Abstract
Introduction
Bosutinib is a dual ABL1 and SRC third generation tyrosine kinase inhibitor (TKI) indicated for the treatment of patients with chronic myelogenous leukemia (CML) resistant to or intolerant of other BCR-ABL1 inhibitors. Bosutinib is active against leukemia cells expressing imatinib-resistant BCR-ABL1 mutations. Mechanistically, this agent may also be beneficial for Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) because in preclinical animal models, SRC accelerates ALL disease development.
Areas Covered
Here we review the current scientific and medical literature on the role of bosutinib for the treatment of CML. We address the unique therapeutic advantages of this agent, specifically its ability to inhibit mutant BCR-ABL1 kinases conferring resistance to other TKIs and its unique safety profile consisting of mainly manageable self-limited diarrhea, not cardiovascular, side effects. Long-term toxicities reported with dasatinib, nilotinib and ponatinib have not been described with bosutinib. Lastly, we present preclinical data demonstrating that bosutinib inhibits a broader range of tyrosine kinases than any other TKI, including those implicated in acute leukemia.
Expert Opinion
We propose that future studies should explore the use of bosutinib in Ph+ ALL due to its multi-kinase inhibitory activity and its relatively long-term safety compared to other second and third generation TKIs.
1. Introduction
Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) is the most common cytogenetic subtype of adult ALL occurring in 20% to 30% of cases.1 This abnormality is formed from a reciprocal translocation between ABL1 on chromosome 9 and the breakpoint cluster region (BCR) on chromosome 22, resulting in the fusion gene, BCR-ABL1.2–4 Until the clinical development of the first BCR-ABL1 tyrosine kinase inhibitor (TKI), imatinib, Ph+ ALL had a very poor prognosis. The only curative potential for such patients was allogeneic stem cell transplant (allo-SCT) in first complete remission (CR1).1 Modern upfront combinations of imatinib and chemotherapy have resulted in substantial improvements in outcome and currently, Ph+ ALL patients achieve remission more than 95% of the time.5 Clinical investigators have looked toward improving upon the first generation TKI, imatinib, with second (dasatinib and nilotinib) and third (bosutinib and ponatinib) generation TKIs, for both chronic myeloid leukemia (CML) and Ph+ ALL.
Despite the initial success of imatinib for Ph+ ALL, clinical follow up studies have demonstrated development of resistance or intolerance to imatinib. Such resistance may be BCR-ABL1 dependent or independent. The most common BCR-ABL1-dependent resistance mechanism is the development of a point mutation within the tyrosine kinase domain.6–8 Another BCR-ABL1-dependent mechanism results from decreased accumulation of the TKI within the cell. Intracellular concentrations of imatinib, for example, are dependent on both efflux [multidrug resistance ATP-binding cassette (MDR-ABC) transporters9 and influx (organic cation protein 1, OCT-1, which is encoded by gene SLC22A1)10 proteins. Common BCR/ABL1-independent mechanisms include activation of pathways which include SRC family kinases,11 as well as other pathways such as those involving CXCR4, which is a C-X-C chemokine receptor type 4, also known as fusion or CD184.12
Bosutinib is a third generation BCR-ABL TKI (see Drug Summary Box) indicated for the treatment of patients with CML have developed resistance to and/or are intolerant of other TKI treatments. This drug’s unique safety profile offers the potential for greater patient compliance with manageable adverse events. 17,19 This review focuses on the currently available clinical data for bosutinib in the treatment of Ph+ CML and discusses its future therapeutic application for Ph+ ALL.
Drug Summary Box 1.
| Drug name (generic) | Bosutinib monohydrate (BOSULIF®) |
|---|---|
| Phase (for indication under discussion) | Bosutinib has been studied for its safety and efficacy in patients with Philadelphia chromosome positive (Ph+) leukemia with resistance or intolerance to prior tyrosine kinase inhibitor therapy. |
| Indication (specific to discussion) | Bosutinib is currentlyFDA approved and indicated for the treatment of chronic, accelerated, or blast phase Ph+ chronic myeloid leukemia in adult patients with resistance or intolerance to prior therapy. |
| Pharmacology description/mechanism of action | Dual Abl/Src tyrosine kinase inhibitor |
| Route of administration | BOSULIF ® tablets are orally administered, available in two strengths, 100mg or 500mg tablets. |
| Chemical structure | C26H29Cl2N5O3 ∙ H2O (monohydrate)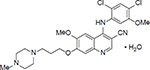 (Image taken from rxlist.com) |
| Pivotal trial(s) | Study 200, BELA Trial 32 |
2. Historical overview
Bosutinib (SKI-606, Bosulif ®) is a third-generation BCR-ABL1 tyrosine kinase inhibitor which was approved by the United States Food and Drug Administration on September 4, 2012, for the treatment of chronic, accelerated, or blast phase Ph+ positive chronic myeloid leukemia in adult patients with resistance or intolerance to prior therapy.13 Bosutinib is an orally active, 4-anilino-3-quinoline cardonitrile, which acts as a dual SRC/ABL1 kinase inhibitor, with lower activity against a few serine-threonine kinases (protein kinase A, CK1 and CK2) and minimal inhibition of platelet derived growth factor and c-KIT.14
3. Biologic effects
Bosutinib prevents tyrosine phosphorylation of BCR-ABL1 at concentrations of 25–50 nM15 and acts as a potent ABL1 tyrosine kinase inhibitor with anti-proliferative activity against CML cells in culture.15 This study also showed that once daily oral administration of bosutinib over a 5-day period eradicated K562 CML tumor xenografts in nude mice.
