Abstract
Corneal endothelium is a cellular monolayer positioned on the Descemet’s membrane at the anterior cornea, and it plays a critical role in maintaining corneal clarity. Our present study examines the feasibility of utilizing our 3-dimensional (3D) corneal stromal construct, which consists of human corneal fibroblasts (HCF) and their self-assembled matrix, to observe the development and maturation of human corneal endothelial cells (HCEndoCs) in a co-culture model. Three-dimensional HCF constructs were created by growing the HCFs on Transwell membranes in Eagles’ minimum essential medium (EMEM) + 10% FBS + 0.5mM Vitamin C (VitC) for about 4 weeks. HCEndoCs, either primary (pHCEndoC) or cell line (HCEndoCL), were either seeded in chamber slides, directly on the Transwell membranes, or on the 3D HCF constructs and cultivated for 5 days or 2 weeks. The HCEndoCs that were seeded directly on the Transwell membranes were exposed indirectly to HCF by culturing the HCF on the plate beneath the membrane. Cultures were examined for morphology and ultrastructure using light and transmission electron microscopy (TEM). In addition, indirect-immunofluorescence microscopy (IF) was used to examine tight junction formation (ZO-1), maturation (ALDH1A1), basement membrane formation (Laminin), cell proliferation (Ki67), cell death (caspase-3), and fibrotic response (CTGF). As expected, both pHCEndoCs and HCEndoCLs formed monolayers on the constructs; however, the morphology of the HCEndoCLs appeared to be similar to that seen in vivo, uniform and closely packed, whereas the pHCEndoCs remained elongated. The IF data showed that laminin localization was present in the HCEndoCs’ cytoplasm as cell-cell contact increased, and when they were grown in the 3D co-culture, the beginnings of what appears to be a continuous DM-like structure was observed. In addition, in co-cultures, ALDH1A1-positive HCEndoCs were present, ZO-1 expression localized within the tight junctions, minimal numbers of HCEndoCs were Ki67- or Caspase-3-positive, and CTGF was positive in both the HCEndoCs cytoplasm and the matrix of the co-culture. Also, laminin localization was stimulated in HCEndoCs upon indirect stimuli secreted by HCF. The present data suggests our 3D co-culture model is useful for studying corneal endothelium maturation in vitro since the co-culture promotes new DM-like formation, HCEndoCs develop in vivo-like characteristics, and the fibrotic response is activated. Our current findings are applicable to understanding the implications of corneal endothelial injection therapy, such as if the abnormal DM has to be removed from the patient, the newly injected endothelial cells will seed onto the wound area and deposit a new DM-like membrane. However, caution should be observed and as much of the normal DM should be left intact since removal of the DM can cause a posterior stromal fibrotic response.
Keywords: Endothelium, Descemet’s membrane, maturation, development, 3D culture model
1. INTRODUCTION
The cornea is a transparent avascular tissue at the front of the eye, which enables the light to transmit and focus on the retina for optimal vision. The human cornea consists of the following layers: epithelium, basement membrane, Bowman’s membrane, stroma, Descemet’s membrane (DM), and endothelium (Nishida and Saika, 2011; Teichmann et al., 2013). All these layers are important for maintaining corneal clarity, structure, and/or function, including the endothelium, which, when in vivo, consists of a monolayer of specialized homogeneous closely-packed flattened hexagonal cells that are attached to the DM at the anterior border and maintained in a non-proliferating state (Joyce, 2003). These cells and their monolayer structure are responsible for regulating corneal hydration, preserving corneal thickness, and maintaining corneal clarity by governing fluid and solute transport across the posterior surface of the cornea through discontinuous tight junctions and ionic “pumps” (Edelhauser, 2006; Parekh et al., 2016), which are crucial for corneal health and vision.
Human corneal endothelial cells (HCEndoCs) cannot fully regenerate in vivo; therefore, their response to cell loss due to aging (Kochar et al., 2016; Laing et al., 1976), trauma (Khan et al., 2017; Motley et al., 2003; Slingsby and Forstot, 1981; Yeniad et al., 2010), or corneal endothelial disorders (Fahmy, 2018; Gagnon et al., 1997; Kheirkhah et al., 2015), such as Fuchs’ dystrophy (Elhalis et al., 2010; Shearer et al., 2016; Syed et al., 2017), is to enlarge and slide along the DM to cover the area previously occupied by the lost cells (Choi et al., 2015; Matsubara and Tanishima, 1983; Tuft and Coster, 1990). Normally, HCEndoCs density in young adults is 3000 to 3500 cells/mm2 (Sanchis-Gimeno et al., 2005), and when this density decreases below a critical number (usually between 400 to 500 cells/mm2), corneal edema ensues, causing vision loss (Shihadeh et al., 2010; Ventura et al., 2001). Currently, the solution to restore vision due to a dysfunctional endothelium is to replace it with a healthy donor endothelium by means of corneal endothelial transplantation (Shihadeh et al., 2010). However, all penetrating transplantations, including penetrating keratoplasty (PKP) (Inoue et al., 2000; Poschl et al., 2013; Sugar and Sugar, 2000), endothelial keratoplasty (Deep Lamellar Endothelial Keratoplasty [DLEK]) (Terry and Ousley, 2001, 2003), Descemet’s stripping with automated endothelial keratoplasty (DSAEK) (Gorovoy, 2006; Koenig and Covert, 2007; Lee et al., 2009), Descemet’s membrane endothelial keratoplasty (DMEK), and Descemet’s membrane automated endothelial keratoplasty (DMAEK) (Dapena et al., 2009; Dapena et al., 2011; Guerra et al., 2011; Maier et al., 2013a; Maier et al., 2013b; Naveiras et al., 2012; Parker et al., 2012; Terry, 2012), require a donor cornea, which is often difficult to obtain due to the global shortage of transplant-grade donor corneal tissue. In addition, in some cases after graft failure in young patients, limited regeneration has been observed (Teichmann et al., 2013). Therefore, alternatives, such as cultured endothelial transplantation therapies, are being investigated (Amano et al., 2005; Ju et al., 2012; Kim et al., 2018; Kruse et al., 2018; Mimura et al., 2013; Wang et al., 2016). The idea behind these therapies is to transplant cultivated HCEndoCs, either as single cells or in a sheet, to the denuded DM of the host (Choi et al., 2010; Hayashi et al., 2009; Hitani et al., 2008; Kinoshita et al., 2018; Lai et al., 2007; Madden et al., 2011; Okumura et al., 2018; Parikumar et al., 2018; Rolev et al., 2018; Sumide et al., 2006; Watanabe et al., 2011). Some additional benefits to this therapy, other than using less donor tissues, would be a reduction in potential side effects, such as scarring, inflammation, and glaucoma, as well as a decrease in corneal endothelial density and immune-mediated graft rejection. Also, conceptually, it may be possible to utilize an autologous transplant of the patient’s own cultivated HCEndoCs.
Cell injection therapy, which is one of the suggested methods for endothelial replacement (Kinoshita et al., 2018; Okumura et al., 2016; Rolev et al., 2018; Shen et al., 2017), involves the injection of cultivated HCEndoCs into the anterior chamber. This therapy raises many unanswered questions: (1) Does the presence of the DM affect the restoration of endothelial function? (2) Is the presence of the DM necessary for the endothelial cells to differentiate? And, (3) do transplanted endothelial cells reform or deposit new DM? Indeed, data from Okumura et al. (Okumura et al., 2018) showed that denuded DM might still be a problem for cell injection therapy because Fuchs’ dystrophy DM exhibits clinically abnormal structural features, and advanced corneal guttae adversely affect vision quality, even in patients without corneal edema. The turnover time of corneal guttae is not certain, so in cases such as these, removal of the abnormal DM may be necessary. However, research by Dr. Chen and colleagues showed that DM supports corneal endothelial cell regeneration in rabbits after endothelial injury (Chen et al., 2017), and Wilson et al. showed that removal of the DM caused severe posterior stromal fibrosis (Medeiros et al., 2018). In addition, in an in vitro human corneal model, the direct transplantation of cultured HCEndoCs to the bare posterior corneal stroma resulted in the formation of an endothelial monolayer and the restoration of stromal hydration, thus restoring the physiological thickness and demonstrating the feasibility of cell therapy in the treatment of corneal endothelial decompensation (Rolev et al., 2018). The mechanisms by which the cells interact after transplantation remain unknown. In the present study, we utilized our cell-based 3-dimensional (3D) corneal stromal construct model (Guo et al., 2007) to examine these mechanisms and test if HCEndoCs can differentiate into mature endothelium and form a DM in co-culture.
