Abstract
Polychlorinated biphenyls (PCBs) are a class of lipophilic endocrine-disrupting chemicals with wide industrial use in the U.S. from the 1930s through 1977. Due to their environmental and biological persistence, low levels of PCBs remain detected in wildlife and humans. Although U.S. studies have shown higher serum PCB concentrations among Black women compared with White women, studies of correlates of PCB exposure among Black women are scarce. We examined predictors of plasma PCB concentrations in a cross-sectional analysis of baseline data from a prospective cohort study of 1,693 premenopausal Black women aged 23-35 years from Detroit, Michigan (2010-2012). We collected demographic, behavioral, dietary, and medical data via self-administered questionnaires, telephone interviews, and in-person clinic visits, as well as non-fasting blood samples. We measured concentrations of 24 PCB congeners in baseline plasma from a subset of 762 participants. We used linear regression for log-transformed lipid-adjusted PCB concentrations to calculate percentage differences across levels of selected predictors. We did this separately for individual PCBs, sum of total PCBs, and sum of PCBs by degree of chlorination and hormonal activity. PCB concentrations were positively associated with age, duration of urban residence, cigarette smoking, heavy alcohol intake, and being breastfed in infancy, and inversely associated with body mass index (BMI) and lactation duration. The strength of some associations varied by degree of chlorination. For example, a 5-kg/m2 higher BMI corresponded to a 2.9% lower summed concentration of tri- and tetra-substituted PCBs (95% CI −4.6%, −1.2%), an 8.3% lower summed concentration of penta- and hexa-substituted PCBs (95% CI −10.0%, −6.5%), and a 12.1% lower summed concentration of hepta-, octa-, nona-, and deca-substituted PCBs (95% CI −13.7%, −10.4%). Likewise, associations for age and being breastfed in infancy were stronger for higher-chlorinated PCBs. Results agree with studies on predictors of PCB body burdens, few of which include large numbers of Black women.
Keywords: polychlorinated biphenyls, predictors, reproductive-age, race
INTRODUCTION
Polychlorinated biphenyls (PCBs) are a class of lipophilic endocrine-disrupting chemicals with wide industrial production and usage in the United States from the 1930s through 1977. They were banned out of concern for environmental contamination and human health. The ban on the manufacture of PCBs resulted in a secular decline in human exposure (LaKind et al., 2009), yet low-level exposure is ongoing, as PCBs have long half-lives (approximately 10-15 years), resist degradation in the environment, and bioaccumulate up the food chain (Agency for Toxic Substances and Disease Registry, 2000).
Several studies have documented large racial and ethnic disparities in PCB exposure, with non-Hispanic Blacks experiencing higher exposures than non-Hispanic Whites (James et al., 2002; Krieger et al., 1994; Lordo et al., 1996; Patterson et al., 2009; Wang et al., 2009; Weintraub and Birnbaum, 2008; Xue et al., 2014). This disparity could reflect differences in diet across racial groups, including intake of certain types of fish (Weintraub and Birnbaum, 2008). It may also stem from a greater concentration of PCB production and disposal sites in communities of color (Taylor, 2014). For example, in Anniston, Alabama, a former PCB manufacturing city, Black residents lived closer to the manufacturing plant and had three times the PCB exposure levels as White residents (Aminov et al., 2014). These patterns of PCB exposure may partially explain racial disparities in health conditions that have been associated with chronic PCB exposure, including diabetes (Ruiz et al., 2018), metabolic syndrome (Gasull et al., 2018), and cancer (International Agency for Research on Cancer, 2016).
Despite evidence of PCB exposure disparities and possible linkage with adverse health outcomes, Black women are underrepresented in studies examining predictors of PCB concentrations in body tissues. This is problematic because the prevalence of predictors and metabolism of toxicants may vary across racial and ethnic groups (Anderson, 2005; Fukami et al., 2005; Moolchan et al., 2006; Muszkat, 2007; Solus et al., 2004). The few studies that have enrolled large numbers of Black women have comprised women who are pregnant (Borrell et al., 2004; Herbstman et al., 2007; McGraw and Waller, 2009; Wolff et al., 2005) or who reside in specific geographic regions with PCB contamination (Pavuk et al., 2014).
The three most consistent predictors of higher PCB concentrations among females across racial/ethnic groups are older age (Artacho-Cordon et al., 2015; Bachelet et al., 2011; Brauner et al., 2011; Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Foster et al., 2012; Gallo et al., 2011; Glynn et al., 2007; Hardell et al., 2010; Herbstman et al., 2007; Humblet et al., 2010; Ibarluzea et al., 2011; Kiviranta et al., 2005; Lewin et al., 2017; McGraw and Waller, 2009; Pavuk et al., 2014; Rylander et al., 2012; Sandanger et al., 2007; Wolff et al., 2005), lower body mass index (BMI) (Bachelet et al., 2011; Brauner et al., 2011; Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Foster et al., 2012; Gallo et al., 2011; Glynn et al., 2007; Hardell et al., 2010; Herbstman et al., 2007; Humblet et al., 2010; Lewin et al., 2017; Pavuk et al., 2014; Sandanger et al., 2007; Wolff et al., 2005), and nulliparity (Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Hardell et al., 2010; Herbstman et al., 2007; Ibarluzea et al., 2011; Lewin et al., 2017; Pavuk et al., 2014). These associations persist after adjustment for other predictors.
The literature on cigarette smoking and PCB exposure is inconsistent: some (Bachelet et al., 2011; Fernandez-Rodriguez et al., 2015; Gallo et al., 2011; Ibarluzea et al., 2011; Lewin et al., 2017; Rylander et al., 2012; Wolff et al., 2005), but not all (Glynn et al., 2007; Herbstman et al., 2007; Humblet et al., 2010; Pavuk et al., 2014; Sandanger et al., 2007) studies have found lower PCB concentrations among current cigarette smokers. Women who breastfed their children for longer durations tend to have lower concentrations of PCBs (Bachelet et al., 2011; Brauner et al., 2011; Hardell et al., 2010; Humblet et al., 2010; Ibarluzea et al., 2011; Kiviranta et al., 2005; Pavuk et al., 2014; Rylander et al., 2012), whereas women who were breastfed as infants may have higher PCB concentrations (Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Gallo et al., 2011; Glynn et al., 2007). Urban residence (Fernandez-Rodriguez et al., 2015; Pavuk et al., 2014) and high intakes of fish (Bachelet et al., 2011; Brauner et al., 2011; Fernandez-Rodriguez et al., 2015; Gallo et al., 2011; Glynn et al., 2007; Humblet et al., 2010; Ibarluzea et al., 2011; Kiviranta et al., 2005; McGraw and Waller, 2009; Pavuk et al., 2014; Rylander et al., 2012; Wolff et al., 2005) and other locally-grown/raised foods (Fernandez-Rodriguez et al., 2015; Pavuk et al., 2014) may be associated with higher PCB concentrations. Lastly, PCB exposure can result from occupational sources (e.g., work with old electrical equipment or building materials that contain PCBs or work involving clean-up of contaminated sites) (Pavuk et al., 2014).
Most studies have assessed predictors of total PCBs and/or individual PCB congeners, particularly the congeners most commonly detected in humans. There has been limited study of predictors of PCB groupings defined by structure or hormonal activity (Gallo et al., 2011; Humblet et al., 2010; McGraw and Waller, 2009). Because individual PCB congeners have varying degrees of persistence (Agency for Toxic Substances and Disease Registry, 2000), it is plausible that important predictors of PCB concentrations may vary across congeners.
We examined selected demographic, behavioral, reproductive, dietary and occupational predictors of plasma PCB concentrations in a cohort of reproductive-aged Black women. We examined PCBs individually, and grouped by structure and hormonal activity.