Bosutinib is also known to be a multi- kinase inhibitor with potent activity against SRC. In an enzyme assay, bosutinib inhibits SRC with an IC50 of 1.2 nM and prevents anchorage-independent growth of SRC-transformed fibroblasts with an IC50 of 100 nM. It also blocks SRC-dependent protein tyrosine phosphorylation at comparable or lower concentrations.15 SRC mediates signals and forms complexes with proteins that regulate cell proliferation, angiogenesis, invasion, metastasis and bone metabolism by phosphorylating and dephosphorylating Tyr530.11 Bosutinib has also been shown to inhibit two SRC related kinases, FGR(a proto-oncogene, aka c-fgr, p55c-fgr) and LYN, at picomolar concentrations, and C-SRC tyrosine kinase, (which acts as an endogenous inhibitor of SRC family protein tyrosine kinases) at concentrations in the nanomolar range.14 This study further investigated the kinase specificity profile of bosutinib by incubating 52 purified proteins belonging to serine-threonine or tyrosine classes. ABL1, C-SRC and LCK kinases demonstrated 95% inhibition and PIM-2 kinase activity was 75% inhibited.14 Several other serine-threonine kinases including AKT, cyclin-dependent kinase 2, apoptosis-linked serine/threonine kinase and calcium/calmodulin-dependent protein kinase II gamma have also been shown to be blocked following exposure to bosutinib.16,17
The potential significance of dual BCR/ABL1 and SRC inhibition in Ph+ ALL was demonstrated by a preclinical study showing that treatment with a SRC inhibitor plus imatinib was superior to imatinib alone in a Ph+ ALL mouse model but not in a CML model.18 In a head-to-head in vitro comparison against BCR/ABL1, bosutinib and dasatinib targeted many of the same kinases.19 There were two Ph+ ALL cell lines that were studied, BV 173 and Z 119. Bosutinib targeted the broadest network of kinases, with varied affinity, although with weaker potency when compared to dasatinib. Many kinases were only interacting with specific drugs, of note, PDGFRB, LK, TESK1 were specific to dasatinib. The kinases CHEK2, MAP2K1 and MAP2K2 were specific to bosutinib. Ultimately, this study concluded overall that dasatinib is most appropriate TKI to treat Ph+ ALL due its strongest impact on tyrosine phosphorylation.
4. Pharmacokinetics
In a study including healthy adult subjects, a single dose of bosutinib (200–800 mg), with food, the Cmax and area under the curve (AUC) increased over the entire dose range.20 Mean clearance and volume of distribution were similar across the doses except at the 800 mg dose. Consumption of a high-fat meal increased drug AUC by approximately 1.5 fold compared to fasting conditions. However, the observed approximately 1.4 fold increase in Cmax with food was not statistically significant compared to fasting state. Further, bosutinib doses were limited to 400 mg under fasting conditions because of multiple adverse events. In addition, the study suggested that food intake increased bosutinib’s exposure and improved tolerability up to 600 mg. Therefore bosutinib should be taken with food.
In another human study, drug solubility appears to be pH dependent as concomitant proton pump inhibitor (lansoprazole) use decreased the Cmax and AUC by 46% and 26%.21 It is therefore recommended that concurrent therapy with bosutinib and gastric acid suppressive therapies, such as proton pump inhibitors be avoided.
A study using healthy adults, 14C radiolabeled bosutinib have shown that only 3% of the administered dose is excreted in the urine, and 91.3% of the dose was recovered in feces.22 Special attention should be paid in dosing bosutinib in patients with hepatic impairment because the AUC in these patients increased 1.9–2.4-fold in patients with Child-Pugh A, B and C.23 Therefore, the manufacturer recommends dose reduction to 200 mg daily in patients with even mild hepatic impairment.23 Despite this clear majority excretion through the biliary system, the manufacturer recommends that bosutinib’s dose should also be reduced in patients with creatinine clearance between 30–50 ml/min if they cannot tolerate the 500 mg dose and be reduced in all patients with creatinine clearance of less than 30 ml/min because the AUC increased by 60% in this patient population.23Interestingly, bosutinib is not a substrate for the multidrug resistance MDR-ABC transporters.24
5. Phase I studies
Bosutinib has been studied primarily for its use in CML. In a Phase I dose-escalation study in healthy adults, Abbas et al20 found that bosutinib’s median tmax was six hours and its t1/2 ranged from 33–39 hours, justifying a once-daily dosing strategy. Cortes et al,26in their run-in phase I, showed that the 600 mg dose in CML patients was associated with increased adverse events, including grade 3 rash, nausea and vomiting, grade 2 alanine aminotransferase elevation, grade 2 rash and grade 3 diarrhea. Based upon higher toxicity at the 600mg dose, bosutinib 500 mg daily was chosen as the recommended Phase II dose.
