (Promega: AS1260). 100ng of purified RNA was hybridized with the PanCancer Immune Profiling Panel (NanoString Technologies) per manufacturer’s instruction in the qPCR core facility at Mount Sinai Medical Center. RCC files from NanoString Digital Analyzer were imported into nSolver2.6 software (NanoString Technologies) and were checked for data quality using default QC settings. All samples were normalized using the geometric mean of multiple housekeeper genes.
NanoString Data Analysis
Statistical analysis was carried out with R-language and packages available through the Bioconductor project. Two groups were compared: WTC PCa patients and non-WTC PCa patients. Differences between the groups were evaluated using Fisher’s exact tests for categorical variables and Wilcoxon Rank Sum Test for continuous variables.
NanoString data (expression values (log2-transformed) were modeled using a linear model with Group (WTC/non-WTC) as a factor. A principal variance component analysis revealed a potential batch effect in NanoString assays (Supplemental Figure 1), which was used as an adjustment factor in all analyses.
Model fitting was carried out using the limma package, and p-values for the comparison between groups were corrected for multiple hypotheses testing using the Benjamini-Hochberg approach, which controls the False Discovery Rate (FDR). Unsupervised clustering of the expression values across patients for selected markers was represented in a heat map and carried out using Pearson correlation as distance and the McQuitty agglomeration scheme.
To evaluate changes at the pathway/gene set levels, we used Gene Set Variation Analysis (GSVA) [10]. We queried the Hallmark, and C7 collections from the MsigDB (http://software.broadinstitute.org/gsea/msigdb) [11], and blood cell type markers from NanoString (Supplemental Data 2).
RNA-seq of Rat Prostate Exposed to WTC Dust
Spontaneously hypertensive rats (SHR) were exposed to the WTC dust or isoflurane (ISO) anesthesia alone in an intra-tracheal inhalation (ITIH) integrated system as described previously [12, 13]. Sets of rats were exposed to WTC53 dust at 33 mg dust/m3 under isoflurane anesthesia for 2 hr/day on two consecutive days. Control rats were exposed to ISO only (1.5-1.8 % in carrier O2 gas). Rat prostate was dissected and snap-frozen in liquid nitrogen, after animals were euthanized by injection with Sleepaway®. Prostate RNA was extracted using Automated Robotic System with Beckman’s Agencourt RNA dv Advance tissue kit. RNA quality was assessed by bioanalyzer nanochips and must have a minimal RIN of 7. RNA sequencing was performed in the NextSeq core facility at Mount Sinai after in-house RNA library preparation with RiboMinus Eukaryote System v2 and Nextflex Rapid Directional RNA-seq kit. Samples were run on an Illumina NextSeq machine using single end run. The software TopHat was used to map the reads to the genome and to calculate the RPKM value for each gene.
RNA-seq Data Analysis
For each gene, we compared the mean expression level (log2 transformed RPKM value) between the WTC group (case) and ISO group (control) using R package limma. We reported both the group mean difference (log fold change) and the nominal p-value of significance.
For each immune cell type in each sample, we calculated an enrichment score (ES) based on cell type-specific gene sets (Supplemental Data 2) using the GSVA method. We then compared the ES between WTC dust exposed and ISO groups for each cell type and report the difference of the group mean, nominal p-value of significance (limma package), and FDR (Bonferroni-Holm method).
RESULTS
NanoString Gene Expression Profiling of Archived WTC PCa Samples
NanoString gene expression data was successfully obtained from 14 patients in the non-WTC group and 15 patients in the WTC group. The PanCancer Immune Profiling Panel allows multiplex gene expression analysis of 770 genes from 24 different immune cell types, common checkpoint inhibitors, C/T antigens, and genes covering both the adaptive and innate immune response.