MATERIALS AND METHODS
Study design
The Study of Environment, Lifestyle, and Fibroids (SELF) is a prospective cohort of Black women age 23-35 years from the Detroit metropolitan area (Baird et al., 2015). Eligible women had an intact uterus and no prior diagnosis of uterine leiomyomata or medically-treated autoimmune disease or cancer. From 2010-2012, 1,693 women participated in the study, which involved completion of computer-assisted telephone- and web-based questionnaires, self-administered hard copy questionnaires, and in-person clinic visits. Using a case-cohort study design, we selected a random sample of participants at baseline (n=659) and all incident cases of uterine leiomyomata through 60 months of follow-up (n=296, including 103 incident cases that were not part of the random subcohort), for a total of 762 women for the present analysis. Institutional Review Boards at the Henry Ford Health System, the National Institute of Environmental Health Sciences, and Boston University Medical Center approved the study and all participants provided written informed consent.
Measurement of polychlorinated biphenyls
At baseline, participants provided non-fasting blood samples, from which plasma was isolated and shipped to the National Institute of Environmental Health Sciences repository and stored at −80 degrees Celsius. We shipped samples on dry ice in three batches to the Centers for Disease Control and Prevention (CDC), where they were analyzed for plasma concentrations of 24 persistent PCBs using high-resolution gas chromatography/isotope-dilution high-resolution mass spectrometry (Sjodin et al., 2004). The involvement of the CDC laboratory did not constitute engagement in human subjects’ research. The samples were analyzed in batches of twenty-four unknowns, three method blanks, and three quality control samples. The samples were extracted using liquid/liquid extraction with hexane/methyl tert-butyl ether after denaturation with hydrochloric acid and methanol. Coextracted plasma lipids were removed using a two layered silica/silica:sulfuric acid cartridge. The limit of detection (LOD) was defined as the higher of three times the standard deviation of method blanks measured in parallel with unknowns or the lowest calibration point having a signal to noise ratio greater than 10:1 (Centers for Disease Control and Prevention, 2016). Quality control criteria considered necessary for valid measurement of analytes were: isotopic ratio of native and 13C-labeled internal standard within 20% of theoretical value, relative retention time within 0.004 of the calibration standard, and recovery between 25% and 150%. In addition, for a batch of samples to be considered valid and reportable, the quality control samples (n=3 per batch) must pass the quality control criteria outlined by Caudill et al. (Caudill et al., 2008). The variability of quality control samples was <10% overall. We measured total lipids in plasma using an enzymatic summation method (Bernert et al., 2007); all PCB concentrations were reported on a lipid-adjusted basis.
Measurement of demographic, behavioral, and other factors
We collected information on age, current marital status, highest education level, current household income, smoking history, alcohol intake in the past year, pregnancy history, residential history, birth order, and occupational history on the baseline questionnaire. We identified occupations with potential PCB exposure, either through exposure to old building materials or through exposure to old x-ray machines (Agency for Toxic Substances and Disease Registry, 2000). We ascertained whether participants were breastfed in infancy and for how long on an early-life questionnaire (for which 85% of participants had help from their mothers). Dietary data, including frequency of fish, meat, poultry, egg, and dairy intakes over the past year, were collected on a self-administered semi-quantitative food frequency questionnaire (Block et al., 1986). During the in-person clinic visit, we measured height and weight, with which we calculated BMI as weight (kilograms) divided by height (meters) squared.
Statistical analysis
All statistical analyses were performed using SAS version 9.4. We used Spearman correlation coefficients to measure the correlation between individual congeners. We set concentrations below the LOD to LOD/sqrt(2) (Hornung and Reed, 1990); the LOD varied by the available plasma and lipid concentration in each individual sample (range: 0.11-5.40 ng/g lipid). We generated PCB groupings by summing individual lipid-adjusted PCB concentrations (Table 1). We grouped PCBs by structure (tri- and tetra-substituted, penta- and hexa-substituted, and hepta-, octa-, nona-, and deca-substituted) and hormonal activity (as classified by Wolff et al. 1997 (Wolff et al., 1997)). We also calculated the sum of: total PCBs; PCBs with >50% detection; PCBs 118, 153, 138/158, and 180 (the congeners most commonly detected in humans); and dioxin-like congeners.
Table 1.
Polychlorinated biphenyl groupings used in the present analysis.
| Group | PCB Congeners Included |
|---|---|
| Wolff Group 1B (WG1B; weak phenobarbital inducers, persistent) | 187 |
| Wolff Group 2A (WG2A; potentially antiestrogenic and immunotoxic, dioxin-like, moderately persistent) | 66, 74, 105, 118, 156, 167 |
| Wolff Group 2B (WG2B; potentially antiestrogenic and immunotoxic, limited dioxin activity, persistent) | 138/158, 170 |
| Wolff Group 3 (WG3; phenobarbital, CYP1A and CYP2B inducers, biologically persistent) | 99, 153, 180, 196/203, 183 |
| Dioxin-like (DL-PCBs) | 105, 114, 118, 156, 157, 167, 189, 170, 180 |
| Tri- and tetra-substituted (TT-PCBs) | 28, 66, 74 |
| Penta- and hexa-substituted (PH-PCBs) | 99, 105, 114, 118, 138/158, 146, 153, 156, 157, 167 |
| Hepta-, octa-, nona-, and deca-substituted (HOND-PCBs) | 170, 178, 180, 183, 187, 189, 194, 196/203, 199, 206, 209 |
| 4 most common PCBs (PCB-4) | 118, 153, 138/158, 180 |
| PCBs >50% detected (PCB>50%) | 28, 66, 74, 99, 105, 118, 138/158, 146, 153, 156, 170, 178, 180, 183, 187, 194, 196/203, 199 |
| Total PCBs | 28, 66, 74, 99, 105, 114, 118, 138/158, 146, 153, 156, 157, 167, 170, 178, 180, 183, 187, 189, 194, 196/203, 199, 206, 209 |
We first summarized the distribution of plasma concentrations of individual PCB congeners. Next, we compared these results with lipid-adjusted serum samples pooled from non-Hispanic Black females participating in the 2005-06, 2007-08, and 2009-10 U.S. National Health and Nutrition Examination Survey (NHANES) cycles in order to compare with a nationally-representative sample (CDC, 2018). We conducted a cross-sectional analysis of demographic, behavioral, dietary, reproductive, and occupational variables ascertained at baseline in relation to baseline plasma PCB concentrations. We selected variables that have been associated with PCB exposure in previous studies or that we hypothesized could plausibly be related to PCB exposure. We examined the following variables as potential predictors of individual and groups of PCBs: age (years, continuous), marital status (never, currently, previously married), education (≤high school or General Education Development (GED), some college or Associate’s degree or technical degree, ≥Bachelor’s degree), annual household income (<$20,000, $20,000-$50,000, >$50,000), cumulative residence in urban area (years, continuous), smoking history (never, former, current <5 cigarettes/day, current ≥5 cigarettes/day), alcohol use in past year (none, moderate [1-5 drinks/day on average on days that they drink and ≥4 drinks in one sitting once per month or less], heavy [≥6 drinks/day on average on days that they drink or ≥4 drinks in one sitting more than once per month]), BMI (kg/m2, continuous), parity and lactation (nulliparous, parity but never breastfed, parous and breastfed <6 months, parous and breastfed ≥6 months), birth order (first, second, third or higher), breastfed in infancy (not, <3 months, ≥3 months), fish intake (grams/day, continuous), meat intake (grams/day, continuous), poultry intake (grams/day, continuous), egg intake (grams/day, continuous), dairy intake (servings/day, continuous), and occupation (ever worked cleaning homes or buildings, as a doctor or medical student, or in a job that required working with x-ray machines (separately) vs. never worked in any of these occupations). We considered country of birth (U.S. vs. other) and ever worked in building construction as a potential predictors, but due to small numbers (n=7 foreign-born and n=7 who ever worked in building construction), results were imprecise and we did not include these variables in the final analysis. We did not examine predictors of individual PCBs with <50% detection, although these congeners were included in the PCB groupings described above, when appropriate.