6. Clinical Studies in Ph+ Leukemia
Multiple clinical studies to date have defined the safety and tolerability of bosutinib in resistant/intolerant 27 and newly diagnosed CML patients.28 Bosutinib induces durable remissions in most CML patients with imatinib resistance or intolerance except those harboring T315I and V299L mutations in the BCR-ABL1kinase.27 Recently, additional mutational BCR-ABL1 events conferring bosutinib-resistance have been identified, including L248R, T315V, F317R, F317V and the compound mutation L248R+F359I.29 Bosutinib’s primary toxicity remains gastrointestinal with a majority of patients developing diarrhea. The severity of this adverse event was mostly low grade, occurring within 2–6 days of drug initiation, with a median duration of 1 to 2 days. Patients were managed with anti-diarrheal medications and dose adjustments 27 Bosutinib results in similar complete cytogenetic responses (CCyR) as compared to imatinib but higher rates of major molecular response (MMR) in newly diagnosed chronic phase CML patients.28
6.1. Treatment of CML following prior BCR-ABL1 TKI therapy
The results from a phase I/II study27 of 288 patients evaluating the efficacy and safety of bosutinib in Ph+ CML who were resistant (n=200) or intolerant (n=88) to imatinib, a phase II study of 87 patients who failed imatinib and were resistant (n=37) or intolerant (n=50) to dasatinib30 and 17 patients with Ph+ ALL31 are summarized in Table 1. Major responses were detected across manyBCR-ABL1 kinase domain mutations, except for T315I. Similar rates of CHR or major cytogenetic response were achieved among patients with and without mutations. The 2-year progression free survival (PFS) was 81%, and the 2-year overall survival (OS) was 91%. Disease progression was the most common cause of death followed by adverse events.
Table 1:
Best cumulative responses to bosutinib among different sub-groups
| Imatinib Resistant (%) N=20026 | Imatinib Intolerant (%) N=8826 | Imatinib + Dasatinib Resistant (%) N=3730 | Imatinib + Dasatinib Intolerant (%) N=5030 | Ph+ ALL (%) N=1731 | |
|---|---|---|---|---|---|
| CHR among patients with no baseline hematologic response | 76 | 83 | 50 | 67 | 15 |
| MCyR among evaluable patients* | 54 | 49 | 31 | 30 | 18 |
| CCyR among evaluable patients* | 41 | 41 | 14 | 30 | NA |
| MMR among patients achieving complete cytogenetic response | 64 | 65 | 3 | 25 | NA |
Abbreviations: ALL, acute lymphoblastic leukemia; CCyR, complete cytogenetic remission; CHR, complete hematologic remission; MCyR, major cytogenetic remission; MMR, major molecular remission
Evaluable patients are those who did not have cytogenetic response at enrollment and had at least 20 metaphase cells to determine response. If fewer than 20 metaphases were available for post baseline assessment, fluorescent in-situ hybridization analysis of bone marrow aspirate with at least 200 nuclei for the presence of BCR/ABL1 gene was acceptable.
6.2. Safety and management of toxicity in patients following prior TKI therapy
The most common non-hematologic treatment-emergent adverse events following bosutinib use in CML patients were gastrointestinal.32 specifically, all grades of diarrhea (82%), nausea (47%), and vomiting (39%). These events occurred very early during the start of treatment and were self-limiting. Pleural effusions occurred overall in 10% of patients, and occurred most frequently in the third line setting. Most patients who developed pleural effusions during bosutinib treatment had been previously exposed to dasatinib. Median time to development of pleural effusion was 541 days. Only 1% of patients discontinued bosutinib due to pleural effusion.
Liver enzyme elevation, specifically in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values, were detected in 17% and 14% of patients. Grade 3 level elevations were reported in 7% of patients and grade 4 in <1%. Only 2% of patients discontinued treatment due to liver enzyme elevations.
Hematologic grade 3/4 adverse events associated with bosutinib included thrombocytopenia in 30% and anemia or neutropenia in 14% of patients.32 Myelosuppressive adverse events were managed primarily by dose modification; only 7% discontinued treatment. Table 2 summarizes the toxicities seen in the 166 advanced phase (accelerated and blast phase) CML and ALL patients. Table 3 summarizes the toxicity seen in the 78 who were resistant or intolerant to imatinib and either resistant or intolerant to dasatinib.