2. MATERIAL AND METHODS
This study adheres to the tenets of the Declaration of Helsinki and all donor eyes were received from the National Disease Research Interchange (NDRI; Philadelphia, PA). The Schepens Eye Research Institute IRB deemed these experiments to be exempt.
2.1. Human Corneal Fibroblast (HCF) and 3D Construct
Human corneal fibroblasts (HCFs) were isolated and cultured as previously described (Guo et al., 2007). Briefly, human corneal stromal explants from donor corneas, were placed in 6-well plates with Eagle’s minimum essential medium (EMEM: ATCC; Manassas, VA) + 10% fetal bovine serum (FBS: Atlantic Biologicals; Lawrenceville, CA) and incubated at 37°C with 5% CO2 until sufficient HCF migrated from the explants. The HCF then were seeded in 6-well Transwell plates containing polycarbonate membrane inserts with 0.4µm pores (Corning Costar; Charlotte, NC) at 106cells/ml in construct medium [EMEM + 10% FBS and 0.5µM 2-O-α-D-glucopyranosyl-L-ascorbic acid (VitC: Wako Chemical USA; Inc.; Richmond, VA)] for ~4 weeks to produce 3D HCF constructs. By this time, the cells within the constructs will be stratified to form multiple layers within a secreted self-assembled matrix (Guo et al., 2007; Ruberti and Zieske, 2008), and the cells in the mid-portion of the construct will have a more dendritic morphology (Ren et al., 2008; Thompson et al., 2013).
2.2. Human Corneal Endothelial Cells (HCEndoCs) and Co-Culture Assembly
Both primary HCEndoCs (pHCEndoC) and cell line (HCEndoCL: a gift from Dr. May Griffith, University of Montreal, Montreal, Canada)(Griffith et al., 1999) were utilized in these studies. To obtain pHCEndoCs, the endothelium and DM were carefully dissected from donor corneas (NDRI), and cultivated using the protocol established in Dr. Joyce’s laboratory (Chen et al., 2001; Joyce, 2005; Zhu and Joyce, 2004). All HCEndoCs were grown in “Chen’s” medium (Zhu and Joyce, 2004), and when a sufficient number of cells were obtained, they were used for the following experiments: direct co-culture, indirect co-culture, and HCEndoCs only. All cultures were processed for indirect-immunofluorescence (IF) (Karamichos et al., 2010) (Zieske et al., 2001) or transmission electron microscopy (TEM) (Gipson et al., 1983). Experiments were performed at least three times for each condition.
2.2.1. Direct Co-culture:
HCEndoC-HCF co-cultures were established by seeding HCEndoCs (5 × 105 cells/construct) on the 4-week 3D HCF construct. “Chen’s” medium was added to the top (or inner) well of the transwell insert to allow the medium to feed the HCEndoCs, while construct medium was applied to the outer well in order to be in contact with the bottom of the transwell membrane, thus feeding the HCF. Cultures were maintained for either 5 days or 2 weeks, and at the appropriate time, they were either frozen, fixed in 4% paraformaldehyde or 1/2 strength Karnovsky’s, and processed for IF or TEM, respectively.
2.2.2. Indirect Co-culture:
In addition to the direct co-culture, HCEndoCs were exposed indirectly to HCF and examined. For these experiments, HCEndoCs were cultivated directly on the polycarbonate membrane of the 6-well transwell inserts ± HCF, which were grown at the bottom of the 6-well plate. “Chen’s” medium was added to the inner well of the transwell and construct medium was added to the outer well. Cultures were maintained for either 5 days or 2 weeks, and at the appropriate time they were either fixed in 4% paraformaldehyde or 1/2 strength Karnovsky’s, and processed for IF or TEM, respectively.
2.2.3. HCEndoCs Only:
In addition to co-culture studies, HCEndoCs only were seeded in glass chamber slides for 2-dimensional (2D) studies and cultivated in “Chen’s” medium. Cells were maintained as either single cell (a few cells with limited cell-cell contact) or confluent (cell-cell contact) cultures, and at the appropriate time, they were fixed with ice-cold methanol for 10 minutes at −20°C and processed for IF.
2.3. Indirect-Immunofluorescence Staining (IF)
To examine for specific endothelial components, samples were incubated with primary antibodies against Laminin (cat# Z0097: DAKO; Carpinteria, CA), Aldehyde dehydrogenase 1 family, member A1 (ALDH1A1, cat# ab23375: Abcam; Cambridge, MA), Connective Tissue Growth Factor (CTGF, cat# ab59681: Abcam), Zonula occludens-1 (ZO-1, cat# 339100: Life Technologies; Grand Island, NY), Caspase-3 (cat# 9661: Cell Signaling; Danvers, MA), and Ki67 (cat# VP-K452: Vector Laboratories Inc.; Burlingame, CA), and then incubated with corresponding secondary antibody (Jackson ImmunoResearch; West Grove, PA), as previously described (Karamichos et al., 2010). TOPRO-3 iodide (642/661, cat# T3605: Thermo Fisher Scientific; Waltham, MA) or DAPI (cat# H-1200: Vector Laboratories) were used as counterstains to mark all cell nuclei. Whole mount samples were observed and imaged using either a confocal TCS-SP2 or TCS-SP5 Leica microscope (Leica Microsystems; Bannockburn, IL). Frozen sections and 2D cultures were observed with a Nikon Eclipse E800 microscope equipped with an Andor Clara E camera and Nikon NIS Elements for Basic Research (Micro Video Instruments; Avon, MA). Negative controls, where primary antibody was omitted, were performed with every experiment, and each experiment was performed at least three times.
2.4. Light Microscopy and Transmission Electron Microscopy (TEM)
The co-culture constructs were collected, fixed in 1/2 strength Karnovsky’s fixative [2% paraformaldehyde and 2.5% glutaraldehyde in cacodylate buffer (pH 7.4)] and processed for TEM by using standard procedures, as described previously (Gipson et al., 1983). A diamond knife ultramicrotome (LKB Ultramicrotome; Bromma, Sweden) was used to cut transverse to the plane of the construct. The sections were collected for both light microscopy and TEM. For light microscopy, optical thick sections of 1 to 2 µm were obtained and stained with phenylenediamine and viewed and photographed with an Eclipse E800 microscope equipped with a SPOT camera (Micro Video Instruments). For TEM, 60- to 90-Å sections were obtained, viewed, and photographed (TEM model 410: Philips Electronics NV; Eindhoven, The Netherlands).
2.5. Statistical Analysis
All experiments were repeated at least 3 times and data was analyzed with Prism (GraphPad Prism v.5.0b; La Jolla, CA) for significance (p<0.05 to p<0.001) using the Student’s t-test and Dunnett’s Multiple Comparison test.