We fit linear regression models with log-transformed PCB concentrations as the dependent variable, adjusted for all other predictors. We calculated the percentage difference in PCB concentrations comparing one category of a predictor with the reference group (or, for continuous variables that demonstrated a linear relationship with PCB congeners, the percentage difference per unit increase in the predictor) by exponentiating beta coefficients (calculated as 100*[exp(beta)-1]). Reported results are adjusted for all other predictors.
We conducted a sensitivity analysis restricting to the women who were randomly sampled at baseline (n=659) to examine the possibility of selection bias by including incident uterine leiomyomata cases that were not part of the random subcohort (n=103).
We used a Markov chain Monte Carlo method to impute missing data (Zhou et al., 2001). We generated five imputation data sets and statistically combined the estimates from each data set. The percentage missing for all variables was low and ranged from 0% (age) to 7% (breastfed in infancy). We imputed PCB concentrations for samples that did not meet quality control criteria (missingness across congeners ranged from 0.0-3.5%).
RESULTS
The mean age of the 762 women in our analysis was 28.6 (range: 23-35 years), and over half had never been married (Table 2). Twenty-seven percent of participants had a Bachelor’s degree or higher and almost half had an annual household income of <$20,000. Current smoking and heavy alcohol use were common and the mean BMI was 33.7 kg/m2, with 60% of participants classified as obese. Sixty-two percent of women were parous and the majority breastfed at least one child. Most participants had lived in an urban area for at least 20 years. History of working in occupations with potential PCB exposure was rare, except for work cleaning houses or buildings.
Table 2.
Characteristics of 762 participants in the Study of Environment, Lifestyle, and Fibroids (SELF) Prospective Cohort Study at Baseline (2010-2012).
| Characteristic | N (%) | Mean (SD) |
|---|---|---|
| Age (years) | 28.6 (3.5) | |
| 23-25 | 182 (23.9) | |
| 26-28 | 189 (24.8) | |
| 29-31 | 204 (26.8) | |
| 32-35 | 187 (24.5) | |
| Marital status | ||
| Never married | 435 (57.1) | |
| Currently married | 217 (28.5) | |
| Previously married | 110 (14.4) | |
| Education | ||
| ≤High school diploma/GED | 163 (21.4) | |
| Some college/Associate’s/Technical | 391 (51.3) | |
| ≥Bachelor’s degree | 208 (27.3) | |
| Annual household income | ||
| <$20,000 | 348 (45.7) | |
| $20,000-$50,000 | 288 (37.8) | |
| >$50,000 | 126 (16.5) | |
| Smoking status | ||
| Never | 558 (73.2) | |
| Past | 59 (7.7) | |
| Current <5 cigarettes/day | 71 (9.3) | |
| Current ≥5 cigarettes/day | 74 (9.7) | |
| Alcohol intake in past year | ||
| None | 217 (28.5) | |
| Moderate | 391 (51.3) | |
| Heavy | 154 (20.2) | |
| BMI (kg/m2) | 33.7 (9.6) | |
| <25 | 144 (18.9) | |
| 25-29 | 160 (21.0) | |
| 30-34 | 150 (19.7) | |
| 35-39 | 128 (16.8) | |
| ≥40 | 180 (23.6) | |
| Parity | 1.2 (1.4) | |
| Nulliparous | 289 (37.9) | |
| 1 | 207 (27.2) | |
| 2 | 130 (17.1) | |
| ≥3 | 136 (17.9) | |
| Duration of lactation (months) | 3.1 (6.1) | |
| 0 | 452 (59.3) | |
| 1-3 | 118 (15.5) | |
| 4-6 | 70 (9.2) | |
| 7-12 | 64 (8.4) | |
| >12 | 58 (7.6) | |
| Birth order | ||
| First | 315 (41.3) | |
| Second | 214 (28.1) | |
| Third or higher | 233 (30.6) | |
| Breastfed in infancy | ||
| No | 541 (71.0) | |
| Yes, ≤3 months | 110 (14.4) | |
| Yes, >3 months | 111 (14.6) | |
| Total years lived in urban area | 21.2 (10.2) | |
| <5 | 73 (9.6) | |
| 5-9 | 42 (5.5) | |
| 10-19 | 158 (20.7) | |
| ≥20 | 489 (64.2) | |
| Fish intake (g/day) | 28.1 (33.9) | |
| <50 | 652 (85.6) | |
| 50-99 | 74 (9.7) | |
| ≥100 | 36 (4.7) | |
| Meat intake (g/day) | 53.5 (63.4) | |
| <50 | 514 (67.5) | |
| 50-99 | 152 (20.0) | |
| ≥100 | 96 (12.6) | |
| Poultry intake (g/day) | 35.7 (37.0) | |
| <50 | 588 (77.2) | |
| 50-99 | 127 (16.7) | |
| ≥100 | 47 (6.2) | |
| Dairy intake (milk equivalents/day) | 1.1 (0.8) | |
| <1.0 | 438 (57.5) | |
| 1.0-1.9 | 240 (31.5) | |
| ≥2.0 | 84 (11.0) | |
| Eggs (g/day) | 14.8 (14.3) | |
| <25 | 632 (82.9) | |
| 25-49 | 103 (13.5) | |
| ≥50 | 27 (3.5) | |
| Ever worked cleaning houses or buildings | 181 (23.8) | |
| Ever worked as doctor/physician/medical student | 52 (6.8) | |
| Ever used x-ray equipment in jobs | 24 (3.2) |
BMI=body mass index; GED=general education diploma
Average plasma concentrations of individual PCBs were lower in SELF than in pooled serum samples from NHANES non-Hispanic Black females (2005-2010; Table 3). Congeners 74 and 99 were detected in 100% of participants; congeners 114, 157, 167, 189, 206, and 209 were detected in <50% of participants and were not analyzed further. Spearman correlation coefficients (r) between individual congeners ranged from 0.14 to 0.98 (Supplemental Table 1). Correlations between PCBs 28 and 66 with other higher-chlorinated congeners were weaker (r=0.12-0.60), whereas most higher-chlorinated congeners had correlation coefficients >0.50. PCB groupings were highly correlated, particularly total PCBs, PCBs with >50% detection, and PCBs 118, 153, 138/158, and 180 (r=0.99).
Table 3.
Distribution of plasma concentrations of PCBs at baseline in a sample of 762 SELF participants (2010-2012) and pooled NHANES serum samples for non-Hispanic Black females age 20-39 years from 2005-2010 (CDC, 2018).