Table 2:
| Adverse event (non-hematologic) | Advanced Phase* CML N (%) |
|---|---|
| Diarrhea | |
| All grade | 122 (74) |
| Grade 3/4 | 8 (5) |
| Nausea | |
| All grade | 80 (48) |
| Grade 3/4 | 3 (2) |
| Vomiting | |
| All grade | 72 (43) |
| Grade 3/4 | 6 (4) |
| Rash | |
| All grade | 51 (31) |
| Grade 3/4 | 6 (4) |
| Pyrexia | |
| All grade | 64 (39) |
| Grade ¾ | 5 (3) |
| Fatigue | |
| All grade | 35 (21) |
| Grade | 7 (4) |
| Abdominalpain | |
| All grade | 35 (21) |
| Grade ¾ | 4 (2) |
| Headache | |
| All grade | 31 (19) |
| Grade 3/4 | 7 (4) |
| Cough | |
| All grade | 34 (21) |
| Grade 3/4 | 0 |
| Elevated ALT | |
| All grade | 17 (10) |
| Grade 3/4 | 7 (4) |
| Upper abdominal pain | 17 (10 |
| All grade | 3 (2) |
| Grade 3/4 | |
| Elevated AST | |
| All grade | 17 (10) |
| Grade 3/4 | 5 (3) |
| Arthralgia | |
| All grade | 22 (13) |
| Grade 3/4 | 1 (1) |
| Decreased appetite | |
| All grade | 21 (13) |
| Grade 3/4 | 0 |
| Constipation | |
| All grade | 27 (16) |
| Grade 3/4 | 1 (1) |
| Dyspnea | |
| All grade | 32 (19) |
| Grade 3/4 | 9 (5) |
| Asthenia | 20 (12) |
| All grade | 1(1) |
| Grade 3/4 | |
| Dizziness | |
| All grade | 21 (13) |
| Grade 3/4 | 1 (1) |
| Peripheral edema | |
| All grade | 17 (10) |
| Grade 3/4 | 1 (1) |
| Extremity pain | |
| All grade | 18(11) |
| Grade 3/4 | 1 (1) |
| Pleural effusion | |
| All grade | 16 (10) |
| Grade 3/4 | 7 (4) |
| Adverse event (hematologic) | |
| Thrombocytopenia | |
| All grade | 74 (45) |
| Grade 3/4 | 65 (39) |
| Anemia | |
| All grade | 64 (39) |
| Grade 3/4 | 42 (25) |
| Neutropenia | |
| All grade | 36 (22) |
| Grade 3/4 | 33 (20) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CML, chronic myeloid leukemia
Advanced phase CML included patients in accelerated phase, blast phase and patients with acute lymphoblastic leukemia
Table 3:
Adverse events (≥10%) following bosutinib in CML patients previously treated with imatinib and dasatinib32 (Reproduced with permission)
| Adverse event (non-hematologic) | Dasatinib-resistant (Total 38 patients) N (%) | Dasatinib-intolerant (Total 50 patients) N (%) |
|---|---|---|
| Diarrhea | ||
| All grade | 30 (79) | 42 (84) |
| Grade 3/4 | 3 (8) | 5 (10) |
| Nausea | ||
| All grade | 20 (53) | 23 (46) |
| Grade 3/4 | 1 (3) | 0 |
| Vomiting | ||
| All grade | 14 (37) | 24 (48) |
| Grade 3/4 | 1 (3) | 0 |
| Rash | ||
| All grade | 10 (26) | 18 (36) |
| Grade 3/4 | 0 | 3 (6) |
| Pyrexia | ||
| All grade | 6 (16) | 8 (16) |
| Grade 3/4 | 0 | 0 |
| Fatigue | ||
| All grade | 8 (21) | 14 (28) |
| Grade 3/4 | 0 | 1 (2) |
| Abdominal pain | ||
| All grade | 9 (24) | 12 (24) |
| Grade 3/4 | 0 | 1 (2) |
| Headache | ||
| All grade | 8 (21) | 14 (28) |
| Grade 3/4 | 1 (3) | 3 (6) |
| Cough | ||
| All grade | 7 (18) | 11 (22) |
| Grade 3/4 | 0 | 0 |
| Elevated ALT | ||
| All grade | 6 (16) | 6 (12) |
| Grade 3/4 | 0 | 4 (8) |
| Upper abdominal pain | ||
| All grade | 8 (21) | 9 (18) |
| Grade 3/4 | 0 | 0 |
| Arthralgia | ||
| All grade | 5 (13) | 10 (20) |
| Grade 3/4 | 0 | 1 (2) |
| Decreased appetite | ||
| All grade | 3 (8) | 7 (14) |
| Grade 3/4 | 0 | 1 (2) |
| Constipation | ||
| All grade | 2 (5) | 8 (16) |
| Grade 3/4 | 0 | 0 |
| Dyspnea | ||
| All grade | 2 (5) | 10 (20 |
| Grade 3/4 | 0 | 1 (2) |
| Back pain | ||
| All grade | 5 (13) | 5 (10) |
| Grade 3/4 | 0 | 2 (4) |
| Dizziness | ||
| All grade | 5 (13) | 8 (16) |
| Grade 3/4 | 0 | 0 |
| Peripheral edema | ||
| All grade | 1 (3) | 5 (10) |
| Grade 3/4 | 0 | 0 |
| Nasopharyngitis | ||
| All grade | 4 (11) | 5 (10 |
| Grade 3/4 | 0 | 0 |
| Extremity pain | ||
| All grade | 1 (3) | 5 (10 |
| Grade 3/4 | 0 | 0 |
| Pleural effusion | ||
| All grade | 5 (13) | 12 (24) |
| Grade 3/4 | 2 (5) | 2 (4) |
| Adverse event (hematologic) | ||
| Thrombocytopenia | ||
| All grade | 11 (29) | 19 (38) |
| Grade 3/4 | 7 (18) | 16 (32) |
| Anemia | ||
| All grade | 8 (21) | 7 (14) |
| Grade 3/4 | 3 (8) | 4 (8) |
| Neutropenia | ||
| All grade | 9 (24) | 7 (14) |
| Grade 3/4 | 6 (16) | 7 (14) |