3. RESULTS
3.1. Formation of an endothelial monolayer on 3D construct
Using the co-culture model, both pHCEndoC and HCEndoCL were seeded on 3D HCF constructs. Since corneal endothelium is normally maintained in aqueous humor, these co-cultures were maintained fully submerged in “Chen’s” medium. The endothelial cells grew slowly, and formed a nearly, if not exact, monolayer on the 3D constructs over time (Fig. 1). By 5 days co-culture (Fig. 1A,B), both the pHCEndoC (Fig. 1A) and HCEndoCL (Fig. 1B) were present on the 3D HCF construct, but they were more elongated than normal endothelial cells. By 14 days (Fig. 1C,D), the endothelial cells were closer together and more evenly distributed on the 3D HCF constructs than at 5 days (Fig 1A,B), indicating that the endothelial cells were proliferating and cell density was increasing with time. When examined by TEM (Fig. 1E), the endothelial cells were shown to have formed a fairly uniform monolayer on the 3D HCF construct. Interestingly, even though both the pHCEndoC and HCEndoCL grew and formed monolayers on the 3D HCF construct, the HCEndoCL consistently produced an endothelium that was more similar to what was observed in vivo. The main reason for this may be that pHCEndoC grew slower than HCEndoCL in culture, which may be resolved by increasing the number of pHCEndoC originally seeded or the length of time in the co-culture. Alternatively, this difference in maturation may be due to the possibility of the pHCEndoCs undergoing apoptosis or transforming into other cell types, which are common difficulties observed in endothelial cell cultures (Kim et al., 2014; Parekh et al., 2017; Peh et al., 2015; Schmedt et al., 2012). In our culture system, apoptosis was noted later in the study and EMT transformation was not observed in the 3D co-culture.
Figure 1.
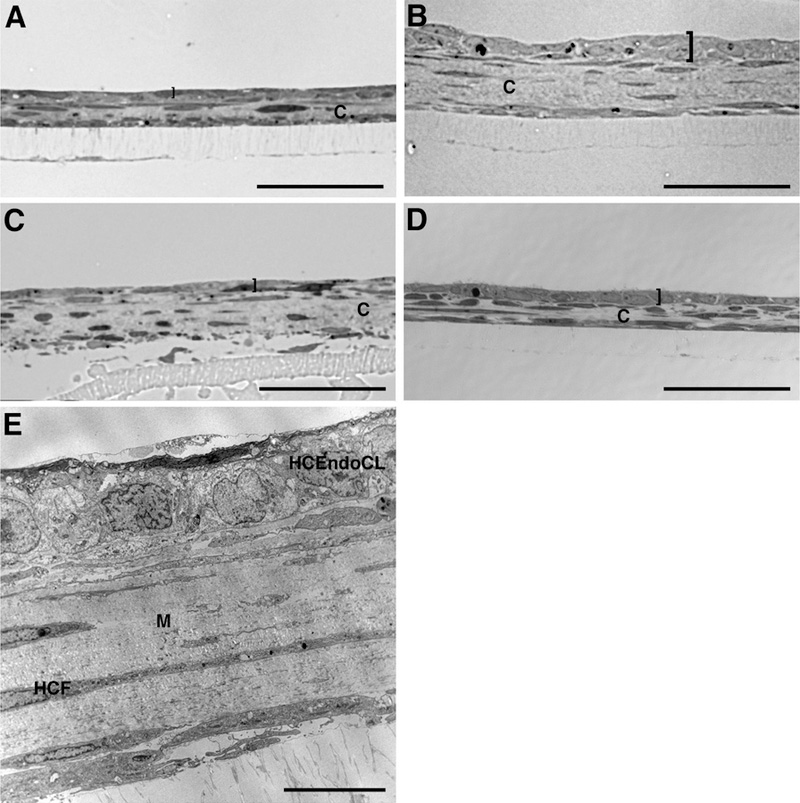
Co-culture of human corneal endothelial cells (HCEndoCs) on 3D human corneal fibroblast (HCF) construct. Brightfield of 5 (A, B) and 14 (C, D) day co-culture showing pHCEndoCs (A, C) and HCEndoCL (B, D) forming a single layer on top of the 3D HCF construct. (E) TEM of HCEndoCL co-culture shows a fairly packed uniform monolayer of endothelium on the 3D HCF construct. “]” indicates HCEndoC layer; C = construct; M = matrix; HCEndoCL = human corneal endothelial cell line; HCF = human corneal fibroblast; Bars = 50 microns (A-D) and 10 microns (E).
3.2. Deposition of New DM-like structure in Co-culture
To examine for DM deposition, endothelial cells in 2D (single and confluent) culture and 3D (5d and 14d) co-culture were assayed by IF for the expression of laminin, a component of DM, and observed by TEM, for DM formation. As seen in Figure 2, immunolocalization of laminin was observed in both the 2D cultures and 3D co-cultures; however, only punctate laminin was observed in the cytoplasm of a few HCEndoCL in the 2D single cell culture (Fig. 2A), whereas uniform cytoplasmic laminin was present in the confluent 2D culture (Fig. 2B), indicating that when the endothelial cells come in contact with one another, laminin production increases within the cells. After 5 days in 3D co-culture (Fig. 2C), laminin was localized in the cytoplasm of the HCEndoCLs, as with the confluent cells (Fig. 2B); however, laminin also appeared as distinct lines between HCEndoCLs and along the interface between the HCEndoCL monolayer and the 3D HCF construct, indicating that the HCEndoCLs were producing laminin and the laminin was beginning to localize in a more specific manner. By 14 days in co-culture (Fig. 2D), the laminin localized in a more continuous band underneath the endothelium at the endothelium/construct interface, indicating the possibility of an early stage formation of DM-like structure. Both 5 and 14 days co-cultures were then examined for DM formation by TEM. By 5 days co-culture, there was an indication of the formation of a membrane-like structure (Fig. 2E, arrow); however, it was patchy. This agrees with the laminin localization observed in Figure 2C. After 14 days in co-culture, a linear membrane-like structure, almost across the entire endothelium/construct interface, was present (Fig. 2F, arrows), agreeing with the laminin localization observed in Figure 2D. Taken together, these data indicate that endothelial cells in contact with one another produce laminin, and when applied to a 3D stroma-like matrix will secret DM components, which over time produce the beginnings of a continuous DM-like structure, suggesting that injected cells will have the ability to produce new DM in vivo.
Figure 2:
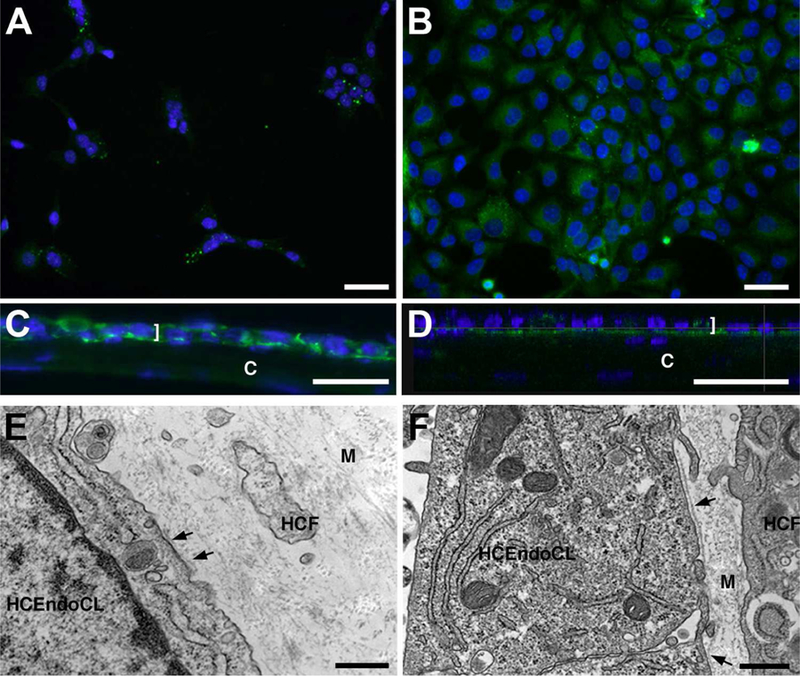
Laminin localization and DM formation. (A and B) 2D culture of HCEndoCL. (A) Single cells, or cells that have little physical cell-cell contact. (B) Near confluent HCEndoCL. (C-F) Co-culture of HCEndoCL with 3D HCF construct. Frozen cross-section (C) and TEM (E) of 5-day HCEndoCL co-culture. Confocal orthoganal (D) and TEM (F) of 14-day HCEndoCL co-culture, (A-D) Bars = 50 microns; (E-F) Bars = 500nm. Arrows indicate the beginning of DM formation (E, F).