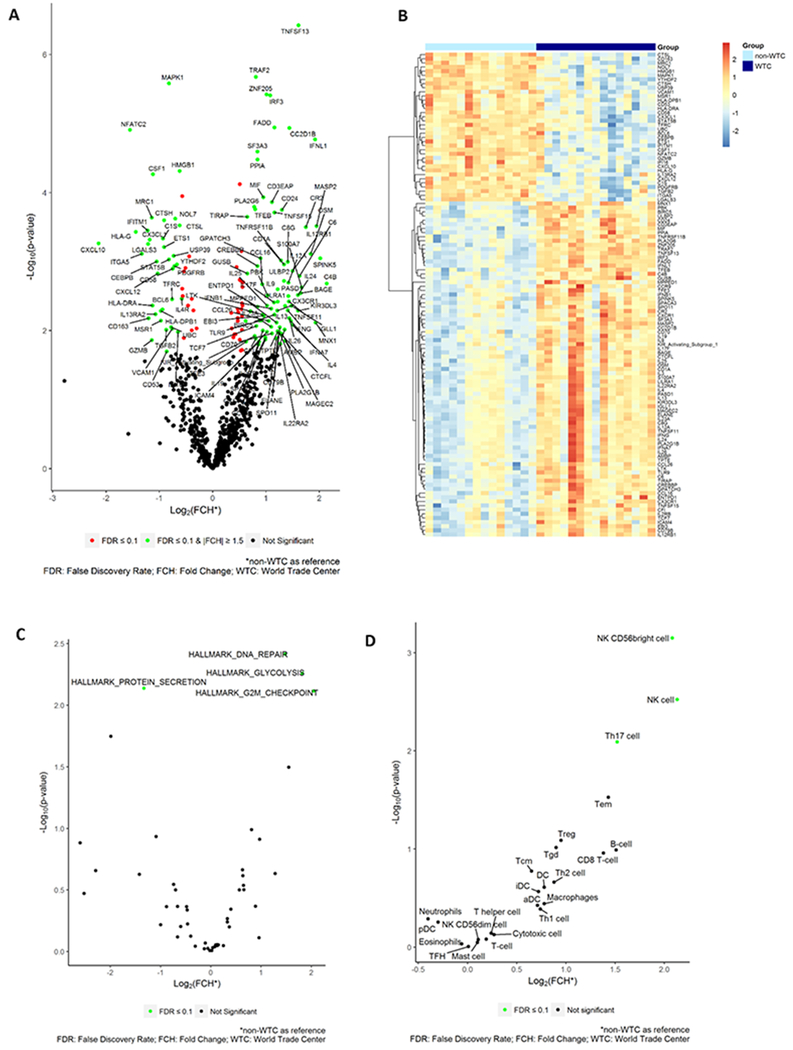
The WTC and non-WTC groups were similar in Gleason scores, Gleason group, TNM classification, race, and age at time of surgery (Table 1). The immune panel gene expression was compared between the WTC and non-WTC cases. In total, there were 117 differentially expressed genes (DEG) with false discovery rate (FDR) ≤0.1 and fold change (FCH) ≥1.5 (using non-WTC as the reference) (Figure 1A). Among them, 81 genes were upregulated and 36 were downregulated. Unsupervised clustering of the gene expression data for these selected markers showed distinct gene expression profiles of WTC and non-WTC samples (Figure 1B). A full list of genes can be found in Supplementary Data 1 Table 1. A subset of the most significantly downregulated genes was related to immune cell chemotaxis and proliferation (CXCL10, CXCL12, CX3CL1, CSF1, and IL4R), whereas the top upregulated genes were related to apoptosis (TNFSF-13, TRAF2, FADD, BIRC5,) and immune modulation (Th1 related genes: IFNG, IL12A, IL12RB1, IL2RB; Th2 related genes: IL4, IL13; Th17 related genes: IL17F, IL22RA2, IL23A).
Table 1:
Description of the population under study
| Variable | Non-WTC (n=14) N (%) |
WTC (n=15) N (%) |
P-value^ | |
|---|---|---|---|---|
| Gleason Score | 6 | 4 (28.6) | 4 (26.7) | 0.912 |
| 7 | 8* (57.1) | 10# (66.7) | ||
| 8 | 1 (7.1) | 1 (6.7) | ||
| 9 | 1 (7.1) | 0 (0) | ||
| T$ | 2c | 9 (64.3) | 10 (71.4) | 0.53 |
| 3a | 2 (14.3) | 3 (21.4) | ||
| 3b | 3 (21.4) | 1 (7.2) | ||
| Race | White | 12 (85.7) | 8 (53.3) | 0.365 |
| Other | 2 (14.3) | 4(26.7) | ||
| Missing | 0 (0) | 3 (20.0) | ||
| Age (years) | mean (SD) | 55.07 (6.17) | 54.7 (6.76) | 0.895 |
| Time from exposure to diagnosis | Months (mean ±SD) | - | 81.4 ±42 | |
| WTC Exposure level$ | Intermediate | 9 (64.3) | ||
| High | 4 (28.6) | |||
| Very high | 1 (7.1) | |||
Fisher’s Exact Test (non-missing values) for categorical variables, Wilcoxon Rank Sum Test for continuous variables
Group Gleason 2 (n= 4); Group Gleason 3 (n=4);
Group Gleason 2 (n= 8); Group Gleason 3 (n=2)