Abbreviations: ALT, alanine aminotransferase; CML, chronic myeloid leukemia
There were 44 deaths within 30 days of the last bosutinib dose. Deaths due to disease progression were seen in 25 patients (4%) and deaths unrelated to bosutinib included pneumonitis (n=5) and cardiac events (n=6). Three bosutinib-related deaths, included gastrointestinal hemorrhage with thrombocytopenia, myocardial infarction and a respiratory failure complicated by sepsis, the latter two occurring in patients with advanced stage disease.32
6.3. Cardiovascular profile of Bosutinib
Bosutinib appears to have a more favorable cardiovascular safety profile as compared to other TKIs. In a bosutinib safety and toxicity management trial of Ph+ leukemia patients, drug related cardiac events were noted in 6% of patients, of whom 2% had grade 3/4 events. Based on electrocardiogram, only one patient experienced on-study grade 3 Fridericia corrected QT interval prolongation. Similarly, based on echocardiogram data, only one patient had a decrease in left ventricular ejection fraction from baseline. Overall, cardiac toxicities on bosutinib were infrequent.32
According to the BELA trial, a study of bosutinib vs imatinib in newly diagnosed chronic phase CML patients, cardiovascular events were reported in 10% of bosutinib patients and 8% of imatinib patients, the most common being hypertension and palpitations. Cardiac failure occurred in <1% of bosutinib patients and 1% of imatinib patients. All patients were managed with dose modifications and 71% of patients on bosutinib and 100% of patients on imatinib were challenged without recurrence of the cardiac adverse events. Four patients on the bosutinib arm and none on the imatinib arm discontinued treatment due to their cardiovascular events.33
In a retrospective analysis that used nilotinib in patients with Ph+ leukemia, 11 out of 179 patients reported cases of severe peripheral arterial occlusive disease (PAOD).34 In another study, 3 out of 24 consecutively treated CML patients presented with severe PAOD, and they did not have previous cardiovascular risk.35 Patients found to have PAOD required angioplasty, among other surgeries. In both studies, there were patients with and without cardiovascular risk factors. These studies suggest that nilotinib can cause vascular events in patients both with and without preexisting arteriosclerotic disease.
Dasatinib has been linked to an increased incidence of pericardial and pleural effusions as well as pulmonary arterial hypertension. In a French study, 9 incident cases of pulmonary hypertension were diagnosed following dasatinib therapy in patients that had never used any previous TKIs. Improvements were noted when withdrawing dasatinib but complete recovery was not, one patient died of cardiac failure.36, 37 Dasatinib has been well tolerated at lower doses but in one study, 4 of 13 patients still reported pulmonary events at doses as low as 50 mg. All patients had previously been treated with another TKI, but none had previous pleural or cardiac risks. 38
6.4. Treatment of newly diagnosed CML patients
A phase III randomized study examining the efficacy of bosutinib in 502 patients with newly diagnosed chronic phase CML was recently reported.28 Patients were randomized to either bosutinib 500 mg orally daily or imatinib 400 mg orally daily. Even though the primary endpoint, CCyR at 12 months was not met (70% for bosutinib versus 68% for imatinib, P=0.601), MMR rate was superior with bosutinib (41%) versus imatinib (27%, P<0.001). There was also a lower rate of disease progression (3% versus 10%) and estimated probability of disease transformation (2% versus 4%) on the bosutinib arm of the study. At 24 months, the rate of CCyR was 58% for bosutinib- and 65% for imatinib-treated patients with MMR of 47% for bosutinib- and 41% for imatinib-treated patients.28 There were no new events of disease acceleration on bosutinib while four occurred on imatinib.