3.3. Differentiation of endothelial cells on 3D constructs
Knowing that endothelial cells produce the beginnings of a DM-like structure over time in the 3D co-culture (Fig. 2C–F), and they produce laminin, a DM component, in 2D culture when they are confluent (Fig. 2B), we examined these cells for signs of differentiation with ALDH1A1 and ZO-1 by IF. ALDH1A1, which is a corneal crystallin that helps maintain the transparency of the cornea (Jester et al., 1999), is a known marker of differentiated, quiescent cells (Obermair et al., 2010; Tanaka et al., 2015). ZO-1 is a tight junction protein that is present in the intercellular borders between differentiated endothelial cells, thereby acting indirectly as a marker of differentiation. As seen in Figure 3, little, if any, ALDH1A1 was present in both 2D cultures (single and confluent: Fig. 3A,B); however, in 3D co-culture, ALDH1A1 localization was observed in the cytoplasm of the HCEndoCLs (Fig. 3C,D), indicating that HCEndoCLs in co-culture were differentiating. Unlike ALDH1A1, ZO-1 was detected in both the 2D culture and 3D co-culture (Fig. 4). In 2D single cell culture, ZO-1 appeared to localize in the cytoplasm of the HCEndoCLs (Fig. 4A); however, when the HCEndoCLs in the 2D culture were allowed to make cell-cell contact, the ZO-1 localized within the tight junctions (Fig. 4B). This localization was also apparent in the HCEndoCLs in co-culture (Fig. 4C,D), suggesting that substrate is not important, but cell density is for ZO-1 expression in HCEndoCLs. Also, based on our ZO-1 data, the HCEndoCs did not transform into EMTs in 3D co-culture. These data indicate that 3D HCF constructs provide a good substrate for endothelial cell differentiation in vitro. In addition, the results imply that cultured single cells have the ability to differentiate to mature endothelium in vivo after cell injection.
Figure 3.
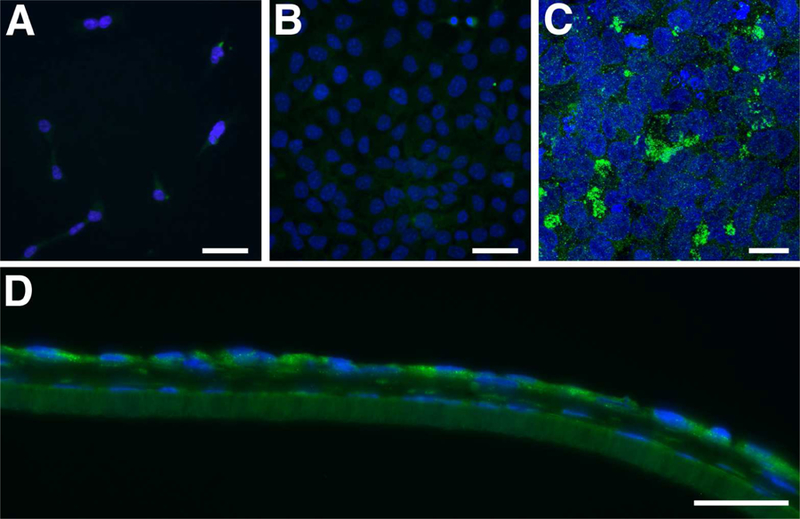
ALDH1A1 localization. (A and B) 2D culture of HCEndoCL. (A) Single cells, or cells that have little physical cell-cell contact. (B) Near confluent HCEndoCL. (C and D) Co-culture of HCEndoCL with 3D HCF construct. Confocal maximum projection of the HCEndoCL co-culture (C) and frozen cross-section of 5-day co-culture (D) Bars = 50 microns; Green = ALDH1A1; Blue = DAPI (A, B, D) or TOPRO3 (C).
Figure 4.
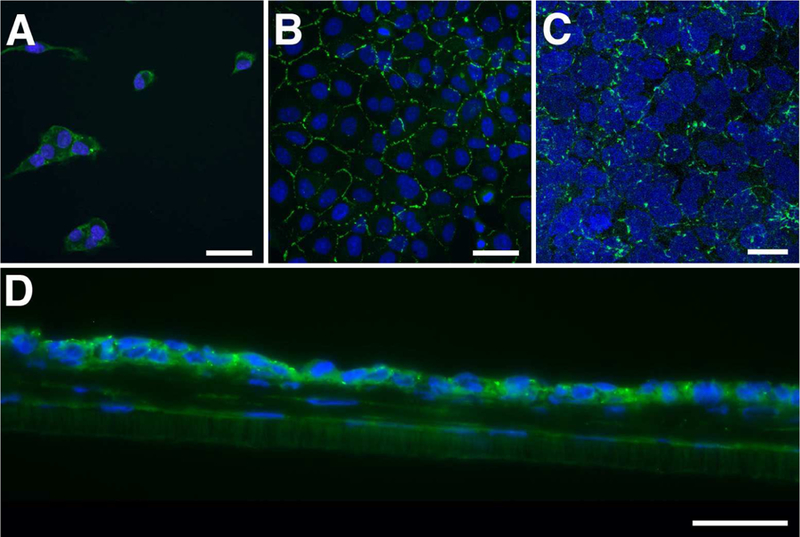
ZO-1 localization. (A and B) 2D culture of HCEndoCL. (A) Single cells, or cells that have little physical cell-cell contact. (B) Near confluent HCEndoCL. (C and D) Co-culture of HCEndoCL with 3D HCF construct. Confocal maximum projection of the HCEndoCL co-culture (C) and frozen cross-section of 5-day co-culture (D) Bars = 50 microns; Green = ALDH1A1; Blue = DAPI.
3.4. Cellular Profile of endothelial cells seeded on 3D constructs
In vivo, human corneal endothelial cells are normally in a quiet, non-proliferating state, even after wounding or disease (Joyce, 2005). However, in order for the endothelial cells that were seeded onto our 3D HCF constructs to repopulate, the cells need to proliferate. This also would be the case for endothelial cells injected into the anterior chamber during endothelial injection therapy. In theory, after a monolayer is reached and the cells have differentiated, they should no longer proliferate since they grow in a contact-inhibited fashion in vitro (Peh et al., 2013; Schmedt et al., 2012). As seen in Figure 5A, 5-day co-culture was immunolocalized with Ki67, a marker of proliferation. A few HCEndoCLs were observed to be Ki67 positive, indicating that some cells were still proliferating and the endothelial cells had yet to produce a complete monolayer. In addition to proliferation, we would expect some of these cells to undergo apoptosis, since it is hard to avoid cell senescence during cell culture (Campisi and d’Adda di Fagagna, 2007). Therefore, 5-day co-culture tissue was examined for apoptosis by immunolocalization with caspase-3. As seen in Figure 5B, a few caspase-3-positive HCEndoCLs were observed, suggesting that some of the endothelial cells were undergoing apoptosis (Fig. 5B). Finally, CTGF, a central mediator of tissue remodeling and fibrosis (Lipson et al., 2012), was observed by IF in the 5-day co-culture (Fig. 5C). CTGF localization was apparent in both the HCEndoCL cytoplasm and the HCF matrix. These results were consistent with the corneal wound repair process, since the 3D co-culture is a model of endothelial wound healing on stroma. Taken together, these results suggest that even though the amount of cells seeded onto the 3D HCF construct were not exactly the number of cells needed to fully cover the construct, they were capable of proliferating to increase the endothelial population in order to form a complete monolayer. In addition, the unhealthy cells were removed by apoptosis, thus allowing the healthy cells to repopulate, differentiate, and mature. Finally, this whole process for the endothelial cells is a wound-healing process, which can activate the fibrotic response; therefore, it is important to make sure that this fibrotic response does not interfere with the clarity of the co-culture. All of these results can be applied to endothelial injection therapy and caution should be observed when removing the DM due to the fibrotic response observed in the co-culture.
Figure 5.