| SELF |
NHANES non-Hispanic Black females (pooled)a |
|||||
|---|---|---|---|---|---|---|
| PCB congener (ng/g lipid) | Range of LOD | % <LOD | Median | 90th percentile | Arithmetic mean (SD) | Arithmetic mean (SD)b |
| PCB28 | 0.12-2.20 | 16.0 | 0.79 | 1.56 | 0.98 (0.92) | 0.83 (0.12) |
| PCB66 | 0.11-1.10 | 18.5 | 0.42 | 0.92 | 0.53 (0.45) | 0.68 (0.11) |
| PCB74 | 0.13-1.10 | 0.0 | 1.47 | 2.70 | 1.69 (1.00) | 1.69 (0.22) |
| PCB99 | 0.11-1.10 | 0.0 | 1.69 | 3.47 | 2.06 (1.66) | 2.07 (0.28) |
| PCB105 | 0.11-1.10 | 4.1 | 0.69 | 1.63 | 0.93 (1.11) | 0.69 (0.11) |
| PCB114 | 0.11-1.10 | 78.6 | 0.18 | 0.33 | --c | --c |
| PCB118 | 0.11-2.10 | 0.3 | 2.78 | 5.92 | 3.54 (3.39) | 2.94 (0.45) |
| PCB138-158 | 0.11-4.20 | 0.7 | 4.32 | 9.98 | 5.44 (4.01) | 6.56 (0.92) |
| PCB146 | 0.11-1.10 | 6.8 | 0.73 | 1.81 | 0.94 (0.74) | 1.23 (0.24) |
| PCB153 | 0.11-5.40 | 0.8 | 5.79 | 13.27 | 7.15 (4.90) | 8.93 (1.48) |
| PCB156 | 0.11-1.10 | 10.1 | 0.60 | 1.40 | 0.77 (0.60) | 0.96 (0.16) |
| PCB157 | 0.11-1.20 | 75.5 | 0.19 | 0.47 | --c | --c |
| PCB167 | 0.11-1.10 | 52.2 | 0.24 | 0.59 | --c | 0.39 (0.05) |
| PCB170 | 0.11-1.10 | 3.5 | 0.18 | 2.90 | 1.51 (1.13) | 2.08 (0.43) |
| PCB178 | 0.11-1.10 | 43.0 | 0.27 | 0.70 | 0.37 (0.29) | 0.55 (0.09) |
| PCB180 | 0.11-1.60 | 0.8 | 2.88 | 7.33 | 3.84 (3.00) | 4.76 (0.81) |
| PCB183 | 0.11-1.10 | 13.9 | 0.52 | 1.26 | 0.66 (0.53) | 1.08 (0.17) |
| PCB187 | 0.11-1.90 | 5.4 | 1.33 | 3.31 | 1.75 (1.49) | 2.41 (0.51) |
| PCB189 | 0.11-1.20 | 97.0 | 0.17 | 0.40 | --c | --c |
| PCB194 | 0.11-1.10 | 13.8 | 0.56 | 1.37 | 0.71 (0.55) | 1.06 (0.20) |
| PCB196-203 | 0.11-1.60 | 8.5 | 0.76 | 1.69 | 0.93 (0.66) | 1.09 (0.20) |
| PCB199 | 0.11-1.10 | 8.3 | 0.68 | 1.61 | 0.86 (0.72) | 1.21 (0.29) |
| PCB206 | 0.19-1.40 | 57.7 | 0.47 | 0.83 | --c | 0.93 (0.18) |
| PCB209 | 0.11-1.40 | 81.0 | 0.25 | 0.45 | --c | 0.57 (0.09) |
Samples were pooled separately in 2005-2006, 2007-2008, and 2009-2010. We report the latest available pooled samples.
Pooled data are comparable to an arithmetic mean of individual samples.
Mean not calculated because >50% of samples were below the LOD.
Age was strongly positively associated with plasma PCB concentrations, even after adjusting for all other predictors (Figure 1a). Adjusted associations were stronger for hepta-, octa-, nona-, and deca-substituted PCBs (mean concentrations were 45.5% higher for each five-year increase in age) than for penta- and hexa-(29.6% higher) and tri- and tetra-substituted PCBs (15.2% higher). Results also varied across hormonally-active groups: associations were stronger for Wolff group 1B (weak phenobarbital inducers) and group 3 (phenobarbital, CYP1A, and CYP2B inducers) than for groups 2A and 2B (potentially antiestrogenic and immunotoxic).
Figure 1.
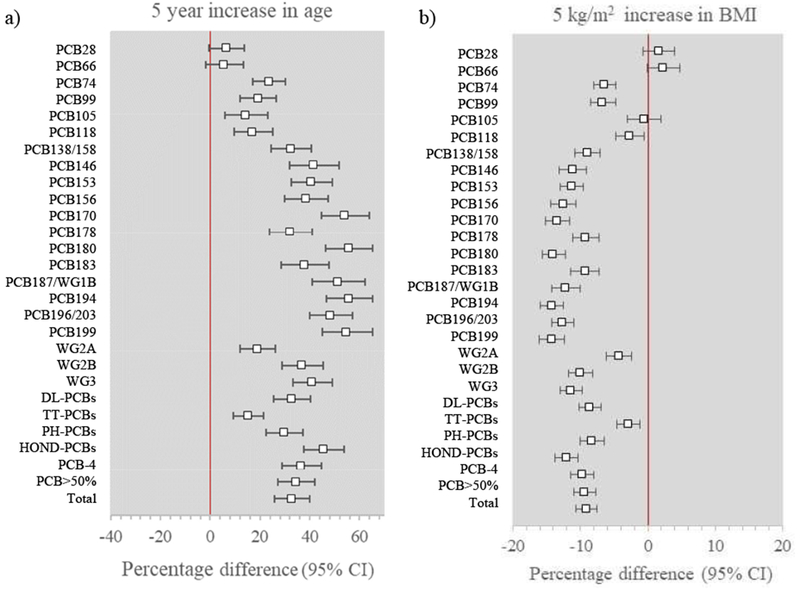
Adjusted percentage difference (95% CI) in plasma PCB concentrations by a) age (percentage difference for a 5 year increase) and b) body mass index (percentage difference for a 5 kg/m2 increase). Results are adjusted for age, marital status, education, income, smoking history, alcohol use, BMI, parity, lactation, birth order, breastfed as an infant, intakes of fish, meat, poultry, eggs, and dairy, and occupation. DL-PCBs=dioxin-like PCBs; HOND-PCBs=hepta, octa=, nona-, deca-substituted PCBs; PCB-4=PCBs 105, 118, 138/158, 180; PCB>50=PCBs with >50% detection in cohort; PH-PCBs=penta, hexa-substituted PCBs; TT-PCBs=tri, tetra-substituted PCBs; WG1B=Wolff group 1B PCBs; WG2A=Wolff group 2A PCBs; WG2B=Wolff group 2B PCBs; WG3=Wolff group 3 PCBs
BMI was strongly inversely associated with plasma PCB concentrations in a linear pattern, except for PCBs 28 and 66, where the association was weakly positive (Figure 1b). The association was stronger for more highly chlorinated PCBs: a 5-kg/m2 increase in BMI was associated with 2.9% lower tri- and tetra-substituted PCBs, 8.3% lower penta- and hexa-substituted PCBs, and 12.1% lower hepta-, octa-, nona-, and deca-substituted PCBs. Results were weakest for Wolff group 2A congeners.
Past and current cigarette smoking tended to be positively associated with plasma PCB concentrations for all congeners except for tri- and tetra-substituted PCBs, which were lower among ever smokers compared with never smokers (Figure 2a, Supplemental Table 2). Associations were strongest for current smokers who smoked ≥5 cigarettes/day (15.0% higher total plasma PCBs compared with never smokers). Associations were significantly weaker in Wolff group 2A congeners compared with other hormonally-defined groups. Heavy but not moderate alcohol use in the past year was consistently positively associated with higher plasma PCB concentrations (11.9% higher concentrations compared with non-drinkers; Figure 2b and Supplemental Table 2). Total years of residence in an urban area was also associated with small increases in plasma PCB concentrations (concentrations increased by 3.4% for a 5-year increase in total time residing in an urban area; Figure 2c).
Figure 2.

Adjusted percentage difference in plasma PCB concentrations by a) cigarette smoking (percentage difference comparing current smokers ≥5 cigarettes/day with never smokers), b) alcohol use in past year (percentage difference comparing heavy users with non-users), and c) total time residing in an urban area (percentage difference for a 5-year increase). Results are adjusted for age, marital status, education, income, smoking history, alcohol use, BMI, parity, lactation, birth order, breastfed as an infant, intakes of fish, meat, poultry, eggs, and dairy, and occupation. DL-PCBs=dioxin-like PCBs; HOND-PCBs=hepta, octa=, nona-, deca-substituted PCBs; PCB-4=PCBs 105, 118, 138/158, 180; PCB>50=PCBs with >50% detection in cohort; PH-PCBs=penta, hexa-substituted PCBs; TT-PCBs=tri, tetra-substituted PCBs; WG1B=Wolff group 1B PCBs; WG2A=Wolff group 2A PCBs; WG2B=Wolff group 2B PCBs; WG3=Wolff group 3 PCBs
Parity was not consistently or strongly associated with plasma PCB concentrations (Supplemental Table 2). However, longer lifetime duration of lactation was associated with lower plasma PCB concentrations (Figure 3a): concentrations were 11.8% lower among parous women who breastfed for ≥6 months compared with nulliparous women, whereas parous women who did not breastfeed or who breastfed for <6 months had similar concentrations to nulliparous women.