6.5. Safety and management of toxicity in newly diagnosed CML patients
Bosutinib was associated with higher incidence (all grades) of diarrhea, 70% of patients on bosutinib compared to 26% of patients on imatinib. The diarrhea occurred within a median of three days and lasted for a median of three days. Treatment included anti-diarrheal medications, temporary interruptions and dose reductions.33 Similarly, all grades of vomiting (33% versus 16%) were more common with bosutinib.
Elevated ALT (33%) and AST (28%) were more common with bosutinib than imatinib (9% and 10%). Concurrent medications, temporary interruptions and dose modifications were used to manage these toxicities without long term toxicities noted. Other laboratory abnormalities included hypophosphatemia (50% on bosutinib versus 69% on imatinib), blood creatinine phosphokinase elevations (45% versus 39%), increased lipase (46% versus 39%) and hypokalemia (18% versus 37%).33
7. Expert Opinion
In conclusion, the efficacy and safety of bosutinib is well established in the treatment of CML patients. Bosutinib has been proven to be effective following the development of dasatinib resistance and/or intolerance in CML patients. The drug’s safety profile is associated mainly with manageable low-grade self-limited diarrhea. Long-term cardiovascular and pulmonary toxicities reported with dasatinib, nilotinib and ponatinib usage have not been described with bosutinib. Given the increasing comorbidities of an overall aging general population and the number of long-term CML survivors, we anticipate increasing numbers of CML patients to be initiated on and/or to be switched to bosutinib therapy over the next few years.
Based on the currently available clinical and preclinical studies, we propose that bosutinib should be actively explored for the treatment of ALL patients. Unfortunately preclinical data showed that neither SKI 606 (bosutinib) nor imatinib treatment eliminated CML or normal progenitor cells, and bosutinib is therefore not expected to have significant activity against Ph+ stem cells. 40 However, this drug may be effective for the treatment of ALL patients due to its ability to inhibit multiple kinases promoting acute leukemia growth. In one study, bosutinib was found to inhibit BCR-ABL kinase activity and SRC phosphorylation more potently than imatinib.40 In another, bosutinib inhibited the broadest range of kinases as compared to all other TKIs. Of interest are the effects of bosutinib on NUP214-ABL1-mediated cell proliferation in T-cell ALL.41 NUP214-ABL1 results from extra-chromosomal circulation of the 500 kb DNA fragment located between ABL1 and NUP214.42 This occurs in 6% of T-ALL. The resultant NUP214-ABL1 tyrosine kinase signals through the SRC family kinase LCK, underscoring a role for the dual ABL1/SRC TKIs, dasatinib and bosutinib, in this subtype of T-ALL.40
Although dasatinib also inhibits BCR-ABL1 and SRC kinases, bosutinib may be a preferred agent for the treatment of Ph+ ALL due to its more favorable side effect profile. Dasatinib has previously been shown to be highly beneficial in inducing cytogenetic and molecular responses in Ph+ ALL patients in combination with standard chemotherapy regimens. However, this TKI has been linked with possible significant long-term toxicity, mainly in the form of pulmonary arterial hypertension and pleural effusions, which have either not been reported to occur or occur with low incidence with bosutinib.37 Similarly, bosutinib’s long-term safety profile is more favorable when compared with both nilotinib’s peripheral arterial occlusive disease38 and ponatinib’s arterial thrombotic events.39 Finally, bosutinib is effective against most resistant BCR-ABL1 mutations emerging following prior imatinib and dasatinib therapy, except for T315I andV299L.27
However, key questions regarding the use of bosutinib for ALL treatment remain. For instance, although the efficacy and safety of bosutinib is well established in the treatment of CML patients, specific data with this drug in Ph+ ALL patients is not clear as individuals with this disease were included together with advanced CML patients in prior clinical trials. Moreover, there is little to no data on whether bosutinib is able to penetrate into the cerebrospinal fluid, a key reservoir of resistant/relapsed ALL disease in patients. In addition, the safety and tolerability of such a broad multi-kinase inhibitor following allogeneic stem cell transplantation is not established.