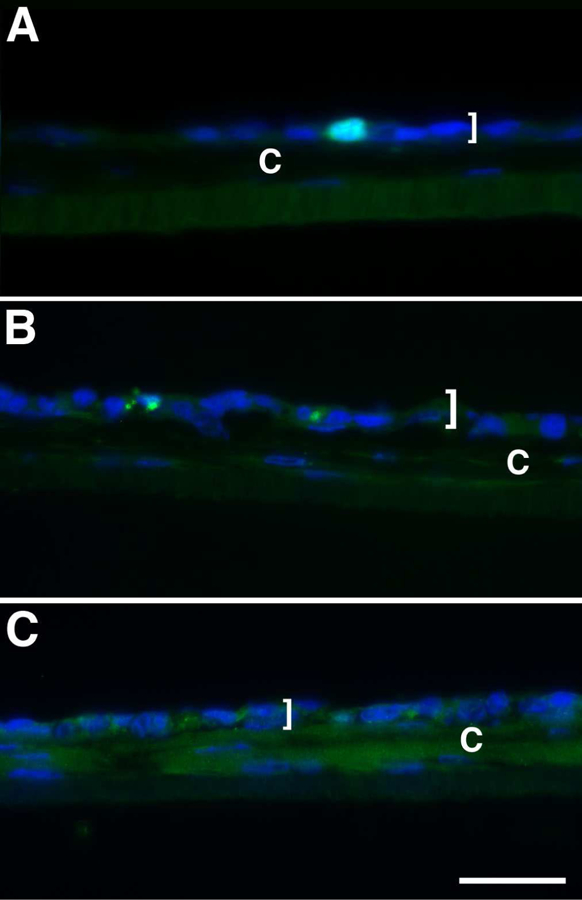
Immunolocalization of (A) Ki67, (B) Caspase-3, and (C) CTGF. Frozen cross-section of 5-day co-culture. “]” indicates HCEndoCL layer; C = construct; Bar = 50 microns.
3.5. Cell-cell contact is not necessary for communication
To examine if cell-cell contact was the only way for HCEndoCs and HCF to communicate, endothelial cells were grown for 2 weeks directly on the transwell membrane and exposed ± HCF growing on the plate surface underneath the membrane. These samples then were examined for laminin expression to observe if the endothelial cells matured in a similar fashion as in co-culture, which had direct contact between cells. As seen in Figure 6, both pHCEndoCs and HCEndoCLs with no exposure to HCF showed only punctate laminin localization in a few cells (Fig. 6A, C, respectively). However, when these cells were exposed to the HCF on the bottom of the culture dish, the laminin localization was much stronger and uniform across the sample (Fig. 6B, D, respectively), indicating that HCF are involved in the secretion of DM components by HCEndoCs, and this regulation may be directed by cell-cell contact or by indirect stimuli from HCF media. This data suggests that before cell injection therapy, the abnormal endothelium and its underlying DM can be removed from the host if necessary, because the stromal cells will assist the newly injected endothelial cells to reform a new DM-like structure; however, keep in mind the possibility of a fibrotic response in the posterior stroma.
Figure 6.
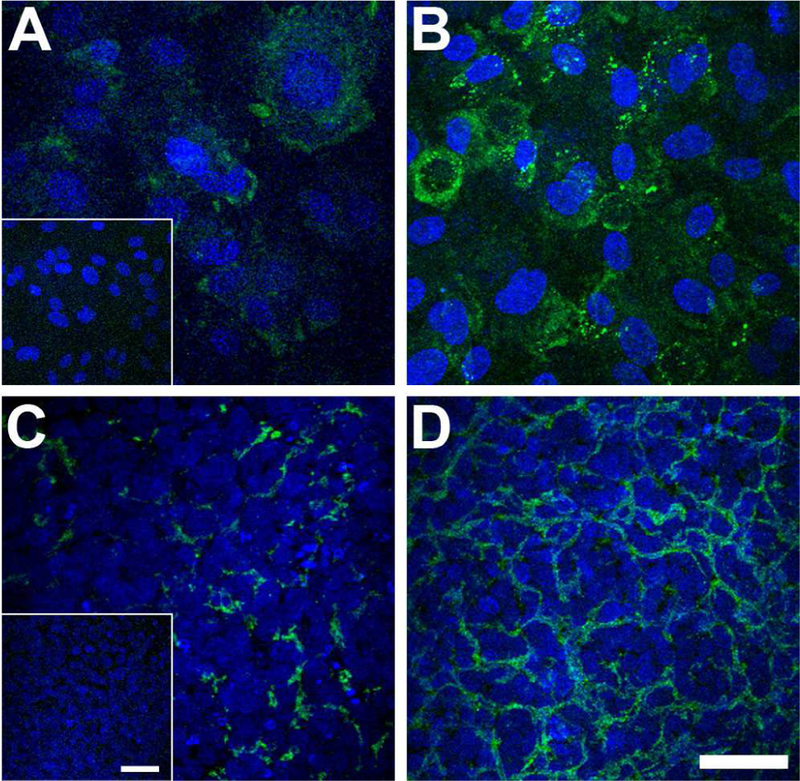
Laminin localization in (A, B) pHCEndoCs and (C, D) HCEndoCLs cultured for 2 weeks on transwell membranes either without (A, C) or with (B, D) indirect exposure to HCF. Inserts = secondary antibody controls. Green = Laminin; Blue = DAPI; Bar = 50 microns.
4. DISCUSSION
In order for corneal endothelial injection therapy to succeed in the recovery and maintenance of corneal transparency, the newly replenished endothelial cells in the host’s anterior chamber must seed onto the posterior stromal surface, and then recover the Descemet’s membrane (DM) and the barrier and pump functions. The mechanisms behind these processes are extremely challenging to study due to the expense and difficulties of performing the experiments in human and/or primates (Tardif et al., 2013). In addition, very often the symptoms and responses to potential treatments seen in other species are dissimilar to those of human patients (Humane Society International, 2018). In the present study, we demonstrated that the cell-based 3D corneal stromal construct developed in our lab provides a good substrate for the differentiation of cultured human corneal endothelial cells and the formation of a new DM-like structure in vitro, thus mimicking the in vivo process of endothelial injection therapy. Ideally, pHCEndoCs would have been used in all experiments; however, multiple problems occur during pHCEndoC expansion, such as the transformation of endothelial cells into a fibroblastic phenotype (Beaulieu Leclerc et al., 2018; Okumura et al., 2013; Peh et al., 2011; Zhu et al., 2012), massive apoptosis during isolation from donor corneas (Numata et al., 2014; Okumura et al., 2009), and limited proliferative ability (Joyce, 2005, 2012; Senoo and Joyce, 2000). Therefore, in addition to pHCEndoC, we also examined an endothelial cell line (HCEndoCL) as an alternative.
In the present study, we demonstrated that when both pHCEndoCs and HCEndoCLs were grown on the 3D HCF construct, they both formed a monolayer sheet (Fig. 1). In addition, we observed that the substrate the HCEndoCs were grown on, as well as cell density, affected cell differentiation. For example, the ALDH1A1 and ZO-1 data (Figs. 3 and 4, respectively) showed that compared with the single cells on glass slides (Figs. 3A and4A, respectively), the cells that were grown at a higher density so that they would make cell-cell contact, increased the ALDH1A1 and ZO-1 localization (Figs. 3B and4B, respectively), thus increased endothelial differentiation. When compared with endothelial cells cultured on the 3D HCF construct for 5 days (Figs. 3C–D and 4C–D, respectively), the differentiation markers’ expression was much stronger than on the glass slide, allowing for ZO-1 to clearly localize on the membrane between cell-cell junctions. These data indicate that the 3D HCF construct provides a good substrate for the study of HCEndoC differentiation in vitro, and the HCEndoC do not transition to EMT. Our observations were consistent with a previous study conducted by Drs. Bourget and Proulx (Bourget and Proulx, 2016), which tested the expression of both Na+/K+ ATPase pumps and Na+/HCO3− co-transporter in corneal endothelial cells seeded on stromal substitutes after 28 days and concluded that self-assembled stromal substitutes support the expression of endothelial cell functionality markers.