Figure 3.
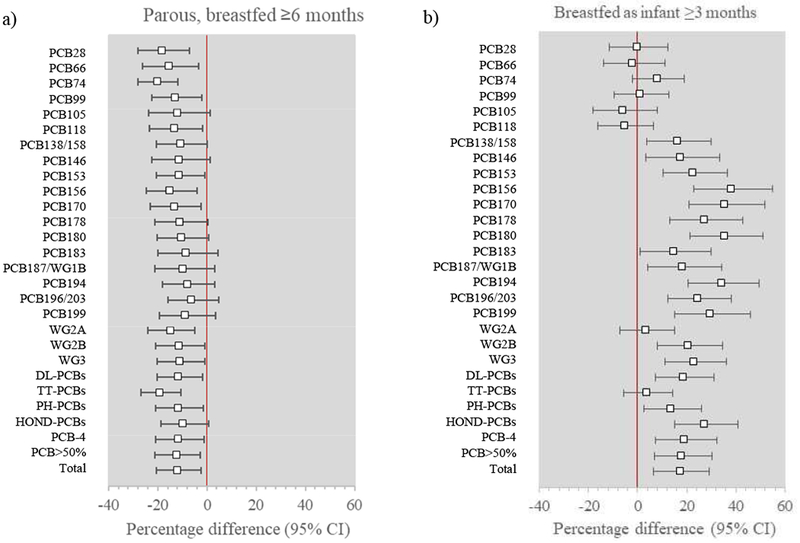
Adjusted percentage difference in plasma PCB concentrations by reproductive and parity/lactation (percentage difference comparing parous women who breastfed ≥6 months with nulliparous women) and b) participant herself breastfed as an infant (percentage difference comparing those breastfed for ≥3 months with those who were not breastfed). Results are adjusted for age, marital status, education, income, smoking history, alcohol use, BMI, parity, lactation, birth order, breastfed as an infant, intakes of fish, meat, poultry, eggs, and dairy, and occupation. DL-PCBs=dioxin-like PCBs; HOND-PCBs=hepta, octa=, nona-, deca-substituted PCBs; PCB-4=PCBs 105, 118, 138/158, 180; PCB>50=PCBs with >50% detection in cohort; PH-PCBs=penta, hexa-substituted PCBs; TT-PCBs=tri, tetra-substituted PCBs; WG1B=Wolff group 1B PCBs; WG2A=Wolff group 2A PCBs; WG2B=Wolff group 2B PCBs; WG3=Wolff group 3 PCBs
Likewise, though we found little evidence of an association between birth order and plasma PCB concentrations (Supplemental Table 2), we observed that women who were breastfed for ≥3 months in infancy had 17.3% higher plasma PCB concentrations than women who were not breastfed (Figure 3b). Women who were breastfed for <3 months had similar concentrations to those not breastfed. The association was stronger for higher-chlorinated PCBs: women breastfed for ≥3 months had 27.2% higher hepta-, octa-, nona-, and deca-substituted PCBs, 13.7% higher penta- and hexa-substituted PCBs, and 3.8% higher tri- and tetra-substituted PCBs compared with women who were not breastfed.
Other factors showed little evidence of an association with PCB concentrations (Supplemental Table 2). Currently married women had slightly lower PCB concentrations than never married women. Plasma PCB concentrations tended to be inversely but not linearly associated with education. Conversely, income was slightly positively associated with plasma PCB concentrations. Fish and poultry intakes were associated with slightly higher concentrations of total plasma PCBs, and meat intake was associated with slightly lower concentrations. We observed some evidence that medical professionals (i.e., doctors, physician’s assistants, and medical students) and occupations with exposure to x-ray machines were associated with higher plasma PCB concentrations, but work cleaning houses or buildings was not appreciably associated with plasma PCB concentrations.
When combined, the predictors explained 30.5% of the variability in total plasma PCB concentrations. Age (13.7%) and BMI (10.0%) were the two individual predictors that explained the largest proportion of the variability; all other variables explained ≤1.0% each.
Although less precise, the magnitude and direction of results were similar when restricting to the 659 women randomly sampled at baseline (data not shown).
DISCUSSION
Reproductive-aged Black women from the Detroit metropolitan area who participated in SELF had lower plasma PCB concentrations than non-Hispanic Black female NHANES participants. Several demographic, behavioral, reproductive, early life, and dietary variables predicted plasma PCB concentrations, in particular, age and BMI. All variables combined explained approximately 30% of the variability in total plasma PCBs. The magnitude of some associations varied by the degree of PCB chlorination.
Plasma PCB concentrations in this cohort of Black women were relatively low, but were still relevant for health outcomes. For example, an increased risk of diabetes (Vasiliu et al., 2006), neurotoxicity (Neugebauer et al., 2015), and low birth weight (Casas et al., 2015; Murphy et al., 2010) have been observed over a range of PCB concentrations comparable to those observed in this cohort. There are three potential explanations for the lower concentrations observed in SELF women compared with non-Hispanic Black women from NHANES (CDC, 2018). The samples in SELF were collected during 2010-2012, whereas those from NHANES women were collected during 2005-2010. Given the decline in environmental PCBs over time, human exposure is expected to decline as well. In addition, NHANES was designed to be representative of the U.S. population, whereas SELF is restricted to the Detroit metropolitan area. Differences in geography and resultant sources of exposure may partially explain the lower concentrations observed in SELF. Lastly, the age ranges in the two populations were not identical: SELF women were 23-35 years old, whereas NHANES samples were collected from women 20-39 years old. If the NHANES sample had more older women than the SELF cohort, we would expect PCB concentrations to be higher.
Our finding of an association between older age and higher plasma PCB concentrations is consistent with previous studies (Artacho-Cordon et al., 2015; Bachelet et al., 2011; Brauner et al., 2011; Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Foster et al., 2012; Gallo et al., 2011; Glynn et al., 2007; Hardell et al., 2010; Herbstman et al., 2007; Humblet et al., 2010; Ibarluzea et al., 2011; Kiviranta et al., 2005; Lewin et al., 2017; McGraw and Waller, 2009; Pavuk et al., 2014; Rylander et al., 2012; Sandanger et al., 2007; Wolff et al., 2005). This likely reflects both an age effect, through bioaccumulation of PCBs over the life course, and a cohort effect, as women born earlier were exposed to higher PCB concentrations in the environment (Laden et al., 1999). We observed stronger positive associations between age and concentrations of higher-chlorinated PCB congeners. The persistence of an individual PCB congener depends partially on its number of chlorine atoms, with higher-chlorinated PCBs exhibiting more persistence than lower chlorinated PCBs (Agency for Toxic Substances and Disease Registry, 2000). Therefore, as individuals age, bioaccumulation of higher-chlorinated PCBs may be greater than for lower-chlorinated PCBs.
Higher BMI was strongly associated with lower plasma PCB concentrations in SELF, particularly with the higher-chlorinated congeners. This finding is consistent with numerous studies that have found lower PCB concentrations among overweight and obese women compared with normal weight women (Bachelet et al., 2011; Brauner et al., 2011; Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Foster et al., 2012; Gallo et al., 2011; Glynn et al., 2007; Hardell et al., 2010; Herbstman et al., 2007; Humblet et al., 2010; Lewin et al., 2017; Pavuk et al., 2014; Sandanger et al., 2007; Wolff et al., 2005) and the observation that higher-chlorinated PCB congeners are more lipophilic than lower-chlorinated PCBs. PCBs and other lipophilic compounds tend to sequester in adipose tissue; therefore, increased adiposity signifies a larger volume of bodily tissue in which PCBs can distribute (La Merrill et al., 2013). An equilibrium exists between PCBs in blood and adipose tissue, and several studies have shown high correlations between lipid-adjusted PCB concentrations in serum and concentrations in adipose (Needham et al., 2011; Patterson et al., 1988).