Similar to prior TKIs in Ph+ALL therapy, prospective trials of bosutinib in combination with other agents for the treatment of newly diagnosed and relapsed/refractory Ph+ ALL should be performed. Bosutinib combined with cytotoxic chemotherapy, such as hyperCVAD, could be investigated in the upfront therapy of younger newly diagnosed Ph+ ALL patients. Because of its extremely favorable toxicity profile, bosutinib also constitutes an ideal agent to combine with current immunotherapeutic approaches (such as blinatumomab or chimeric T-cell therapy) for the treatment of relapsed/refractory Ph+ B-cell ALL patients, particularly older individuals. At present, a phase I/II study of bosutinib combined with the anti-CD22 antibody-drug conjugate, inotuzumab ozogamicin, for the treatment of CD22-positive Ph+ ALL and CML patients is currently accruing patients.
Acknowledgement
Unfortunately Dr. Wetzler passed away during the preparation of this manuscript. Because he had contributed significantly to its writing, he is still is listed as an author
Declaration of Interest
This work was supported partially by grants from the National Cancer Institute Grant CA16056, the Szefel Foundation, Roswell Park Cancer Institute, the Leonard S. LuVullo Endowment for Leukemia Research, the Nancy C. Cully Endowment for Leukemia Research, the Babcock Family Endowment and the Heidi Leukemia Research Fund, Buffalo, NY. ESW is also supported by Cancer Clinical Investigator Team Leadership Award (CCITLA) awarded by National Cancer Institute through a supplement to P30CA016056. Drs. Griffiths and Wang have served as consultants for Ariad. Dr. Wetzler served as consultant for Novartis, Ariad and Teva. He was a principal onvestigator of clinical trials with Bristol Myers Squibb and Teva. Drs. Varallo-Rodriguez, Freyer and Ontiveros, have no financial relationships to report.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer 2011; 117(8): 1583–94. [DOI] [PubMed] [Google Scholar]
- 2.Chan LC, Karhi KK, Rayter SI, et al. A novel abl protein expressed in Philadelphia chromosome positive acute lymphoblastic leukaemia. Nature 1987; 325(6105): 635–7. [DOI] [PubMed] [Google Scholar]
- 3.Kurzrock R, Shtalrid M, Romero P, et al. A novel c-abl protein product in Philadelphia-positive acute lymphoblastic leukaemia.Nature 1987; 325(6105): 631–5. [DOI] [PubMed] [Google Scholar]
- 4.Westbrook CA, Rubin CM, Carrino JJ, Le Beau MM, Bernards A, Rowley JD. Long-range mapping of the Philadelphia chromosome by pulsed-field gel electrophoresis. Blood 1988; 71(3): 697–702. [PubMed] [Google Scholar]
- 5.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 2004; 103(12): 4396–407. [DOI] [PubMed] [Google Scholar]
- 6.Jones D, Thomas D, Yin CC, et al. Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer 2008; 113(5): 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann WK, Jones LC, Lemp NA, et al. Ph(+) acute lymphoblastic leukemia resistant to the tyrosine kinase inhibitor STI571 has a unique BCR-ABL gene mutation. Blood 2002; 99(5): 1860–2. [DOI] [PubMed] [Google Scholar]
- 8.Soverini S, De Benedittis C, Papayannidis C, et al. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era: The main changes are in the type of mutations, but not in the frequency of mutation involvement. Cancer 2014; 120(7): 1002–9. [DOI] [PubMed] [Google Scholar]
- 9.Mahon FX, Belloc F, Lagarde V, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 2003; 101(6): 2368–73. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther 2008; 83(2): 258–64. [DOI] [PubMed] [Google Scholar]
- 11.Li S Src-family kinases in the development and therapy of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk Lymphoma 2008; 49(1): 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei F, Stoddart S, Muschen M, Kim YM, Groffen J, Heisterkamp N. Development of resistance to dasatinib in Bcr/Abl-positive acute lymphoblastic leukemia. Leukemia 2010; 24(4): 813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amsberg GK, Koschmieder S. Profile of bosutinib and its clinical potential in the treatment of chronic myeloid leukemia. OncoTargets and Therapy 2013; 6: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res 2006; 66(23): 11314–22. [DOI] [PubMed] [Google Scholar]
- 15.Golas JM, Arndt K, Etienne C, et al. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 2003; 63(2): 375–81. [PubMed] [Google Scholar]
- 16.Mancini M, Brusa G, Zuffa E, et al. Persistent Cdk2 inactivation drives growth arrest of BCR-ABL-expressing cells in response to dual inhibitor of SRC and ABL kinases SKI606. Leuk Res 2007; 31(7): 979–87. [DOI] [PubMed] [Google Scholar]
- 17.Remsing Rix LL, Rix U, Colinge J, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia 2009; 23(3): 477–85. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Liu Y, Pelletier S, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet 2004; 36(5): 453–61.•• This study demonstrates that dual inhibition of SRC and BCR-ABL1 was superior to BCR-ABL1 inhibition (imatinib) alone in mouse models of Ph+ ALL, but not CML.