DM is synthesized by corneal endothelium during both prenatal and postnatal periods of life (Murphy et al., 1984; Wulle, 1972). It helps maintain the corneal endothelial phenotype and functions under physiological conditions (Chen et al., 2017). So far, little is known how DM contributes to corneal endothelial wound healing. Corneal endothelial injection therapy is a process that transplants cultivated human corneal endothelial cells to the denuded DM of the host by injection into the anterior chamber. However, it is not clear if the presence of the DM is necessary for the endothelial cells to differentiate and mature. The data from Okumura et al. (Okumura et al., 2018) showed that denuded DM might still be a problem, which disagrees with Chen et al., who showed that DM supports corneal endothelial cell regeneration in rabbits after endothelial injury (Chen et al., 2017). However, studies by Dr. Steve Wilson’s laboratory (Medeiros et al., 2018)) showed that removal of the DM led to severe posterior fibrosis of the cornea within a month of surgery, which may be due to an increase in TGF-β that penetrated into the stroma from the aqueous humor causing the keratocytes and bone marrow-derived fibrocytes to transform into myofibroblasts. Since the DM is important for corneal endothelial functioning, as well as the prevention of corneal fibrosis, and there is no direct evidence to answer if transplanted endothelial cells reform the DM in vivo, it would be a huge risk to strip the DM from the diseased area along with the endothelium. In order to investigate whether HCEndoCs can deposit a new DM on a denuded matrix, we examined our 3D co-cultures for laminin, which is one of a group of key proteins found in the DM. We found that by 14 days in co-culture, an almost continuous band of laminin was present at the endothelium/construct interface (Fig. 2D). This was confirmed by TEM, which showed the presence of an early stage DM-like structure (Fig. 2F), indicating that HCEndoCs seeded directly onto the 3D HCF construct in vitro can secret laminin and potentially form a new DM-like structure. Our observation is consistent with the study by Drs. Bourget and Proulx (Bourget and Proulx, 2016), who observed a thin line of type IV collagen, another DM component, underneath the endothelial cells after 28 days in culture on an engineered stroma. These data indicate that for corneal endothelial injection therapy, if the removal of the diseased DM is necessary, the injected single epithelial cells will generate a DM-like structure on the denuded host’s matrix in vivo, which potentially may restore the ability of the host’s cornea to modulate TGF-β penetrating from the aqueous humor to the stroma. However, more experiments will need to be performed in order to establish the relationship between injected cells, DM, and corneal fibrosis.
Interactions amongst neighboring cells are essential for most functions of the cornea, including the development, regeneration, and homeostasis. Cell-cell communication has been thought to consist of the release of numerous soluble growth factors and cytokines to direct corneal wound repair. In addition to local cell-cell communication, secreted factors also play a key role in the interaction amongst cells located further apart (Becker et al., 2016; Han et al., 2017; Kalluri, 2016). By growing HCEndoCs on transwell membranes for 2 weeks with HCF growing on the surface of the plate underneath, we were able to examine distal communication between the HCEndoCs and HCF while avoiding cell-cell contact, and we found that when HCEndoCs were exposed to HCFs, the laminin localization was stronger and more uniform, indicating that HCF are involved in the secretion of DM components by the HCEndoCs, and this regulation may be direct, via cell-cell contact (Fig. 2), or indirect, by stimuli from HCF media (Fig. 6). Now that we know that the HCFs influence the HCEndoCs, the next step in our studies will be to determine what the stimuli produced by the HCF might be: growth factors, cytokines, extracellular vesicles, or something else.
In conclusion, our current findings that our 3D HCF construct stimulates the seeded corneal endothelial cells to differentiate and mature, promote new DM-like structure formation, and enhance HCF regulation of stromal degradation or turnover are applicable to understanding the clinical implications of corneal endothelial injection therapy. Our data also proves that our 3D co-culture model will be useful in studying corneal endothelium in vitro. Further studies will aim at understanding the mechanisms of endothelial differentiation in vitro, and how HCF and endothelial cells regulate one another.
HIGHLIGHTS.
Endothelial cells form a monolayer on 3D constructs, similar to in vivo.
DM-like structure was observed when endothelial cells were grown on the 3D constructs.
Endothelial cells matured in co-culture as seen by ALDH1A1 and ZO-1 localization.
Endothelial cells activated the fibrotic response in 3D constructs in co-culture.
Cell communication occurs through direct contact and indirect stimuli.
ACKNOWLEDGMENTS
This study was funded by grants from National Institute of Health (P30EY03790 and R21EY025833).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial interests to disclose.
REFERENCES
- Amano S, Mimura T, Yamagami S, Osakabe Y, Miyata K, 2005. Properties of corneas reconstructed with cultured human corneal endothelial cells and human corneal stroma. Japanese journal of ophthalmology 49, 448–452. [DOI] [PubMed] [Google Scholar]
- Beaulieu Leclerc V, Roy O, Santerre K, Proulx S, 2018. TGF-beta1 promotes cell barrier function upon maturation of corneal endothelial cells. Scientific reports 8, 4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D, 2016. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer cell 30, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourget JM, Proulx S, 2016. Characterization of a corneal endothelium engineered on a self-assembled stromal substitute. Experimental eye research 145, 125–129. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F, 2007. Cellular senescence: when bad things happen to good cells. Nature reviews. Molecular cell biology 8, 729–740. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Z, Zhang L, Ou S, Wang Y, He X, Zou D, Jia C, Hu Q, Yang S, Li X, Li J, Wang J, Sun H, Chen Y, Zhu YT, Tseng SCG, Liu Z, Li W, 2017. Descemet’s Membrane Supports Corneal Endothelial Cell Regeneration in Rabbits. Scientific reports 7, 6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Azar D, Joyce NC, 2001. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea 20, 731–737. [DOI] [PubMed] [Google Scholar]
- Choi JS, Williams JK, Greven M, Walter KA, Laber PW, Khang G, Soker S, 2010. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 31, 6738–6745. [DOI] [PubMed] [Google Scholar]
- Choi SO, Jeon HS, Hyon JY, Oh YJ, Wee WR, Chung TY, Shin YJ, Kim JW, 2015. Recovery of Corneal Endothelial Cells from Periphery after Injury. PloS one 10, e0138076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapena I, Ham L, Melles GR, 2009. Endothelial keratoplasty: DSEK/DSAEK or DMEK--the thinner the better? Current opinion in ophthalmology 20, 299–307. [DOI] [PubMed] [Google Scholar]
- Dapena I, Moutsouris K, Droutsas K, Ham L, van Dijk K, Melles GR, 2011. Standardized “no-touch” technique for descemet membrane endothelial keratoplasty. Arch Ophthalmol 129, 88–94. [DOI] [PubMed] [Google Scholar]
- Edelhauser HF, 2006. The balance between corneal transparency and edema: the Proctor Lecture. Investigative ophthalmology & visual science 47, 1754–1767. [DOI] [PubMed] [Google Scholar]
- Elhalis H, Azizi B, Jurkunas UV, 2010. Fuchs endothelial corneal dystrophy. The ocular surface 8, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy R, 2018. Correlation between corneal endothelial cell characteristics and dry eye disease. Med Surg Ophthal Res 1, 1–7. [Google Scholar]
- Gagnon MM, Boisjoly HM, Brunette I, Charest M, Amyot M, 1997. Corneal endothelial cell density in glaucoma. Cornea 16, 314–318. [PubMed] [Google Scholar]
- Gipson IK, Grill SM, Spurr SJ, Brennan SJ, 1983. Hemidesmosome formation in vitro. The Journal of cell biology 97, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovoy MS, 2006. Descemet-stripping automated endothelial keratoplasty. Cornea 25, 886–889. [DOI] [PubMed] [Google Scholar]