Associations of PCBs with other predictors were relatively weak, compared with age and BMI. Relative to never smokers, we found that current and past smokers had higher plasma PCB concentrations, with the exception of tri- and tetra-chlorinated PCBs. This result is consistent with some (Herbstman et al., 2007; Humblet et al., 2010; Pavuk et al., 2014), but not all (Bachelet et al., 2011; Fernandez-Rodriguez et al., 2015; Gallo et al., 2011; Ibarluzea et al., 2011; Lewin et al., 2017; Rylander et al., 2012) previous studies. Organochlorine compounds like PCBs are metabolized by the cytochrome P-450 oxidase system, as are nicotine and its breakdown products. Competition or negative feedback in metabolism between the two groups of compounds may contribute to the higher plasma PCB concentrations observed among smokers, particularly heavy smokers. Lower-chlorinated PCBs are metabolized more quickly than higher-chlorinated PCBs (Kato et al., 1980; Matthews and Anderson, 1975; Mills et al., 1985), which could explain the lack of positive association between smoking and plasma PCB concentrations for lower-chlorinated congeners. A study of a PCB-contaminated area in Anniston, Alabama also reported positive associations between plasma concentrations of most PCB congeners and current smoking, but an inverse association between lower-chlorinated PCBs 28 and 66 and current smoking (Pavuk et al., 2014).
Heavy but not moderate alcohol intake was associated with higher plasma PCB concentrations, consistent with other studies (Miyashita et al., 2015; Pavuk et al., 2014). Alcohol intake may interfere with hepatic drug-metabolizing enzymes, which could slow the rate of elimination of PCBs from the body (Miyashita et al., 2015). Rich, fatty foods of animal origin, in which PCBs may bioaccumulate, are often consumed with alcohol and, although we controlled for some dietary factors, residual confounding is possible and could partially account for the observed positive association between alcohol intake and plasma PCBs (Arisawa et al., 2011).
Longer time spent residing in an urban area was associated with higher plasma PCB concentrations. This finding agrees with several studies showing positive associations between residing near industrial areas and PCB exposure (Fernandez-Rodriguez et al., 2015; Pavuk et al., 2014). Because all participants are current residents of the Detroit metropolitan area and 62% reported living in Detroit their entire lives, most of the “time spent living in an urban area” variable corresponded with time spent living in Detroit. Historically, Detroit is an industrial city, which could explain the correlation of time spent in an urban area with PCB concentrations; these findings may not be generalizable to other cities.
Pregnancy and lactation are major routes of elimination of PCBs (Barr et al., 2005). However, we found that lactation was more important than parity in predicting PCB concentrations. The inverse association between breastfeeding duration and plasma PCB concentrations is relatively well-established (Bachelet et al., 2011; Brauner et al., 2011; Hardell et al., 2010; Humblet et al., 2010; Ibarluzea et al., 2011; Kiviranta et al., 2005; Pavuk et al., 2014; Rylander et al., 2012), but ours is one of only a few studies to report that having been breastfed in infancy is associated with higher plasma PCB concentrations in adulthood (Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Gallo et al., 2011; Glynn et al., 2007). Conversely, we did not observe strong associations between parity or birth order and plasma PCB concentrations, with the exception of a small inverse association between parity and tri- and tetra-chlorinated PCBs. This finding, which is unexpected and inconsistent with previous literature (Caspersen et al., 2016; Fernandez-Rodriguez et al., 2015; Hardell et al., 2010; Herbstman et al., 2007; Ibarluzea et al., 2011; Lewin et al., 2017; Pavuk et al., 2014), may be due to chance or may relate to the fact that some previous studies do not control for lactation.
Fatty fish intake is widely recognized as a source of PCB exposure in the general population, as PCBs are present in the environment and bioaccumulate (Bachelet et al., 2011; Brauner et al., 2011; Fernandez-Rodriguez et al., 2015; Gallo et al., 2011; Glynn et al., 2007; Humblet et al., 2010; Ibarluzea et al., 2011; Kiviranta et al., 2005; McGraw and Waller, 2009; Pavuk et al., 2014; Rylander et al., 2012; Wolff et al., 2005). We observed weak positive associations between total fish intake and plasma PCB concentrations. We did not collect information on type of fish (i.e., fatty or not) or the source of fish (i.e., from potentially contaminated waters or not), which could have contributed to measurement error and attenuated our results. We hypothesized that intake of other foods containing animal fat would be associated with higher plasma PCB concentrations; however, we only observed this pattern for poultry intake, not meat, egg, or dairy intake. As with fish intake, we did not collect information on the source of the individual foods (i.e., whether they originated in regions with higher PCB contamination).
Our analytic sample was selected from the full SELF cohort using a case-cohort design (to study risk of uterine leiomyomata). We measured PCBs in the plasma of a random sample of participants at baseline (i.e., the subcohort) as well as all incident cases of uterine leiomyomata (some of whom were part of the subcohort). If PCBs and the predictors of interest are associated with uterine leiomyomata risk, we may have introduced selection bias into our analysis. However, we conducted a sensitivity analysis restricted to the 659 women who we randomly sampled at baseline and found similar results.
Grouping individual congeners together may attenuate findings if associations are driven by one particular PCB rather than the group. For example, the inverse association between PCBs 28 and 66 with current cigarette smoking are masked when these PCBs are grouped with other lower-chlorinated congeners. However, grouping congeners has the advantage of utilizing knowledge about the structure and hormonal activity of PCBs to provide some insight into biological mechanisms of observed correlations and potential connections to health outcomes. For example, the identification of a stronger association between PCBs and BMI with increasing chlorination may indicate increased storage of PCBs in adipose tissue or slower metabolism, given that higher-chlorinated PCBs are more lipophilic.
We conducted a cross-sectional analysis using data collected at baseline only. Because current plasma PCB concentrations reflect exposures throughout the life course, some of the variables in SELF (e.g., fish intake over the last 12 months) may not have been measured in the ideal time window, generally resulting in some attenuation of our findings. In addition, there may have been exposure misclassification due to long periods of recall, particularly for the early-life variables (e.g., breastfed in infancy). We did not have information on how far participants live from Superfund or other toxic waste sites, which are potentially important sources of exposure. We presented results adjusted for a wide range of demographic, lifestyle, and medical covariates for the purpose of hypothesis generation rather than estimating the effects of each covariate on PCB exposure. Some associations may be adjusted for covariates that plausibly lie on a causal pathway to exposure; others may be affected by residual or unmeasured confounding (Westreich and Greenland, 2013). Lastly, the SELF cohort was restricted to women age 23-35 years from the Detroit metropolitan area, and results may not be generalizable to women of other ages and geographic regions.
Major strengths of our study include our focus on Black women, a racial group traditionally understudied in environmental health research (Burchard et al., 2015; Oh et al., 2015). We collected data on a wide range of potentially predictive variables. We used multivariable regression to control for confounding by other predictors, although given the cross-sectional nature of our study, the temporality of the predictors in reference to each other is unclear, so we may have over-adjusted for causal intermediates. In addition, we used state-of-the-art methods with rigorous quality control to measure PCB concentrations in plasma (Sjodin et al., 2004).
CONCLUSIONS
Our observation that PCB concentrations were positively associated with age, duration of urban residence, cigarette smoking, heavy alcohol intake, and having been breastfed in infancy, and inversely associated with BMI and lactation duration are generally consistent with the literature. This study expands on previous work by filling the data gap for Black women and examining predictors of both individual and groups of PCBs which revealed patterns of correlations by extent of chlorination.