Information missing in 1 WTC case
1 WTC case was N+; all cases were M negative.
Figure 1. NanoString PanCancer Immune Profiling Panel Analysis of archived WTC and non-WTC PCa samples.
A. Differentially expressed genes (DEG) with FDR≤0.1 and Fold Change(FCH)≥1.5 (using non-WTC as the reference) represented in a volcano plot with batch-adjusted expression values. X axis: fold changes in log2. Y axis: −p value in log 10. B. Unsupervised clustering of the expression values for selected genes was represented in a heat map. C. Differentially expressed pathways in Hallmark collection. D. Differentially regulated cell types in cell type enrichment analysis. X axis: fold changes in log2. Y axis: −p value in log 10.
Pathway enrichment analysis showed 3 significantly upregulated pathways (FDR≤0.1), specifically, DNA Repair, G2M Checkpoint and Glycolysis, and 1 downregulated pathway, Protein Secretion (Figure 1C and Supplementary Data 1 Table 2). A full list of genes used in pathway analysis can be found in Supplementary Data 1 Table 3.
In cell type enrichment analysis using blood cell type markers provided by NanoString as reference gene sets (Supplemental Data 2), we found three cell types significantly upregulated in WTC cases: CD56bright subset of NK cells (FDR=0.017), total NK cells (FDR= 0.036), and Th17 cells (FDR=0.065) (Figure 1D and Supplementary Data 1 Table 4).
Gene Expression Profiling of Rat Prostate After WTC Dust Exposure
To evaluate the influence of WTC dust inhalation on gene expression in the normal prostate, we performed RNA-seq on the prostate of the SHR rats that were acutely exposed to WTC dust as described in the Methods. The dust dose was mimicking a relatively moderate to high exposure faced by WTC first responders during the initial three days at Ground Zero. The rat prostate was harvested at 1 day and 30 days after exposure in order to assess immediate and delayed responses to WTC exposure. Prostate of rats treated with isoflurane (ISO) anesthesia alone was used as controls.
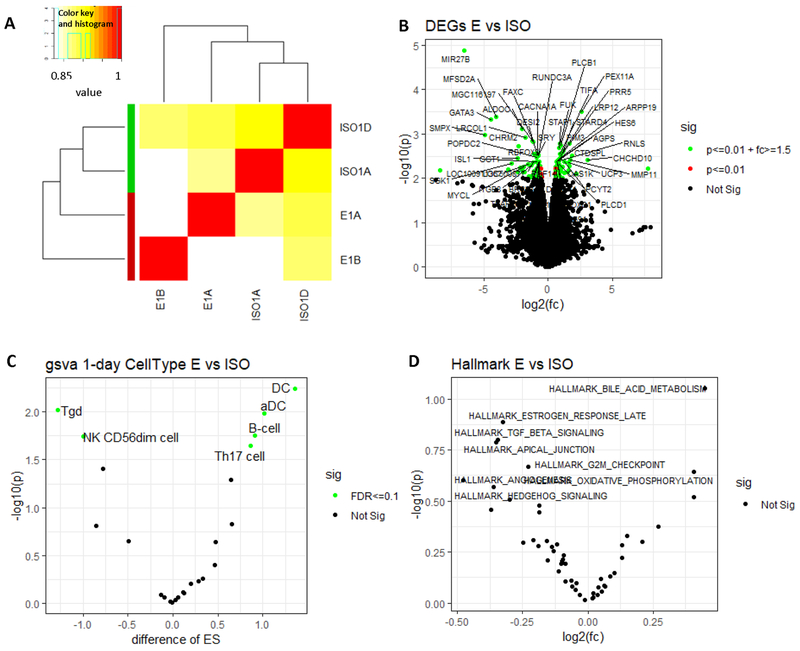
Unsupervised clustering of RNA-seq data revealed that WTC dust-treated rats showed unique gene expression pattern in the prostate compared to ISO group at one-day post exposure (Figure 2A). We identified 299 upregulated and 287 downregulated genes compared to ISO group (p<0.05) (Figure 2B, Supplemental Data 3). Cell type enrichment analysis revealed that the changes in the gene expression profile reflected significant upregulation of Th17 cells, activated dendritic cells (aDC), dendritic cells (DC cells) and B cells, and downregulation of Tgd and NK CD56dim cells (Figure 2C and Supplemental Figure 2). Note that CD56bright NK cells, which were upregulated in archived human samples, were not significantly changed here. In pathway analysis, the top three deregulated pathways were bile acid metabolism, hedgehog signaling and oxidative phosphorylation (Figure 2D); however, the changes did not reach significant levels; this may be due to small number of animals used in this preliminary study.