- Griffith M, Osborne R, Munger R, Xiong X, Doillon CJ, Laycock NL, Hakim M, Song Y, Watsky MA, 1999. Functional human corneal equivalents constructed from cell lines. Science 286, 2169–2172. [DOI] [PubMed] [Google Scholar]
- Guerra FP, Anshu A, Price MO, Giebel AW, Price FW, 2011. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology 118, 2368–2373. [DOI] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW, 2007. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Investigative ophthalmology & visual science 48, 4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KY, Tran JA, Chang JH, Azar DT, Zieske JD, 2017. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Scientific reports 7, 40548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamagami S, Tanaka K, Yokoo S, Usui T, Amano S, Mizuki N, 2009. Immunologic mechanisms of corneal allografts reconstituted from cultured allogeneic endothelial cells in an immune-privileged site. Investigative ophthalmology & visual science 50, 3151–3158. [DOI] [PubMed] [Google Scholar]
- Hitani K, Yokoo S, Honda N, Usui T, Yamagami S, Amano S, 2008. Transplantation of a sheet of human corneal endothelial cell in a rabbit model. Molecular vision 14, 1–9. [PMC free article] [PubMed] [Google Scholar]
- Humane Society International, H.S., 2018. About Animal Testing, Humane Society International http://www.hsi.org/campaigns/end_animal_testing/qa/about.html
- Inoue K, Amano S, Oshika T, Sawa M, Tsuru T, 2000. A 10-year review of penetrating keratoplasty. Japanese journal of ophthalmology 44, 139–145. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. Journal of cell science 112 ( Pt 5), 613–622. [DOI] [PubMed] [Google Scholar]
- Joyce NC, 2003. Proliferative capacity of the corneal endothelium. Progress in retinal and eye research 22, 359–389. [DOI] [PubMed] [Google Scholar]
- Joyce NC, 2005. Cell cycle status in human corneal endothelium. Experimental eye research 81, 629–638. [DOI] [PubMed] [Google Scholar]
- Joyce NC, 2012. Proliferative capacity of corneal endothelial cells. Experimental eye research 95, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Gao L, Wu X, Pang K, 2012. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. The Indian journal of medical research 135, 887–894. [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, 2016. The biology and function of exosomes in cancer. The Journal of clinical investigation 126, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AE, Zieske JD, 2010. Human corneal fibrosis: an in vitro model. Investigative ophthalmology & visual science 51, 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MS, Habib A, Ishaq M, Yaqub MA, 2017. Effect of Femtosecond Laser-Assisted Cataract Surgery (FLACS) on Endothelial Cell Count. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP 27, 763–766. [PubMed] [Google Scholar]
- Kheirkhah A, Saboo US, Abud TB, Dohlman TH, Arnoldner MA, Hamrah P, Dana R, 2015. Reduced Corneal Endothelial Cell Density in Patients With Dry Eye Disease. American journal of ophthalmology 159, 1022–1026 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Sim BR, Kim JI, Khang G, 2018. Functionalized silk fibroin film scaffold using beta-Carotene for cornea endothelial cell regeneration. Colloids and surfaces. B, Biointerfaces 164, 340–346. [DOI] [PubMed] [Google Scholar]
- Kim E, Kim JJ, Hyon JY, Chung ES, Chung TY, Yi K, Wee WR, Shin YJ, 2014. The effects of different culture media on human corneal endothelial cells. Investigative ophthalmology & visual science 55, 5099–5108. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Koizumi N, Ueno M, Okumura N, Imai K, Tanaka H, Yamamoto Y, Nakamura T, Inatomi T, Bush J, Toda M, Hagiya M, Yokota I, Teramukai S, Sotozono C, Hamuro J, 2018. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. The New England journal of medicine 378, 995–1003. [DOI] [PubMed] [Google Scholar]
- Kochar A, Bhargava P, Agarwal P, Maurya L, 2016. Comparison of corneal endothelial cell parameters in four different groups by specular microscope. Int J of Med Sci and Public Health 5, 1863–1868. [Google Scholar]
- Koenig SB, Covert DJ, 2007. Early results of small-incision Descemet’s stripping and automated endothelial keratoplasty. Ophthalmology 114, 221–226. [DOI] [PubMed] [Google Scholar]
- Kruse M, Walter P, Bauer B, Rutten S, Schaefer K, Plange N, Gries T, Jockenhoevel S, Fuest M, 2018. Electro-spun Membranes as Scaffolds for Human Corneal Endothelial Cells. Current eye research 43, 1–11. [DOI] [PubMed] [Google Scholar]
- Lai JY, Chen KH, Hsiue GH, 2007. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 84, 1222–1232. [DOI] [PubMed] [Google Scholar]
- Laing RA, Sanstrom MM, Berrospi AR, Leibowitz HM, 1976. Changes in the corneal endothelium as a function of age. Experimental eye research 22, 587–594. [DOI] [PubMed] [Google Scholar]
- Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM, 2009. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology 116, 1818–1830. [DOI] [PubMed] [Google Scholar]
- Lipson KE, Wong C, Teng Y, Spong S, 2012. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis & tissue repair 5, S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden PW, Lai JN, George KA, Giovenco T, Harkin DG, Chirila TV, 2011. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 32, 4076–4084. [DOI] [PubMed] [Google Scholar]
- Maier AK, Gundlach E, Gonnermann J, Klamann MK, Eulufi C, Bertelmann E, Joussen AM, Torun N, 2013a. Fellow Eye Comparison of Descemet Membrane Endothelial Keratoplasty and Penetrating Keratoplasty. Cornea 32, 1344–1348. [DOI] [PubMed] [Google Scholar]
- Maier P, Reinhard T, Cursiefen C, 2013b. Descemet stripping endothelial keratoplasty--rapid recovery of visual acuity. Deutsches Arzteblatt international 110, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara M, Tanishima T, 1983. Wound-healing of corneal endothelium in monkey: an autoradiographic study. Japanese journal of ophthalmology 27, 444–450. [PubMed] [Google Scholar]
- Medeiros CS, Marino GK, Santhiago MR, Wilson SE, 2018. The Corneal Basement Membranes and Stromal Fibrosis. Investigative ophthalmology & visual science 59, 4044–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Yamagami S, Amano S, 2013. Corneal endothelial regeneration and tissue engineering. Progress in retinal and eye research 35, 1–17. [DOI] [PubMed] [Google Scholar]
- Motley WW 3rd, Kaufman AH, West CE, 2003. Pediatric airbag-associated ocular trauma and endothelial cell loss. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus 7, 380–383. [DOI] [PubMed] [Google Scholar]
- Murphy C, Alvarado J, Juster R, 1984. Prenatal and postnatal growth of the human Descemet’s membrane. Investigative ophthalmology & visual science 25, 1402–1415. [PubMed] [Google Scholar]
- Naveiras M, Dirisamer M, Parker J, Ham L, van Dijk K, Dapena I, Melles GR, 2012. Causes of glaucoma after descemet membrane endothelial keratoplasty. American journal of ophthalmology 153, 958–966 e951. [DOI] [PubMed] [Google Scholar]
- Nishida T, Saika S, 2011. Part I - Basic Science: Cornea, Sclera, Ocular Adnexa Anatomy, PHysiology and Pathophysiologic Responses, in: Krachmer JH, Mannis MJ, Holland EJ (Eds.), Cornea: Fundamentals and Medical Aspects of Cornea and External Disease, 3rd ed. Mosby/Elsevier, China. [Google Scholar]
- Numata R, Okumura N, Nakahara M, Ueno M, Kinoshita S, Kanematsu D, Kanemura Y, Sasai Y, Koizumi N, 2014. Cultivation of corneal endothelial cells on a pericellular matrix prepared from human decidua-derived mesenchymal cells. PloS one 9, e88169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair FJ, Fiorelli R, Schroeter A, Beyeler S, Blatti C, Zoerner B, Thallmair M, 2010. A novel classification of quiescent and transit amplifying adult neural stem cells by surface and metabolic markers permits a defined simultaneous isolation. Stem cell research 5, 131–143. [DOI] [PubMed] [Google Scholar]