Supplementary Material
Acknowledgements:
This research was funded primarily by the extramural program of the National Institute of Environmental Health Sciences (R01-ES024749). In addition, the research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences and in part by funds allocated for health research by the American Recovery and Reinvestment Act. The funding sources had no role in study design; collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Abbreviations:
- BMI
body mass index
- CDC
Center for Disease Control and Prevention
- LOD
limit of detection
- NHANES
National Health and Nutrition Examination Survey
- PCB
polychlorinated biphenyls
- SELF
Study of the Environment, Lifestyle, and Fibroids
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Declaration of interests: none.
REFERENCES
- Agency for Toxic Substances and Disease Registry, 2000. Toxicological profile for Polychlorinated Biphenyls (PCBs). U.S. Department of Health and Human Services, Atlanta, GA. [PubMed] [Google Scholar]
- Aminov Z, Haase R, Olson JR, Pavuk M, Carpenter DO, Anniston Environmental Health Research, C., 2014. Racial differences in levels of serum lipids and effects of exposure to persistent organic pollutants on lipid levels in residents of Anniston, Alabama. Environ Int 73, 216–223. [DOI] [PubMed] [Google Scholar]
- Anderson GD, 2005. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 14, 19–29. [DOI] [PubMed] [Google Scholar]
- Arisawa K, Uemura H, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Nishioka Y, Hasegawa M, Tanto M, Satoh H, Shima M, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, 2011. Dietary patterns and blood levels of PCDDs, PCDFs, and dioxinlike PCBs in 1656 Japanese individuals. Chemosphere 82, 656–662. [DOI] [PubMed] [Google Scholar]
- Artacho-Cordon F, Belhassen H, Arrebola JP, Ghali R, Amira D, Jimenez-Diaz I, Perez-Lobato R, H B, A H, Olea N, 2015. Serum levels of persistent organic pollutants and predictors of exposure in Tunisian women. Sci Total Environ 511, 530–534. [DOI] [PubMed] [Google Scholar]
- Bachelet D, Truong T, Verner MA, Arveux P, Kerbrat P, Charlier C, Guihenneuc-Jouyaux C, Guenel P, 2011. Determinants of serum concentrations of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls among French women in the CECILE study. Environ Res 111, 861–870. [DOI] [PubMed] [Google Scholar]
- Baird DD, Harmon QE, Upson K, Moore KR, Barker-Cummings C, Baker S, Cooper T, Wegienka G, 2015. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J Womens Health (Larchmt) 24, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wang RY, Needham LL, 2005. Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children’s Study. Environ Health Perspect 113, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Turner WE, Patterson DG Jr., Needham LL, 2007. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 68, 824–831. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L, 1986. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124, 453–469. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Factor-Litvak P, Wolff MS, Susser E, Matte TD, 2004. Effect of socioeconomic status on exposures to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE) among pregnant African-American women. Arch Environ Health 59, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner EV, Raaschou-Nielsen O, Gaudreau E, LeBlanc A, Tjonneland A, Overvad K, Sorensen M, 2011. Predictors of polychlorinated biphenyl concentrations in adipose tissue in a general Danish population. Environ Sci Technol 45, 679–685. [DOI] [PubMed] [Google Scholar]
- Burchard EG, Oh SS, Foreman MG, Celedon JC, 2015. Moving toward true inclusion of racial/ethnic minorities in federally funded studies. A key step for achieving respiratory health equality in the United States. Am J Respir Crit Care Med 191, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Nieuwenhuijsen M, Martinez D, Ballester F, Basagana X, Basterrechea M, Chatzi L, Chevrier C, Eggesbo M, Fernandez MF, Govarts E, Guxens M, Grimalt JO, Hertz-Picciotto I, Iszatt N, Kasper-Sonnenberg M, Kiviranta H, Kogevinas M,Palkovicova L, Ranft U, Schoeters G, Patelarou E, Petersen MS, Torrent M, Trnovec T, Valvi D, Toft GV, Weihe P, Weisglas-Kuperus N, Wilhelm M, Wittsiepe J, Vrijheid M, Bonde JP, 2015. Prenatal exposure to PCB-153, p,p’-DDE and birth outcomes in 9000 mother-child pairs: exposure-response relationship and effect modifiers. Environ Int 74, 23–31. [DOI] [PubMed] [Google Scholar]
- Caspersen IH, Kvalem HE, Haugen M, Brantsaeter AL, Meltzer HM, Alexander J, Thomsen C, Froshaug M, Bremnes NM, Broadwell SL, Granum B, Kogevinas M, Knutsen HK, 2016. Determinants of plasma PCB, brominated flame retardants, and organochlorine pesticides in pregnant women and 3 year old children in The Norwegian Mother and Child Cohort Study. Environ Res 146, 136–144. [DOI] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL, 2008. Multi-rule quality control for the age-related eye disease study. Stat Med 27, 4094–4106. [DOI] [PubMed] [Google Scholar]
- CDC, U.S., 2018. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention, 2016. Laboratory Procedure Manual: Polybrominated diphenyl ethers (PBDEs), Polybrominated biphenyls (PBBs), Polychlorinated biphenyls (PCBs) and Persistent Pesticides (PPs). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Fernandez-Rodriguez M, Arrebola JP, Artacho-Cordon F, Amaya E, Aragones N, Llorca J, Perez-Gomez B, Ardanaz E, Kogevinas M, Castano-Vinyals G, Pollan M, Olea N, 2015. Levels and predictors of persistent organic pollutants in an adult population from four Spanish regions. Sci Total Environ 538, 152–161. [DOI] [PubMed] [Google Scholar]
- Foster WG, Cheung AP, Davis K, Graves G, Jarrell J, Leblanc A, Liang CL, Leech T, Walker M, Weber JP, Van Oostdam J, 2012. Circulating metals and persistent organic pollutant concentrations in Canadian and non-Canadian born primiparous women from five Canadian centres: results of a pilot biomonitoring study. Sci Total Environ 435-436, 326–336. [DOI] [PubMed] [Google Scholar]
- Fukami T, Nakajima M, Higashi E, Yamanaka H, McLeod HL, Yokoi T, 2005. A novel CYP2A6*20 allele found in African-American population produces a truncated protein lacking enzymatic activity. Biochem Pharmacol 70, 801–808. [DOI] [PubMed] [Google Scholar]
- Gallo MV, Schell LM, DeCaprio AP, Jacobs A, 2011. Levels of persistent organic pollutant and their predictors among young adults. Chemosphere 83, 1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull M, Castell C, Pallares N, Miret C, Pumarega J, Te Llez-Plaza M, Lopez T, Salas-Salvado J, Lee DH, Goday A, Porta M, 2018. Blood Concentrations of Persistent Organic Pollutants and Unhealthy Metabolic Phenotypes in Normal-Weight, Overweight, and Obese Individuals. Am J Epidemiol 187, 494–506. [DOI] [PubMed] [Google Scholar]
- Glynn A, Aune M, Darnerud PO, Cnattingius S, Bjerselius R, Becker W, Lignell S, 2007. Determinants of serum concentrations of organochlorine compounds in Swedish pregnant women: a cross-sectional study. Environ Health 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardell E, Carlberg M, Nordstrom M, van Bavel B, 2010. Time trends of persistent organic pollutants in Sweden during 1993-2007 and relation to age, gender, body mass index, breastfeeding and parity. Sci Total Environ 408, 4412–4419. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR, 2007. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect 115, 1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed L, 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5, 46–51. [Google Scholar]
- Humblet O, Williams PL, Korrick SA, Sergeyev O, Emond C, Birnbaum LS, Burns JS, Altshul L, Patterson DG, Turner WE, Lee MM, Revich B, Hauser R, 2010. Predictors of serum dioxin, furan, and PCB concentrations among women from Chapaevsk, Russia. Environ Sci Technol 44, 5633–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarluzea J, Alvarez-Pedrerol M, Guxens M, Marina LS, Basterrechea M, Lertxundi A, Etxeandia A, Goni F, Vioque J, Ballester F, Sunyer J, 2011. Sociodemographic, reproductive and dietary predictors of organochlorine compounds levels in pregnant women in Spain. Chemosphere 82, 114–120. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2016. Polychlorinated Biphenyls and Polybrominated Biphenyls. IARC Monogr Eval Carcinog Risks Hum 107, 9–500. [PMC free article] [PubMed] [Google Scholar]