Figure 2. Gene expression profiling by RNA-seq of rat prostate harvested at 1 day after WTC exposure.
A. Unsupervised clustering of RNA-seq data obtained from rat prostate samples collected at 1-day post dust exposure. Sample ID is represented as group (E for WTC group, ISO for control group) followed by sample harvest day (1 for 1-day post exposure) and animal ID number within the group (A, B, C or D). B. Differential expressed genes (DEGs) in WTC dust-treated group (E) compared to control group (ISO), represented in a volcano plot (p<0.01). X axis: fold changes in log2. Y axis: −p value in log 10. C. Cell type analysis in WTC dust- treated (E) vs. control rat prostate (ISO). X axis: cell type enrichment score (ES). Y axis: −p value in log 10. D. Differences in pathway activities in WTC dusts treated vs. control rat prostate. X axis: fold changes in log2. Y axis: −p value in log 10.
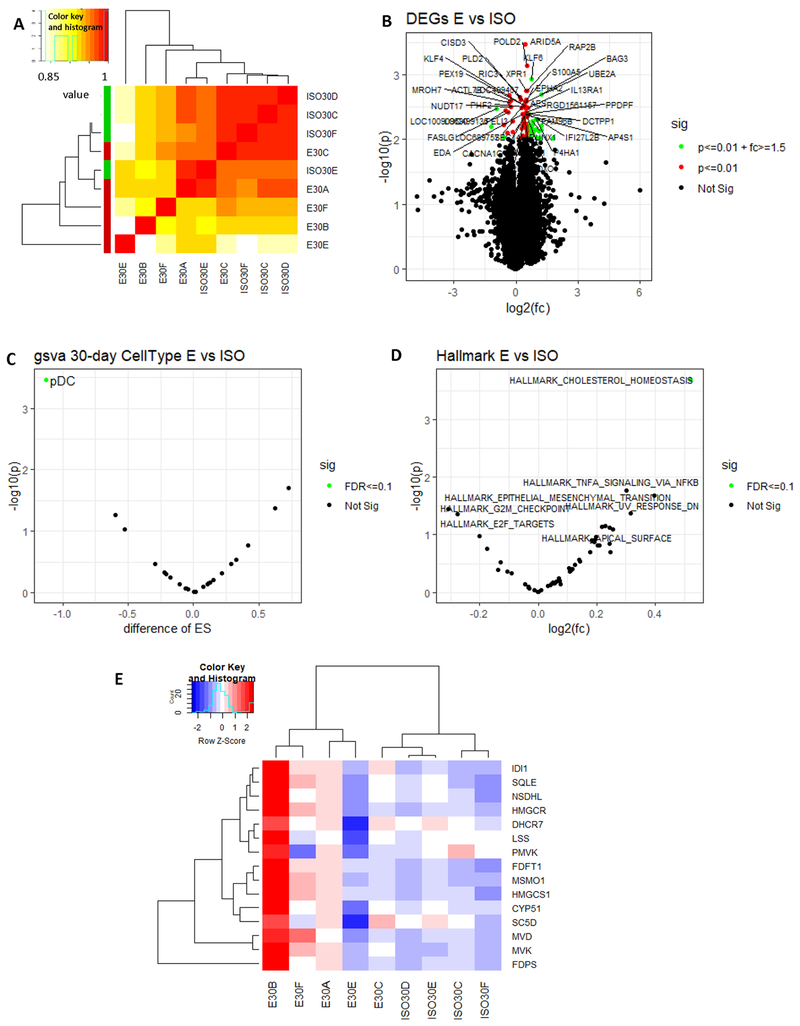
Unsupervised clustering of gene expression data obtained at 30 days after exposure revealed that WTC samples showed distinct but also heterogeneous gene expression profiles, compared to ISO controls (Figure 3A), which could be due to the variation in the actual dust exposure, retention and clearance in individual animals. DEG analysis identified 602 upregulated and 171 downregulated genes at day 30 (p<0.05) (Figure 3B, Supplemental Data 3). In cell type enrichment analysis, plasmacytoid dendritic cells (pDC) were downregulated (Figure 3C and Supplemental Figure 3). In pathway analysis, cholesterol homeostasis appeared to be the only significantly altered pathway in WTC samples (Figure 3D). Gene expression levels of the enzymes directly involved in cholesterol synthesis are shown in Figure 3E. Again, ISO group had relatively uniform expression of the pathway genes whereas WTC dust-treated rats displayed elevated but also heterogeneous pattern of gene expression. Expression of 3-hydroxy-3-methyl-glutaryl–CoA reductase (HMGCR), a crucial enzyme in the mevalonate pathway for sterol biosynthesis, was elevated in 3/5 WTC dust exposed rats.