- 19.Rix U, Colinge J, Blatt K, et al. A target-disease network model of second-generation BCR-ABL inhibitor action in Ph+ ALL. PloS One 2013; 8(10): e77155.•• This study demonstrates that bosutinib exhibits the broadest range of kinase inhibition as compared with all other available TKIs.
- 20.Abbas R, Hug BA, Leister C, Gaaloul ME, Chalon S, Sonnichsen D. A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemotherapy and Pharmacology 2012; 69(1): 221–7. [DOI] [PubMed] [Google Scholar]
- 21.Abbas R, Leister C, Sonnichsen D. A clinical study to examine the potential effect of lansoprazole on the pharmacokinetics of bosutinib when administered concomitantly to healthy subjects. Clinical Drug Investigation 2013; 33(8): 589–95. [DOI] [PubMed] [Google Scholar]
- 22.Abbas-Borhan R, Chaudhary I, Hug BA, et al. Mass balance, metabolic disposition, metabolite characterization, and pharmacokinetics of oral 14C-labeled bosutinib in healthy subjects. The 9th Triennial Meeting of the International Society for the Study of Xenobiotics; September 4–8, 2010; Istanbul, Turkey 2010: Abstract P350. [Google Scholar]
- 23.Pfizer. Bosutinib prescribing information. 2014.
- 24.Hegedus C, Ozvegy-Laczka C, Apati A, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. British Journal of Pharmacology 2009; 158(4): 1153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perini P Role of the drug transporters in the multidrug resistance affecting bosutinib treatment. BICOCCA; 2011. [Google Scholar]
- 26.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011; 118(17): 4567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gambacorti-Passerini C, Brummendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: Minimum 24-month follow-up. Am J Hematol 2014; 89(7): 732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brummendorf TH, Cortes JE, de Souza CA, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol 2015; 168(1): 69–81.•• The BELA trial established that bosutinin treatment of newly diagnosed chronic phase CML patients resulted in similar rates of cytogenetic and molecular responses. Higher rates of early response were reported following bosutinib therapy with no accelerated-/blast-phase transformations following the 12-month primary analysis.
- 29.Redaelli S, Mologni L, Rostagno R, et al. Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol 2012; 87(11): E125–8.• This mutational analysis study identified specific bosutinib-resistant mutations.
- 30.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood 2012; 119(15): 3403–12.•• This study demonstrates the efficacy of bosutinib in patients with known BCR-ABL1 mutations, some of which confer resistance to dasatinib and nilotinib.
- 31.Gambacorti-Passerini C, Cortes J, Kantarjian H, et al. Bosutinib (SKI-606) shows high tolerability and clinical activity in patients with Philadelphia chromosome positive leukemias. Haematologica 2008; 93 ((sl)): 160–1. [Google Scholar]
- 32.Kantarjian HM, Cortes JE, Kim DW, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood 2014; 123(9): 1309–18.•• This study is the pivotal study that led to approval of bosutinib for CML. This summarizes the safety profile of bosutinib and compiles the current available data available in CML patients resistant to or intolerant of other TKIs.
- 33.Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol 2014; 89(10): 947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Courte P, Rea D, Abruzzese E, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst 2011; 103(17): 1347–1348. [DOI] [PubMed] [Google Scholar]
- 35.Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol 2011; 86(7): 533–9. [DOI] [PubMed] [Google Scholar]
- 36.Montani D, Bergor E, Günther s, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation 2012; 125(17): 2128–37. [DOI] [PubMed] [Google Scholar]
- 37.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123(4): 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krauth MT, Herndlhofer S, Schmook MT, Mitterbauer-Hohndanner G, Schlogl E, Valent P, et al. Extensive pleural and pericardial effusion in chronic myeloid leukemia during treatment with dasatinib at 100mg or 500mg daily. Haematologica 2011; 96(1); 163–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GD, Bruno BJ, Lim CS. Resistant mutations in CML and Ph (+)ALL - role of ponatinib. Biologics: Targets & Therapy 2014; 8: 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konig H, Holyoake TL, Bhatia R. Effective and selective inhibition of chronic myeloid leukemia primitive hematopoietic progenitors by the dual Src/Abl kinase inhibitor SKI-606. Blood 2008; 111(4): 2329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Keersmaecker K, Porcu M, Cox L, et al. NUP214-ABL1-mediated cell proliferation in T-cell acute lymphoblastic leukemia is dependent on the LCK kinase and various interacting proteins. Haematologica 2014; 99(1): 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graux C, Cools J, Melotte C, et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nat Genet 2004; 36(10): 1084–9. [DOI] [PubMed] [Google Scholar]
- 43.Phase I/II Study of Bosutinib in Combination With Inotuzumab Ozogamicin in CD22-positive Philadelphia-Chromosome (PC) Positive Acute Lymphoblastic Leukemia (ALL) and Chronic Myeloid Leukemia (CML) found at https://clinicaltrials.gov/ct2/show/NCT02311998.