- James RA, Hertz-Picciotto I, Willman E, Keller JA, Charles MJ, 2002. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the child health and development study cohort, 1963-1967. Environ Health Perspect 110, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Tani T, Yoshida A, 1980. Effect of dietary level of protein on liver microsomal drug-metabolizing enzymes, urinary ascorbic acid and lipid metabolism in rats fed PCB-containing diets. J Nutr 110, 1686–1694. [DOI] [PubMed] [Google Scholar]
- Kiviranta H, Tuomisto JT, Tuomisto J, Tukiainen E, Vartiainen T, 2005. Polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in the general population in Finland. Chemosphere 60, 854–869. [DOI] [PubMed] [Google Scholar]
- Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N, 1994. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst 86, 589–599. [DOI] [PubMed] [Google Scholar]
- La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clement K, Birnbaum LS, Barouki R, 2013. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect 121, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, Wolff MS, Hunter DJ, 1999. Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environ Health Perspect 107, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Hays SM, Aylward LL, Naiman DQ, 2009. Perspective on serum dioxin levels in the United States: an evaluation of the NHANES data. J Expo Sci Environ Epidemiol 19, 435–441. [DOI] [PubMed] [Google Scholar]
- Lewin A, Arbuckle TE, Fisher M, Liang CL, Marro L, Davis K, Abdelouahab N, Fraser WD, 2017. Univariate predictors of maternal concentrations of environmental chemicals: The MIREC study. Int J Hyg Environ Health 220, 77–85. [DOI] [PubMed] [Google Scholar]
- Lordo RA, Dinh KT, Schwemberger JG, 1996. Semivolatile organic compounds in adipose tissue: estimated averages for the US population and selected subpopulations. Am J Public Health 86, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HB, Anderson MW, 1975. The distribution and excretion of 2,4,5,2’,5’-pentachlorobiphenyl in the rat. Drug Metab Dispos 3, 211–219. [PubMed] [Google Scholar]
- McGraw JE, Waller DP, 2009. Fish ingestion and congener specific polychlorinated biphenyl and p,p’-dichlorodiphenyldichloroethylene serum concentrations in a great lakes cohort of pregnant African American women. Environ Int 35, 557–565. [DOI] [PubMed] [Google Scholar]
- Mills RA, Millis CD, Dannan GA, Guengerich FP, Aust SD, 1985. Studies on the structure-activity relationships for the metabolism of polybrominated biphenyls by rat liver microsomes. Toxicol Appl Pharmacol 78, 96–104. [DOI] [PubMed] [Google Scholar]
- Miyashita C, Sasaki S, Saijo Y, Okada E, Kobayashi S, Baba T, Kajiwara J, Todaka T, Iwasaki Y, Nakazawa H, Hachiya N, Yasutake A, Murata K, Kishi R, 2015. Demographic, behavioral, dietary, and socioeconomic characteristics related to persistent organic pollutants and mercury levels in pregnant women in Japan. Chemosphere 133, 13–21. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Franken FH, Jaszyna-Gasior M, 2006. Adolescent nicotine metabolism: ethnoracial differences among dependent smokers. Ethn Dis 16, 239–243. [PubMed] [Google Scholar]
- Murphy LE, Gollenberg AL, Buck Louis GM, Kostyniak PJ, Sundaram R, 2010. Maternal serum preconception polychlorinated biphenyl concentrations and infant birth weight. Environ Health Perspect 118, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkat M, 2007. Interethnic differences in drug response: the contribution of genetic variability in beta adrenergic receptor and cytochrome P4502C9. Clin Pharmacol Ther 82, 215–218. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Nielsen F, Patterson DG Jr., Sjodin A, Turner WE, Weihe P, 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol 45, 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schoneck N, Scholmerich A, Wilhelm M, 2015. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health 218, 153–162. [DOI] [PubMed] [Google Scholar]
- Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, de Bruin DM, Greenblatt RM, Bibbins-Domingo K, Wu AH, Borrell LN, Gunter C, Powe NR, Burchard EG, 2015. Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Med 12, e1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DG Jr., Needham LL, Pirkle JL, Roberts DW, Bagby J, Garrett WA, Andrews JS Jr., Falk H, Bernert JT, Sampson EJ, et al. , 1988. Correlation between serum and adipose tissue levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin in 50 persons from Missouri. Arch Environ Contam Toxicol 17, 139–143. [DOI] [PubMed] [Google Scholar]
- Patterson DG Jr., Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC,Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL, 2009. Levels in the U.S. population of those persistent organic pollutants (2003-2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol 43, 1211–1218. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Olson JR, Wattigney WA, Dutton ND, Sjodin A, Shelton C, Turner WE, Bartell SM, 2014. Predictors of serum polychlorinated biphenyl concentrations in Anniston residents. Sci Total Environ 496, 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz D, Becerra M, Jagai JS, Ard K, Sargis RM, 2018. Disparities in Environmental Exposures to Endocrine-Disrupting Chemicals and Diabetes Risk in Vulnerable Populations. Diabetes Care 41, 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander C, Lund E, Froyland L, Sandanger TM, 2012. Predictors of PCP, OH-PCBs, PCBs and chlorinated pesticides in a general female Norwegian population. Environ Int 43, 13–20. [DOI] [PubMed] [Google Scholar]
- Sandanger TM, Sinotte M, Dumas P, Marchand M, Sandau CD, Pereg D, Berube S, Brisson J, Ayotte P, 2007. Plasma concentrations of selected organobromine compounds and polychlorinated biphenyls in postmenopausal women of Quebec, Canada. Environ Health perspectives 115, 1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE 3rd, Patterson DG Jr., 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem 76, 1921–1927. [DOI] [PubMed] [Google Scholar]
- Solus JF, Arietta BJ, Harris JR, Sexton DP, Steward JQ, McMunn C, Ihrie P, Mehall JM, Edwards TL, Dawson EP, 2004. Genetic variation in eleven phase I drug metabolism genes in an ethnically diverse population. Pharmacogenomics 5, 895–931. [DOI] [PubMed] [Google Scholar]
- Taylor D, 2014. Toxic Communities: Environmental Racism, Industrial Pollution, and Residential Mobility. New York University Press, Ney York. [Google Scholar]
- Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W, 2006. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology 17, 352–359. [DOI] [PubMed] [Google Scholar]
- Wang RY, Jain RB, Wolkin AF, Rubin CH, Needham LL, 2009. Serum concentrations of selected persistent organic pollutants in a sample of pregnant females and changes in their concentrations during gestation. Environ Health Perspect 117, 1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub M, Birnbaum LS, 2008. Catfish consumption as a contributor to elevated PCB levels in a non-Hispanic black subpopulation. Environ Res 107, 412–417. [DOI] [PubMed] [Google Scholar]
- Westreich D, Greenland S, 2013. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 177, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Camann D, Gammon M, Stellman SD, 1997. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect 105, 13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Deych E, Ojo F, Berkowitz GS, 2005. Predictors of organochlorines in New York City pregnant women, 1998-2001. Environ Res 97, 170–177. [DOI] [PubMed] [Google Scholar]
- Xue J, Liu SV, Zartarian VG, Geller AM, Schultz BD, 2014. Analysis of NHANES measured blood PCBs in the general US population and application of SHEDS model to identify key exposure factors. J Expo Sci Environ Epidemiol 24, 615–621. [DOI] [PubMed] [Google Scholar]
- Zhou XH, Eckert GJ, Tierney WM, 2001. Multiple imputation in public health research. Stat Med 20, 1541–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