Figure 3. Gene expression profiling by RNA-seq of rat prostate harvested at 30 days after WTC exposure.
A. Unsupervised clustering of RNA-seq data obtained from rat prostate samples collected at 30-days post dust exposure. Sample ID is represented as group (E for WTC group, ISO for control group) followed by sample harvest day (30 for 30-days post exposure) and animal ID number within the group (A, B, C, D, E or F ). B. Differential expressed genes (DEGs) in WTC dust-treated group (E) compared to control group (ISO), represented in a volcano plot (p<0.01). X axis: fold changes in log2. Y axis: −p value in log 10. C. Cell type analysis in WTC dust- treated (E) vs. control rat prostate (ISO). X axis: cell type enrichment score (ES). Y axis: −p value in log 10. D. Differences in pathway activities in WTC dust-treated vs. control rat prostate. X axis: fold changes in log2. Y axis: −p value in log 10. E. A heat map of gene expression levels of enzymes directly involved in cholesterol synthesis.
DISCUSSION
Epidemiological studies reveal an increased incidence of PCa in WTC responders. In this study, we aimed to uncover the specific molecular and cellular events associated with WTC dust exposure that could contribute to prostate carcinogenesis. By comparing gene expression profiles of archived prostate tissues from WTC PCa and non-WTC patients, we found a distinct pattern of gene expression in WTC samples characterized by upregulation of genes related to DNA damage repair pathway, G2/M arrest, and glycolysis. When the types of cellular infiltrates were assessed, a clear increase in natural killer (NK) cells and Th17 was noted in WTC cases. In addition, SHR rats acutely exposed to a moderate to high dose of WTC dust through respiration displayed unique gene expression changes in the prostate. Among them, upregulation of antigen presenting cells (aDC and DC) and B cells indicated activation of adaptive immune response in response to antigens present in WTC dust. Upregulation of Th17 cells is consistent with the data obtained from human samples and suggests a local inflammatory cascade induced by acute dust exposure. Our results provide first line of evidence to support the hypothesis that acute WTC dust exposure through inhalation can profoundly disturb gene expression and immune cell infiltration in the prostate. Our study also suggests that acute dust exposure may have a long-lasting effect in the prostate, as demonstrated by deregulated gene expression pattern 30 days after dust exposure in rats. An interesting question remains about the retention and elimination rates of WTC dust particles and their distribution amongst different organs. The long–lasting inflammatory effect in prostate revealed by our study calls for further study of the exposure effect in other organs such as kidney, CNS and etc. So far, the dust retention study in the lung reported by Cohen et al has shown that WTC dust of the same composition significantly damaged ciliated cells [14]; therefore greatly increased retention of the dust in the lung. Over a 1-yr period after the final exposure, there was only a nominal non-significant decrease (6–11%) of inhaled dust burden in the lung of experimental rats. These results provide support for our current hypothesis that exposure to WTC dusts will cause chronic changes in the body.