- Okumura N, Kay EP, Nakahara M, Hamuro J, Kinoshita S, Koizumi N, 2013. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PloS one 8, e58000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Matsumoto D, Fukui Y, Teramoto M, Imai H, Kurosawa T, Shimada T, Kruse F, Schlotzer-Schrehardt U, Kinoshita S, Koizumi N, 2018. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PloS one 13, e0191306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Sakamoto Y, Fujii K, Kitano J, Nakano S, Tsujimoto Y, Nakamura S, Ueno M, Hagiya M, Hamuro J, Matsuyama A, Suzuki S, Shiina T, Kinoshita S, Koizumi N, 2016. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Scientific reports 6, 26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Ueno M, Koizumi N, Sakamoto Y, Hirata K, Hamuro J, Kinoshita S, 2009. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investigative ophthalmology & visual science 50, 3680–3687. [DOI] [PubMed] [Google Scholar]
- Parekh M, Ahmad S, Ruzza A, Ferrari S, 2017. Human Corneal Endothelial Cell Cultivation From Old Donor Corneas With Forced Attachment. Scientific reports 7, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh M, Ferrari S, Sheridan C, Kaye S, Ahmad S, 2016. Concise Review: An Update on the Culture of Human Corneal Endothelial Cells for Transplantation. Stem cells translational medicine 5, 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikumar P, Haraguchi K, Senthilkumar R, Abraham SJ, 2018. Human corneal endothelial cell transplantation using nanocomposite gel sheet in bullous keratopathy. American journal of stem cells 7, 18–24. [PMC free article] [PubMed] [Google Scholar]
- Parker J, Dirisamer M, Naveiras M, Tse WH, van Dijk K, Frank LE, Ham L, Melles GR, 2012. Outcomes of Descemet membrane endothelial keratoplasty in phakic eyes. Journal of cataract and refractive surgery 38, 871–877. [DOI] [PubMed] [Google Scholar]
- Peh GS, Chng Z, Ang HP, Cheng TY, Adnan K, Seah XY, George BL, Toh KP, Tan DT, Yam GH, Colman A, Mehta JS, 2015. Propagation of human corneal endothelial cells: a novel dual media approach. Cell transplantation 24, 287–304. [DOI] [PubMed] [Google Scholar]
- Peh GS, Toh KP, Ang HP, Seah XY, George BL, Mehta JS, 2013. Optimization of human corneal endothelial cell culture: density dependency of successful cultures in vitro. BMC research notes 6, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh GS, Toh KP, Wu FY, Tan DT, Mehta JS, 2011. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PloS one 6, e28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl EM, El-Shabrawi Y, Ardjomand N, 2013. Central corneal haze after wedge resection following penetrating keratoplasty and photorefractive keratectomy. Eye (Lond) 27, 679–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Hutcheon AE, Guo XQ, Saeidi N, Melotti SA, Ruberti JW, Zieske JD, Trinkaus-Randall V, 2008. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Developmental dynamics : an official publication of the American Association of Anatomists 237, 2705–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolev K, O’Donovan DG, Coussons P, King L, Rajan MS, 2018. Feasibility Study of Human Corneal Endothelial Cell Transplantation Using an In Vitro Human Corneal Model. Cornea 37, 778–784. [DOI] [PubMed] [Google Scholar]
- Ruberti JW, Zieske JD, 2008. Prelude to corneal tissue engineering - gaining control of collagen organization. Progress in retinal and eye research 27, 549–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gimeno JA, Lleo-Perez A, Alonso L, Rahhal MS, Martinez Soriano F, 2005. Corneal endothelial cell density decreases with age in emmetropic eyes. Histology and histopathology 20, 423–427. [DOI] [PubMed] [Google Scholar]
- Schmedt T, Chen Y, Nguyen TT, Li S, Bonanno JA, Jurkunas UV, 2012. Telomerase immortalization of human corneal endothelial cells yields functional hexagonal monolayers. PloS one 7, e51427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo T, Joyce NC, 2000. Cell cycle kinetics in corneal endothelium from old and young donors. Investigative ophthalmology & visual science 41, 660–667. [PubMed] [Google Scholar]
- Shearer TR, Chamberlain WD, Fujii A, Azuma M, 2016. Selecting Fuchs patients for drug trials involving endothelial cell proliferation. European journal of ophthalmology 26, 536–539. [DOI] [PubMed] [Google Scholar]
- Shen L, Sun P, Zhang C, Yang L, Du L, Wu X, 2017. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Scientific reports 7, 13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh WA, Abdelkader A, Kaufman HE, 2010. Corneal Physiology, in: John T (Ed.), Corneal Endothelial Transplant (DSAEK, DMEK & DLEK), 1st ed. Jaypee Brothers Medical Publishers, New Delhi, India, pp. 13–21. [Google Scholar]
- Slingsby JG, Forstot SL, 1981. Effect of blunt trauma on the corneal endothelium. Arch Ophthalmol 99, 1041–1043. [DOI] [PubMed] [Google Scholar]
- Sugar A, Sugar J, 2000. Techniques in penetrating keratoplasty: a quarter century of development. Cornea 19, 603–610. [DOI] [PubMed] [Google Scholar]
- Sumide T, Nishida K, Yamato M, Ide T, Hayashida Y, Watanabe K, Yang J, Kohno C, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y, 2006. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20, 392–394. [DOI] [PubMed] [Google Scholar]
- Syed ZA, Tran JA, Jurkunas UV, 2017. Peripheral Endothelial Cell Count Is a Predictor of Disease Severity in Advanced Fuchs Endothelial Corneal Dystrophy. Cornea 36, 1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Tomita H, Hisamatsu K, Nakashima T, Hatano Y, Sasaki Y, Osada S, Tanaka T, Miyazaki T, Yoshida K, Hara A, 2015. ALDH1A1-overexpressing cells are differentiated cells but not cancer stem or progenitor cells in human hepatocellular carcinoma. Oncotarget 6, 24722–24732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Coleman K, Hobbs TR, Lutz C, 2013. IACUC review of nonhuman primate research. ILAR journal 54, 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann J, Valtink M, Nitschke M, Gramm S, Funk RH, Engelmann K, Werner C, 2013. Tissue engineering of the corneal endothelium: a review of carrier materials. Journal of functional biomaterials 4, 178–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MA, 2012. Endothelial keratoplasty: why aren’t we all doing Descemet membrane endothelial keratoplasty? Cornea 31, 469–471. [DOI] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ, 2001. Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea 20, 239–243. [DOI] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ, 2003. Replacing the endothelium without corneal surface incisions or sutures: the first United States clinical series using the deep lamellar endothelial keratoplasty procedure. Ophthalmology 110, 755–764; discussion 764. [DOI] [PubMed] [Google Scholar]
- Thompson RE, Boraas LC, Sowder M, Bechtel MK, Orwin EJ, 2013. Three-dimensional cell culture environment promotes partial recovery of the native corneal keratocyte phenotype from a subcultured population. Tissue engineering. Part A 19, 1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuft SJ, Coster DJ, 1990. The corneal endothelium. Eye (Lond) 4 ( Pt 3), 389–424. [DOI] [PubMed] [Google Scholar]
- Ventura AC, Walti R, Bohnke M, 2001. Corneal thickness and endothelial density before and after cataract surgery. The British journal of ophthalmology 85, 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Wang IJ, Hu FR, Young TH, 2016. Applications of Biomaterials in Corneal Endothelial Tissue Engineering. Cornea 35 Suppl 1, S25–S30. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Hayashi R, Kimura Y, Tanaka Y, Kageyama T, Hara S, Tabata Y, Nishida K, 2011. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue engineering. Part A 17, 2213–2219. [DOI] [PubMed] [Google Scholar]
- Wulle KG, 1972. Electron microscopy of the fetal development of the corneal endothelium and Descemet’s membrane of the human eye. Investigative ophthalmology 11, 897–904. [PubMed] [Google Scholar]
- Yeniad B, Corum I, Ozgun C, 2010. The effects of blunt trauma and cataract surgery on corneal endothelial cell density. Middle East African journal of ophthalmology 17, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Joyce NC, 2004. Proliferative response of corneal endothelial cells from young and older donors. Investigative ophthalmology & visual science 45, 1743–1751. [DOI] [PubMed] [Google Scholar]
- Zhu YT, Chen HC, Chen SY, Tseng SC, 2012. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. Journal of cell science 125, 3636–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AE, 2001. Kinetics of keratocyte proliferation in response to epithelial debridement. Experimental eye research 72, 33–39. [DOI] [PubMed] [Google Scholar]