An upregulation of DNA damage repair pathway and the G2M checkpoint was observed in archived WTC PCa cases. Components of WTC dust including benzene, asbestos, polycyclic aromatic hydrocarbons (PAHs), dioxins are among the most potent carcinogens. Given the fine particulate nature of the WTC dust [15], it is likely that these components could enter the blood circulation through lung and eventually reach distal organs such as prostate, where their chemical composition determines the long-term retention and toxic potential. Exposure to WTC carcinogens not only directly induce DNA damage, but also induce reactive oxygen species (ROS) production by interfering with normal cellular functions. The high lipid content of mitochondrial membrane facilitates accumulation of lipophilic compounds such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and polychlorinated dibenzofurans (PCDFs) found in WTC dust [16]. Accumulation of these toxic substances may interfere with normal mitochondrial function by inducing ROS production [17]. The chronically elevated oxidative byproducts may contribute to different stages of cancer development by inducing DNA damage and inflammation.
Th17 cells are consistently upregulated in WTC group in both human and rat experiments. Th17 cells represent a subset of helper T cells characterized by the production of IL17 [6]. They are significant mediators of inflammation in the tumor microenvironment, and have been detected in several cancer types [18–20]. The upregulation of Th17 cascade (IL17F, IL22RA2 and IL23A) observed in our study further supports a tumor-promoting role of IL17-mediated inflammation in PCa pathogenesis.
In human peripheral blood, there are at least two major subtypes of NK cells, referred to as CD56bright and CD56dim NK cells [21]. While the CD56dim NK cells represent majority (~ 90%) of all peripheral blood NK cells and display significantly higher cytotoxicity than CD56bright NK cells, the latter ones are the most efficient cytokine producers. The cytokines released by CD56bright NK cells include interferon-γ, TNFα, GM-SCF, IL-10 and IL-13. Our cell type enrichment analysis indicated significant upregulation of CD56bright NK cells in archived WTC PCa cases, which might have contributed to the upregulation of inflammatory cytokines such as IFNG and IL-13. In WTC dust-treated rat prostate, we did not observe such as change; instead, we observed downregulation of CD56dim NK cells. The discrepancy in the NK cell response is not fully understood but it might reflect the distinct nature of the two studies (human vs. rats, acute response vs. chronic response). Further studies are needed to understand the response of NK cells to WTC dust exposure.
Cholesterol is a sterol lipid synthesized by all animal cells and serves as a precursor to steroid hormones such as androgen. The relationship between cholesterol and PCa has been studied for decades. Compared to normal prostate cells, prostate tumors have increased levels of cholesterol synthesis [22]. High levels of cellular cholesterol are generally associated with cancer progression and less favorable outcomes [23–25]. Our RNA-seq data identified the cholesterol synthesis pathway as the single most upregulated pathway in WTC dust-treated rat cohort, suggesting the potential role of WTC dust in activating this pathway to promote PCa development.
Taken together, our results suggest that WTC PCa cases have a distinct gene expression pattern that may be the result of exposure to specific carcinogens during the WTC attacks. WTC dust-exposed rat prostate displayed unique changes in gene expression and immune cell infiltrates after acute dust exposure, suggesting that the effect of exposure may be measured locally in target organs such as prostate. In addition, some of the genes overexpressed in rat normal prostates as a consequence of exposure are also overexpressed in human PCa tissues, suggesting a link between exposure, local immune dysregulation, and PCa development. However, from the preliminary data generated by these experiments, no specific genetic patterns suggestive of a more aggressive cancer phenotype can be identified. A larger study targeting the genes and cell types that were overexpressed in this hypothesis-generating work should be conducted in order to address if the WTC prostate cases have a distinct signature of aggressiveness in comparison to non-WTC cases.
This work has larger implications to population exposed to environmental particulates such as emissions from motor vehicles, industrial processes, power generation and the household combustion of solid fuel, since inflammation could be the common pathway driving an increase in cancer occurrence [26].
Supplementary Material
IMPLICATIONS.
WTC-related PCa displayed distinct gene expression pattern that could be the result of exposure to specific carcinogens. Our data warrant further epidemiological and cellular mechanistic studies to better understand the consequences of WTC dust exposure.
Acknowledgments
Financial Support: This work was supported by CDC/NIOSH Grant UO1 OH010396 (Taioli), CDC UO1 OHO11328 (Aaronson), National Institute of Health Grant R21 ES026731 (Chen), CDC/NIOSH Grant OH010921 (Cohen), and in part, by NIEHS Center Grant ES00260 (Cohen).
Footnotes
The authors have no conflicts of interests to declare.
References
- 1.L.P. J and PANOS G, The Anatomy of the Exposures That Occurred around the World Trade Center Site. Annals of the New York Academy of Sciences, 2006. 1076(1): p. 54–79. [DOI] [PubMed] [Google Scholar]
- 2.de la Hoz RE, et al. , Occupational toxicant inhalation injury: the World Trade Center (WTC) experience. Int Arch Occup Environ Health, 2008. 81(4): p. 479–85. [DOI] [PubMed] [Google Scholar]
- 3.Fireman EM, et al. , Induced Sputum Assessment in New York City Firefighters Exposed to World Trade Center Dust. Environmental Health Perspectives, 2004. 112(15): p. 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashim D, et al. , Prostate cancer characteristics in the World Trade Center cohort, 2002–2013. Eur J Cancer Prev, 2018. 27(4): p. 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, and Karin M, Immunity, Inflammation, and Cancer. Cell, 2010. 140(6): p. 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Marzo AM, et al. , Inflammation in prostate carcinogenesis. Nature Reviews Cancer, 2007. 7: p. 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiden MD, et al. , Comparison of WTC Dust Size on Macrophage Inflammatory Cytokine Release In vivo and In vitro. PLoS ONE, 2012. 7(7): p. e40016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunil VR, et al. , World Trade Center (WTC) dust exposure in mice is associated with inflammation, oxidative stress and epigenetic changes in the lung. Experimental and molecular pathology, 2017. 102(1): p. 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisnivesky JP, et al. , Persistence of multiple illnesses in World Trade Center rescue and recovery workers: a cohort study. The Lancet, 2011. 378(9794): p. 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hänzelmann S, Castelo R, and Guinney J, GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics, 2013. 14(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brannon AR, et al. , Molecular Stratification of Clear Cell Renal Cell Carcinoma by Consensus Clustering Reveals Distinct Subtypes and Survival Patterns. Genes & cancer, 2010. 1(2): p. 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MD, et al. , Impact of acute exposure to WTC dust on ciliated and goblet cells in lungs of rats. Inhalation toxicology, 2015. 27(7): p. 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MD, et al. , Acute high-level exposure to WTC particles alters expression of genes associated with oxidative stress and immune function in the lung. J Immunotoxicol, 2015. 12(2): p. 140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MD, et al. , Impact of acute exposure to WTC dust on ciliated and goblet cells in lungs of rats. Inhal Toxicol, 2015. 27(7): p. 354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGee JK, et al. , Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ Health Perspect, 2003. 111(7): p. 972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross, Meredith F, et al. , Accumulation of lipophilic dications by mitochondria and cells. Biochemical Journal, 2006. 400(Pt 1): p. 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X, Crawford DL, and Oleksiak MF, Effects of Anthropogenic Pollution on the Oxidative Phosphorylation Pathway of Hepatocytes from Natural Populations of Fundulus heteroclitus. Aquat Toxicol, 2015. 165: p. 231–40. [DOI] [PubMed] [Google Scholar]
- 18.Sfanos KS, et al. , Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res, 2008. 14(11): p. 3254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evelyna D, et al. , Pretreatment frequency of circulating IL‐17+CD4+ T‐cells, but not Tregs, correlates with clinical response to whole‐cell vaccination in prostate cancer patients. International Journal of Cancer, 2009. 125(6): p. 1372–1379. [DOI] [PubMed] [Google Scholar]
- 20.Steiner GE, et al. , Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate, 2003. 56(3): p. 171–82. [DOI] [PubMed] [Google Scholar]
- 21.Poli A, et al. , CD56bright natural killer (NK) cells: an important NK cell subset. Immunology, 2009. 126(4): p. 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stopsack KH, et al. , Cholesterol uptake and regulation in high-grade and lethal prostate cancers. Carcinogenesis, 2017. 38(8): p. 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang L, et al. , Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. The Journal of clinical investigation, 2005. 115(4): p. 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allott EH, et al. , Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol Biomarkers Prev, 2014. 23(11): p. 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtola TJ, et al. , Serum cholesterol and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Prostate Cancer and Prostatic Diseases, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Straif K, Cohen A, and Samet J, Air polution and cancer, in IARC Scientific. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.